Abstract
Research on bisphenol A (BPA) as an environmental contaminant has now major regulatory implications toward the ecosystem health, and hence it is incumbent on scientists to do their research to the highest standards possible, in order that the most appropriate decisions are made to mitigate the impacts to aquatic wildlife. However, the contribution given so far appears rather fragmented. The present overview aims to collect available information on the effects of BPA on aquatic vertebrates and invertebrates to provide a general scenario and to suggest future developments toward more comprehensive approaches useful for aquatic species protection.
Keywords: BPA, endocrine disrupting chemicals (EDC), dose–response, fish, amphibians, invertebrates
Effects of BPA on Vertebrate Species
As for many other chemicals, rivers and lakes are the major sinks for bisphenol A (BPA); therefore, the majority of data on the impact of BPA on wildlife come from studies on aquatic vertebrates, fish in particular and, to a minor extent, amphibians, with some available data also in reptiles and birds. Independent of the species, most studies were carried out in controlled settings, while few of them really focused on wild populations. However, since in the aquatic environment, (i) BPA concentrations vary dramatically, (ii) BPA is part of a complex mixture of chemical stressors, and (iii) the different vertebrate classes and species have different sensitivities to xenobiotics, undoubtedly laboratory experiments provide better insights toward the understanding of the mode of action of BPA, its dose response, and its effects on animal physiology. Some particularly sensitive species, tissues, and life stages have been addressed and useful for further investigations. Tests for screening and assessment of BPA toxicity based on fish are also under development in relation to regulatory aspects, aiming to the possible replacement of BPA with more ecocompatible compounds.
Vertebrates as Test Organisms for the Effects of BPA
Overall, BPA is reported to act as a teratogen and endocrine disruptor in vertebrate animals (Flint et al. 2012). Teratogenic effects were found at high (1-10 mg/L range; Iwamuro et al. 2003; Sone et al. 2004), while endocrine and pleiotropic effects have been observed at lower doses (within the µg/L range; Flint et al. 2012), possibly reflecting the effective concentrations present in the environment.
To establish what concentrations of BPA are to be considered as environmentally relevant has been a difficult task. The highest amounts of BPA are reported in landfill leachate and pulp mill effluents (up to 17 mg/L; Flint et al. 2012), while concentrations in the low µg/L range are found in surface waters (Crain et al. 2007; Flint et al. 2012). BPA has been detected in river and marine sediments (eg, 43 and up to 191 g/kg dw; Flint et al. 2012 and Koh et al. 2006, respectively) and at a concentration of about 100 µg/kg dw in soils; however, not enough data are available to discuss the relationships between these concentrations and the real exposure/uptake in different animals. According to a vast collection of data, some authors defined about 12 µg/L or lower as the environmentally relevant concentration in surface waters (Flint et al. 2012). This value may be adopted to discriminate among the effects in aquatic vertebrates described subsequently, in order to acknowledge particular importance to the responses observed at environmental concentrations.
Possible Mechanisms of Action of BPA as an Endocrine Disrupting Chemical in Vertebrates
In comparison to mammals, information on the mechanisms of action of BPA in nonmammalian vertebrates is far behind. Only a few studies were addressed to the interaction of BPA with cellular receptors and signaling. Furthermore, model organisms and experimental designs were different among studies, and dose–response effects were rarely measured. Available evidence suggests that BPA interactions similar to those observed in mammals can be hypothesized; however, since the exposure is more prolonged and routes of exposure to BPA are different, especially in the aquatic environment, more focused investigations are needed.
As a matter of fact, the main route of exposure in fish is not the diet, but inhalation through the gills, and metabolism of BPA via this route is not as efficient as in the liver. Thus, it is not surprising that waterborne BPA consistently produces more relevant estrogenic effects in fish, including the induction of egg yolk protein precursor vitellogenin (vtg) in males (Kang et al. 2007).
The general consensus is that the estrogenic activity of BPA is mediated through its binding to estrogen receptors (ERs) in fish (Gibert et al. 2011) as well as in frogs (Lutz and Kloas, 1999; Suzuki et al. 2004b).
A relative estrogenic activity of BPA, calculated to be about 0.008% of estradiol (E2) activity, was observed in Xenopus hepatocytes (Mitsui et al. 2007). These estimates are within the range of those reported in the hepatocyte vtg assays on fish species (for a review, Navas and Segner, 2006). It was also found that BPA has a significant antagonistic activity on ER (approximately 0.8% of tamoxifene activity). This evidence would also explain why the BPA-ER complex did not cause the postranscriptional enhancement of vtg synthesis in the Xenopus system (Mitsui et al. 2007).
More recent advances were obtained in zebrafish, where it was demonstrated that the orphan nuclear estrogen-related receptor y (ERRy) is the mediator of BPA-induced malformation of the otoliths (Tohmé et al. 2014). By blocking the ERRy function, the effects of BPA on otoliths were abolished, further supporting this hypothesis. According to this evidence, the effects induced by BPA in fish may be wider than anticipated. In particular, the metabolic effects of BPA through ERRy, that in mammals regulates the expression of gluconeogenesis genes, is also implicated in heart and skeletal muscle metabolism and in the control of insulin secretion. If this were the case also for nonmammalian vertebrates needs to be more widely addressed.
Further data suggest that BPA could also act by using mechanisms independent of ER. Waterborne BPA showed antagonism on androgen receptors (ARs) in fish (Ekman et al. 2012). At 10 and 100 µg/L, it reduced the masculinization effect of trembolone, a potent toxicant that binds fish AR (Ankley et al. 2003) and also reduced the effect of trembolone on hepatic metabolome in female fathead minnows, providing evidence for AR antagonism (Ekman et al. 2012). In vitro, BPA, in the range of 0.1 to 100 µmol/L, inhibited the binding of 3 nmol/L dihydrotestosterone to AR in a dose-dependent manner (Ekman et al. 2012). Previous studies based on both mammalian and fish AR indicated that BPA could act as an antiandrogen, and the potency of BPA as an AR antagonist in vitro was similar for human and fathead minnow ARs (Sultan et al. 2001; Ekman et al. 2012). These data can be explained on the basis of AR conservation in vertebrates; however, in vivo studies never confirmed the antiandrogenic effects in mammals (Bonefeld and Jorgensen, 2007; Howdeshell et al. 2008).
Based on its structural similarity to thyroid hormones (THs) due to its 2 benzoic rings, BPA has been proposed to act as a TH antagonist or agonist and to cause disruption of the thyroid system (Zoeller et al. 2005; Hiroi et al. 2006; Jung et al. 2007). These data were mainly obtained in models of amphibian metamorphosis. Using larval stages of Xenopus, Iwamuro et al. (2006) found that BPA blocked both spontaneous and TH-induced metamorphosis in vivo, as well as the TH-induced tail resorption in tail cell culture in vitro. According to Goto et al. (2006), Rana rugosa tadpole tails displayed marked apoptotic features by triiodothyronine (T3) treatment, which were blocked by a simultaneous presence of BPA. The compound also suppressed corticotropin-releasing factor (CRF)-inducible release of thyroid-stimulating hormone (TSH) and thyrotropin-releasing hormone (TRH)-inducible release of both TSH and prolactin from the pituitary gland. Estradiol did not regulate the release of TSH and prolactin in tadpoles, confirming that the effects of BPA on the release of the pituitary hormones were not related to ER binding. Iwamuro et al. (2006) also showed that BPA decreased the expression of genes related to metamorphosis in a tail cell culture, reinforcing the hypothesis that BPA induces its effect by directly binding to thyroid hormone receptors (TR; Iwamuro et al. 2003, reviewed by Zoeller, 2005).
However, BPA was shown to be a weak ligand to liver TR in rodents, while being a potent inhibitor of T3 binding to human TH-binding proteins. So, as in mammals, also in other vertebrates, the TH antagonism could be played not at the receptor level, but through the ability of the chemical to suppress TR-mediated transcription and consequently to reduce TR availability (Mathieu-Denoncourt et al. 2014).
On these basis, we can conclude that BPA, besides its estrogenic effects and ability to bind ERRy, possesses anti-TH and antiandrogen hormone activities on aquatic vertebrates.
Mode of Action and Pleiotropic Effects of BPA in Vertebrates: Some Examples in Aquatic Animals
Sex and reproduction
Abnormal sex ratios (up to 11 females: 1 male) were found in zebrafish fries fed with food containing BPA, at 2000 mg /kg, which is an ecologically unrealistic dose. No effects were observed below 500 mg BPA/kg (Drastichova et al. 2005).
Treatment with 22.8 µg/L BPA induced approximately 62% to 70% of females in Xenopus laevis (12 week-treatment; Kloas et al. 1999; 2-week treatment, Levy et al. 2004). In contrast, a 90-day treatment in the range of 0.81 to 4,971 µg/L BPA did not affect frog sex ratio (Pickford et al. 2003). Inhibition of metamorphosis was observed in the frog Silurana tropicalis after 9 days of exposure at 2.3 µg/L BPA (Kashiwagi et al. 2008). The BPA effects at low doses were observed on the development of the viviparous swordtail fish, where exposure to 7 µg/L BPA for 60 days reduced tail length (Kwak et al. 2001).
Vitellogenin is a large (250-600 kDa mm) calcium-binding phospholipoglycoprotein precursor of egg yolk proteins that is common to all oviparous vertebrates and required for normal oocyte maturation. It is produced by the liver upon estrogen stimulation and released into the blood. The Vtg production is normally restricted to mature females, and little, if any, vtg is normally detected in males or sexually immature females. However, exposure to estrogenic compounds can trigger its expression in males, since they carry the vtg gene (Sumpter and Jobling, 1995). For monitoring purposes, vtg can be measured in the liver, blood, and mucus of male and female fish as well as in primary hepatocyte cultures (Navas and Segner, 2006).
BPA induced synthesis of vtg and expression of vtg messenger RNA (mRNA) in multiple species of fish and amphibians at concentrations ranging from 10 to 2000 μg/L (Hatef et al. 2012; Mihaich et al. 2012; Arukwe et al. 2000; Sohoni et al. 2001; Kloas et al. 1999; Gye and Kim 2005). No increases were observed in goldfish at concentrations below 5 μg/L (Hatef et al. 2012). However, increased vtg levels were measured in the plasma of female and male carp (Cyprinus carpio) after a 2-week exposure to BPA concentrations as low as 1 µg/L, with further increases at 10 to 1000 µg/L (Mandich et al. 2007).
The dose–response curves of BPA on vtg expression are available for adult fathead minnows and zebrafish (Villeneuve et al. 2012). Fish were exposed to 0, 0.01, 0.1, 1.0, 10, or 100 μg/L BPA for 96 hours, and the compound significantly increased plasma vtg concentrations in both males and females of fathead minnows, at concentrations higher than 10 μg/L. Moreover, E2 concentrations were significantly decreased in plasma of females, while circulating testosterone levels were significantly reduced in males, consistently with a negative feedback response to an increasing estrogenic signal. Zebrafish appeared less sensitive to BPA, and increases in vtg were observed only in males (at 10 and 100 μg/L BPA), although to a much lower extent than in fathead minnows (Villeneuve et al. 2012).
In the same work, the ovarian transcription profiles for fathead minnows and zebrafish were evaluated (Villeneuve et al. 2012). At the lowest BPA concentrations, enrichment of differentially expressed genes associated with cell cycle control and mitosis was observed only in zebrafish. In fathead minnows, the expression of genes with functions related to intracellular signaling processes was upregulated. Conversely, reduced expression of oocyte growth-related genes, together with genes associated with cholesterol uptake, and genes coding for various matrix metalloproteinases, which play a role in ovulation, was reported in BPA-treated fish versus controls.
Expression of genes related to steroid biosynthesis was induced in fish exposed to the lowest concentrations of BPA compared to controls (Villeneuve et al. 2012). Overall, from this work it emerged that after 4 days of exposure to 0.01 μg/L BPA, approximately 4% and 2% of microarray features for fathead minnow and zebrafish females, respectively, had expression levels significantly different from controls. The level of impact on the ovarian transcriptome declined in the 0.1 and 1.0 μg BPA/L treatments and then increased dramatically at the 10 μg/L, before falling to values similar to controls at 100 μg/L BPA. It is worth noting that the effects observed at 10 μg/L are somehow consistent with the effects observed in vtg or hormone production; however, the changes in microarray features at 100 μg/L BPA are in clear contrast. Such apparent inconsistency reduces the importance of the information, at present. However, toxicogenomics data clearly exemplify an inverted U dose–response pattern to BPA, thus advising for additional studies more specifically focused on the low doses.
At upper levels of the hierarchy of the reproductive axis, a complex network of neuroendocrine systems act coordinately to regulate the reproductive functions. Gonadotropin releasing hormone (GnRH) decapeptides are produced in the hypothalamus and bind to GnRH receptors on the cell surface of gonadotrope cells. Here they stimulate the synthesis and release of the gonadotropins, which in turn act on the gonads to stimulate maturation, gametogenesis, and steroidogenesis. Adult rare minnows Gobiocypris rarus exposed for 35 days to environmental concentrations of BPA (5 and 15 μg/L) showed significant downregulation of cyp19a1a mRNA expression, which suggests a reduced synthesis of estrogen in the ovary of female fish because of lower aromatase activity (Qin et al. 2013). Both GnRH3 and GnRH1A receptors were significantly upregulated in the brains of females exposed to 15 μg/L BPA. The potential negative feedback on the GnRH system in response to the alteration of downstream patterns of the brain–pituitary–gonadal axis is then suggested, although the mechanism underlying the modulation of neuroendocrine systems by BPA has not been clarified.
Data from the field highlighted that BPA contributes to intersex conditions. Immature barbels (Barbus sp) living in a polluted river had a high incidence of intersexuality, with gonads containing oogonia and previtellogenic oocytes and also spermatogonia, spermatoctyes, and spermatids (Viganò et al. 2006). The highest BPA content in the water was about 0.3 µg/L (Viganò et al. 2006), and the results were therefore not consistent with those from laboratory tests on the Japanese medaka (Oryzias latipes), where similar feminized testes occurred after 3 weeks of exposure at BPA concentrations of about 800 µg/L or higher (Kang et al. 2002). Such evidence suggests that the environmental concentrations of BPA per se were probably not able to induce intersex conditions, instead, additive or synergistic effects by a number of different compounds, possibly present at low concentrations, could be hypothesized in this case.
Finally, examples of sex disruption by BPA are also provided in aquatic reptiles. In some species, gender is determined by the temperature of egg incubation during a critical window of development. While control embryos of Caiman latirostris incubated at 30°C all developed ovaries and at 33°C all developed testes, in ovo exposure to BPA (about 1000 µg/egg) determined that all resultant alligator offspring were females, regardless of the incubation temperature (Stoker et al. 2003). Further investigations (Durando et al. 2013) indicated that after in ovo exposure to lower BPA concentrations (about 90 µg/egg) at 33°C, all the hatched individuals were males; however, their testes showed disrupted seminiferous tubules. No changes in the expression of genes that regulate sex determination and differentiation in neonatal caimans were found at the above conditions (Durando et al. 2013).
Gonad functionality
Several reports are available supporting the ability of BPA to affect gonad functionality at environmentally relevant concentrations (<12 µg/L). At about 1 µg/L BPA showed a reduced sperm density and mobility in brown trout, Salmo trutta (Lahnsteiner et al. 2005,) and a reduction in sperm production was reported in fathead minnows Pimephales promelas (Sohoni et al. 2001). Three months of fish exposure to about 2 and 5 µg/L caused delayed ovulation and no ovulation, respectively (Lahnsteiner et al. 2005).
In the carp C. carpio, a 2-week exposure to 1 µg/L caused alterations in male gonad structure and atresic oocytes in females (Mandich et al. 2007). Changes in gonad endocrine activity were deduced from the reduction of sex hormones in plasma after a 2-week exposure of fish to BPA. In males, exposure to 1 to 10 µg/L BPA strongly reduced E2 plasma levels, which returned to basal levels at 100 µg/L and increased at 1000 µg/L. Conversely, testosterone plasma levels were increased with respect to controls at 1 µg/L and reduced at higher concentrations. In females, a strong decrease in E2 plasma levels was observed at 1 up to 100 µg/L, followed by recovery at 1000 µg/L. Testosterone levels instead were increased at 1 µg/L and decreased at 100 and 1000 µg/L (Mandich et al. 2007). In male goldfish exposed to 0.6, 4.5, and 11 µg/L BPA, testosterone and 11-ketotestosterone were not affected at day 10 but were significantly decreased after 20 and 30 days. In particular, testosterone levels were decreased at 4.5 and 11 µg/L, while 11-ketotestosterone levels were decreased at 0.6 and 4.5 µg/L (Hatef et al. 2012).
After a 14-day exposure to 10, 100, and 1000 µg/L BPA, the gonads of zebrafish had a normal external appearance; however, multiple alterations in the ovaries with degeneration of the cell components and a significant increase in atresic follicles were induced at 100 and 1000 µg/L BPA (Molina et al. 2013). These data highlight that an exposure to BPA for 2 weeks may be sufficient to affect gonadal function in the zebrafish, although the active concentrations are above the average environmental levels.
Bisphenol A has been shown to affect maturation and quality of eggs and semen in adult brown trout (Lahnsteiner et al. 2005); however, the same species seemed to be relatively insensitive to the exposure concentration in the period from fertilization until larvae swim-up (Bachmann-Bjerregaard et al. 2008). Brown trout embryos and larvae were exposed to BPA at environmentally realistic concentrations (from 1.75 µg/L) from 0 to 63 days postfertilization (dpf) corresponding to the swim stage, and then gonad development was followed up to 400 dpf. No alterations were observed after BPA exposure in these experimental conditions (Bachmann-Bjerregaard et al. 2008).
Fathead minnows exposed to nominal concentrations of 1, 16, 64, 160, and 640 µg/L BPA for 164 days (Mihaich et al. 2013) showed male gonad cell type frequencies significantly different from controls and a slight decrease in spermatocytes at 160 µg/L or higher concentrations. No further changes in growth, gonadosomatic index, or reproduction variables (eg, number of eggs, hatchability) were detected after 164 days (Mihaich et al. 2013).
Development
Acute exposure of rainbow trout eggs to 30 and 100 µg/L BPA for 3 hours prior to fertilization, in order to mimick accumulation of the compound by maternal transfer, did not modify fertilization rate but delayed hatching, yolk reabsorption, larval growth, and first feeding of larvae of about 7 days compared to controls (Aluru et al. 2010). Amounts of 32 and 420 ng BPA/oocyte were detected after a 3-day exposure and they dropped by at least 90% after 13 days postfertilization. Although BPA was cleared from the embryos within 31 days postfertilization, growth suppression persisted in juvenile fish. Moreover, adult developed from BPA-exposed oocytes displayed a disturbed plasma cortisol and glucose profile in response to stressors, suggesting the disruption of the hypothalamus–pituitary–interrenal axis and metabolic disfunctions during early embryogenesis (Aluru et al. 2010).
BPA showed dramatic effects on amphibian metamorphosis. The time of development was delayed, and body length and weight were reduced in tadpoles of Rana chensinensis continuously exposed to 10 µmol/L BPA till metamorphosis; delayed or arrested development, reduced body size, persistent yolk plug, microcephaly, underdeveloped gills, mouth malformations, and further alterations were documented in Rhinella arenarum exposed to BPA in the range of 1.25 and 40 mg/L (Wolkowicz et al. 2014).
Thyroid system
Transcriptome analyses of TH-regulated genes in zebrafish during the eleutheroembryonic stage (days 2-5 postfertilization) were performed to detect potential markers of thyroid disruption. Bisphenol A (0.1, 0.4, 1, 2, and 4 mg/L) acted as a weak T3 agonist when tested alone, but it strongly enhanced the effect of subsaturating concentrations of T3, with effects at 2 and 4 mg/L (Pelayo et al. 2012). The chemical did not prevent the ability of thyroid follicles to synthesize thyroxine, a landmark for substances able to suppress thyroid gland functions.
Exposure to 200 µg/L BPA accelerated early embryonic development within 24 hours of exposure, attenuated body growth, and advanced the times of hatching and reproductive maturation in the medaka, O latipes (Ramakrishnan and Wayne 2008). The acceleration in embryonic development and time to hatch were blocked by the thyroid-hormone receptor antagonist amiodarone, suggesting that BPA alters global developmental timing through a thyroid-hormone pathway.
Conversely, BPA showed lethality in zebrafish larvae (96 hour-LC50 was 8.04 mg/L) and hatching success for zebrafish embryos was reduced by about 50% after 96 hours of exposure at BPA 5.25 mg/L (Chan and Chan 2012). However, at these high concentrations, the chemical did not change the expression of any of the mRNA related to the thyroid system, for example, thyroglobulin, TH receptors, and transthyretin (Chan and Chan 2012). By using the microarray technology, it was shown that 4 days of premetamorphic X laevis tadpoles treatment with BPA (22.8 and 228 μg/L) caused a downregulation of about 33% of the T3-induced genes. Many of these genes were early and/or direct target genes of T3 necessary for apoptosis, tissue morphogenesis, and further modifications (Heimeier et al. 2009). Since the TH T3 is the causative agent of amphibian development, these data indicate that decreases in transcription of T3-regulated genes is the main cause for the inhibition of amphibian metamorphosis by BPA.
In vivo and in vitro studies on adult bullfrog (Rana catesbeiana) pituitary demonstrated that neither BPA nor T3 affected the spontaneous release of TSH, while 100 µmol/L BPA partially blocked the CRF-inducible TSH release; this inhibition was additive with respect to T3 or T4 (Kaneko et al. 2008). Moreover, 10 and 100 µmol/L BPA markedly suppressed the TRH-inducible PRL release, and the effect was not played through ERs.
We may note that the above-mentioned effects of BPA were caused at relatively high concentrations, and the different effects and active concentrations found in the different approaches seem to depend on the type of tissue and animal species under investigation. Therefore, whether BPA may affect the animal thyroid system in the natural environment needs to be ascertained.
Metabolism
Human obesity and related disorders have increased dramatically over the past decades, reaching epidemic proportions. The worldwide prevalence of obesity more than doubled between 1980 and 2014 (WHO 2015). Among the suggested causes is the increasing exposure to chemicals (obesogens) that more appropriately are addressed to as metabolic disrupters. This class of compounds affect mainly adipocyte physiology and alter adipocyte mass and energy homeostasis (Regnier and Sargis 2014). A common obesogen target is the peroxisome proliferator activated receptor γ (PPARγ), a nuclear receptor family member which has widely been recognized as a master regulator of adipogenesis. Some evidence for obesogenic effects of BPA has been obtained also in lower vertebrates. Zebrafish embryo exposure to BPA activated PPARγ and induced lipid accumulation as well as late-onset weight gain in juvenile zebrafish (Riu et al. 2014). Recently, a laboratory test for examining the effects potential obesogens taken through the diet and exposure to environmental contaminants has been developed in zebrafish larvae (Tingaud-Sequeira et al. 2011). The assay proved able to evaluate the obesogenic effect of PPARγ agonists and tributyltin (a well-known environmental obesogen), being useful also for BPA. However, the knowledge on the modulation of satiety, energy balance, and their main regulators (eg, leptin, a nonglycosylated peptide target of estrogens with a key role in fish appetite; Salmeron et al. 2015) in nonmammalian fauna is scarce and its relationship with BPA exposure has not been investigated yet.
Immune function
Besides being an endocrine disruptor, BPA increased lymphocyte proliferation at 5 µg/L to 50 mg/L concentrations in aquacultured goldfish, Carassius auratus. At higher doses (500-1000 mg/L), it inhibited macrophage proliferation (Yin et al. 2007). In yellow perch, a 7-day BPA exposure at environmentally relevant concentrations (2-8 µg/L) significantly increased leukocyte counts (Rogers and Mirza 2013). In the short term, such immunostimulatory response may be beneficial; however, further studies are needed to establish its potential consequences in the long term.
In primary macrophages from head kidney of the common carp (C. carpio), it was observed that BPA exposure significantly enhanced the antibacterial activity at 0.1, 1, or 10 μg/L but induced cell apoptosis in the range of 100 to 10 000 μg/L. The proinflammatory effect of BPA exposure was concomitant with the increase in nitrogen monoxide (NO) and reactive oxygen species, the induction of mRNA for interleukin 1β, NF-κB, and other NF-κB-associated immune genes. It has been hypothesized that increases in oxidant condition may be responsible for the negative effects observed at highest BPA doses (Yang et al. 2015).
Oxidative stress
Concentrations of BPA that can be encountered in nature are capable to induce oxidative stress, leading to impaired sperm quality and DNA fragmentation in fish. Higher levels of protein and lipid oxidation and superoxide dismutase activity were observed in sterlet (Acipenser ruthenus) spermatozoa after exposure to BPA at concentrations 1.75 to 10 μg/L for 2 hours, reflecting oxidative stress. Concomitant decrease in the fish spermatozoa motility and velocity was also shown at the same concentrations, and a dramatic increase in DNA fragmentation was recorded by Comet assay (Hulak et al. 2013).
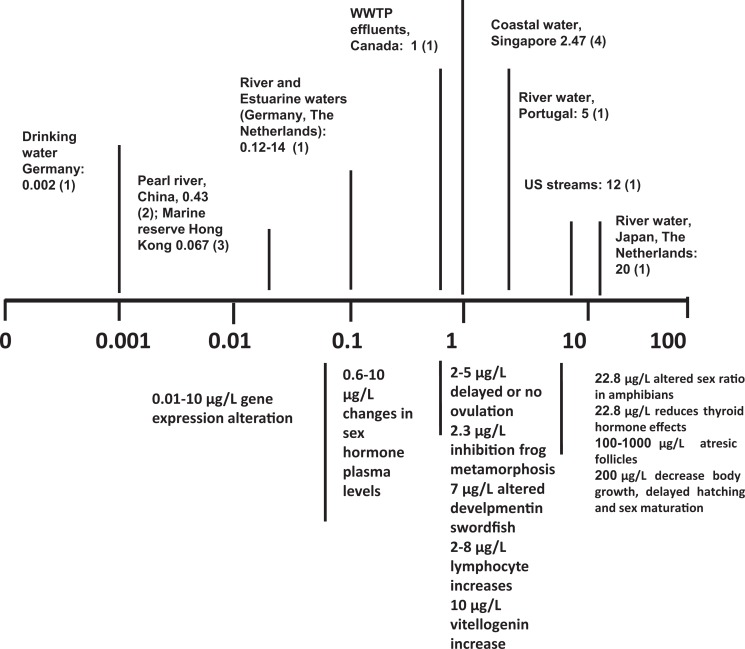
Overall, available data provide convincing evidence that BPA affects physiological homeostasis in aquatic vertebrates, also at environmental (≤12 µg/L) concentrations. A graphical summary is provided in Figure 1. The existence of main windows of susceptibility (development) and targets (gonad functionality) can be deduced, together with a dose- and time-dependent BPA effectiveness. In particular, nonmonotonic dose responses are highlighted, where low doses, encompassing the environmental range, are the most effective.
Figure 1.
Scale bar of effects of environmentally relevant concentrations of bisphenol A (BPA; µg/L) in aquatic vertebrates. Upper panel: Values represent examples of BPA concentrations detected in different types of environmental samples. Lower panel: Values represent examples from laboratory experiments of BPA exposure with different vertebrate models. (1) Flint et al., 2012 and reference therein; (2) Xu W, Yan W, Huang W, Miao L, Zhong L.2014. Endocrine-disrupting chemicals in the Pearl River Delta and coastal environment: sources, transfer, and implications. Environ Geochem Health. 36:1095-1104. (3) Xu EG, Morton B, Lee JH, Leung KM., 2015. Environmental fate and ecological risks of nonylphenols and bisphenol A in the Cape D’Aguilar Marine Reserve, Hong Kong. Mar Pollut Bull. 91:128-138. (4) Basheer C, Lee HK, Tan KS, 2004 Endocrine disrupting alkylphenols and bisphenol-A in coastal waters and supermarket seafood from Singapore. Mar Pollut Bull. 48:1161-1167. (5) Villeneuve et al. 2012. (6) Mandich et al. 2007. (7) Lahsteiner et al. 2005. (8) Kashiwagi et al. 2008. (9) Kwak et al. 2001. (10) Roger and Mirza, 2013. (11) Haterf et al. 2012. (12) Kloas et al. 1999. (13) Heimeier et al. 2009. (14) Molina et al. 2013.
Bisphenol A Bioaccumulation/Elimination in Vertebrates
It is reported that relatively little BPA occurs in animal tissues, with maximum bioconcentration factors (BCFs) well below 1000, which is the limit of concern according to United States Environmental Protection Agency (USEPA) (Staples et al. 1998). Renz et al. (2013) assessed the presence BPA in the brain of 44 of the 58 fish collected from the Greater Pittsburgh area, with a maximum value of 120 pg/g w/w, and a calculated BCF ranging from 0.7 to 143 in different species. In general, bioaccumulation is estimated to occur only at high doses of BPA (Staples et al. 1998), while low doses are biodegraded or metabolized. BPA was detectable in plasma, liver, and muscle of rainbow trout after 2 hours of exposure to 100 µg/L BPA, reaching a steady state within 6 to 12 hours and BCF values of about 3.5-5.5 (Lindholst et al. 2001). Rainbow trout injected with 154 µmol BPA/kg w/w excreted the compound mainly as BPA–glucuronid acid, and a half-life of 3.75 hours was calculated for BPA (Lindholst et al. 2001). The accumulation of BPA–glucuronid acid in the gall fluid before excretion was reported in rainbow trout (Larsson et al. 1999) and in carp (Mandich et al. 2007). Although able of readily excrete BPA, aquatic organisms may be chronically exposed to the compound, and in these cases excretion may be overwhelmed. For example, the field BCF calculated for the bile of the common carp C. linnaeus was higher than those generally obtained in laboratory studies for other fish; however, field and laboratory BCF values of BPA in fish bile are unavailable for comparison (Yang et al. 2014).
Furthermore, metabolism of BPA is known to produce xenoestrogenic compounds as potent as the parent molecule or even more potent, as reported in medaka, O. latipes (Yamaguchi et al. 2005).
Bisphenol A uptake was demonstrated in X. laevis (Fini et al. 2009), which rapidly took up radioactive BPA within 2 hours of exposure, reaching a plateau at 4 hours. After 24 hours from the uptake, about 80% of the BPA was excreted in water showing that tadpoles metabolize the compound similarly to mammals, mainly producing glucuronid- and sulfate derivatives. The percent excretion was reduced accordingly to the lower exposure and uptake, indicating that metabolism is stimulated at higher doses.
Effects of BPA on Invertebrate Species
Invertebrates as Test Organisms for the Effects of BPA
In the Final Report of the State of the Art Assessment of Endocrine Disrupters (Kortenkamp et al. 2011), it was stated that assays utilizing invertebrates offer some advantages over vertebrate models, since their use involves fewer ethical considerations, doses are easier to deliver in the aquatic medium, and their small size and inexpensive cultural requirements allow larger data sets to be collected. Unfortunately, although invertebrates represent 95% of animal species, and important components of aquatic ecosystems, and are, therefore, at considerable risk of exposure, scattered information is available on the biological effects of endocrine disruptive chemicals (EDCs) in these organisms. This is due to the large number of species and diversity of endocrine systems in different phyla and scarcity of data on the synthesis, effects, and mechanisms of action of hormones, steroids in particular, in comparison with those available in vertebrates (Porte et al. 2006; Crain et al. 2007; Scott 2013).
From the first observations indicating that prosobranch molluscs are prone to the endocrine disrupting effects of BPA (Ohelmann et al. 2000), the possible adverse effects of this compound have been investigated in different invertebrate species. However, these studies indicated the absence of reproductive effects of BPA at environmental concentrations in most invertebrate groups, including cnidarians, nematodes, and crustaceans (reviewed in Crain et al. 2007). In contrast, chronic exposure to low levels of BPA induced a superfeminization syndrome (ie, increased egg mass/number) in the freshwater rumshorn snail Marisa cornuarietis (Ohëlmann et al. 2006). On one hand, these observations stimulated a considerable debate over the possible detrimental impact of this compound on invertebrate reproduction (Dietrich et al. 2006; Forbes et al. 2007) on the other, they greatly contributed to focus the attention on aquatic invertebrates as possible targets of BPA (Crain et al. 2007; Kang et al. 2007).
The controversy surrounding the validity of these studies, regarding the failure to compare data obtained in different laboratories, was partly ascribed to differences in the experimental design. In fact, different attempts to reproduce these data underlined the importance of taking into account the influence of both endogenous and environmental variables (ie, seasonal differences in reproductive stage and effects of temperature; Sieratowiczs et al. 2011).
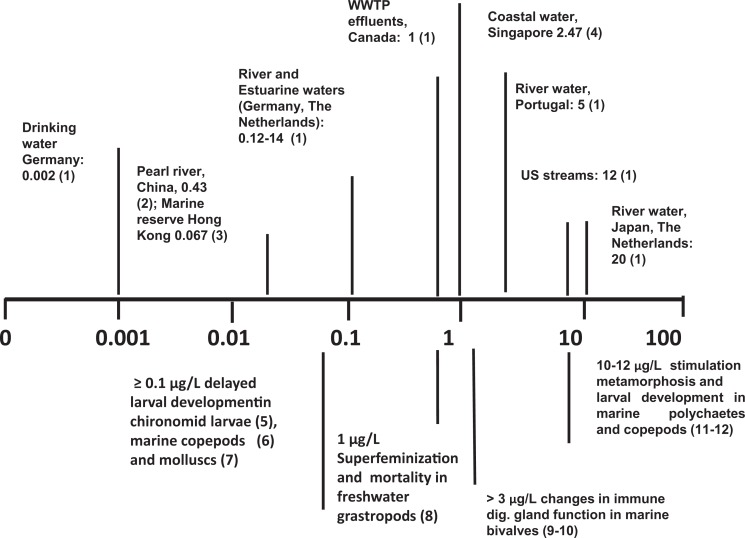
A large number of laboratory studies have been carried out to evaluate the effects of BPA on invertebrate development and reproduction at different concentrations and times of exposure. The results have been recently reviewed by Flint et al. (2012), leading to the conclusion that the effect of BPA appears to vary considerably among related taxa. Although the results obtained from laboratory experiments are obtained at different times of exposure and involve evaluation of different biological endpoints in different species, the available data underline how some invertebrates appear to be quite sensitive to BPA (freshwater and marine molluscs, insect larvae, marine copepods in particular), with effects documented at environmentally relevant concentrations (Figure 2). Differences in sensitivity to BPA can be explained by the differences in endocrine regulation in different groups, in physiological adaptations to different environments, as well as to the diverse modes of action of this compound at the cellular level.
Figure 2.
Scale bar of effects of environmentally relevant concentrations of bisphenol A (BPA; µg/L) in aquatic invertebrates. Upper panel, Values represent examples of maximal BPA concentrations detected in different types of environmental water samples. WWTP indicates waste water treatment plants. Lower panel, Values represent examples from laboratory experiments of BPA exposure with different invertebrate models. For reference 1-4 see Figure 1. (5) Watts et al. 2003. (6) Marcial, H.S., Hagiwara, A., Snell, T.W., 2003. Estrogenic compounds affect development of harpacticoid copepod Tigriopus japonicus. Environ. Toxicol. Chem. 22:3025-3030. (7) Fabbri et al. 2014. (8) Oehlmann, J., Schulte-Oehlmann, U., Kloas, W., Jagnytsch, O., Lutz, I., Kusk, K.O., Wollenberger, L., Santos, E.M., Paull, G.C., Van Look, K.J., Tyler, C.R., 2009. A critical analysis of the biological impacts of plasticizers on wildlife. Phil. Trans. R. Soc. B 364, 2047-2062. (9) Canesi et al. 2005. (10) Canesi et al. 2007. (11) Biggers, W.J., Laufer, H., 2004. Identification of juvenile hormone-active alkylphenols in the lobster Homarus americanus and in marine sediments. Biol. Bull. 206:13-24.(12) Mariager, L., 2001. Effects of Environmental Endocrine Disrupters on a Freshwater and a Marine Crustacean. Master Thesis, Aarhus University Dept. Zool., Inst. Of Biol. Sci., Aarhus, Denmark.
Possible Mechanisms of Action of BPA as an ED in Invertebrate Cells
A great deal of efforts has been made to identify the mechanisms of action of BPA at the cellular level in invertebrate models. The most comprehensively described endocrine system within invertebrates is that of insects, where different hormones act through nuclear receptors (NRs) that control multiple functions at different life stages. The midge Chironomus riparius is a test species with aquatic larval development widely used in aquatic toxicology and identified as a suitable model organism for testing the effects of EDCs in invertebrates (Segner et al. 2003). In C. riparius, BPA significantly increased the mRNA level of the ecdysone receptor, an NR well characterized in insects (Planellò et al. 2008), as well as BPA expression and activity of estrogen-related receptor (Park and Kwak, 2010).
However, the general assumption that BPA acts as a xenoestrogen in vertebrate cells through binding to nuclear ERs, leading to changes in gene transcription (through “classical,” genomic, or receptor-dependent mechanisms of action) is in contrast with the failure in identifying functional ERs in invertebrates (Thornton et al. 2003). The ER-like sequences have been identified in different invertebrate groups, molluscs in particular; however, molluscan ERs generally show constitutive transcriptional activity and are not activated by either natural estrogens or estrogenic compounds (Bingham et al. 2014 and references quoted therein). An accurate analysis on the literature on the effects of steroids and EDCs recently concluded that there is no indisputable bioassay evidence that vertebrate sex steroids (or their agonist/antagonists) have endocrinological or reproductive roles in molluscs (Scott 2013b). A recent genetic and structural analysis of oyster ERs clearly demonstrated that the estrogen-binding site is occluded by hydrophobic residues and it is too small to accommodate the physiological agonist 17β-E2 or other similar compounds. Evolution of constitutive activity was due to 2 substitutions and subsequent mutations filled in the hormone-binding cavity, in a process of vestigialization (Bingham et al. 2014). This implies that molluscan ER is an authentically ligand-independent transcriptional activator, which exists in the active conformation in the absence of ligand or other apparent modifications. Therefore, if estrogen-like compounds, endogenous or environmental, affect mollusc reproduction and physiology, these impacts must be mediated by mechanisms other than ER activation.
Mode of Action and Pleiotropic Effects of BPA in Invertebrates: The Example of the Marine Bivalve Mytilus
The marine bivalve Mytilus is tolerant to fluctuations in abiotic and biotic factors and shows a wide range of responses to environmental stressors and therefore it is worldwide utilized as a model organism for investigating the effects of environmental contaminants, as well as a sentinel species for biomonitoring of coastal areas (Viarengo et al. 2007).
The effects of the natural estrogen 17β-E2 and environmental estrogens, including BPA, have been thoroughly investigated in mussel immune cells, the hemocytes, both in vitro, in short-term exposure experiments (0-60 minutes), and in vivo, in the hemocytes sampled from mussels exposed to BPA for longer periods of time (6-24 hours). In vitro, E2 induces rapid changes in a number of functional parameters, including lysosomal membrane stability, extracellular lysozyme release and phagocytosis, activates the oxidative burst, and stimulates NO production (Canesi et al. 2006). In the hemocyte, the lysosomal function represents a sensitive target for the action of the hormone both in vitro and in vivo. The effects of E2 were prevented by the antiestrogen Tamoxifen and were mediated by rapid, nongenomic mechanisms of action similar to those identified in mammalian cells (Ropero et al. 2006), involving activation of cytosolic kinases such as mitogen-activated protein kinases (MAPKs) and protein kinase C (PKC), and phosphorylation of the transcription factors signal transducers and activators of transcription (STATs) and cyclic adenosine monophosphate responsive element binding protein (CREB; Canesi et al. 2006). The effects of E2 showed an inverted U-shaped response in the nmol/L range, with low concentrations being immunostimulatory and higher concentrations resulting in immunotoxic effects, due to disruption of cell signaling and lysosomal function. These results demonstrated that, like in mammalian cells, E2 can also act through receptor-independent, nongenomic mechanisms of action, involving rapid activation of membrane and cytosolic signaling pathways. These data opened the possibility that, like E2, also EDCs, including BPA, could act through ER-independent mechanisms of action in invertebrate cells like in mammalian cells.
In vitro BPA, like E2, induced destabilization of lysosomal membranes in mussel hemocytes, and the effect was prevented by the antiestrogen Tamoxifen and by the PKC inhibitor GF109203X (Canesi et al. 2004); however, BPA was effective at concentrations about 1000 times higher than those of E2, with a Lowest Observed Effect Concentration (LOEC) of 5 μmol/L, an EC50 of 34 μmol/L, and an E2 equivalency factor (ie, the ratio EC50E2/EC50 test compound, conventionally set at 1 for E2) of 0.38 × 10−3 (Canesi et al. 2007a). Bisphenol A also induced distinct effects on phosphorylation of signaling components, in particular leading to dephosphorylation of the stress-activated p38/MAPK and STAT5. These effects was confirmed in vivo in the hemocytes of mussels injected with much lower concentrations of BPA (nmol/L, from 3 μg/L) and sampled at different times postinjection (6, 12, and 24 hours; Canesi et al. 2005). Moreover, BPA induced a dramatic dephosphorylation of the transcription factor CREB. Taken together, the results demonstrate that in mussel hemocytes, BPA can downregulate signaling components that are crucial in activation of the immune response.
In the same in vivo experimental conditions, significant effects of BPA were also observed in the digestive gland (hepatopancreas), a tissue involved in the control of metabolism and gamete maturation. Bisphenol A affected gene expression, including that of the Mytilus ER2, antioxidant enzyme activities, and lysosomal function, with similar and distinct effect with respect to E2 (Canesi et al. 2007b). Overall, these data indicate that BPA, at environmentally relevant concentrations, can have both estrogen-like and distinct effects in invertebrates like in vertebrates, modulating both immune and digestive gland function.
In mussel digestive gland, BPA was recently shown to affect 17 β-hydroxysteroid dehydrogenases (17β-HSDs), multifunctional enzymes involved in the metabolism of steroids, fatty acids, retinoids, and bile acid (Zhang et al. 2014). Expression of 2 novel types of 17β-HSDs (MgHsd17b10 and MgHsd17b12) identified in Mytilus galloprovincialis was largely but transiently decreased by 24-hour exposure to BPA (1 and 10 μg/L) in both sexes. A recent metabolomics study investigated the effects of chronic exposure (1 month) to BPA on the gonad of M galloprovincialis (Ji et al. 2014). The results showed that female mussels were sensitive to BPA (1 and 10 μg/L), whereas no significant metabolic responses were observed in males. Both concentrations of BPA induced changes in aminoacid and glycogen content in the female gonad, suggesting gender-related differences, and indicating osmotic stress and changes in energy metabolism, although with distinct effects at different concentrations.
Effects of BPA on Invertebrate Development
Developmental effects of BPA have been observed in invertebrates, in different exposure models, but only in few species at environmental concentrations (see Flint, 2012 and reference quoted therein). In the gastropod Haliotis diversicolor, BPA affected different stages of larval development, with an EC50 of 1.02 μg/L at 96 hours (completion of metamorphosis); proteomic analysis indicated that both chemicals interfered with different physiological pathways including energy and substance metabolism, cell signalling, formation of cytoskeleton and cilium, immune, and stress responses at the same time, leading to the failure of metamorphosis (Liu et al. 2011). In the bivalve M. galloprovincialis, exposure of fertilized eggs to BPA affected the formation of fully developed D-larvae at 48 hours, indicating a delay in development, with an EC50 of 3.68 μg/L (Fabbri et al. 2014). Interestingly, similar effect were observed with E2 (Canesi, unpublished observations). These results support the hypothesis that environmentally relevant levels of BPA could affect embryogenesis in molluscan species.
Bisphenol A Bioaccumulation/Elimination in Invertebrates
Bisphenol A discharge can occur from the migration of BPA-based products into rivers and marine waters, and in particular from effluents from wastewater treatment plants and landfill sites. However, in the aquatic environment, BPA can undergo photodegradation or biodegradation through microbial or plant activities and metabolism by aquatic animals. As a result of this, relatively little environmental BPA occurs in biota compared to nonbiotic environmental compartments (reviewed by Flint, 2012). Sparse reports on BPA concentrations in aquatic invertebrates are available (Nurulnadia et al. 2014 and references quoted therein), with levels in the order of ng/g w/w, generally lower than those found for other alkhylphenols such as Nonyl-phenol. Information on accumulation and metabolism of BPA is available in molluscs. In freshwater, bivalves (Pisidium amnicum) exposed to environmentally relevant BPA concentrations temperature-dependent BCFs ranged from 110 to 144 (Heinonen et al. 2002). A comparable bioconcentration was observed in the brackish bivalve Corbicula japonica, where BPA is mainly accumulated in the visceral mass, rapidly reaching a steady state in 4 hours, where it is metabolized to mono- and disulfate concentrations found in aquatic invertebrates (Hayashi et al. 2008)
Conclusions
Laboratory studies indicate that in non-mammalian vertebrates, in particular aquatic species, BPA causes developmental and reproductive effects, including reduction of male hormones, death of testicular cells, decreased sperm density and motility, inhibition of spermatogenesis and egg production, along with delayed or absent ovulation, and impairment of sex ratio. Furthermore, the chemical proved able to affect other systems, for example, disturbed immune function and metabolism. Therefore, the compound appears to be a multifunctional endocrine disrupter. The whole animal may respond to BPA in a different way according to its developmental and physiological status, which would justify the difficulties encountered when drawing possible mechanisms of action.
With regard to invertebrate species, according to the USEPA final Report (2014), BPA showed high chronic aquatic toxicity; however, experimental studies located for daphnid used as an invertebrate test species were of insufficient exposure duration to be utilized to assign the hazard concern. Taken together, the available results indicate that neither ecotoxicity tests carried out in model species nor studies focused on a mechanistic approach based on interactions of BPA with ERs are sufficient to explain and predict the effects of BPA, or other EDCs, in different invertebrate systems. The greater susceptibility of certain invertebrate species may be explained with multiple mechanisms of action, as now recognized also in vertebrates (Rubin et al. 2011). Moreover, knowledge on the physiological status and the responses to abiotic variables of the test organism is crucial in evaluating the impact BPA in invertebrate groups. However, taken together available data support the hypothesis that environmental concentrations of BPA (low μg/L) can elicit significant responses on certain groups of aquatic invertebrates, mollusks, and copepods in particular.
Overall, from available data in both vertebrates and invertebrates, there is agreement on the fact that BPA is a chemical of potential concern for the ecosystem.
In many cases, the concentrations of BPA necessary to cause such effects exceeded the average concentrations in the environment or were in the upper range. The laboratory studies cannot account for the life-long animal exposure to BPA, since it is continuously released in large amounts. Thus, an underestimation of the effects is conceivable, also considering that wildlife species may be exposed to higher BPA concentrations in specific matrices (leachates, plants effluents, river, and marine sediments).
The present review highlights that a few reports address dose–response effects of BPA exposure. In the absence of clear dose response curves, and due to the shortcomings in experimental designs, a conclusion cannot be reached regarding the BPA impact on ecosystem. These studies would have major regulatory implications, and hence it is incumbent on scientists to do their research to the highest standards possible, in order that the most appropriate decisions are taken.
Recommendations
Develop a full toxicological assessment on BPA to determine an acceptable freshwater and marine exposure level.
Identify which animal species are most at risk to environmental BPA levels.
Perform more studies in the natural environment to evaluate real concentrations and long-term exposures.
Investigate the relative importance of different exposure pathway to BPA (digestive tract, tegument, and respiratory surfaces) for wildlife.
Evaluate the bioaccumulation potential of BPA, especially in edible species, also in the light of the latest EFSA reevaluation of BPA exposure and toxicity and concomitant reduction of the safe highest dietary exposure to BPA from 50 to 4 µg/kg of bw/d (EFSA, 2015)
Assess effects at low BPA doses and avoid narrow dose ranges; testing 1 or 2 doses may lead to erroneous conclusions since nonmonotonic dose–response curves are encountered frequently in basic endocrinology.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- Aluru N, Leatherland JF, Vijayan MM. 2010. Bisphenol A in oocytes leads to growth suppression and altered stress performance in juvenile rainbow trout. Plos One 2010 5(5): e10741 doi: 10.1371/journal.pone.0010741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley GT, Jensen KM, Makynen EA, Kahl MD, Korte JJ, Hornung MW, Henry TR, Denny JS, Leino RL, Wilson VS, Cardon MC, Hartig PC, Gray LE. 2003. Effects of the androgenic growth promoter 17-beta-trenbolone on fecundity and reproductive endocrinology of the fathead minnow. Environ Toxicol Chem 22:1350–1360. [PubMed] [Google Scholar]
- Arukwe A, Celius T, Walther BT, Goksøyr A. 2000. Effects of xenoestrogen treatment on zona radiata protein and vitellogenin expression in Atlantic salmon (Salmo salar). Aquat Toxicol 49:159–170. [DOI] [PubMed] [Google Scholar]
- Bjerregaard LB, Lindholst C, Korsgaard B, Bjerregaard P. 2008. Sex hormone concentrations and gonad histology in brown trout (Salmo trutta) exposed to 17beta-estradiol and bisphenol A. Ecotoxicology 17:252–263. [DOI] [PubMed] [Google Scholar]
- Bonefeld-Jørgensen EC, Long M, Hofmeister MV, Vinggaard AM. 2007. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect 115 Suppl 1:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridgham JT, Keay J, Ortlund EA, Thornton JW. 2014. Vestigialization of an allosteric switch: genetic and structural mechanisms for the evolution of constitutive activity in a steroid hormone receptor. PLoS Genet 10: e1004058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canesi L, Betti M, Lorusso LC, Ciacci C, Gallo G. 2005. ‘In vivo’ effects of Bisphenol A in Mytilus hemocytes: modulation of kinase-mediated signalling pathways. Aquat Toxicol 71:73–84. [DOI] [PubMed] [Google Scholar]
- Canesi L, Borghi C, Ciacci C, Fabbri R, Vergani L, Gallo G. 2007a. Bisphenol-A alters gene expression and functional parameters in molluscan hepatopancreas. Mol Cell Endocrinol. 276: 36–44. [DOI] [PubMed] [Google Scholar]
- Canesi L, Ciacci C, Lorusso LC, Betti M, Guarnieri T, Tavolari S, Gallo G. 2006. Immunomodulation by 17beta-estradiol in bivalve hemocytes. Am J Physiol Regul Integr Comp Physiol. 291: R664–673. [DOI] [PubMed] [Google Scholar]
- Canesi L, Lorusso LC, Ciacci C, Betti M, Rocchi M, Pojana G, Marcomini A. 2007b. Immunomodulation of Mytilus hemocytes by individual estrogenic chemicals and environmentally relevant mixtures of estrogens: in vitro and in vivo studies. Aquat Toxicol 81:36–44 [DOI] [PubMed] [Google Scholar]
- Canesi L, Lorusso LC, Ciacci C, Betti M, Zampini M, Gallo G. 2004. Environmental estrogens can affect the function of mussel hemocytes through rapid modulation of kinase pathways. Gen Comp Endocrinol 138:58–69. [DOI] [PubMed] [Google Scholar]
- Chan WK, Chan KM. 2012. Disruption of the hypothalamic-pituitary-thyroid axis in zebrafish embryo-larvae following waterborne exposure to BDE-47, TBBPA and BPA. Aquat Toxicol 108:106–111. [DOI] [PubMed] [Google Scholar]
- Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, Guillette LJ., Jr 2007. An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol 24:225–239. [DOI] [PubMed] [Google Scholar]
- Dietrich DR, O’Brien E, Hoffmann S, Balaguer P, Nicolas JC, Seinen W, Depledge M. 2006. Effects of BPA in snails. Environ Health Perspect. 114: A340–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drastichova J, Svobodova Z, Groenland M, Dobsikova R, Zlabek V, Weissova D. 2005. Effect of exposure to bisphenol A and 17β-estradiol on the sex differentiation in zebrafish (Danio rerio). Acta Vet Brno 74:287–291. [Google Scholar]
- Durando M, Cocito L, Rodríguez HA, Varayoud J, Ramos JG, Luque EH, Muñoz-de-Toro M. 2013. Neonatal expression of amh, sox9 and sf-1 mRNA in Caiman latirostris and effects of in ovo exposure to endocrine disrupting chemicals. Gen Comp Endocrinol 191:31–38. [DOI] [PubMed] [Google Scholar]
- Ekman DR, Hartig PC, Cardon M, Skelton DM, Teng Q, Durhan EJ, Jensen KM, Kahl MD, Villeneuve DL, Gray LE, Jr, Collette TW, Ankley GT. 2012. Metabolite profiling and a transcriptional activation assay provide direct evidence of androgen receptor antagonism by bisphenol A in fish. Environ Sci Technol 46: 9673–9680. [DOI] [PubMed] [Google Scholar]
- EPA Final Report BISPHENOL A ALTERNATIVES IN THERMAL PAPER, January 2014. http://www.epa.gov/dfe/pubs/projects/bpa/bpa-report-complete.pdf.
- Fabbri R, Montagna M, Balbi T, Raffo E, Palumbo F, Canesi L. 2014. Adaptation of the bivalve embryotoxicity assay for the high throughput screening of emerging contaminants in Mytilus galloprovincialis . Mar Environ Res 99:1–8 [DOI] [PubMed] [Google Scholar]
- Fini JB, Dolo L, Cravedi JP, Demeneix B, Zalko D. 2009. Metabolism of the endocrine disruptor BPA by Xenopus laevis tadpoles. Ann N Y Acad Sci. 1163:394–397. [DOI] [PubMed] [Google Scholar]
- Flint S, Markle T, Thompson S, Wallace E. 2012. Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manage 104:19–34. [DOI] [PubMed] [Google Scholar]
- Forbes VE, Aufderheide J, Warbritton R, van der Hoeven N, Caspers N. 2007. Does bisphenol A induce superfeminization in Marisa cornuarietis? Part II: toxicity test results and requirements for statistical power analyses. Ecotoxicol Environ Saf 66:319–325. [DOI] [PubMed] [Google Scholar]
- Gibert Y, Sassi-Messai S, Fini JB, Bernard L, Zalko D, Cravedi JP, Balaguer P, Andersson-Lendahl M, Demeneix B, Laudet V. 2011. Bisphenol A induces otolith malformations during vertebrate embryogenesis. BMC Dev Biol. 11:4 doi: 10.1186/1471-213X-11-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Kitamura S, Kashiwagi K, Oofusa K, Tooi O, Yoshizato Y, Sato J, Ohta S, Kashiwagi A. 2006. Suppression of amphibian metamorphosis by bisphenol A and related chemical substances. J Health Sci 52:160–168. [Google Scholar]
- Gye MC, Kim DH. 2005. Bisphenol A induces hepatic vitellogenin mRNA in male Bombina orientalis. Bull. Environ. Contam. Toxicol 75: 1–6. [DOI] [PubMed] [Google Scholar]
- Hatef A, Zare A, Alavi SM, Habibi HR, Linhart O. 2012. Modulations in androgen and estrogen mediating genes and testicular response in male goldfish exposed to bisphenol A. Environ Toxicol Chem 31:2069–2077. [DOI] [PubMed] [Google Scholar]
- Hayashi O, Kameshiro M, Masuda M, Satoh K. 2008. Bioaccumulation and metabolism of [14C]bisphenol A in the brackish water bivalve Corbicula japonica . Biosci Biotechnol Biochem 7212:3219–3224. [DOI] [PubMed] [Google Scholar]
- Heimeier RA, Das B, Buchholz DR, Shi YB. 2009. The xenoestrogen bisphenol A inhibits postembryonic vertebrate development by antagonizing gene regulation by thyroid hormone. Endocrinology 150:2964–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinonen J, Honkanen J, Kukkonen JV, Holopainen IJ. 2002. Bisphenol A accumulation in the freshwater clam Pisidium amnicum at low temperatures. Arch Environ Contam Toxicol 43:50–55. [DOI] [PubMed] [Google Scholar]
- Hiroi T, Okada K, Imaoka S, Osada M, Funae Y. 2006. Bisphenol A binds to protein disulfide isomerase and inhibits its enzymatic and hormone-binding activities. Endocrinology 147:2773–2780. [DOI] [PubMed] [Google Scholar]
- Howdeshell KL, Furr J, Lambright CR, Wilson VS, Ryan BC, Gray LE., Jr 2008. Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol Sci 102:371–382. [DOI] [PubMed] [Google Scholar]
- Hulak M, Gazo I, Shaliutina A, Linhartova P. 2013. In vitro effects of bisphenol A on the quality parameters, oxidative stress, DNA integrity and adenosine triphosphate content in sterlet (Acipenser ruthenus) spermatozoa. Comp Biochem Physiol C Toxicol Pharmacol 158:64–71. [DOI] [PubMed] [Google Scholar]
- Iwamuro S, Sakakibara M, Terao M, Ozawa A, Kurobe C, Shigeura T, Kato M, Kikuyama S. 2003. Teratogenic and anti-metamorphic effects of bisphenol A on embryonic and larval Xenopus laevis . Gen Comp Endocrinol 133:189–198 [DOI] [PubMed] [Google Scholar]
- Iwamuro S, Yamada M, Kato M, Kikuyama S. 2006. Effects of bisphenol A on thyroid hormone-dependent up-regulation of thyroid hormone receptor alpha and beta and down-regulation of retinoid X receptor gamma in Xenopus tail culture. Life Sci. 79:2165–2171. [DOI] [PubMed] [Google Scholar]
- Ji C, Wei L, Zhao J, Wu H. 2014. Metabolomic analysis revealed that female mussel Mytilus galloprovincialis was sensitive tobisphenol A exposures. Environ Toxicol Pharmacol 37:844–849 [DOI] [PubMed] [Google Scholar]
- Jung KK, Kim SY, Kim TG, Kang JH, Kang SY, Cho JY, Kim SH. 2007. Differential regulation of thyroid hormone receptor-mediated function by endocrine disruptors. Arch Pharm Res. 30:616–623. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Okada R, Yamamoto K, Nakamura M, Mosconi G, Polzonetti-Magni AM, Kikuyama S. 2008. Bisphenol A acts differently from and independently of thyroid hormone in suppressing thyrotropin release from the bullfrog pituitary. Gen Comp Endocrinol 155:574–580 [DOI] [PubMed] [Google Scholar]
- Kang IJ, Yokota H, Oshima Y, Tsuruda Y, Oe T, Imada N, Tadokoro H, Honjo T. 2002. Effects of bisphenol a on the reproduction of Japanese medaka (Oryzias latipes). Environ Toxicol Chem 21:2394–2400. [PubMed] [Google Scholar]
- Kang JH, Asai D, Katayama Y.2007. Bisphenol A in the aquatic environment and its endocrine-disruptive effects on aquatic organisms. Crit Rev Toxicol 37:607–625. [DOI] [PubMed] [Google Scholar]
- Kashiwagi K, Utsumi K, Kashiwagi K, Ohta S, Sugihara K, Hanada H, Kitamura S. 2008. Effects of Endocrine Disrupting Chemicals on amphibian metamorphosis and mitochondrial membrane permeability transition. J. Health Sci. 54: 273–280. [Google Scholar]
- Kloas W, Lutz I, Einspanier R. 1999. Amphibians as a model to study endocrine disruptors: II. Estrogenic activity of environmental chemicals in vitro and in vivo . Sci Total Environ. 225:59–68. [DOI] [PubMed] [Google Scholar]
- Koh CH, Khim JS, Villeneuve DL, Kannan K, Giesy JP. 2006. Characterization of trace organic contaminants in marine sediment from Yeongil Bay, Korea: 1. Instrumental analyses. Environ Pollut. 142: 39–47. [DOI] [PubMed] [Google Scholar]
- Kortenkamp A, Martin O, Faust M, Evans R, McKinlay R, Orton F, et al. 2011. State of the Art Assessment of Endocrine Disruptors. Final Report. http://ec.europa.eu/environment/endocrine/documents/4_SOTA%20EDC%20Final%20Report%20V3%206%20Feb%2012.pdf [DOI] [PubMed]
- Kwak HI, Bae MO, Lee MH, Lee YS, Lee BJ, Kang KS, Chae CH, Sung HJ, Shin JS, Kim JH, Mar WC, Sheen YY, Cho MH. 2001. Effects of nonylphenol, bisphenol A, and their mixture on the viviparous swordtail fish (Xiphophorus helleri). Environ Toxicol Chem. 20:787–795. [PubMed] [Google Scholar]
- Lahnsteiner F, Berger B, Kletzl M, Weismann T. 2005. Effect of bisphenol A on maturation and quality of semen and eggs in the brown trout, Salmo trutta f. fario. Aquat Toxicol. 75:213–224. [DOI] [PubMed] [Google Scholar]
- Larsson DGJ, Adolfsson-Erici M, Parkkonen J, Pettersson M, Berg AH, Olsson P-E. 1999. Ethinyloestradiol—an undesired fish contraceptive? Aquat Toxicol 45:91–97. [Google Scholar]
- Levy G, Lutz I, Krüger A, Kloas W. 2004. Bisphenol A induces feminization in Xenopus laevis tadpoles. Environ Res 94:102–111 [DOI] [PubMed] [Google Scholar]
- Lindholst C, Pedersen KL, Pedersen SN. 2000. Estrogenic response of bisphenol A in rainbow trout (Oncorhynchus mykiss). Aquat Toxicol 48:87–94. [DOI] [PubMed] [Google Scholar]
- Liu Y, Tam NF, Guan Y, Yasojima M, Zhou J, Gao B. 2011. Acute toxicity of nonylphenols and bisphenol A to the embryonic development of the abalone Haliotis diversicolor supertexta. Ecotoxicology 20:1233–1245. [DOI] [PubMed] [Google Scholar]
- Lutz I, Kloas W. 1999. Amphibians as a model to study endocrine disruptors: I. Environmental pollution and estrogen receptor binding. Sci Total Environ 225:49–57. [DOI] [PubMed] [Google Scholar]
- Mandich A, Bottero S, Benfenati E, Cevasco A, Erratico C, Maggioni S, Massari A, Pedemonte F, Viganò L. 2007. In vivo exposure of carp to graded concentrations of bisphenol A. Gen Comp Endocrinol 153:15–24. [DOI] [PubMed] [Google Scholar]
- Mathieu-Denoncourt J, Wallace SJ, de Solla SR, Langlois VS. In press Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. Gen Comp Endocrinol. doi: 10.1016/j.ygcen.2014.11.003. Review. [DOI] [PubMed] [Google Scholar]
- Mihaich E, Rhodes J, Wolf J, van der Hoeven N, Dietrich D, Hall AT, Caspers N, Ortego L, Staples C, Dimond S, Hentges S. 2012. Adult fathead minnow, Pimephales promelas, partial life-cycle reproductive and gonadal histopathology study with bisphenol A. Environ Toxicol Chem 31: 2525–2535. [DOI] [PubMed] [Google Scholar]
- Mitsui N, Tooi O, Kawahara A. 2007. Vitellogenin-inducing activities of natural, synthetic, and environmental estrogens in primary cultured Xenopus laevis hepatocytes. Comp Biochem Physiol C Toxicol Pharmacol 146:581–587 [DOI] [PubMed] [Google Scholar]
- Molina AM, Lora AJ, Blanco A, Monterde JG, Ayala N, Moyano R. 2013. Endocrine-active compound evaluation: qualitative and quantitative histomorphological assessment of zebrafish gonads after bisphenol-A exposure. Ecotoxicol Environ Saf 88:155–162. [DOI] [PubMed] [Google Scholar]
- Navas JM, Segner H. 2006. Vitellogenin synthesis in primary cultures of fish liver cells as endpoint for in vitro screening of the (anti)estrogenic activity of chemical substances. Aquat Toxicol. 80:1–22. [DOI] [PubMed] [Google Scholar]
- Nurulnadia MY, Koyama J, Uno S, Kito A, Kokushi E, Bacolod ET, Ito K, Chuman Y. 2014. Accumulation of endocrine disrupting chemicals (EDCs) in the polychaete Paraprionospio sp. from the Yodo River mouth, Osaka Bay, Japan. Environ Monit Assess 186:1453–1463. [DOI] [PubMed] [Google Scholar]
- Oehlmann J, Schulte-Oehlmann U, Bachmann J, Oetken M, Lutz I, Kloas W, Ternes TA. 2006. Bisphenol A induces superfeminization in the ramshorn snail Marisa cornuarietis (Gastropoda: Prosobranchia) at environmentally relevant concentrations. Environ Health Perspect 114 Suppl 1:127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehlmann J, Schulte-Oehlmann U, Tillmann M, Markert B. 2000. Effects of endocrine disruptors on prosobranch snails (Mollusca: Gastropoda) in the laboratory. Part I: Bisphenol A and octylphenol as xeno-estrogens. Ecotoxicology 9:383–397 [DOI] [PubMed] [Google Scholar]
- Park K, Kwak IS. 2010. Molecular effects of endocrine-disrupting chemicals on the Chironomus riparius estrogen-related receptor gene. Chemosphere 79:934–941 [DOI] [PubMed] [Google Scholar]
- Pelayo S, Oliveira E, Thienpont B, Babin PJ, Raldúa D, André M, Piña B. 2012. Triiodothyronine-induced changes in the zebrafish transcriptome during the eleutheroembryonic stage: implications for bisphenol A developmental toxicity. Aquat Toxicol. 110-111:114–122. [DOI] [PubMed] [Google Scholar]
- Pickford DB, Hetheridge MJ, Caunter JE, Hall AT, Hutchinson TH. 2003. Assessing chronic toxicity of bisphenol A to larvae of the African clawed frog (Xenopus laevis) in a flow-through exposure system. Chemosphere. 53:223–235 [DOI] [PubMed] [Google Scholar]
- Planelló R, Martínez-Guitarte JL, Morcillo G. 2008. The endocrine disruptor bisphenol A increases the expression of HSP70 and ecdysone receptor genes in the aquatic larvae of Chironomus riparius . Chemosphere 71:1870–1876. [DOI] [PubMed] [Google Scholar]
- Porte C, Janer G, Lorusso LC, Ortiz-Zarragoitia M, Cajaraville MP, Fossi MC, Canesi L. 2006. Endocrine disruptors in marine organisms: approaches and perspectives. Comp Biochem Physiol C Toxicol Pharmacol 143:303–315. [DOI] [PubMed] [Google Scholar]
- Qin F, Wang L, Wang X, Liu S, Xu P, Wang H, Wu T, Zhang Y, Zheng Y, Li M, Zhang X, Yuan C, Hu G, Wang Z. 2013. Bisphenol A affects gene expression of gonadotropin-releasing hormones and type I GnRH receptors in brains of adult rare minnow Gobiocypris rarus . Comp Biochem Physiol C Toxicol Pharmacol 157:192–202. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan S, Wayne NL. 2008. Impact of bisphenol-A on early embryonic development and reproductive maturation. Reprod Toxicol 25:177–183. [DOI] [PubMed] [Google Scholar]
- Regnier SM, Sargis RM. 2014. Adipocytes under assault: environmental disruption of adipose physiology. Biochim Biophys Acta 1842(3):520–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz L, Volz C, Michanowicz D, Ferrar K, Christian C, Lenzner D, El-Hefnawy T. 2013. A study of parabens and bisphenol A in surface water and fish brain tissue from the Greater Pittsburgh Area. Ecotoxicology 22:632–641. [DOI] [PubMed] [Google Scholar]
- Rogers JA, Mirza RS. 2013. The Effects of Bisphenol-A on the Immune System of Wild Yellow Perch, Perca flavescens . Water Air Soil Pollut 224:1728–1734 [Google Scholar]
- Ropero AB, Alonso-Magdalena P, Ripoll C, Fuentes E, Nadal A. 2006. Rapid endocrine disruption: environmental estrogen actions triggered outside the nucleus. J Steroid Biochem Mol Biol 102:163–169. [DOI] [PubMed] [Google Scholar]
- Rubin BS. 2011. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol 127:27–34 [DOI] [PubMed] [Google Scholar]
- Salmerón C, Johansson M, Angotzi AR, Rønnestad I, Jönsson E, Björnsson BT, Gutiérrez J, Navarro I, Capilla E. 2015. Effects of nutritional status on plasma leptin levels and in vitro regulation of adipocyte leptin expression and secretion in rainbow trout. Gen Comp Endocrinol 210:114–123. [DOI] [PubMed] [Google Scholar]
- Scott AP. 2013. Do mollusks use vertebrate sex steroids as reproductive hormones? II. Critical review of the evidence that steroids have biological effects. Steroids. 78:268–281. [DOI] [PubMed] [Google Scholar]
- Segner H, Caroll K, Fenske M, Janssen CR, Maack G, Pascoe D, Schäfers C, Vandenbergh GF, Watts M, Wenzel A. 2003. Identification of endocrine-disrupting effects in aquatic vertebrates and invertebrates: report from the European IDEA project. Ecotoxicol Environ Saf 54:302–314. [DOI] [PubMed] [Google Scholar]
- Sieratowicz A, Stange D, Schulte-Oehlmann U, Oehlmann J. 2011. Reproductive toxicity of bisphenol A and cadmium in Potamopyrgus antipodarum and modulation of bisphenol A effects by different test temperature. Environ Pollut 159:2766–2774 [DOI] [PubMed] [Google Scholar]
- Sohoni P, Tyler CR, Hurd K, Caunter J, Hetheridge M, Williams T, Woods C, Evans M, Toy R, Gargas M, Sumpter JP. 2001. Reproductive effects of long-term exposure to Bisphenol A in the fathead minnow (Pimephales promelas). Environ Sci Technol 35:2917–2925. [DOI] [PubMed] [Google Scholar]
- Sone K, Hinago M, Kitayama A, Morokuma J, Ueno N, Watanabe H, Iguchi T. 2004. Effects of 17beta-estradiol, nonylphenol, and bisphenol-A on developing Xenopus laevis embryos. Gen Comp Endocrinol. 138:228–236. [DOI] [PubMed] [Google Scholar]
- Staples CA, Dorn PB, Klecka GM, O’Block ST, Harris LR. 1998. A review of the environmental fate, effects, and exposures of bisphenol A. Chemosphere 36:2149–2173. [DOI] [PubMed] [Google Scholar]
- Stoker C, Rey F, Rodriguez H, Ramos JG, Sirosky P, Larriera A, Luque EH, Muñoz-de-Toro M. 2003. Sex reversal effects on Caiman latirostris exposed to environmentally relevant doses of the xenoestrogen bisphenol A. Gen Comp Endocrinol 133:287–296. [DOI] [PubMed] [Google Scholar]
- Sultan C, Balaguer P, Terouanne B, Georget V, Paris F, Jeandel C, Lumbroso S, Nicolas J. 2001. Environmental xenoestrogens, antiandrogens and disorders of male sexual differentiation. Mol Cell Endocrinol 178:99–105 [DOI] [PubMed] [Google Scholar]
- Sumpter JP, Jobling S. 1995. Vitellogenesis as a biomarker for estrogenic contamination of the aquatic environment. Environ Health Perspect 103 Suppl 7:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Nakagawa Y, Takano I, Yaguchi K, Yasuda K. 2004. Environmental fate of bisphenol A and its biological metabolites in river water and their xeno-estrogenic activity. Environ Sci Technol. 38:2389–2396 [DOI] [PubMed] [Google Scholar]
- Thornton JW, Need E, Crews D. 2003. Resurrecting the ancestral steroid receptor: ancient origin of estrogen signaling. Science 301:1714–1717. [DOI] [PubMed] [Google Scholar]
- Tingaud-Sequeira A, Ouadah N, Babin PJ. 2001. Zebrafish obesogenic test: a tool for screening molecules that target adiposity. J Lipid Res. 52:1765–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tohmé M, Prud’homme SM, Boulahtouf A, Samarut E, Brunet F, Bernard L, Bourguet W, Gibert Y, Balaguer P, Laudet V. 2014. Estrogen-related receptor γ is an in vivo receptor of bisphenol A. FASEB J 28:3124–3133. [DOI] [PubMed] [Google Scholar]
- Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler E. 2007. The use of biomarkers in biomonitoring: A 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Comp Biochem Physiol C Toxicol Pharmacol 146: 281–300. [DOI] [PubMed] [Google Scholar]
- Viganò L, Mandich A, Benfenati E, Bertolotti R, Bottero S, Porazzi E, Agradi E. 2006. Investigating the estrogenic risk along the river Po and its intermediate section. Arch Environ Contam Toxicol 51:641–651. [DOI] [PubMed] [Google Scholar]
- Villeneuve DL, Garcia-Reyero N, Escalon BL, Jensen KM, Cavallin JE, Makynen EA, Durhan EJ, Kahl MD, Thomas LM, Perkins EJ, Ankley GT. 2012. Ecotoxicogenomics to support ecological risk assessment: a case study with bisphenol A in fish. Environ Sci Technol 46:51–59. [DOI] [PubMed] [Google Scholar]
- WHO Factsheets: Obesity and overweight. Fact sheet N°311 Updated January 2015. (http://www.who.int/mediacentre/factsheets/fs311/en/).
- Wolkowicz IR, Herkovits J, Pérez Coll CS. 2014. Stage-dependent toxicity of bisphenol a on Rhinella arenarum (anura, bufonidae) embryos and larvae. Environ Toxicol. 29:146–154. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Ishibashi H, Kohra S, Arizono K, Tominaga N. 2005. Short-term effects of endocrine-disrupting chemicals on the expression of estrogen-responsive genes in male medaka (Oryzias latipes). Aquat Toxicol. 72:239–249. [DOI] [PubMed] [Google Scholar]
- Yang J, Li H, Ran Y, Chan K. 2014. Distribution and bioconcentration of endocrine disrupting chemicals in surface water and fish bile of the Pearl River Delta, South China. Chemosphere 107:439–446. [DOI] [PubMed] [Google Scholar]
- Yang M, Qiu W, Chen B, Chen J, Liu S, Wu M, Wang KJ. 2015. The in vitro immune modulatory effect of bisphenol A on fish macrophages via estrogen receptor α and nuclear factor-κB signaling. Environ Sci Technol. 49:1888–1895 [DOI] [PubMed] [Google Scholar]
- Yin DQ, Hu SQ, Gu Y, Wei L, Liu SS, Zhang AQ. 2007. Immunotoxicity of bisphenol A to Carassius auratus lymphocytes and macrophages following in vitro exposure. J Environ Sci (China). 19:232–237 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang Q, Ji Y, Zhang Q, Wu H, Xie J, Zhao J. 2014. Identification and mRNA expression of two 17β-hydroxysteroid dehydrogenase genes in the marine mussel Mytilus galloprovincialis following exposure to endocrine disrupting chemicals. Environ Toxicol Pharmacol 37:1243–1255. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C. 2005. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology 146:607–612. [DOI] [PubMed] [Google Scholar]