Abstract
Reverse hybrid therapy is an 1-step 2-phase treatment for Helicobacter pylori (H. pylori) infection with less cost than standard triple therapy. We conducted a randomized, controlled study to compare the efficacies of standard triple therapy and reverse hybrid therapy in the treatment of H. pylori infection.
From October 2012 to March 2015, consecutive H. pylori-infected subjects were randomly allocated to receive either a reverse hybrid therapy (pantoprazole plus amoxicillin for 12 days and clarithromycin plus metronidazole for the initial 7 days) or a standard triple therapy (pantoprazole plus amoxicillin and clarithromycin for 12 days). H. pylori status was assessed 6 weeks after treatment. Additionally, antibiotic resistances and host CYP2C19 genotypes were examined and analyzed.
A total of 440 H. pylori-infected patients were randomly assigned to receive either a reverse hybrid (n = 220) or a standard triple therapy (n = 220). The reverse hybrid group had a higher eradication rate than standard triple group either by intention-to-treat (93.6% vs. 86.8%; P = 0.016) or per-protocol analysis (95.7% vs. 88.3%; P = 0.005). The 2 patient groups exhibited similar frequencies of overall adverse events (14.1% vs. 9.5%) and drug compliance (96.8% vs. 98.6%). Clarithromycin resistance was an independent risk factor predicting eradication failure in standard triple group (P < 0.001), but not in reverse hybrid group. CYP2C19 genotypes did not affect the eradication rates in both groups.
Reverse hybrid therapy can be considered for first-line treatment of H. pylori infection since the new therapy achieves a higher eradication rate than standard triple therapy with similar tolerability and less pharmaceutical cost.
INTRODUCTION
Helicobacter pylori (H. pylori) infection is well recognized as the leading cause of chronic gastritis, peptic ulcer disease, and gastric cancer.1–5 Currently, H. pylori treatment remains a challenge for physicians as antimicrobial resistance has continued to increase worldwide. Although standard triple therapy has been recommended as the first-line therapy in many guidelines,6–9 its eradication rate has decreased to unacceptable level in most parts of the world.10–15 The growing treatment failure rate is generally attributed to an increasing prevalence of resistance to clarithromycin, a basic component of standard triple therapy.10–12,15 An updated consensus report8 has therefore proposed a bismuth-containing quadruple therapy or non-bismuth quadruple therapy (sequential or concomitant therapy)16–18 as first-line treatment in settings with clarithromycin resistance rates greater than 15% to 20%. Although levofloxacin-based triple therapy can achieved a high eradication rate in populations with clarithromycin resistance greater than 20% and quinolone resistance less than 10%,19 it is not generally recommended as a first-line therapy on concerns of the rapid development of resistant strains.
Hybrid (dual–quadruple) therapy developed by Hsu et al. consists of a proton pump inhibitor (PPI) and amoxicillin for 14 days with addition of clarithromycin and metronidazole for the final 7 days.20 In its pilot study, hybrid therapy generated excellent eradication rates of 99% and 97% according to per-protocol (PP) and intention-to-treat (ITT) analyses. Subsequent randomized trials demonstrated that hybrid regimens were either comparable with or more effective than sequential therapies.21–23 Recently, a large multicenter randomized trial also showed that both 14-day hybrid and 14-day concomitant therapies cured more than 90% of the patients with H. pylori infection in areas with high clarithromycin and metronidazole resistance.24 Many experts have recommended hybrid therapy as a treatment option for H. pylori in areas with moderate or high clarithromycin resistance.25–27
Recently, we further tested whether the duration of hybrid therapy could be reduced while maintaining the high eradication rate.28 Two hundred twenty H. pylori-infected subjects were randomized to receive a 10-, 12-, or 14-day hybrid therapy. The eradication rates with PP analyses were similar: 95.0% for 10-day, 95.1% for 12-day, and 93.4% for 14-day hybrid therapies.28 The results suggested that the duration of hybrid therapy could be reduced to 12 days or less while maintaining the high eradication rate.
From the perspective of clinical practice, reversing the drug administration sequence in hybrid therapy (a quadruple regimen followed by a dual regimen) simplifies the treatment and makes it an 1-step 2-phase therapy (Fig. 1). Although whether changing the administration sequence would reduce the efficacy of hybrid therapy is unclear, 1 of our recent studies demonstrated similar efficacies of 10-day reverse and standard sequential therapies in the treatment of H. pylori infection.29
FIGURE 1.
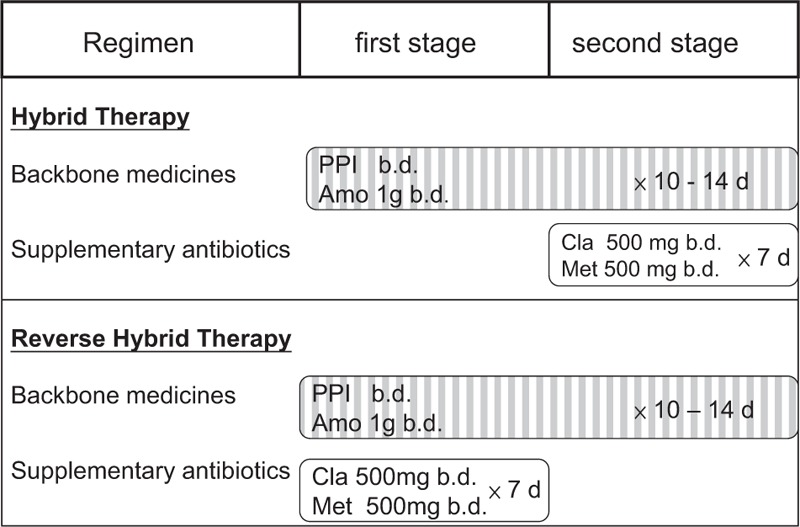
Hybrid regimen and reverse hybrid regimen. Hybrid therapy (a 2-step 2-phase therapy) consists of a proton pump inhibitor and amoxicillin (backbone drugs) for 10 to 14 days, with addition of clarithromycin and metronidazole for the final 7 days; reverse hybrid therapy (an 1-step 2-phase therapy) consists of a proton pump inhibitor and amoxicillin for 10 to 14 days, with addition of clarithromycin and metronidazole for the first 7 days.
Currently, whether hybrid therapy is superior to standard triple therapy remains unanswered. To address this issue, we conducted this multicentre, randomized controlled trial to compare the efficacies of 12-day reverse hybrid therapy and 12-day standard triple therapy. Furthermore, we also investigated the impact of antibiotic resistance and polymorphism of CYP2C19 on the eradication rates of the 2 first-line treatments.
METHODS
Participants
This study was a multicentre, single-blind, randomized trial conducted in conducted in the gastroenterology clinics at the Kaohsiung Veterans General Hospital, Kaohsiung Medical University Hospital, and Kaohsiung Municipal Hsiao-Kang Hospital in Taiwan between October 2012 and March 2015. The study was approved by the Institutional Review Board of each hospital. It was registered at ClinicalTrials.gov, trial NCT02359435.
All consecutive H. pylori-infected adult patients with endoscopically proven peptic ulcer diseases or gastritis were eligible for recruitment. The diagnosis of H. pylori was based on at least 2 positive results of rapid urease test, histology, and culture. Exclusion criteria were as follows: age younger than 20 years; prior H. pylori eradication, allergy to any of the medications used in the trial, presence of severe comorbidities (for example, decompensated liver cirrhosis, uremia), ingestion of antibiotics or bismuth within the prior 4 weeks, and pregnancy.
Randomization
Using a computer-generated number sequence, we randomly allocated patients at a 1:1 ratio to either a 12-day reverse hybrid therapy (pantoprazole 40 mg plus amoxicillin 1 g twice daily for 12 days, and clarithromycin 500 mg plus metronidazole 500 mg twice daily for the first 7 days) or a 12-day standard triple therapy (pantoprazole 40 mg plus clarithromycin 500 mg and amoxicillin 1 g twice daily for 12 days). All drugs were taken 1 hour before breakfast and dinner. An independent research assistant at the Kaohsiung Veterans General Hospital generated the computerized random number sequence. She prepared the study medicines and instructions for drug administration according to the number sequence and concealed them in an opaque envelope. The opaque envelopes labeled with sequence numbers outside were kept by research nurses at each study hospital.
After written informed consent was obtained from each participant, the independent research assistant at the Kaohsiung Veterans General Hospital reported by telephone each patient's treatment allocation to the research nurses at each study hospital. The patients took medicines according to the instructions in the envelopes. Both physicians and research nurses were blind to the treatment arm.
Patients were followed in outpatient clinics to investigate adverse effects and drug compliance at the second week. An additional 4-week pantoprazole (40 mg once daily) therapy was administered following eradication treatment for the patients with peptic ulcer disease. In contrast, the patients with gastritis only received 4-week antacid therapy following H. pylori eradication.
Trial Design
Participants completed a standard questionnaire which contained questions regarding demographic data and personal history of smoking and alcohol consumption. For the analysis of CYP2C19 genotypes, blood sampling was performed on enrollment. DNA was extracted from the leucocytes with a commercially available kit (Qiagen K.K., Tokyo, Japan). Genotyping procedures for identifying the CYP2C19 wild-type gene and 2 mutated alleles, CYP2C19 m1 and CYP2C19 m2, were performed by polymerase chain reaction-based restriction fragment length polymorphism as previous description.30 Genotypes were classified into 3 distinct groups: homogeneous extensive metabolizer (homEM); heterogeneous extensive metabolizer (hetEM); poor metabolizer (PM).
Adverse events were assessed by a specific questionnaire fulfilled at the end of eradication therapy. The severity of adverse events were recorded according to a 4-point (none; mild; moderate; severe) scale system as previous description.31 Compliance with therapy was determined via pill counts.
Patients with gastric ulcer on enrollment received a follow-up endoscopy with rapid urease test, histological examination and culture 6 weeks after the end of eradication therapy. In contrast, patients with gastritis or duodenal ulcer underwent a urea breath test to assess final H. pylori status. Cure of H. pylori infection was defined as a negative result of urea breath test, or negative results of all histology, urease test, and bacterial culture.32
Because of facility problems, H. pylori culture was only conducted in the Kaohsiung Veterans General Hospital. We took a specimen from the antrum for culture of H. pylori.33 and then rubbed the specimen on the surface of an agar plate (Campy-BAP agar plate; Brucella agar + 10% whole sheep blood + IsoVitalex). The agar plate was incubated at 37°C with microaerobic condition for 5 days. If one or more colonies of Gram-negative bacilli were positive in urease, catalase, and oxidase tests, the culture result was considered positive. The E-test (AB Biodisk, Solna, Sweden) was used to evaluate the resistance to antibiotics according to minimum inhibitory concentration (MIC) values of >1, >0.5, and >8 μg/mL for clarithromycin, amoxicillin, and metronidazole, respectively.34
Statistical Methods
Eradication rate was the primary outcome of this study. It was evaluated by ITT, modified ITT and PP analyses. ITT analysis included all eligible patients enrolled in the study regardless of compliance with the study protocol. Patients with unavailable data following treatment were assumed to have been treated unsuccessfully. Modified ITT analysis included all those receiving at least 1 dose of drug and undergoing follow-up tests for H. pylori infection. PP analysis only included patients with good drug compliance and excluded patients with unknown H. pylori status following therapy. The second outcomes were the frequency of adverse events and drug compliance. Compliance was defined as good (taking at least 80% of eradication medication) or poor (taking less than 80% of the total medication). Differences in demographic data, eradication rates, and adverse events among different arms were determined by Chi-square test or Fisher exact test, as appropriate. The Student t test was used for the comparison of continuous data. SPSS (version 12.0 for Microsoft Windows) were used for all statistical analyses. A P-value of <0.05 was considered statistically significant.
According to previous studies,35 the eradication rate of 12-day standard triple therapy was 85%. It was estimated that at least 220 participants in each group were required to achieve a statistic power of 90% with a type I error of 0.05, assuming a 15% loss to follow-up and 4% of participant with poor medication compliance.
To identify the independent factors determining the eradication rate, clinical, genetic, and bacterial parameters were initially analyzed by univariate analysis. The parameters with significant differences in univariate analysis were then further analyzed by a logistic regression method to search the factors independently predicting eradication rate.
RESULTS
Patients
From October 2012 to March 2015, a total of 440 H. pylori-infected patients were recruited for the study and randomly allocated to the reverse hybrid (n = 220) or standard triple group (n = 220). The baseline demographic and clinical characteristics of patients in both groups are shown in Table 1. There were no differences in all parameters between groups. The flow of patients through the study is shown in Figure 2. In the reverse hybrid therapy group, 10 participants were excluded from PP analysis for loss to follow-up (n = 4) or poor compliance (n = 6). In the standard triple therapy group, 6 participants were excluded from PP analysis for loss to follow-up (n = 3), poor compliance (n = 2), or both (n = 1).
TABLE 1.
Participant Characteristics by Treatment Group
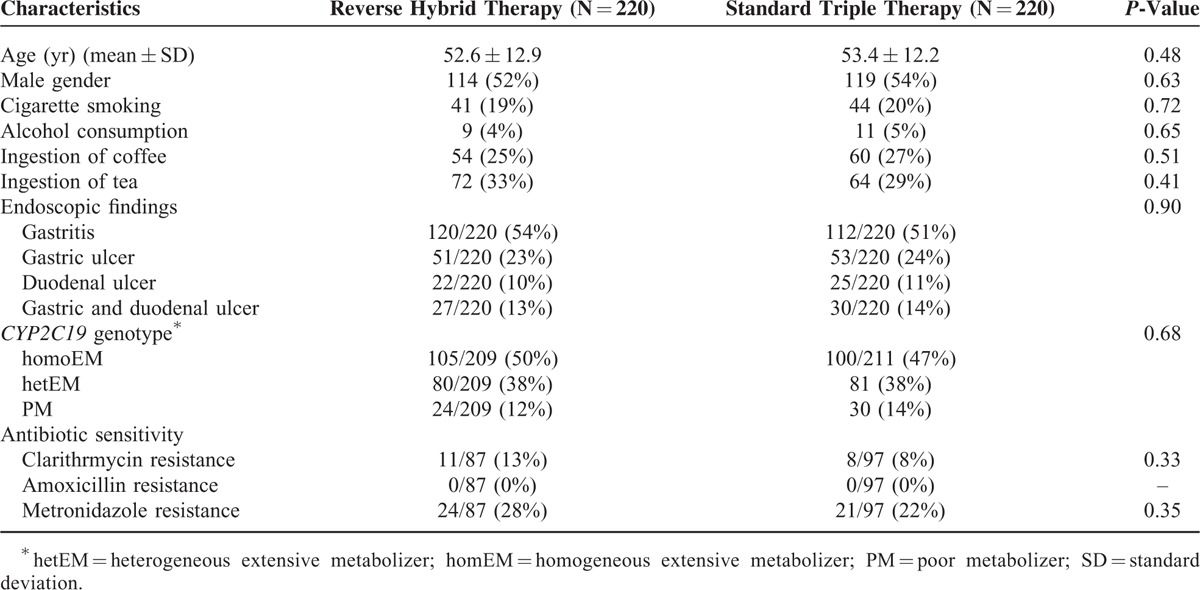
FIGURE 2.
Trial profile. ITT = intention-to-treat; PP = per-protocol.
Eradication of H. pylori
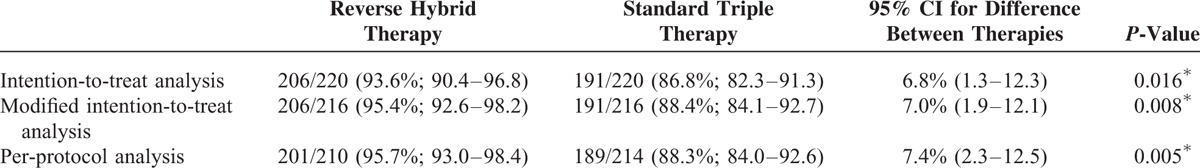
The outcomes of treatments are listed in Table 2. The ITT eradication rates were 93.6% (95% confidence interval [CI], 90.4–96.8%) and 86.8% (95% CI, 82.3–91.3%) for reverse hybrid and standard triple therapies, respectively. Reverse hybrid therapy achieved a higher eradication rate than standard triple therapy (95% CI, 1.3–12.3%; P = 0.016). The modified ITT (95.4% vs. 88.4%) and PP analyses (95.7% vs. 88.3%) yielded similar results (P = 0.008 and 0.005, respectively).
TABLE 2.
Eradication Rates in the ITT and PP Populations
Eradication Rates in Resistant Strains
H. pylori culture was performed in 230 patients at initial endoscopy. H. pylori strains were isolated from 184 (80.6%) of them. Antibiotic resistance rates in each group are listed in Table 1. The overall clarithromycin, amoxicillin and metronidazole resistant rates of H. pylori strains isolated in this trial were 10.3% (19/184), 0.0% (0/184) and 24.5% (45/184), respectively. Reverse hybrid therapy achieved a higher eradication rate for clarithromycin-resistant strains than standard triple therapy (90% vs. 25%, P = 0.01). However, the 2 therapies had comparable eradication efficacy for clarithromycin-sensitive strains (99% vs. 97%). There were no differences in treatment efficacy between the 2 therapies for either metronidazole-sensitive (100% vs. 93%) or resistant strains (91% vs. 81%). In the reverse hybrid therapy group, the eradication rates of the strains with nonresistance, single clarithromycin resistance, single metronidazole resistance, and dual resistances were 100%, 100%, 95%, and 67%, respectively. The corresponding eradication rates in the standard therapy were 99%, 33%, 90%, and 0%, respectively. Reverse hybrid therapy had a higher eradication rate for single clarithromycin-resistant strains than standard triple therapy (P = 0.021).
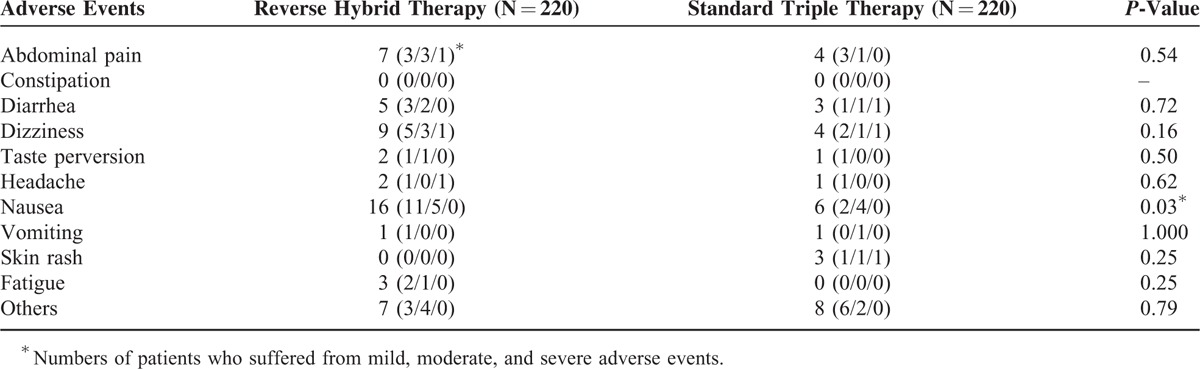
Adverse Events and Compliances
The incidences of adverse events in the participants receiving reverse hybrid and standard triple therapies were 14.1% (95% CI, 9.2–19.0%) and 9.5% (95% CI, 5.6–13.4%), respectively. The 2 therapies exhibited similar frequencies of overall adverse events (P = 0.14).
Table 3 lists the profiles of adverse events of the 2 eradication therapies. Nausea was the most common adverse event in all the 2 treatment groups (7.3% and 2.7% in reverse hybrid and standard triple groups, respectively). The former had a higher frequency of nausea than the latter (P = 0.03). There were no significant differences in the frequencies of other adverse events between groups. Three patients in the hybrid group stopped the anti-H. pylori medication because of abdominal pain (n = 1), headache (n = 1), and dizziness (n = 1). In the standard triple group, 3 patients discontinued treatment owing to diarrhea (n = 1), dizziness (n = 1), and skin rash (n = 1). Reverse hybrid and standard triple groups displayed similar compliance rates (96.8% [95% CI, 94.5–99.1%] and 98.6% [95% CI, 97.1–100.2%], respectively).
TABLE 3.
Adverse Events of Reverse Hybrid and Standard Triple Therapies
Factors Influencing Efficacy of Anti-H. pylori Therapy
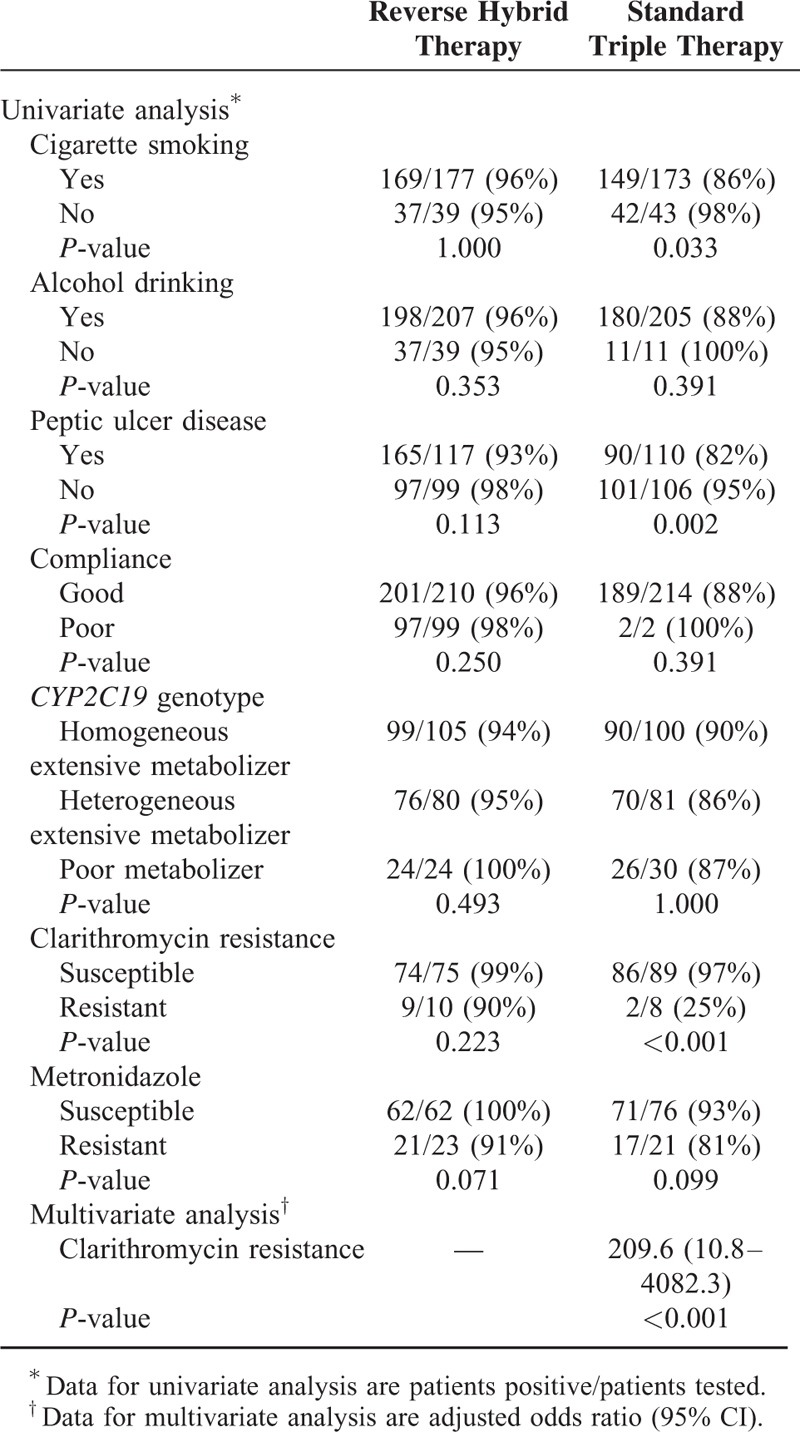
Univariate analysis for the clinical, host, and bacterial factors did not identify any risk factors associated with treatment failure by reverse hybrid therapy (Table 4). The eradication rate of standard triple therapy was affected by the history of smoking (P = 0.033), presence of peptic ulcer (P = 0.002), and clarithromycin resistance (P < 0.001), but not by host CYP2C19 genotype and other clinical factors (Table 4). Multiple regression analysis showed that clarithromycin resistance was the only independent factor predicting eradication failure with an odds ratio of 209.6 (95% CI, 10.8–4082.3; P < 0.001).
TABLE 4.
Factors Affecting Eradication in Reverse Hybrid and Standard Triple Therapies
DISCUSSION
With the rising prevalence of antimicrobial resistance, the treatment success of standard triple therapy has recently declined to less than 80% in many countries. An ideal treatment for H. pylori infection should be highly effective, well tolerated, simple, and inexpensive. This study is the first to conduct a head-to-head, randomized, controlled trial to assess the efficacy of 12-day standard triple therapy and that of 12-day reverse hybrid therapy for H. pylori eradication. The results clearly showed that 12-day reverse hybrid therapy achieved a higher eradication rate than 12-day triple therapy, whether by ITT (93.6% vs. 86.8%), modified ITT (95.4% vs. 88.4%), or PP analyses (95.7% vs. 88.3%). The 2 treatments were well tolerated and shared comparable total adverse events (14.1% and 9.5%) and compliance (96.8% and 98.6%). With regard to pharmaceutical cost, 12-day reverse hybrid therapy was cheaper than 12-day triple therapy (0.37.2 vs. 0.44.0 in Taiwan). The data support the use of reverse hybrid therapy as the standard first-line treatment for H. pylori infection.
As a general rule for the treatment of other infectious diseases, clinicians should prescribe therapeutic regimens that have a PP eradication rate ≥90% for anti-H. pylori therapy.10,25–27,35 Numerous previous reports11–13 disclosed that clarithromycin resistance is the key factor determining the treatment efficacy of standard tripe therapy. According to the theoretical effect of increasing clarithromycin resistance on the success of standard triple therapy, PP eradication rate falls to 90% with 5% clarithromycin resistance for 7-day therapy and 15% resistance for 14-day therapy.36 Two recent randomized controlled trials confirmed that the 7-day standard triple therapy had a cure rate of 82% in an area with 18% clarithromycin resistance,18 and 14-day standard triple therapy achieved an eradication rate of 87% in an area with 11% clarthromycin resistance.37 In the present study, the clarithromycin resistant rate of H. pylori strains in the study population was 10.3%. The 12-day standard triple therapy failed to surpass a 90% eradication rate (only 88 3% by PP analysis). In contrast, 12-day reverse hybrid therapy achieved an eradication rate ≥95% (95.7% by PP analysis). Molina-Infante and his colleagues24 also confirmed that 14-day hybrid therapy cured more than 90% of H. pylori infections in areas with high clarithromycin (24%) and metronidazole resistance (34%). The aforementioned data strongly indicate that reverse hybrid therapy can replace the standard triple therapy as the first-line treatment for H. pylori infection in areas with clarithromycin resistance ≥10% since the new therapy achieves a higher eradication rate than standard triple therapy with similar tolerability and less pharmaceutical cost.
Many antibiotics applied in H. pylori eradication therapy are acid-sensitive. PPIs can increase the activity of some antibiotics by reducing gastric acid secretion and possess direct anti-H. pylori activity.38 Most of them are metabolized by the hepatic cytochrome P450 system, especially CYP2C19.38 Several previous reports demonstrated that the CYP2C19 homEM genotype was an independent factor determining the success rate of H. pylori eradication therapy.38,39 In this study, the subjects with CYP2C19 homEM genotype had a lower eradication rate than those with PM genotype (100% vs. 94%) in the reverse hybrid therapy group. However, the difference did not reach statistical significance. In the stand triple therapy group, there were also no significant differences in eradication rates among the subjects with homEM, hetEM, and PM genotypes.
To simplify eradication regimen, the sequence of drug administration in hybrid therapy was reversed in this study (Fig. 1). Patients took 4 drugs in the initial phase and then took the remaining 2 antibiotics in the second treatment phase. This altered drug administration sequence rendered the hybrid therapy an 1-step 2-phase treatment. Whether reverse hybrid (a 4 + 2 regimen) and standard hybrid therapies (a 2 + 4 regimen) achieve a similar eradication rate remains unclear. However, the eradication rates of 12-day reverse hybrid therapy in this study and 12-day standard hybrid therapy in one of our previous studies28 were comparable (95.7% vs. 95.1% by PP analysis). The data suggest that both reverse and standard hybrid regimens can achieve an eradication rate exceeding 90% in the first-line treatment of H. pylori infection.
Our study had several limitations. First, the number of H. pylori strains with antibiotic susceptibility data in either reverse hybrid or standard triple therapy group was too small to make a robust conclusion for the impacts of antibiotic resistances on the eradication rates of each therapy. Second, the study was conducted in a single country. The results are therefore needed to be confirmed in other countries with different patterns of antibiotic resistances. However, this study is the first trial comparing reverse hybrid therapy and standard triple therapy in the treatment of H. pylori infection. Additionally, it is a randomized-controlled trial with large sample size (>400 participants).
In conclusion, reverse hybrid therapy can replace the standard triple therapy as the first-line treatment for H. pylori infection in areas with clarithromycin resistance ≥10% since the new one-step therapy achieves a higher eradication rate than standard triple therapy with similar tolerability and less pharmaceutical cost.
Acknowledgments
The authors are indebted to Drs. Wang, C.C. Lee, K.M. Wang, S.N. Chang, and J.L. Ou for recruiting the patients and performing the endoscopies; to Prof. L.P. Ger for statistic calculations.
Footnotes
Abbreviations: bd = twice a day, CI = confidence interval, H. pylori = Helicobacter pylori, hetEM = heterogeneous extensive metabolizer, homEM = homogeneous extensive metabolizer, ITT = intention-to-treat, MALToma = mucosa-associated lymphoid tissue lymphoma, PM = poor metabolizer, PP = per-protocol, PPI = proton pump inhibitor.
This study was funded by research grant (NSC 101-2314-B-075B-002-MY2) from the National Science Council.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Sung JJY, Chung SCS, Ling TKW, et al. Antibacterial treatment of gastric ulcer associated with Helicobacter pylori. N Engl J Med 1995; 332:139–142. [DOI] [PubMed] [Google Scholar]
- 2.Hunt RH, Lam SK. Helicobacter pylori: from art to a science. J Gastroenterol Hepatol 1998; 13:21–28. [DOI] [PubMed] [Google Scholar]
- 3.Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002; 347:1175–1186. [DOI] [PubMed] [Google Scholar]
- 4.Isaacson PG. Recent developments in our understanding of gastric lymphomas. Am J Surg Pathol 1996; 20 Suppl. 1:1–7. [DOI] [PubMed] [Google Scholar]
- 5.Wong BC, Lam SK, Wong WM, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004; 291:189–194. [DOI] [PubMed] [Google Scholar]
- 6.Fock KM, Katelaris P, Sugano K, et al. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol 2009; 24:1587–1600. [DOI] [PubMed] [Google Scholar]
- 7.Asaka M, Kato M, Takahashi S, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 2010; 15:1–20. [DOI] [PubMed] [Google Scholar]
- 8.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut 2012; 61:646–664. [DOI] [PubMed] [Google Scholar]
- 9.Chey WD, Wong BCY. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102:1808–1825. [DOI] [PubMed] [Google Scholar]
- 10.Graham DY, Akiko S. New concepts of resistance in the treatment of Helicobacter pylori infections. Nat Clin Pract Gastroenterol Hepatol 2008; 5:321–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Megraud F. H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 2004; 53:1374–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luther J, Higgins PD, Schoenfeld PS, et al. Empiric quadruple vs triple therapy for primary treatment of Helicobacter pylori infection: systematic review and meta-analysis of efficacy and tolerability. Am J Gastroenterl 2010; 105:65–73. [DOI] [PubMed] [Google Scholar]
- 13.Gumurdulu Y, Serin E, Ozer B, et al. Low eradication rate of Helicobacter pylori with triple 7-14 days and quadruple therapy in Turkey. World J Gastroenterol 2004; 10:668–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bigard MA, Delchier JC, Riachi G, et al. One-week triple therapy using omeprazole, amoxycillin and clarithromycin for the eradication of Helicobacter pylori in patients with non-ulcer dyspepsia: influence of dosage of omeprazole and clarithromycin. Aliment Pharmacol Ther 1998; 12:383–388. [DOI] [PubMed] [Google Scholar]
- 15.De Francesco V, Margiotta M, Zullo A, et al. Prevalence of primary clarithromycin resistance in Helicobacter pylori strains over a 15-year period in Italy. J Antimicrob Chemother 2007; 59:783–785. [DOI] [PubMed] [Google Scholar]
- 16.Zullo A, De Francesco V, Hassan C, et al. The sequential therapy regimen for Helicobacter pylori eradication: a pooled-data analysis. Gut 2007; 56:1353–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu PI, Wu DC, Chen WC, et al. Comparison of 7-day triple, 10-day sequential and 7-day concomitant therapies for Helicobacter pylori infection—a randomized controlled trial. Antimicrob Agents Chemother 2014; 214:5936–5942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gisbert JP, Calvet X. Non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Aliment Pharmacol Ther 2011; 34:604–617. [DOI] [PubMed] [Google Scholar]
- 19.Berning M, Krasz S, Miehlke S. Should quinolones come first in Helicobacter pylori therapy? Therap Adv Gastroenterol 2011; 4:103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu PI, Wu DC, Wu JY, et al. Modified sequential Helicobacter pylori therapy: proton pump inhibitor and amoxicillin for 14 days with clarithromycin and metronidazole added as a quadruple (hybrid) therapy for the final 7 days. Helicobacter 2011; 16:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sardarian H, Fakheri H, Hosseini V, et al. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter 2013; 18:129–134. [DOI] [PubMed] [Google Scholar]
- 22.De Francesco V, Hassan C, Ridola L, et al. Sequential, concomitant and hybrid first-line therapies for Helicobacter pylori eradication: a prospective randomized study. J Med Microbiol 2014; 63:748–752. [DOI] [PubMed] [Google Scholar]
- 23.Oh DH, Lee DH, Kang KK, et al. The efficacy of hybrid therapy as first-line regimen for Helicobacter pylori infection compared with sequential therapy. J Gastroenterol Hepatol 2014; 29:1171–1176. [DOI] [PubMed] [Google Scholar]
- 24.Molina-Infante J, Romano M, Fernandez-Bermejo M, et al. Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 2013; 145:121–128. [DOI] [PubMed] [Google Scholar]
- 25.Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol 2011; 8:79–88. [DOI] [PubMed] [Google Scholar]
- 26.Chuah SK, Tsay FW, Hsu PI, et al. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol 2011; 17:3971–3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Georgopoulos SD, Papastergiou V, Karatapanis S. Helicobacter pylori eradication therapies in the era of increasing antibiotic resistance: a paradigm shift to improved efficacy. Gastroenterol Res Pract 2012; 757926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu JY, Hsu PI, Wu DC, et al. Feasibility of shortening 14-day hybrid therapy while maintaining an excellent Helicobacter pylori eradication rate. Helicobacter 2014; 19:207–213. [DOI] [PubMed] [Google Scholar]
- 29.Tsay FW, Wu DC, Kao SS, et al. Reverse sequential therapy achieves a similar eradication rate as standard sequential therapy for Helicobacter pylori eradication: a randomized controlled trial. Helicobacter 2015; 20:71–77. [DOI] [PubMed] [Google Scholar]
- 30.Hsu PI, Lai KH, Lau CP. Esomeprazole with clopidogrel reduces peptic ulcer recurrence, compared with clopidogrel alone, in patients with atherosclerosis. Gastroenterology 2011; 140:791–798. [DOI] [PubMed] [Google Scholar]
- 31.Wu DC, Hsu PI, Tseng HH, et al. Helicobacter pylori Infection: a randomized, controlled study comparing 2 rescue therapies after failure of standard triple therapies. Medicine 2011; 90:180–185. [DOI] [PubMed] [Google Scholar]
- 32.Hsu PI, Wu DC, Wu JY, et al. Is there a benefit to extending the duration of Helicobacter pylori sequential therapy to 14 days? Helicobacter 2011; 16:146–152. [DOI] [PubMed] [Google Scholar]
- 33.Hsu PI, Lai KH, Lin CK, et al. A prospective randomized trial of esomeprazole-versus pantoprazole based triple therapy for Helicobacter pylori eradication. Am J Gastroenterol 2005; 100:2387–2392. [DOI] [PubMed] [Google Scholar]
- 34.Wu DC, Hsu PI, Wu JY, et al. Sequential and concomitant therapy with 4 drugs are equally effective for eradication of H. pylori infection. Clin Gastroenterol Hepatol 2010; 8:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Federico A, Gravina AG, Miranda A, et al. Eradication of Helicobacter pylori infection: which regimen first? World J Gastroenterol 2014; 20665–20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graham DY, Lee YC, Wu MS. Rational Helicobacter pylori therapy: evidence-based medicine rather than medicine-based evidence. Clin Gastroenterol Hepatol 2014; 12:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liou JM, Chen CC, Chen MJ, et al. Sequential versus triple therapy for the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 2012; 381:205–213. [DOI] [PubMed] [Google Scholar]
- 38.Furuta T, Shirai N, Sugimoto M, et al. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab Pharmacokinet 2005; 20:153–167. [DOI] [PubMed] [Google Scholar]
- 39.Kuo CH, Hu HM, Kuo FC, et al. Efficacy of levofloxacin-based rescue therapy for Helicobacter pylori infection after standard triple therapy: a randomized controlled trial. J Antimicrob Chemother 2009; 63:1017–1024. [DOI] [PubMed] [Google Scholar]


