Abstract
Hormetic response is an adaptive mechanism for a cell or organism surviving in an unfavorable environment. It has been an intriguing subject of researches covering a broad range of biological and medical disciplines, in which the underlying significance and molecular mechanisms are under intensive investigation. In the present study, we demonstrated that topoisomerase I inhibitor camptothecin (CPT), a potent anticancer agent, induced an obvious hormetic response in rat pheochromocytoma PC12 cells. Camptothecin inhibited PC12 cell growth at relative high doses as generally acknowledged while stimulated the cell growth by as much as 39% at low doses. Moreover, low doses of CPT protected the cells from hydrogen peroxide (H2O2)-induced cell death. Phosphoinositide 3-kinase (PI3K)/Akt and nuclear factor-E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathways were reported playing pivotal roles in protecting cells from oxidative stress. We observed that these 2 pathways were upregulated by low doses of CPT, as evidenced by increased levels of phosphorylated PI3K, phosphorylated Akt, phosphorylated mammalian target of rapamycin, Nrf2, and HO-1; and abolishment of the growth-promoting and neuroprotective effects of CPT by LY294002, a PI3K inhibitor. These results suggest that the hormetic and neuroprotective effects of CPT at low doses on PC12 cells were attributable, at least partially, to upregulated PI3K/Akt and Nrf2/HO-1 pathways.
Keywords: hormesis, neuroprotection, topoisomerase inhibitor, PC12 cells
Introduction
Hormesis is defined as a process in which exposure to a low dose of a chemical agent or environmental factor that is damaging at higher doses induces an adaptive beneficial effect on the cell or organism.1,2 It occurs in essentially all species of microbes, plants, vertebrates, and invertebrates.3 The phenomenon of hormesis has been typified for many agents and seems to be important in pharmacology. It appears when the same drug modulates the growth of cultured cells in a biphasic fashion, stimulating at lower concentrations but inhibiting the growth at higher doses. It was proposed that physical and chemical stressors at low doses may have long-term beneficial effects as a way of adapting an organism toward enhanced stress responses.2,4 However, it may be a potential hazard for patients who take chemotherapeutic agents, which stimulate cell proliferation of human tumor at low doses. The concept of hormetic response is important in areas of clinical pharmacology, especially in the discovery of drug for antiaging, neuroprotection, drug addiction, pain, and so on.5
Recently, the hormesis concept has been receiving increasing attention in neural science. A class of stressors markedly increased the neuronal resistance to more drastic stresses. These hormetic stressors include excitatory neurotransmission,6 exercise,7 and dietary restriction.8 Various phytochemicals, including resveratrol, sulforaphane, curcumins, lycopene, catechins, allium, ginkgo biloba, ginsenosides, and hypericin, were observed to exhibit neuroprotective activities at low doses through hormetic mechanisms.9 In addition, amyloid-β protein, a neurotoxic peptide that is implicated in the pathogenesis of Alzheimer’s disease, may actually have a protective effect on neurons at physiological concentration.10 Hormesis in neural cells opens a new window to study neuroprotection, however, more evidences are required to evaluate the phenomena and the underlying mechanisms.
In the current study, we investigate whether low doses of topoisomerase (topo) inhibitors could induce hormetic and neuroprotective effects in rat pheochromocytoma PC12 cell line, which is a dopaminergic neuronal cell line with typical neuron features, and has been widely used in a variety of neurotoxicology and neuropharmacology studies.11–13 Topoisomerases, enzymes involved in removing supercoiling and play a key role in DNA replication, have been excellent targets for anticancer drug discovery, and the inhibitors constitute an important class of the current anticancer agents.14 Camptothecin (CPT), a monoterpene indole alkaloid from Camptotheca acuminata, inhibits topo I by stabilizing the enzyme–DNA complex.15 Camptothecin has been regarded as one of the most promising anticancer agents.16 Despite its cytotoxicity at relative high concentration, we found that low doses of CPT exhibited overt hormetic and neuroprotective effects in PC12 cells.
Materials and Methods
Reagents
Camptothecin, doxorubicin hydrochloride, etoposide, and hydrogen peroxide (H2O2) were purchased from Sigma (St. Louis, Missouri). F-12K Medium was supplied by Gibco (Bethesda, Maryland). Fetal bovine serum (FBS) and horse serum were obtained from Invitrogen (Carlsbad, California). 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from Molecular Probes (Eugene, Oregon). Primary antibodies against phosphorylated phosphoinositide 3-kinase (p-PI3K; p-FAK and Tyr925), phosphorylated Akt (p-Akt; Ser473), phosphorylated mammalian target of rapamycin (p-mTOR), phosphatase with tensin homology (PTEN), heme oxygenase-1 (HO-1), nuclear factor-E2-related factor 2 (Nrf2), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and secondary antibodies were purchased from Cell Signaling Technology (Danvers, Massachusetts). Hoechst 33342 staining kit and LY294002 were purchased from Jiangsu Beyotime Institute of Biotechnology. The enhanced chemiluminescence (ECL) detection kit was purchased from Amersham Pharmacia Biotech (Buckinghamshire, United Kingdom). All other chemicals of analytical grade were purchased from local sources.
Cell Culture and Treatments
The PC12 cells were obtained from the American Type Culture Collection (ATCC, Manassas, Virginia) and maintained in ATCC-formulated F-12K medium supplemented with 15% horse serum, 2.5% heat-inactivated FBS, and 100 U/mL penicillin-streptomycin, and incubated at 37°C in a humidified atmosphere containing 5% CO2. The culture medium was changed every 2 days.
Cell Viability Assay
Cell viability was evaluated by MTT colorimetric assay, which provides accurate and reliable quantification of viable cell number and is a frequently used method for measuring neural cytotoxicity.17,18 Briefly, PC12 cells (6 × 103 cells/0.1 mL per well) were treated with a wide range of concentrations of topo inhibitors for 24 hours in a 96-microwell plate. To test the protective effect of CPT at low doses against H2O2-induced cell death, PC12 cells were pretreated with or without CPT for 1 hour prior to the treatment of 2 µmol/L H2O2 for another 24 hours. The treated cells were incubated in fresh culture medium containing 0.2 mg/mL MTT for another 4 hours at 37°C. The supernatants were then aspirated off, and 100 μL dimethyl sulfoxide (DMSO) was added to dissolve the formazan crystals. After shaking for 10 minutes in the dark, the absorbance was determined using a microplate reader at 570 nm (BioTek, Winooski, Vermont). The relative viability of treated cells was expressed as percentage of control untreated cells. In addition, cell morphology was visualized and photographed using the InCell 2000 confocal microscope (GE Biosciences, Piscataway, New Jersey).
Western Blotting
PC12 cells (4.0 × 105 cells/mL) were treated with different concentrations of CPT. Total proteins were extracted with radioimmunoprecipitation assay lysis buffer, and the sample protein concentrations were determined using a bicinchoninic acid assay. Equal amounts of proteins from each group were subjected to sodium dodecylsulfate polyacrylamide gel electrophoresis, and the gels were transferred onto polyvinylidene fluoride membranes. The membranes were blocked with 5% nonfat dry milk in Tris-buffered saline buffer for 90 minutes at room temperature, followed by incubation overnight at 4°C with primary antibody (1:1000). The membranes were washed with Tris-buffered saline with 0.1% Tween-20 (TBST) and incubated with secondary antibodies (1:5000) for 2 hours at room temperature. Signals were developed using an ECL detection kit. Densitometric measurement of band intensity was performed with the Quantity One Software (Bio-Rad, Hercules, California).
Statistical Analysis
All data were expressed as means ± standard deviation (SD) for at least 3 independent experiments. Statistical analysis was performed using one-way analysis of variance followed by Tukey post hoc analysis using GraphPad Prism 5 software package (GraphPad Software, San Diego, California). A P value of less than .05 was considered to be statistically significant.
Results
Hormetic Effect of CPT Protected PC12 Cells Against H2O2-Induced Cell Death
To examine the dose response, PC12 cells were treated with CPT at concentrations ranging from 0.01 to 14 μmol/L for 24 hours. The cell viability of CPT was assessed by MTT assay. As shown in Figure 1A, CPT at a concentration of 0.22 μmol/L increased cell proliferation by 38.7% and did not show cytotoxicity up to the concentration of 0.44 μmol/L. In contrast, treatment with CPT at a concentration of 14 μmol/L significantly reduced proliferation. The cytotoxicity of CPT increased in a concentration-dependent manner when the concentrations were above 0.88 μmol/L. Moreover, with prolonged incubation time (48 hours or 72 hours), the proliferation-stimulating effect declined (data not shown). The effects of topo II inhibitors doxorubicin hydrochloride and etoposide on proliferation of PC12 cells in vitro were also evaluated using the MTT assay. As shown in Supplementary Figures S1 and S2, doxorubicin hydrochloride and etoposide increased cell proliferation at concentrations ranging from 0.16 to 1.25 μmol/L and from 0.78 to 12.5 μmol/L, respectively, while higher concentrations inhibited cell proliferation. These results indicated that topo inhibitors CPT, doxorubicin, and etoposide exhibited obvious hormetic effects1,5 on PC12 cells. Since CPT induced the strongest hormetic effect, we chose CPT for further experiments.
Figure 1.
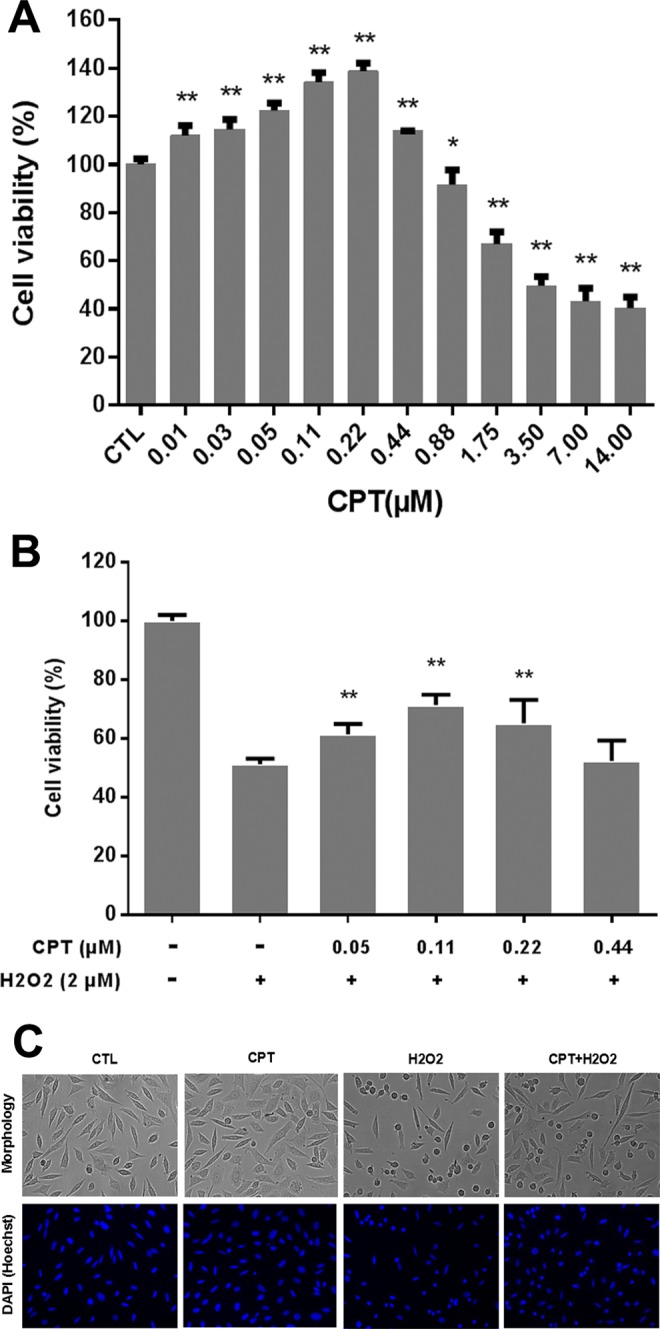
Camptothecin (CPT) induced hormetic effect in PC12 cells and protected against hydrogen peroxide (H2O2)-induced cell death. A, The PC12 cells were treated with indicated concentrations of CPT for 24 hours. B, PC12 cells were treated with 2 μmol/L H2O2 alone or in combination with CPT (0.05, 0.11, 0.22, and 0.44 μmol/L) for 24 hours. *P < .05, **P < .01, compared to control (CTL) or H2O2 alone. Cell viability was determined by MTT assay as described in Materials and Methods section. Data are represented as means ± SD of 3 to 5 independent experiments. C, PC12 cells were treated with 2 μmol/L H2O2 alone or in combination with 0.11 μmol/L CPT for 24 hours. Cells were photographed using the InCell 2000 confocal microscope.
We further examined whether CPT at low doses could protect PC12 cells against H2O2-induced cell death. Cell viability was tested by MTT assay as previous described. H2O2 induced PC12 cell death in a dose-dependent manner and about 60% of PC12 cells were killed by H2O2 at a concentration of 2 μmol/L for 24 hours (data not shown). To tested the protective effects, PC12 cells were treated with H2O2 (2 μmol/L) alone or in combination with low doses of CPT (0.05, 0.11, 0.22, and 0.44 μmol/L) for 24 hours. As shown in Figure 1B, CPT at low doses except 0.44 μmol/L significantly protected PC12 cells from H2O2-induced cell death. Notably, CPT of 0.11 μmol/L exhibited the highest protective activity of 20.1% as compared to H2O2 alone. The hormetic and neuroprotective effects of CPT at low doses were further confirmed by cell morphology (Figure 1C), which was consistent with the results shown in Figure 1A and B.
Low Doses of CPT Upregulated PI3K/Akt/mTOR and Nrf2/HO-1 Pathways
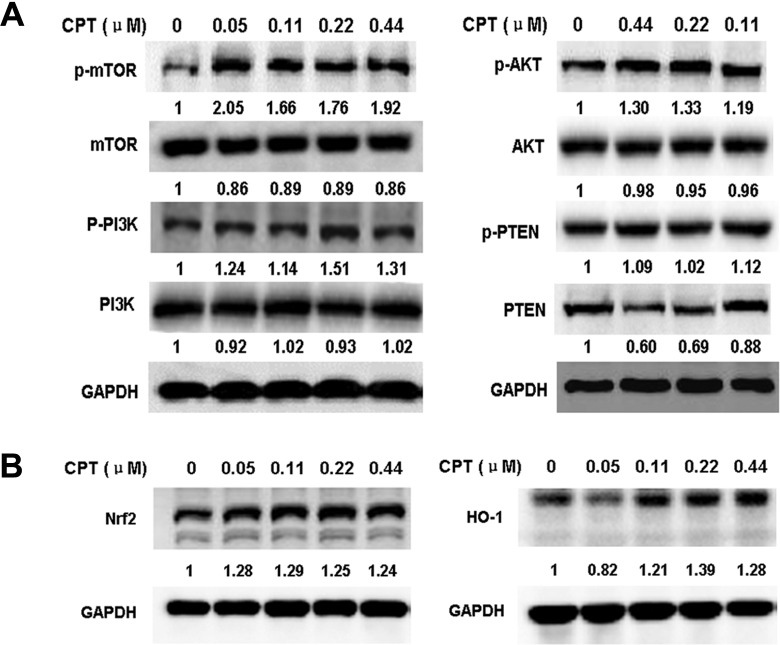
To investigate the underlying molecular mechanisms of the hormetic and neuroprotective effects of CPT, we tested the effects of CPT at low doses on the key components of PI3K/Akt/mTOR cell proliferation/survival pathway and antioxidant Nrf2/HO-1 pathway by Western blotting assay. The results showed that low doses of CPT upregulated the levels of p-PI3K, p-Akt, and p-mTOR, and the expression levels of HO-1 and Nrf2 proteins, and downregulated the expression of PTEN protein in PC12 cells (Figure 2A and B). These results strongly suggested that the hormetic and neuroprotective effects of CPT at low doses on PC12 cells were through upregulating PI3K/Akt/mTOR and Nrf2/HO-1 pathways.
Figure 2.
Effects of camptothecin (CPT) on the expression level of proteins of phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) (A) and nuclear factor-E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) (B) pathways in PC12 cells. Total protein was extracted from PC12 cells treated with indicated concentrations of CPT for 3 hours (A) or 18 hours (B). The protein levels were determined by Western blotting as described in Materials and Methods section. The indicated number under each band was the fold changes of expression level compared to that in the control group (0 µmol/L CPT). GAPDH was used as the internal standard for protein loading.
Inhibition of PI3K Pathway Attenuated the Hormetic and Neuroprotective Effects of CPT
Since the PI3K pathway plays a pivotal role in cell proliferation and cell survival,19 we further tested its role in the hormetic and neuroprotective effects of CPT in PC12 cells. The MTT colorimetric assay revealed that CPT stimulated proliferation of PC12 cells by 40.6% (dose of 0.22 μmol/L) and 35.3% (0.11 μmol/L), which was similar to the results shown in Figure 1A. However, co-treatment of CPT and LY294002 (2.5 μmol/L), a PI3K inhibitor, abolished the proliferation-promoting effect of CPT (Figure 3A) and the protective effect of CPT against H2O2-induced cell death in PC12 cells (Figure 3B), suggesting that PI3K pathway is involved in the hormetic and neuroprotective effects of CPT at low doses on PC12 cells.
Figure 3.
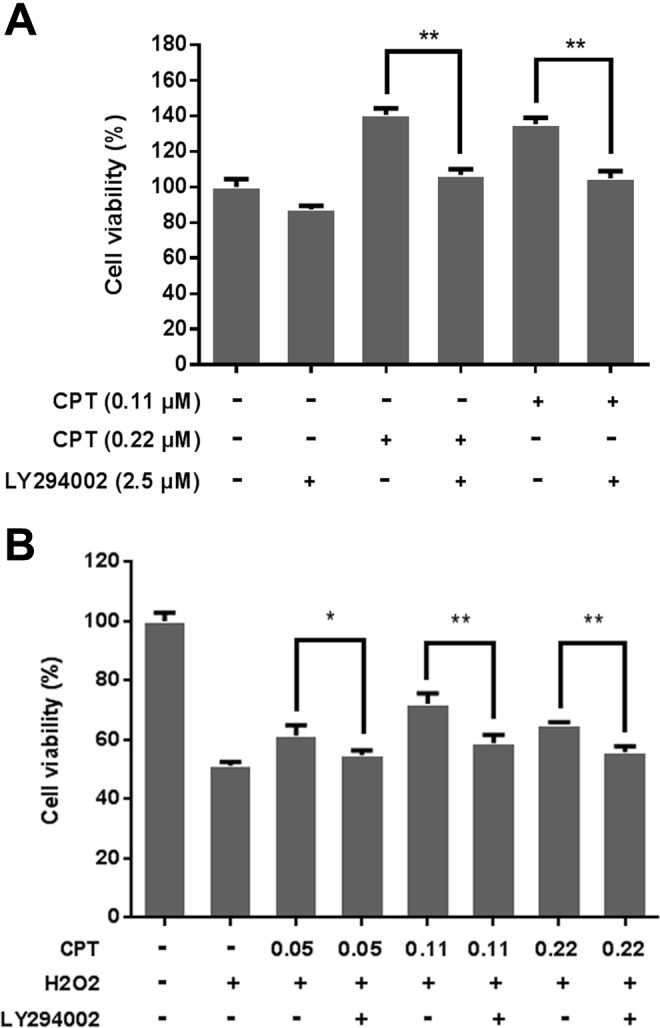
Inhibition of PI3K activity attenuated the hormetic and neuroprotective effects of camptothecin (CPT) at low doses. A, PC12 cells were treated with 2.5 μmol/L LY294002 (a PI3K inhibitor) alone or in combination with 0.11 and 0.22 μmol/L CPT for 24 hours. B, PC12 cells were pretreated with or without LY294002 for 2 hours. Cells were then treated with 2 μmol/L H2O2 alone or in combination with 0.05, 0.11, and 0.22 μmol/L CPT for 24 hours. Cell viability was determined by MTT assay as described in Materials and Methods section. Data are represented as means ± SD of 3 to 5 independent experiments. **P < .01, compared to CPT-treated groups.
Discussion
In this study, we found that topo I inhibitor CPT showed an overt hormetic and neuroprotective effects in PC12 cells. The characteristic of hormesis is a biphasic dose–response curve, with beneficial or stimulatory effects at low doses and adverse or inhibitory effects at high doses. The range of maximum stimulatory responses is generally within 30% to 60% greater than control value, and the width of the stimulatory dosage is within 10- to 1000-fold below the threshold value.5 Our results indicated that the highest stimulatory rate of cell growth is 39% by CPT of 0.22 μmol/L, and the range of the stimulatory dosage is about 88-fold below the threshold value (Figure 1A). Furthermore, we found that topo II inhibitors doxorubicin and etoposide at low doses also enhanced the growth of PC12 cells to a lesser extent (Supplementary Figures S1 and S2). These results demonstrated that topo inhibitors exhibited typical hormetic effects in PC12 cells. Vichi and Tritton reported a significant stimulation of cellular proliferation in cancer and normal cells by doxorubicin,20 suggesting a universal hormetic phenomenon induced by topo inhibitors in various cells.
The growth stimulation of topo inhibitors raises clinical concerns when cancer cell growth were enhanced during chemotherapy, while on the other hand, myelosuppression or other side effects of chemotherapy could be counteracted. Stimulatory phase of hormesis at low doses of agents may benefit neurological diseases by enhancing neural cell growth or protecting neural cells from further damaging. As evidenced in our results, CPT at doses of inducing stimulatory responses significantly protected PC12 cells from cytotoxicity of H2O2 (Figure 1B). Kainic acid, domoic acid, and excitotoxins showed similar hormetic effects and protected neurons from ischemic and excitotoxic death at subneurotoxic doses.21 In addition, Goldoni et al reported that pretreatment of low doses of methylmercury protected PC12 cells from a neurotoxic food contaminant PCB153.22 Large numbers of articles reported neuroprotective effects of compounds in natural products, particularly the antioxidants.23 In the meantime, the same compounds were reported possessing cytotoxic activities to cancer cells.24 Heat shock protein 90 (Hsp90) inhibitors are in clinical trials for multiple indications in cancer, however, low doses of the Hsp90 inhibitor HSP990 and 17-allylamino-17- demethoxygeldanamycin protected against inherited retinal degeneration and neurodegenerative disorders, respectively.25,26 The seemingly contradictory functions of many compounds might be due to different range of concentrations be used in defined studies. Our results further suggest that hormetic responses should generally be taken into account in pharmacological studies of anticancer, neuroprotection, and others. Actually, the concept of hormesis provides a new vision for investigating neuroprotective agents as well.
The PI3K/Akt/mTOR pathway plays a central role in the regulation of metabolism, cell proliferation, and cell survival in oxidative stress.19,27 In response to mild stress (DNA damage, oxidation, etc.), insulin and insulin-like growth factor trigger self-phosphorylation of the receptors with intrinsic tyrosine kinase activity. The activated receptor phosphorylates the insulin receptor substrate protein, by which PI3K signaling pathway is then activated. The serine/threonine kinase Akt is a key mediator of the PI3K signaling pathway. The p-Akt by PI3K promotes cell survival and proliferation through activating forkhead box O (FOXO) transcription factor and mTOR. FOXO upregulates the expression of antioxidant proteins, while mTOR activates 40S ribosomal protein S6 kinase, thus enhancing the expression of cell proliferation-related proteins.
Our data showed that CPT at low doses significantly increased the levels of p-PI3K, p-Akt, and p-mTOR (Figure 2A), suggesting the hormetic and neuroprotective effects of CPT on PC12 cells were through activating PI3K/Akt/mTOR pathway. LY294002, a PI3K inhibitor,28 reversed the proliferation-promoting effect of CPT and its protection against H2O2-induced cell death in PC12 cells (Figure 3A and B), confirming this hypothesis. The tumor-suppressor PTEN negatively regulates PI3K signaling by dephosphorylating phosphatidylinositol 3,4,5-trisphosphate, converting it back to phosphatidylinositol 4,5-bisphosphate, then inhibits the activation of Akt.29 In our result, PTEN was downregulated by low dose of CPT (Figure 2A), which is consistent with the activation of PI3K by CPT. The pharmacological inhibition of PTEN was reported to reduce neurological damage after ischemic brain injury by upregulating PI3K/Akt activity.30
In addition to PI3K pathway, Nrf2 is another important effector protecting cells from oxidative damage.31,32 Oxidative stress causes the release of Nrf2 from Keap1 and translocation to the nucleus, where it triggers the expression of antioxidant response element (ARE)-target genes, such as NAD(P)H: quinine oxidoreductase 1, glutathione S-transferase, glutamate-cysteine ligase, and HO-1.33,34 Accumulating evidences indicated that Nrf2 activation exerted robust protective effects against cytotoxicity caused by various injuries,35,36 suggesting that targeting the Nrf2 pathway could be a promising approach for therapeutic intervention of oxidative damage-related diseases, particularly neurodegenerative diseases. Indeed, several phytochemicals with neuroprotective activities have been shown to upregulate the Nrf2 activity.37,38 In our results, low dose CPT significantly increased the levels of Nrf2 and its downstream target HO-1 (Figure 2B), indicating their roles in hormetic and thereby neuroprotective effects of low dose CPT in PC12 cells. This is in accordance with the suggestion that Nrf2/ARE is one of the major signaling pathways participating in hormesis.39 Since PI3K/Akt and Nrf2/HO-1 pathways were parallelly activated by low dose CPT in PC12 cells, we speculate that there might be association or crosstalk between these 2 pathways. Although there was no evidence that PI3K or Akt directly interacted with Nrf2, translocation and accumulation of Nrf2 in nucleus and thereby expression of antioxidant response genes were dependent on PI3K/Akt/mTOR activation.40,41
In summary, this study demonstrated that topo I inhibitor CPT at low doses induced hormetic effect in PC12 cells and protected the cells from oxidative damage. The hormetic and neuroprotective effects of low dose CPT on PC12 cells were attributable, at least partially, to upregulated PI3K/Akt/mTOR cell proliferation/survival and Nrf2/HO-1 antioxidant pathways (as summarized in Figure 4). Our results suppose that hormetic effects induced by challenging factors might be beneficial to the prevention and therapeutic intervention of neurodegenerative disorders.
Figure 4.
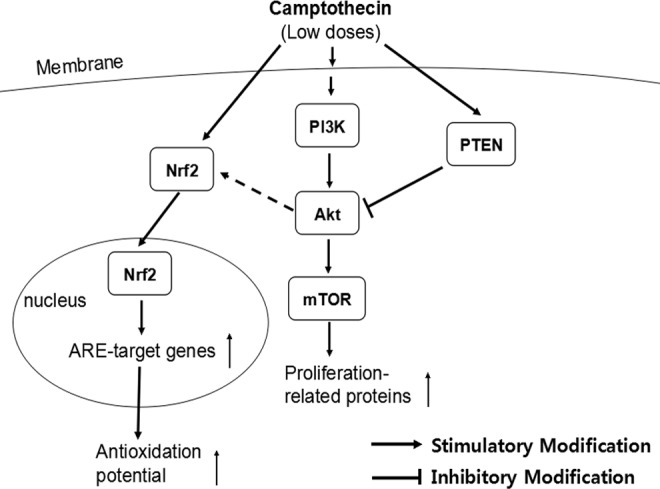
A schematic model of upregulated phosphoinositide 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) proliferation/survival pathway and nuclear factor-E2-related factor 2 (Nrf2)/HO-1 antioxidant pathway by low doses of camptothecin (CPT) in PC12 cells.
Footnotes
Authors’ Note: Chao Zhang and Shenghui Chen contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Macao Science and Technology Development Fund (074/2013/A) and the Research Fund of the University of Macau (MYRG107(Y1-L3)-ICMS13-HCW, MRG013/HCW/2014/ICMS, MYRG2015-00081-ICMS-QRCM).
Supplemental Material: The supplementary figures are available at http://dos.sagepub.com/supplemental.
References
- 1. Calabrese EJ, Bachmann KA, Bailer AJ, et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol Appl Pharmacol. 2007;222(1):122–128. [DOI] [PubMed] [Google Scholar]
- 2. Mattson MP. Hormesis defined. Ageing Res Rev. 2008;7(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davies JMS, Lowry CV, Davies KJA. Transient adaptation to oxidative stress in yeast. Arch Biochem Biophys. 1995;317(1):1–6. [DOI] [PubMed] [Google Scholar]
- 4. Liu YC, Wu ZY, Feng SB, Yang XN, Huang DJ. Hormesis of glyceollin I, an induced phytoalexin from soybean, on budding yeast chronological lifespan extension. Molecules. 2014;19(1):568–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calabrese EJ. Hormesis and medicine. Brit J Clin Pharmacol. 2008;66(5):594–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jiang XY, Zhu DM, Okagaki P, et al. N-methyl-D-aspartate and TrkB receptor activation in cerebellar granule cells—An in vitro model of preconditioning to stimulate intrinsic survival pathways in neurons. Ann Ny Acad Sci. 2003;993:134–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ding Y, Li J, Luan X, et al. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience. 2004;124(3):583–591. [DOI] [PubMed] [Google Scholar]
- 8. Mattson MP. Energy intake, meal frequency, and health: a neurobiological perspective. Annu Rev Nutr. 2005;25:237–260. [DOI] [PubMed] [Google Scholar]
- 9. Mattson MP, Cheng A. Neurohormetic phytochemicals: Low-dose toxins that induce adaptive neuronal stress responses. Trends Neurosci. 2006;29(11):632–639. [DOI] [PubMed] [Google Scholar]
- 10. Ittner LM, Gotz J. Amyloid-beta and tau-a toxic pas de deux in Alzheimer’s disease. Nat Rev Neurosci. 2011;12(2):67–72. [DOI] [PubMed] [Google Scholar]
- 11. Zhong L, Sarafian T, Kane DJ, et al. Bcl-2 inhibits death of central neural cells induced by multiple agents. Proc Natl Acad Sci. 1993;90(10):4533–4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shearman MS, Ragan CI, Iversen LL. Inhibition of PC12 cell redox activity is a specific, early indicator of the mechanism of beta-amyloid-mediated cell death. Proc Natl Acad Sci. 1994;91(4):1470–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levites Y, Amit T, Mandel S, Youdim MB. Neuroprotection and neurorescue against Aβ toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (-)-epigallocatechin-3-gallate. FASEB J. 2003;17(8):952–954. [DOI] [PubMed] [Google Scholar]
- 14. Pourquier P, Lansiaux A. Molecular determinants of response to topoisomerase I inhibitors. B Cancer. 2011;98(11):1287–1298. [DOI] [PubMed] [Google Scholar]
- 15. Wall ME, Wani MC. Camptothecin and Taxol-Discovery to Clinic-13th Bruce-F-Cain-Memorial-Award-Lecture. Cancer Res. 1995;55(4):753–60. [PubMed] [Google Scholar]
- 16. Kathiravan MK, Khilare MM, Nikoomanesh K, Chothe AS, Jain KS. Topoisomerase as target for antibacterial and anticancer drug discovery. J Enzyme Inhib Med Chem. 2013;28(3):419–435. [DOI] [PubMed] [Google Scholar]
- 17. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65(1-2):55–63. [DOI] [PubMed] [Google Scholar]
- 18. Liu Y, Peterson DA, Kimura H, Schubert D. Mechanism of cellular 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction. J Neurochem. 1997;69(2):581–593. [DOI] [PubMed] [Google Scholar]
- 19. Liu DX, Hou P, Liu Z, Wu GJ, Xing MZ. Genetic Alterations in the Phosphoinositide 3-Kinase/Akt Signaling Pathway Confer Sensitivity of Thyroid Cancer Cells to Therapeutic Targeting of Akt and Mammalian Target of Rapamycin. Cancer Res. 2009;69(18):7311–7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vichi P, Tritton TR. Stimulation of growth in human and murine cells by adriamycin. Cancer Res. 1989;49(10):2679–2682. [PubMed] [Google Scholar]
- 21. Plamondon H, Blondeau N, Heurteaux C, Lazdunski M. Mutually protective actions of kainic acid epileptic preconditioning and sublethal global ischemia on hippocampal neuronal death: Involvement of adenosine A(1) receptors and K-ATP channels. J Cerebr Blood F Met. 1999;19(12):1296–1308. [DOI] [PubMed] [Google Scholar]
- 22. Goldoni M, Caglieri A, Poli D, Vettori MV, Ceccatelli S, Mutti A. Methylmercury at low doses modulates the toxicity of PCB153 on PC12 neuronal cell line in asynchronous combination experiments. Food Chem Toxicol. 2008;46(2):808–811. [DOI] [PubMed] [Google Scholar]
- 23. Levi MS, Brimble MA. A review of neuroprotective agents. Curr Med Chem. 2004;11(18):2383–2397. [DOI] [PubMed] [Google Scholar]
- 24. Ahmad A, Biersack B, Li YW, et al. Perspectives on the role of isoflavones in prostate cancer. Aaps J. 2013;15(4):991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aguila M, Bevilacqua D, McCulley C, et al. Hsp90 inhibition protects against inherited retinal degeneration. Hum Mol Genet. 2014;23(8):2164–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang G, Krishnamurthy K, Tangpisuthipongsa D. Protection of murine neural progenitor cells by the Hsp90 inhibitor 17-allylamino-17-demethoxygeldanamycin in the low nanomolar concentration range. J Neurochem. 2011;117(4):703–711. [DOI] [PubMed] [Google Scholar]
- 27. Kimura R, Okouchi M, Fujioka H, et al. Glucagon-Like Peptide-1 (Glp-1) Protects against Methylglyoxal-Induced Pc12 Cell Apoptosis through the Pi3k/Akt/Mtor/Gclc/Redox Signaling Pathway. Neuroscience. 2009;162(4):1212–1219. [DOI] [PubMed] [Google Scholar]
- 28. Imai Y, Yamagishi H, Ono Y, Ueda Y. Versatile inhibitory effects of the flavonoid-derived PI3K/Akt inhibitor, LY294002, on ATP-binding cassette transporters that characterize stem cells. Clin Trans Med. 2012;1(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3 K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6(3):184–192. [DOI] [PubMed] [Google Scholar]
- 30. Matsuda S, Nakanishi A, Wada Y, Kitagishi Y. Roles of PI3K/AKT/PTEN Pathway as a Target for Pharmaceutical Therapy. Open Med Chem J. 2013;7:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Itoh K, Chiba T, Takahashi S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236(2):313–322. [DOI] [PubMed] [Google Scholar]
- 32. Umemura K, Itoh T, Hamada N, et al. Preconditioning by sesquiterpene lactone enhances H2O2-induced Nrf2/ARE activation. Biochem Biophys Res Commun. 2008;368(4):948–954. [DOI] [PubMed] [Google Scholar]
- 33. Ouyang Y, Chen Z, Tan M, et al. Carvedilol, a third-generation beta-blocker prevents oxidative stress-induced neuronal death and activates Nrf2/ARE pathway in HT22 cells. Biochem Biophys Res Commun. 2013;441(4):917–922. [DOI] [PubMed] [Google Scholar]
- 34. Sosa V, Moline T, Somoza R, Paciucci R, Kondoh H, ME LL. Oxidative stress and cancer: an overview. Ageing Res Rev. 2013;12(1):376–390. [DOI] [PubMed] [Google Scholar]
- 35. Zhang N, Shu HY, Huang T, et al. Nrf2 signaling contributes to the neuroprotective effects of urate against 6-OHDA toxicity. PloS One. 2014;9(6):e100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao X, Aronowski J. Nrf2 to pre-condition the brain against injury caused by products of hemolysis after ICH. Transl Stroke Res. 2013;4(1):71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Linker RA, Lee DH, Ryan S, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;134(pt 3):678–692. [DOI] [PubMed] [Google Scholar]
- 38. Leonardo CC, Dore S. Dietary flavonoids are neuroprotective through Nrf2-coordinated induction of endogenous cytoprotective proteins. Nutr Neurosci. 2011;14(5):226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mattson MP, Calabrese EJ. Hormesis: A Revolution in Biology, Toxicology and Medicine. Totowa: Humana Press Inc; 2010. [Google Scholar]
- 40. Gorrini C, Gang BP, Bassi C, et al. Estrogen controls the survival of BRCA1-deficient cells via a PI3K-NRF2-regulated pathway. Proc Natl Acad Sci U S A. 2014;111(12):4472–4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Das BN, Kim YW, Keum YS. Mechanisms of Nrf2/Keap1-dependent phase II cytoprotective and detoxifying gene expression and potential cellular targets of chemopreventive isothiocyanates. Oxid Med cell longev. 2013;2013:839409. [DOI] [PMC free article] [PubMed] [Google Scholar]