Abstract
India is one of the endemic areas where control of malaria has become a formidable task. Artesunate is the current antimalarial drug used to treat malaria, especially chloroquine resistant. The objective of the present study was to investigate the dose-dependent effect of oral administration of artesunate on the oxidative parameters in testes of adult male Swiss albino mice and ameliorative efficacy of curcumin, a widely used antioxidant. An oral dose of 150 mg/kg body weight (bwt; low dose) and 300 mg/kg bwt (high dose) of artesunate was administered for a period of 45 days to male mice, and ameliorative efficacy of curcumin was also assessed. The results revealed that artesunate caused significant alteration in oxidative parameters in dose-dependent manner. Administration of artesunate brought about significant decrease in activities of superoxide dismutase, glutathione, glutathione peroxidase, and glutathione reductase, whereas lipid peroxidation and glutathione-S-transferase activity were found to be significantly increased. The results obtained show that oxidative insult is incurred upon the intracellular antioxidant system of testis tissue by artesunate treatment. Further, administration of curcumin at the dose level of 80 mg/kg bwt along with both doses of artesunate attenuated adverse effects in male mice.
Keywords: antimalarial drug, artesunate, curcumin, antioxidant, testis
Introduction
Malaria is a leading cause of mortality and morbidity in developing areas of the world (Breman et al. 2004). It is endemic in at least 87 countries, placing approximately 2.5 billion people at risk (Hay et al. 2004; Guerra et al. 2008). The consequences of severe malaria include coma and death if untreated, young children and pregnant women being most vulnerable. The use of antimalarial drugs as prophylaxis has generated drug resistance, which is of great concern. The development of resistance to chloroquine phosphate and other agents further necessitated the development of new antimalarial compounds for use as monotherapy or in combination with existing methods of treatment. Artemisinin is currently the most widely used antimalarial drug against drug-resistant malaria. It is a Chinese herb that has been used over thousands of years for the treatment of fever. Artemisinin, also known as quinghaosu, is a sesquiterpene lactone extracted from the leaves of Artemisia annua (sweet wormwood).
Artesunate, a water-soluble semisynthetic hemisuccinate derivative of artemisinin, is a drug used to treat malaria, especially chloroquine-resistant malaria. It is synthesized by reacting dihydroartemisinin and succinic acid anhydride in an alkaline medium. Artesunate and its active metabolite dihydroartemisinin are potent blood schizonticides, highly effective against multidrug-resistant strains of Plasmodium falciparum. Hence, it is increasingly used widely for the treatment and management of malaria. A combination of mefloquine and artesunate is highly effective in multidrug-resistant malaria (van Agtmael et al. 1999). The effectiveness of artesunate is due to its rapid and complete hydrolysis to form dihydroartemisinin that is 3- to 5-folds more active than the parent compound (Li et al. 2002). Recent observations indicate that oxidative stress also plays a role in the activity of artemisinins against cancer cells (Efferth et al. 2004). Furthermore, artesunate is able to induce oxidative DNA damage in mammalian cells (Berdelle et al. 2011), and the DNA damage induced by artesunate also contributes to its therapeutic effect against cancer cells (Paul et al. 2008). Further, artesunate is capable of ameliorating airway inflammation and oxidative lung damage via the suppression of pro-oxidant production and restoration of antioxidant expression and their activities in the lung (Ho et al. 2012).
However, it has been stated that the use of these drugs should be controlled and restricted only to proven multidrug resistance or severe malaria in order to preserve their efficacy (Mulenga 1998). The possibility of administration of overdose and misappropriation in the usage of antimalarials is very common, as drugs though useful in the treatment of disease condition could also produce adverse side effects in the individual. These untoward effects may be harmful to the patient. Following increased resistance of malarial parasites to conventional drugs in the malarial regions of the world, the World Health Organization has been promoting artemisinin-based combination therapy for treating uncomplicated malaria (Ogbonna and Uneke 2008; Lin et al. 2010). Recent studies show that coadministration of artesunate and amodiaquine altered lipid profile parameters and liver function markers but spared the kidney. The drugs also increased the risk of erythrocyte oxidative damage as evidenced by the elevated concentration of malonyldialdehyde (MDA), an oxidative stress marker. Hence, artesunate causes adverse effects when the drug is misused, and therefore it should be taken with prescription only when there is a clinical evidence of malarial parasite infection (Abolaji et al. 2014).
Many antimalarial drugs have been associated with male reproductive dysfunction. Chloroquine has been reported to reduce sperm motility and hence fertility by a reduction in the average number of fetuses of cohabited female rats (Adeeko and Dada 1998). Artesunate has also been shown to cause a decrease in sperm motility in guinea pigs (Obianime and Aprioku 2009). Sertoli cells play a key role in spermatogenesis (Sharpe 1993), and Leydig cells are the main source of androgen production (Al-Hazmi et al. 2005). Both types of cells can be readily affected by toxicants and chemical drugs (Papadakis et al. 1999). This study was therefore undertaken to further examine the effects of artesunate on the oxidative parameters of the testis in adult male Swiss albino mice.
Recent studies suggest that artesunate could cause some histometrical changes in the rat testes by the sloughing of the germinal epithelium from the basement membrane and reduction in the population of the germ cells. These changes were apparently dose dependent (Jewo et al. 2008; Izunya et al. 2010).
Further, certain reports have shown that long-term administration of artesunate could induce reversible infertility in rats, which may act via distortion of blood–testis barrier formed by Sertoli cells (Olumide and Raji 2011).
Histological studies carried out in our laboratory also revealed that artesunate caused significant alterations in testes of Swiss albino mice in a dose-dependent manner. The histological findings after hematoxylin and eosin staining indicated that the treated section of the testis showed some varying degree of cell clustering, cellular hypertrophy, and intercellular vacuolations, specifically in the germinal cell layer, resulting in a decline in sperm production (Rajput et al. 2012).
The global use of this antimalarial drug thus made its detailed investigation on male fertility imperative. Thus, there is a need to determine whether these observations may be applicable to humans, and in this regard, one can suggest that artesunate at normal dose could be a potential male antiferftility agent. We therefore recommend that further studies be carried out in humans to corroborate these findings and that self-medication involving artesunate should be discouraged.
Curcumin, an active component of turmeric (Curcuma longa), exhibits antioxidant property. It is a yellow-colored phenolic pigment obtained from the rhizome of turmeric (family Zingiberaceae). Curcumin is considered to be an effective antioxidant against oxidative tissue damage. It has been considered to be mediated via its beneficial effects on the antioxidant defense system, the scavenging of free radicals and/or via preventing lipid peroxidation (LPO; Kandemir et al. 2011). Recent study revealed that antioxidant supplements such as curcumin can inhibit the harmful effects induced by nicotine on the hormones and quality and quantity of reproductive indices (Jalili et al. 2014). It can significantly inhibit the generation of reactive oxygen species (ROS) both in vitro and in vivo. (Joe and Lokesh 1994). Administration of curcumin to cadmium-treated rats prevents the cadmium-induced spermatogenic damage, decreased sperm count, reduced testosterone level, and generation of free radicals by inducing antioxidant defense mechanism (Salama and El-Bahr 2007). Curcumin was shown to be a potent scavenger of a variety of reactive oxygen species including hydroxyl radicals, nitrogen dioxide radicals, and superoxide radicals (Payton et al. 2007; Goel et al. 2008). Curcumin has also been shown to alleviate various forms of male reproductive disorders in experimental animals, thereby enhancing fertility (Ilbey et al. 2009; Noorafshan et al. 2011; Khorsandi et al. 2013). Additionally, as curcumin is used as a spice in human diet and is easily obtained in many shops, it is almost orally given by human and is used as an antioxidative agent. Therefore, in the present study, curcumin was selected as an antidote against artesunate-induced oxidative damage in testis of adult male Swiss albino mice.
Materials and Methods
Animals
Healthy adult male albino mice, Mus musculus of Swiss strain weighing between 30 and 35 gm, were obtained from Cadila Pharaceuticals, Dholka, Gujarat, India. All the animals were acclimatized 7 days prior to the commencement of treatment and were maintained under controlled condition with 12-hour light and 12-hour dark cycles at temperature of 26°C ± 2°C and relative humidity of 30% to 70%. They were divided into 7 groups (A, B, C, D, E, and F) of 5 mice each. Group B mice were given 150 mg/kg body weight (bwt) of artesunate daily for 45 days, group C mice were given 300 mg/kg bwt of artesunate daily for 45 days, and group D mice were given 80 mg/kg bwt of curcumin for 45 days. Groups E and F mice were given 150 mg/kg bwt of artesunate + 80 mg/kg bwt of curcumin daily for 45 days and 300 mg/kg bwt of artesunate + 80 mg/kg bwt of curcumin daily for 45 days, respectively. Group A served as the control and were given only distilled water. The animals were fed on a commercial pellet supplied by Amrut mice feed (Pranav Agro Industries, Vadodara, Gujarat, India) and water ad libitum. Experiments were conducted in accordance with the guidelines set by the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), India, and experimental protocols were approved by the institutional animals’ ethics committee (167/1999/CPCSEA).
Experimental Design
Artesunate was prepared in double-distilled water and orally given to mice via feeding canula with a hypodermic syringe.
Rationale for Selection of Doses
In the present study, the dose of 150 mg/kg bwt (low dose) and 300 mg/kg bwt (high dose) were selected based on literature report of Angus et al. (2002). All the doses for artesunate were derived from its equivalent to the therapeutic dose of artesunate for the treatment of uncomplicated malaria (Angus et al. 2002). The dose for amelioration by curcumin (80 mg/kg bwt) was selected based on earlier reports by Ilbey et al. (2009), Noorafshan et al. (2011), and Khorsandi et al. (2013).
Experimental Protocol
| Group | Group Name | Dosage | Duration |
|---|---|---|---|
| A | Control | – | 45 days |
| B | Low dose treated | 150 mg/kg bwt of artesunate | 45 days |
| C | High dose treated | 300 mg/kg bwt of artesunate | 45 days |
| D | Curcumin | 80 mg/kg bwt of curcumin | 45 days |
| E | Low dose + curcumin | 150 mg/kg bwt of artesunate + 80 mg/kg bwt of curcumin | 45 days |
| F | High dose + curcumin | 300 mg/kg bwt of artesunate + 80 mg/kg bwt of curcumin | 45 days |
Vehicle: distilled water. Number of animals in each group (n) = 6.
Artesunate was administered per the experimental protocol. At the end of each treatment, animals were euthanized, and testis was carefully dissected out and weighed. Tissue was then processed for biochemical evaluation.
Hypothesis
Null hypothesis: Curcumin shows no ameliorative effects on artesunate-induced subchronic toxicity in testis of Swiss albino male mice.
Alternative hypothesis: Curcumin shows ameliorative effects on artesunate-induced subchronic toxicity in testis of Swiss albino male mice.
Lipid Peroxidation Thiobarbutiric Acid Reactive Species
The thiobarbutiric acid reactive species (TBARS) levels in testis of control and all treated animals were determined by the method of Ohkawa et al. (1979). The method is based on the formation of a red chromophore that absorbs at 532 nm following the reaction of thiobarbituric acid with MDA and other breakdown products of peroxidized lipids collectively called as TBARS.
Superoxide Dismutase
The activity of superoxide dismutase (SOD) in testis of control and all treated animals was assayed by the modified spectrophotometric method of Kakkar et al. (1984). In this method, the formazan formed at the end of the reaction indicates the presence of the enzyme. One unit of enzyme activity is defined as the enzyme concentration required to inhibit 50% of the optical density of chromogen formed in 1 minute at 560 nm under the assay condition.
Glutathione
The concentration of glutathione (GSH) in testis of control and all treated groups of mice was assayed by the method of Ellman (1959). Glutathione present in the tissue oxidizes 5,5′-dithiobis-(2-nitrobenzoic acid) to form yellow-colored complex that can be read at 412 nm. The absorbance is proportional to the amount of GSH.
Glutathione Peroxidase
Activity of glutathione peroxidase (G-Px) was estimated in testis of control and treated animals by the method of Rotruck et al. (1973). Glutathione peroxidase acts on hydrogen peroxide (H2O2) and splits it into 2 water molecules. While doing so it consumes 2 hydrogen atoms from 2 molecules of GSH. As a by-product, 1 molecule of reduced glutathione (GS-SG) is obtained. Thus, the amount of GSH consumed per 10 minutes is related to activity of G-Px.
Glutathione Reductase
The estimation of glutathione reductase (G-Rx) was done by the method of Carlberg and Mannervik (1985). Glutathione reductase can be measured by measuring the rate of nicotinamide adenine dinucleotide phosphate (NADPH) oxidation. The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm. Since G-Rx is present at rate-limiting concentrations, the rate of decrease is directly proportional to the G-Rx activity in the sample.
Glutathione-S-Transferase
Glutathione-S-transferase (GST) activity was measured in testis of control and treated group animals by modified method of Habig et al. (1974). Glutathione-S-transferase catalyze the conjugation of GS-SG—via a sulfhydryl group—to electrophilic centers on a wide variety of substrates. Hence, the quantification of GSH–CDNB (1-Chloro-2,4-dinitrobenzene) conjugate formed by reaction of CDNB and GSH in presence of enzyme source may be used to measure the activity of GST.
Statistical Analysis
All the data are presented as mean ± standard error. Statistical analysis was performed using the trial version of SPSS software package version 16.0. Comparison between the groups was made by 1-way analysis of variance (ANOVA) taking significance at P < .05 followed by Student t test taking significance at ***P < .001, **P < .005, and *P < .01. Tukey honestly significance difference post hoc test was used for comparison among different treatment groups (P < .05).
Results
Biochemical Analysis
Oral administration of low dose of artesunate for 45 days produced a significant increase (P < .005) in LPO in testis (group B) when compared to the control group (Table 1). At higher dose, artesunate exhibited significant increase (P < .001) in LPO in testis of 45-day treated group (group C). On the other hand, supplementation of curcumin (80 mg/kg bwt) did not show significant LPO after 45 days (group D). Further, curcumin when supplemented along with artesunate low-dose and high-dose treatment showed nonsignificant decrease in LPO after 45 days of treatment (groups E and F; Table 1, Figure 1).
Table 1.
Alteration in LPO (TBARS) and SOD Content in Testis of Control and Treated Animals.
| Groups | LPO (nanomoles of MDA/mg tw/ 60 min) | SOD (units of SOD/mg protein) | |||
|---|---|---|---|---|---|
| Control (A) | 62.5 ± 1.62 | 0.35 ± 0.02 | |||
| Artesunate (150 mg/kg bwt) (B) | 73.3 ± 2.85b | 0.28 ± 0.03b | |||
| Artesunate (300 mg/kg bwt) (C) | 76.6 ± 2.38c | 0.25 ± 0.03c | |||
| Curcumin (80 mg/kg bwt) (D) | 61.1 ± 2.50 NS | 0.37 ± 0.01 NS | |||
| Artesunate (150 mg/kg bwt) + Curcumin (80 mg/kg bwt) (E) | 62.9 ± 3.10 NS | 0.33 ± 0.03 NS | |||
| Artesunate (300 mg/kg bwt) + Curcumin (80 mg/kg bwt) (F) | 67.1 ± 2.47 NS | 0.30 ± 0.02 NS | |||
| Two-way ANOVA | |||||
| Source of Variation | SS | df | MS | F Value | P Value |
| Between the groups (dosage) | 0.2129 | 3 | 0.07097 | 6.041 | .0005 |
| Within the groups (duration) | 4.887 | 416 | 0.01175 | ||
Abbreviations: ANOVA, analysis of variance; bwt, body weight; LPO-TBARS, lipid peroxidation thiobarbutiric acid reactive species; MDA, malonyldialdehyde; SE, standard error; SOD, superoxide dismutase; NS, nonsignificant analysis of variance at P < .05 significance level.
aValues are mean ± SE.
b P < .005.
c P < .001.
Figure 1.
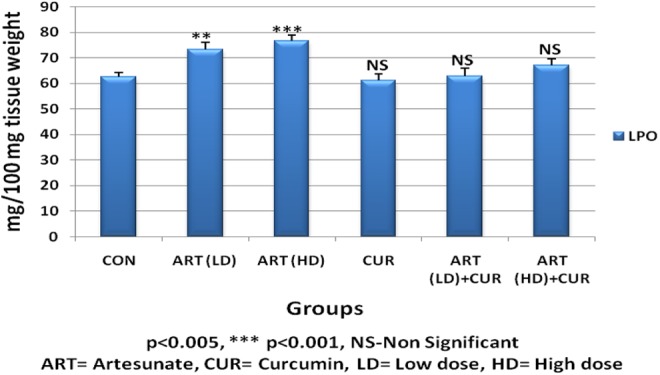
Alteration in lipid peroxidation (LPO) thiobarbutiric acid reactive species (TBARS) content in testis of control and treated animals.
Administration of artesunate at high dose (300 mg/kg bwt) for 45 days exhibited significantly decreased (P < .001) SOD activity in testis. Similarly, low dose of artesunate at 45 days showed significant reduction (P < .005) in SOD activity as compared with the control group values (Table 2). Supplementation of curcumin along with low or high dose of artesunate brought about a recovery from the decrease in SOD activity after 45 days in the treated groups (Table 1, Figure 2).
Table 2.
Alteration in Oxidative Parameters GSH and GST Levels in Testis of Control and Treated Animals.a
| Groups | GSH (µg/100 mg tissue wt) | GST (units/mg protein) | |||
|---|---|---|---|---|---|
| Control (A) | 37.11 ± 0.63 | 0.297 ± 0.03 | |||
| Artesunate (150 mg/kg bwt) (B) | 27.20 ± 0.32b | 0.317 ± 0.05b | |||
| Artesunate (300 mg/kg bwt) (C) | 24.68 ± 0.63c | 0.360 ± 0.07c | |||
| Curcumin (80 mg/kg bwt) (D) | 42.50 ± 0.30 NS | 0.280 ± 0.04 NS | |||
| Artesunate (150 mg/kg bwt) + curcumin (80 mg/kg bwt) (E) | 31.91 ± 0.50 NS | 0.288 ± 0.01 NS | |||
| Artesunate (300 mg/kg bwt) + curcumin (80 mg/kg bwt) (F) | 28.62 ± 0.25 NS | 0.288 ± 0.03 NS | |||
| Two-way ANOVA Analysis | |||||
| Source of Variation | SS | df | MS | F Value | P Value |
| Between the groups (dosage) | 961.5 | 3 | 320.5 | 15.35 | .0001 |
| Within the groups (duration) | 8687 | 416 | 20.88 | ||
Abbreviations: ANOVA, analysis of variance; bwt, body weight; GSH, glutathione; GST, glutathione S transferase; NS, nonsignificant analysis of variance at P < .05 significance level; SE, standard deviation.
aValues are mean ± SE.
b P < .005.
c P < .001.
Figure 2.
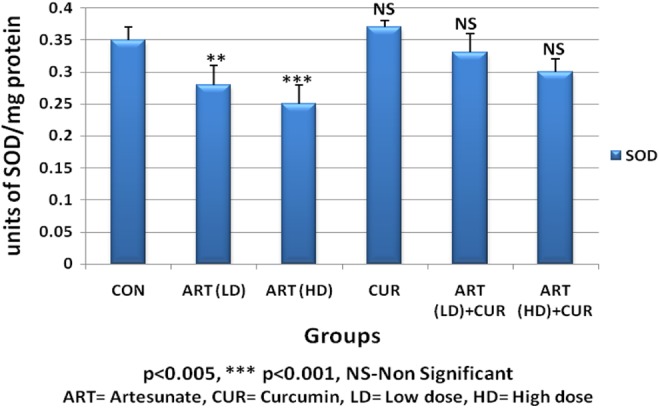
Alteration in superoxide dismutase (SOD) content in testis of control and treated animals.
Similarly, the activity levels of GSH, G-Px, and G-Rx showed marked decline (P < .005) after administration of low-dose artesunate for 45 days. Further, highly significant decrease was noted with administration of high-dose artesunate (P < .001) in GSH, G-Px, and G-Rx after 45 days of treatment. Curcumin administered along with the artesunate low dose and high dose manifested an ameliorative effect, with recovery in activities of GSH, G-Px, and G-Rx in the testis after 45 days of treatment (Tables 2 and 3).
Table 3.
Alteration in Oxidative Parameters G-Px and G-Rx Levels in Testis of Control and Treated Animals.a
| Groups | G-Px (GSH Consumed/mg Protein) | G-Rx (Moles NADPH Oxidized/min/mg Protein) | |||
|---|---|---|---|---|---|
| Control (A) | 11.98 ± 0.43 | 0.93 ± 0.14 | |||
| Artesunate (150 mg/kg bwt) (B) | 8.72 ± 0.43b | 0.69 ± 0.03b | |||
| Artesunate (300 mg/kg bwt) (C) | 7.90 ± 0.26c | 0.66 ± 0.04c | |||
| Curcumin (80 mg/kg bwt) (D) | 14.06 ± 0.27 NS | 1.17 ± 0.14 NS | |||
| Artesunate (150 mg/kg bwt) + curcumin (80 mg/kg bwt) (E) | 9.37 ± 0.27 NS | 0.74 ± 0.02 NS | |||
| Artesunate (300 mg/kg bwt) + curcumin (80 mg/kg bwt) (F) | 9.42 ± 0.25 NS | 0.71 ± 0.02 NS | |||
| Two-way ANOVA Analysis | |||||
| Source of Variation | SS | df | MS | F Value | P Value |
| Between the groups (dosage) | 90.64 | 3 | 30.21 | 11.08 | .0001 |
| Within the groups (duration) | 1134 | 416 | 2.726 | ||
Abbreviations: ANOVA, analysis of variance; bwt, body weight; G-Px, glutathione peroxidase; G-Rx, glutathione reductase; GSH, glutathione; GST, glutathione S transferase; NS, nonsignificant analysis of variance at P < .05 significance level; SE, standard deviation.
aValues are mean ± SE.
b P < .005.
c P < .001.
Contrary to this, the GST levels recorded significant increase (P < .005) with low dose of artesunate at 45-day interval. The higher dosage also showed similar increase (P < .001) after 45 days (Table 2). Amelioration with curcumin revealed nonsignificant decrease in GST levels.
Based on the above-observed results, the null hypothesis has been rejected and the alternative hypothesis has been accepted, which suggests that curcumin shows ameliorative effects on artesunate-induced toxicity.
Discussion
Despite the efforts to control malarial infection, it still prevails and perpetuates to pose great challenge to those living in endemic areas. Several factors have been reported to be responsible, including development of drug resistance to the potent and affordable drugs by the parasite (Okeola et al. 2011).
To combat these, use of artesunate as an alternative drug has been widely accepted. Artesunate exerts its antimalarial activity by the generation of reactive oxygen species (ROS) from its endoperoxide bond (Maggs et al. 1988) by iron (II)-heme produced during hemoglobin degradation, resulting in the generation of ROS (Jefford 2001; Robert et al. 2002) and leading to LPO (Robert et al. 2001). The accumulation of lipid peroxides is toxic to the membrane structure, leading to a change in permeability and disintegration of cellular organelles (Muller et al. 1982). In several situations, the rate of generation of ROS exceeds their removal, resulting in oxidative stress (Giordano et al. 2005). Similarly, studies of artemisinins on embryonic stem cells in mice have shown that these compounds raised intracellular levels of ROS (Wartenberg et al. 2003).
Oxidative stress also has long-term impact on male germ cell differentiation. It has been reported that oxidative stress inhibits spermatogenesis, decreases SOD activity, and induces DNA damage as well as apoptosis (Celino et al. 2012). Under normal circumstances, the antioxidant enzymes of the cell get activated and effectively scavenge the free radicals restoring the normal cell environment. However, artesunate is known to inhibit protein synthesis in most mammalian systems by exposing the heptocyte to unprotectable oxidative injury, thus accounting for the degree of oxidative damage done by the accumulated artesunate and its metabolites (Xu et al. 2007). Therefore, the significant decrease in GSH and SOD observed following artesunate treatment could result from enhanced utilization without augmented production. Artesunate has been reported to be a substrate of the GST detoxification system (Efferth et al. 2005). Most antimalarial agents had been shown to be toxic to male gonadal functions (Orisakwe et al. 2003; Raji et al. 2005). Furthermore, various studies on the effects of artemisinins in male reproductive dysfunction had been centered on single artemisinin agents, using mice and albino rats (Raji et al. 2005; Nwanjo et al. 2007).
Results of the present study revealed that treatment with antimalarial drug artesunate at low dose (150 mg/kg bwt) as well as high dose (300 mg/kg bwt) caused a significant depletion in the activities of various antioxidant enzymes such as SOD, GSH, G-Px, and G-Rx in testis tissue of the experimental male mice. Enhanced levels of LPO and GST were also observed in artesunate-treated mice. Moreover, the analysis of data using 2-way ANOVA at the P < .005 level revealed that there was a statistically significant difference between the groups (based on dose), suggesting a dose-dependent toxic effect on administration of artesunate.
Superoxide radicals are one of the most important reactive oxygen free radicals constantly produced in the living cells (Naik et al. 2005). Superoxide dismutase has antitoxic effect against the superoxide anion. Thus, the primary defense mechanism against superoxide anion is due to SOD activity and prevents further generation of free radicals (E1-Demerdash et al. 2009). In this context, the results of the present study showed that the SOD activity in the artesunate-treated mice decreased significantly due to oxidative stress in the testis.
The decreased SOD activity in turn could be responsible for increased LPO as well as decreased GSH level. The GSH/GST system is a critical factor in protection of cells and organs against toxicity. The thiol-containing metabolites reacts with GSH which are then converted into less harmful and more water-soluble molecules, and the reaction is catalyzed by enzyme GST. Artesunate treatment at both low and high dose brought about a significant reduction in GSH activity of testis of treated mice which clearly indicates the toxic effect of this antimalarial drug on body’s defense mechanism.
There are several isoforms of G-Px in testis that use GSH as a source of electrons to reduce H2O2 to water. The phospholipid hydroperoxide G-Px is one of the most important G-Px isoforms in a testicular tissue and is highly expressed in both spermatogenic and Leydig cells (Baek et al. 2007). In accordance with this report, the result of present study depicts the observed decline in G-Px levels.
Glutathione reductase is concerned with the maintenance of cellular level of GSH by affecting fast reduction in oxidized GSH to reduced form (Lenzi et al. 1994). A decrease in the activity of G-Rx would further decrease the concentration of ascorbic acid. Our present finding showed decrease in activity of G-Rx which corroborates with that of earlier findings (Chitra et al. 2003).
Results of our investigation revealed a significant increase in the LPO in testis of mice treated with low- and high-dose artesunate. This suggests an increased peroxidation of lipids with concomitant loss of cellular functions in the body by artesunate treatment. Similar effects have been shown with fluoride toxicity in testis (Rao and Bhatt 2012).
Glutathione-S-transferase is a phase II detoxification antioxidant enzyme playing a key role in cellular detoxification by catalyzing the reaction of GSH with the toxicant to form an S-substituted GSH (Townsend and Tew 2003). In the present study, the increase in the GST activity along with LPO suggests that GST is unable to utilize GSH and protect the tissue against the artesunate-induced toxicity.
Curcumin as an antioxidant may be an important component of an effective artesunate intoxication treatment. Farombi et al. (2007) indicated that curcumin protected against testicular oxidative damage in rats induced by di-n-butylphthalate. Furthermore, Mathuria and Verma (2007) have reported that curcumin ameliorated aflatoxin-induced LPO in liver, kidney, and testis of mice. Because of its safety record and multiple mechanisms of action, curcumin is useful as a valuable protective agent to ameliorate spermatogenesis dysfunction and cell loss (Khorsandi et al. 2013). The restoration of the concentration of testicular protein following the administration of the aqueous curcumin could enhance sperm maturation, indicating the androgenic potential of the plant. The significant reductions in the testicular total agree with previous studies of Kamel et al. (2014). Oral combination with curcumin could effectively counteract artesunate-induced oxidative testicular dysfunction as represented through ameolerating all oxidative stress and improving antioxidant defense system and prevent all toxic effect of artesunate.
The present investigation was carried out to explore the reproductive and oxidative effects of artesunate and the possible ameliorative role of curcumin in testis. Curcumin with low-dose and high-dose treatment significantly improved the level of enzymatic (SOD, G-Px, G-Rx, and GST) and nonenzymatic (GSH and LPO) components of the defense system. Results from this study indicate that curcumin might have protective effect against artesunate-induced oxidative stress in mice.
Conclusion
Oral administration of curcumin could effectively stabilize artesunate-induced testicular dysfunction as represented through reduction in oxidative stress, improving antioxidant defense system and counteracting the toxic effects of artesunate. Thus, it was observed that artesunate caused a dose-dependent toxic effect in testis function in adult mice which has statistical confirmation from the 2-way ANOVA results. However, at an effective dose of 80 mg/kg bwt, curcumin brought about significant ameliorative recovery in the testicular toxicity.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Abolaji AO, Osedeme F, Olusemire O. 2014. Artesunate–amodiaquine combination therapy in the absence of malarial parasite infection induces oxidative damage in female rats. Cell Biochem Funct 32:303–308 [DOI] [PubMed] [Google Scholar]
- Adeeko AO, Dada OA. 1998. Chloroquine reduces fertilizing capacity of epididyma sperm in rats. Afr J Med Med Sci 27(1-2):63–64 [PubMed] [Google Scholar]
- Al-Hazmi E, Shakar S, Al-Sagaff S, Khoraium S. 2005. Histological changes of testis in mice after administration of doxorubicin HCL (Adribastina), cytotoxic drug. Proceedings of 2nd Saudi Science Conference, Faculty of Science, KAU, Part 1:99–117 [Google Scholar]
- Angus BJ, Thaiaporn I, Chanthapadith K, Suputtamongkol Y, White NJ. 2002. Oral artesunate dose-response relationship in acute Falciparum malaria. Antimicrob. Agents Chemother 46:778–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek IJ, Yon JM, Lee R, Jin Y, Kim MR, Ahn B, Hong JT, Choo YK, Lee BJ, Yun YW, Nam SY. 2007. Effects of endocrine disrupting chemicals on expression of phospholipid hydroperoxide glutathione peroxidase mRNA in rat testes. J Vet Sci 8(3):213–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdelle N, Nikolova T, Quiros S, Efferth T, Kaina B. 2011. Artesunate Induces Oxidative DNA Damage, Sustained DNA Double-Strand Breaks, and the ATM/ATR Damage Response in Cancer Cells. Mol Cancer Ther 10(12):2224–2233. [DOI] [PubMed] [Google Scholar]
- Breman JG, Alilio MS, Mills A. 2004. Conquering the Intolerable Burden of Malaria: What’s New, What’s Needed: A Summary. American Journal of Tropical Medicine and Hygiene 71(2):1–15 [PubMed] [Google Scholar]
- Carlberg I, Mannervik B. 1985. Glutathione reductase. Methods Enzymol 113:484–490 [DOI] [PubMed] [Google Scholar]
- Celino FT, Yamaguchi-Shimizu S, Miura C, Miura T. 2012. Proliferating spermatogonia are susceptible to reactive oxygen species attack in Japanese eel (Anguilla japonica). Biol Rep 87(70):1–9 [DOI] [PubMed] [Google Scholar]
- Chitra KC, Rao KR, Mathur P. 2003. Effect of bisphenol A and co-administration of bisphenol A and vitamin C on epididymis of adult rats: a histopathological and biochemical study. Asian J Androl 5:203–208 [PubMed] [Google Scholar]
- Efferth T, Benakis A, Romero MR, et al. 2004. Enhancement of cytotoxicity of artemisinins toward cancer cells by ferrous iron. Free Radic Biol Med 27:998–1009. [DOI] [PubMed] [Google Scholar]
- Efferth T, Volm M. 2005. Glutathione-related enzymes contribute to resistance of tumor cells and low toxicity in normal organs to artesunate. In Vivo 19:225–232 [PubMed] [Google Scholar]
- EI-demerdash FM, Yousef IM, Radwan ME. 2009. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food and Chemical Toxicology 47:249–254 [DOI] [PubMed] [Google Scholar]
- Ellman GL. 1959. Tissue Sulfhydryl groups. Arch Biochem Biophys 82:70–77 [DOI] [PubMed] [Google Scholar]
- Farombi EO, Abarikwu SO, Adedara IA, Oyeyemi MO. 2007. Curcumin and kolaviron ameliorate di-n-butylphthalate-induced testicular damage in rats. Basic Clin Pharmacol Toxicol 100:43–48 [DOI] [PubMed] [Google Scholar]
- Giordano FJ. 2005. Oxygen, oxidative stress, hypoxia and heart failure. J Clin Invest 115:500–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Jhurani S, Aggarwal BB. 2008. Multi-targeted therapy by curcumin: how spicy is it? J Nat Prod 70(2):143–146 [DOI] [PubMed] [Google Scholar]
- Guerra CA, Gikandi PW, Tatem AJ, Noor AM, Smith, et al. 2008. The limits and intensity of Plasmodium falciparum transmission: Implications for malaria control and elimination worldwide. PLoS Med 5(2):300–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habig WH, Pabst MJ, Jakoby WB. 1974. Glutathione S-transferases: The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139 [PubMed] [Google Scholar]
- Hay SI, Guerra CA, Tatem AJ, Noor AM, Snow RW. 2004. The global distribution and population at risk of malaria: past, present, and future. Lancet Infect Dis 4:327–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WE, Cheng C, Peh HY, Xu F, Tannenbaum SR, Ong CN, Fred Wong W.S. 2012. Anti-malarial drug artesunate ameliorates oxidative lung damage in experimental allergic asthma. Free Radical Biology and Medicine 53:498–507 [DOI] [PubMed] [Google Scholar]
- Ilbey YO, Ozbek E, Simsek A, Otunctemur A, Cekmen M, Somay A. 2009. Potential chemoprotective effect of melatonin in cyclophosphamide and cisplatin-induced testicular damage in rats. Fertil Steril 92(3):1124–1132 [DOI] [PubMed] [Google Scholar]
- Izunya AM, Nwaopara AO, Aigbiremolen AE, Odike MAC, Oaikhena GA, Bankole JK. 2010. Histological studies of the toxicity of artesunate on the testes in Wistar rats. Biology and Medicine 2(2):49–56 [Google Scholar]
- Jalili C, Khani F, Salahshoor MR, Roshankhah S. 2014. Protective effect of Curcumin against Nicotine-induced damage on reproductive parameters in male mice. Int. J. Morphol 32(3):844–849 [Google Scholar]
- Jefford CW. 2001. Why artemisinin and certain synthetic peroxides are potent antimalarials: Implications for the mode of action. Curr Med Chem 8:1803–1826 [DOI] [PubMed] [Google Scholar]
- Jewo PI, Fadeyibi IP, Saalu LC, Amole OO, Izegbu MC, Ashiru OA, 2008. Effects of Short and Medium Term use of Artesunate on Fertility in Male Rats. Nigerian Journal of Health and Biomedical Sciences, 7 (2). [Google Scholar]
- Joe B, Lokesh BR. 1994. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochem Biophys Acta 1224:255–263 [DOI] [PubMed] [Google Scholar]
- Kakkar P, Das B, Viswanathan PN. 1984. A modified spectrophotometric assay of superoxide dismutase. Ind J Biochem Biophysics 4:130–132 [PubMed] [Google Scholar]
- Kamel KM, Abd El-Raouf OM, Metwally SA, Abd El-Latif HA, El-Sayed ME. 2014. Hesperidin and rutin, antioxidant citrus flavonoids, attenuate Cisplatin-induced nephrotoxicity in rats. J Biochem Mol Toxicol 28(7):312–319 [DOI] [PubMed] [Google Scholar]
- Kandemir FM, Benzer F, Yildirim NC, Ozdemir N. 2011. Compensatory effects of curcumin on cisplatin-induced toxicity in rabbit testis. Journal of Medicinal Plants Research 5(3):456–461 [Google Scholar]
- Khanna NM. 1999. Turmeric-nature’s precious gift. CUlT Sci 76:1351–1356 [Google Scholar]
- Khorsandi L, Mirhoseini M, Mohamadpour M, Orazizadeh M, Khaghani S. 2013. Effect of curcumin on dexamethasone-induced testicular toxicity in mice. Pharm Biol 51(2):206–212 [DOI] [PubMed] [Google Scholar]
- Lenzi A, Picardo M, Gandini L, Lombardo F, Terminali O, Passi S, Dondero F. 1994. Glutathione treatment of dyspermia: effect on the lipoperoxidation process. Hum Reprod 9:2044–2050 [DOI] [PubMed] [Google Scholar]
- Li QG, Mog SR, Si YZ, Kyle DE, Gettayacamin M, Milhous WK. 2002. Neurotoxicity and efficacy of arteether related to its exposure times and exposure levels in rodents. Am J Trop Med Hyg 66:516–525 [DOI] [PubMed] [Google Scholar]
- Lin T, Juliano JJ, Wongsrichanalai C. 2010. Drug-resistant Malaria: the era of ACT. Current Infectious Disease Reports 12(3):165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggs JL, Tingle MD, Kitteringham NR, Park BK. 1988. Drug-protein conjugates--XIV. Mechanisms of formation of protein-arylating intermediates from amodiaquine, a myelotoxin and hepatotoxin in man. Biochem Pharmacol 37(2):303–311 [DOI] [PubMed] [Google Scholar]
- Mathuria N, Verma RJ. 2007. Curcumin ameliorates aflatoxin-induced lipid peroxidation in liver, kidney and testis of mice-an in vitro study. Acta Pol Pharm 64:413–416. [PubMed] [Google Scholar]
- Mulenga M. 1998. Facing drug resistance therapeutic option for treatment of uncomplicated plasmodium Falciparum malaria in adult Zambians. J of med and health science 2:11–20 [Google Scholar]
- Muller L, Ohnesorge FK. 1982. Difference response of liver parenchymal cells from starvedand fed rats to cadmium. Toxicology 25:141–150 [DOI] [PubMed] [Google Scholar]
- Naik GH, Priyadarsini KI, Bhagirathi RG, Mishra B, Mishra KP, Banavalikar MM, Mohan H. 2005. In vitro antioxidant studies and free radical reactions of triphala, an ayurvedic formulation and its constituents. Phytother Res 19:582–586 [DOI] [PubMed] [Google Scholar]
- Noorafshan A, Karbalay-Doust S, Valizadeh A, Aliabadi E. 2011. Ameliorative effects of curcumin on the structural parameters of seminiferous tubules and Leydig cells in metronidazole-treated mice: a stereological approach. Exp Toxicol Pathol 63(7):627–633. [DOI] [PubMed] [Google Scholar]
- Nwanjo HU, Iroagba II, Nnatuanya IN, Eze NA. 2007. Antifertility activity of Dihydroartemisinin in male albino rats. The Internet J Endocrinol 4(1):3 [Google Scholar]
- Obianime AW, Aprioku JS. 2009. Comparative study of artesunate, ACTs and their combinants on the spermatic parameters of the male guinea pig. Niger J Physiol Sci 24:1–6 [PubMed] [Google Scholar]
- Ogbonna A, Uneke CJ. 2008. Artemisinin-based combination therapy for uncomplicated malaria in sub-Saharan Africa: the efficacy, safety, resistance and policy implementation since Abuja 2000. Transactions of the Royal Society of Tropical Medicine and Hygiene 102(7):621–627 [DOI] [PubMed] [Google Scholar]
- Ohkawa H, Ohishi N, Yagi K. 1979. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal Biochem 95:351–358 [DOI] [PubMed] [Google Scholar]
- Okeola VO, Adaramoye OA, Nneji CM, Falade CO, Farombi EO, Ademowo OG. 2011. Antimalarial and antioxidant activities of methanolic extract of Nigella sativa seeds (black cumin) in mice infected with Plasmodium yoelli nigeriensis. Parasitol Res 108:1507–1512 [DOI] [PubMed] [Google Scholar]
- Olumide SA, Raji Y. 2011. Long-Term Administration of Artesunate Induces Reproductive Toxicity in Male Rats. J Reprod Infertil 12(4):249–260 [PMC free article] [PubMed] [Google Scholar]
- Orisakwe OE, Obi E, Udemezue OO. 2003. Effect of halofantrin on testicular architecture and testosterone level in guinea pigs. Eur Bull Drug Res 11:105–109 [Google Scholar]
- Papadakis VE, Vlachopapadopoulu K, Syckle K, Tan C, Sklar C. 1999. Gonadal function in young patients successfully treated for Hodgkin disease. Journal of Pediatric Oncology 32(5):366–372 [DOI] [PubMed] [Google Scholar]
- Paul CH, Elena L, Wynand P, Małgorzata Z, Bernd K, Thomas E. 2008. Artesunate Derived from Traditional Chinese Medicine Induces DNA Damage and Repair. Cancer Res 68(11):4347–4351 [DOI] [PubMed] [Google Scholar]
- Payton F, Sandusky P, Alworth WL, RMN. 2007. Study of the SolutionStructure of Curcumin. J Nat Prod 70(2):144–146 [DOI] [PubMed] [Google Scholar]
- Raji Y, Osonuga TO, Akinsomisoye OS, Osonuga OA, Mewoyeka OO. 2005. Gonadotoxicity evaluation of oral artemisinin derivatives in male rats. J Med Sci 5(4):303–306 [Google Scholar]
- Rajput DK, Mehta DS, George LB, Desai KR. 2012. Histological and Biochemical alterations on oral administration of artesunate on the testis of male mice. International Journal of Pharmaceutical and Biological Research (IJPBR) 3(3):113–121 [Google Scholar]
- Rao MV, Bhatt RN. 2012. Protective effect of melatonin on fluoride-induced oxidative stress and testicular dysfunction in rats. Fluoride 45(2):116–124 [Google Scholar]
- Robert A, Dechy-Cabaret O, Cazelles J, Meunier B. 2002. From mechanistic studies on artemisinin derivatives to new modular antimalarial drugs. Acc Chem Res 35:167–174 [DOI] [PubMed] [Google Scholar]
- Robert AF, Benoit-Vical Dechy-Cabaret O, Meunier B. 2001. From classical antimalarial drugs to new compounds based on the mechanism of action of artemisinin. Pur Appl Che 73(7):1173–1188 [Google Scholar]
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. 1973. Selenium: Biochemical Role as a Component of Glutathione Peroxidase. Science 179:588–590 [DOI] [PubMed] [Google Scholar]
- Salama AF, El-Bahr SM. 2007. Effect of Curcumin on Cadmium-Induced Oxidative Testicular Damage in Rats. Journal of Medical Research Institute (JMRI) 28(2):167–173 [Google Scholar]
- Sharpe RM. 1993. Experimental evidence for Sertoli-germ cell and Sertoli-Leydig interactions. In The Sertoli Cell (ed. Russell L. D., Griswold) M. D., Cache River Press, Clearwater FL:391–418 [Google Scholar]
- Townsend DM, Tew KD. 2003. The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22(47):7369–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Agtmael M, Bouchaud O, Malvy D, et al. 1999. The comparative efficacy and tolerability of CGP 56697 (artemether + lumefantrine) versus halofantrine in the treatment of uncomplicated falciparum malaria in travelers returning from the tropics to the Netherlands and France. Int J Antimicrob Agents 12:159–69 [DOI] [PubMed] [Google Scholar]
- Wartenberg M, Wolf S, Budde P, Grünheck F, Acker H, Hescheler J, Wartenberg G, Sauer H. 2003. The antimalarial agent artemisinin exerts antiangiogenic effects in mouse embryonic stem cell-derived embryoid bodies. Lab Invest 83:1647–1655 [DOI] [PubMed] [Google Scholar]
- Xu H, He Y, Yang X, Liang L, Zhan Z, Ye Y, Yang X, Lian F, Sun L. 2007. Anti-malarial agent artesunate inhibits TNF-alpha-induced production of proinflammatory cytokines via inhibition of NF-kappaB and PI3 kinase/Akt signal pathway inhuman rheumatoid arthritis fibroblast-like synoviocytes. Rheumatology 46:920–926 [DOI] [PubMed] [Google Scholar]


