Abstract
To explore the efficacy of the revised Atlanta classification (RACAP) and the determinant-based classification of acute pancreatitis severity (DBCAPS) on the basis of clinical data and feedback from patients with acute pancreatitis (AP).
The authors retrospectively investigated a total of 573 patients with AP admitted to our hospital between December 2011 and December 2014. The definitions of severity and local complications in AP using RACAP and DBCAPS are presented and common points and mutual differences between the 2 groups are analyzed and discussed.
Classification according to RACAP and DBCAPS found 86 (15%) and 178 (31.1%) mild cases (P < 0.01), 269 (46.9%) and 176 (30.7%) moderate cases (P < 0.01), and 218 (38.0%) and 219 (38.2%) severe cases (P = 0.95), respectively. A major contribution of DBCAPS is the introduction of a new type of severe AP, critical AP, identified in 4 patients (0.7%). Complications were observed in 313 RACAP-defined cases and 153 DBCAPS-defined cases (P < 0.01). Among the 313 RACAP-defined cases, acute peripancreatic fluid collection (236 patients, 75.40%), pancreatic pseudocysts (20 patients, 6.4%), acute necrotic collection (42 patients, 13.4%), and walled-off necrosis (15 patients, 4.8%) were observed. Among the 153 DBCAPS-defined cases, sterile peripancreatic necrosis (105 patients, 68.6%), sterile pancreatic necrosis (44 patients, 28.8%), infected peripancreatic necrosis (2 patients, 1.3%), and infected pancreatic necrosis (2/153 patients, 1.3%) were observed.
Both classifications adopted organ failure and complications as determinants of severity. Revised Atlanta classification refined local complications and DBCAPS modified severity to include critical AP. In accordance with the demands of precision medicine, a combination of the 2 could be important for further clinical practice and scientific research.
INTRODUCTION
In 1992, the Atlanta symposium offered a global consensus and a universally applicable classification system for acute pancreatitis (AP) named the Atlanta consensus of acute pancreatitis.1 It was the advent of this system that improved the chaotic nature of diagnosis and management of AP. In addition, the system eliminates the obstacle of international academic communication. As the pathophysiology of the biochemical mechanisms that cause local and systemic manifestations of the underlying inflammatory process are not clearly understood, the use of empirical observations for the classification and management of AP is required.2,3
After decades of research and clinical trials, there is a need to develop a new international classification for AP severity based on a sound framework, a comprehensive review of the published evidence, and worldwide consultation. Fortunately, 2 new projects were proposed by 2 groups in 2012: the revised Atlanta classification (RACAP) 4 system and the determinant-based classification of acute pancreatitis severity (DBCAPS) 5 system. Revised Atlanta classification was recommended and reported by Banks et al, and when compared with the original Atlanta classification, the most significant difference was that RACAP focused on a series of definitions and classifications of AP whereas DBCAPS (published by Bruno and colleagues) takes only the Atlanta classification of severity and introduces a new definition of critical AP.
Certainly, there is a fine distinction between the 2 (Tables 1 and 2) and these differences could potentially affect their clinical application. Therefore, it is necessary to analyze and discuss these 2 methods of classification.
TABLE 1.
Definitions Comparison of Severity in Acute Pancreatitis in Revised Atlanta Classification and Determinant-Based Classification of Acute Pancreatitis Severity
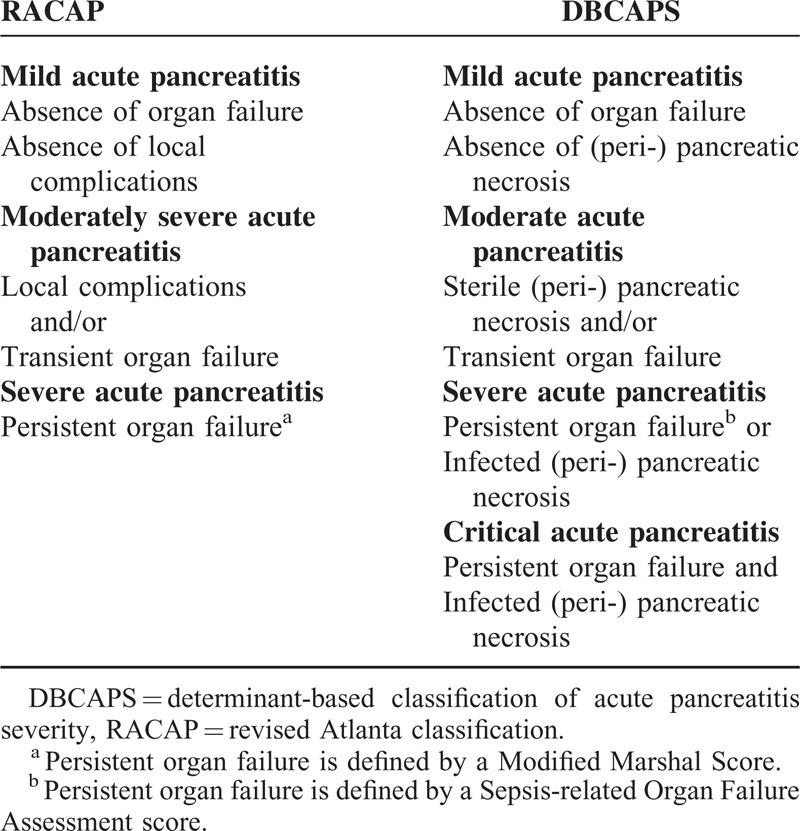
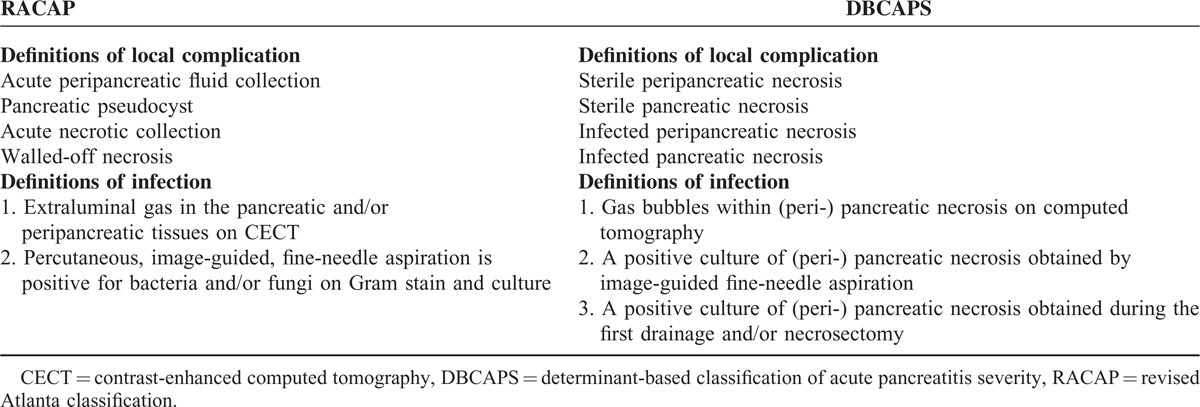
TABLE 2.
Definitions Comparison of Local Complication in Acute Pancreatitis in Revised Atlanta Classification and Determinant-Based Classification of Acute Pancreatitis Severity
MATERIALS AND METHODS
This literature was reported according to the Standards for Quality Improvement Reporting Excellence, and did not breach any ethical guidelines.
Patients
A total of 582 patients with AP were retrospectively analyzed at Lanzhou University's Second Hospital between December 2011 and December 2014. Combined with the current general diagnostic criteria in China,6 295 patients were diagnosed with mild AP and 278 patients were diagnosed with severe AP. Exclusion criteria included patients with incomplete or inconsistent data, patients where doubt existed regarding diagnosis, and patients aged <18 years. Nine patients were excluded from the study and a total of 573 patients were ultimately enrolled. Patient characteristics, including age, sex, and outcomes data were extracted.
Outcomes Measures
Outcome measures were determined in accordance with the 2 classifications RACAP and DBCAPS and included 3 sections: severity; such as the proportions of mild, moderate, severe, and critical; complications; including acute peripancreatic fluid collection (APFC), pancreatic pseudocyst, acute necrotic collection (ANC), and walled-off necrosis (WON) in RACAP; and sterile peripancreatic necrosis, sterile pancreatic necrosis, infected peripancreatic necrosis, and infected pancreatic necrosis in DBCAPS; and prognosis; including recovery, progress, and death.
Statistical Analysis
The software package SPSS (Version 17.0, San Francisco, CA) was used for data analysis. Continuous variables were described using the median (range) and dichotomous data were presented as the number of cases (percentage). Comparisons between the groups were made using the χ2 test and a P-value<0.05 was considered statistically significant.
RESULTS
A total of 573 patients with AP were enrolled in this study. Of these, 346 patients were male and 227 patients were female (M/F, 1.53). The median age was 49 years (range: 20–89 years). No significant differences were observed in cases of mild and moderate AP when comparing the 2 systems; where 86 (15%) and 178 (31.1%) cases were classified as mild (P < 0.01), 269 (46.9%) and 176 (30.7%) cases were classified as moderate (P < 0.01), and 218 (38.0%) and 219 (38.2%) cases were classified as severe (P = 0.95) in accordance with RACAP and DBCAPS, respectively. The significant difference observed for DBCAPS introduced a new type of severe AP known as critical AP, observed in 4 patients (0.7%).
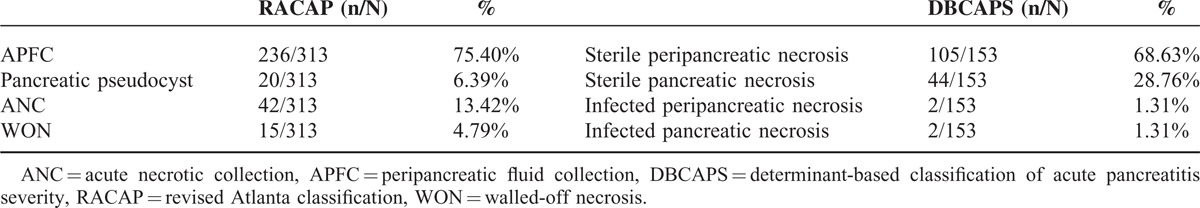
Complications of RACAP were observed in 313 patients and included 236 patients of APFC (75.40%), 20 patients of pancreatic pseudocysts (6.4%), 42 patients of ANC (13.4%), and 15 patients of WON (4.8%). Complications of DBCAPS were observed in 153 patients, including 105 patients of sterile peripancreatic necrosis (68.6%), 44 patients of sterile pancreatic necrosis (28.8%), 2 patients of infected peripancreatic necrosis (1.3%), and 2 patients of infected pancreatic necrosis (1.3%). The total number of complications observed with RACAP was significantly higher than that with DBCAPS (P < 0.01; Table 3).
TABLE 3.
Complications Incidence Based on Revised Atlanta Classification and Determinant-Based Classification of Acute Pancreatitis Severity
According to the 2 classifications, we further analyzed the prognosis of moderate and severe AP. For moderate AP patients classified by RACAP, 249 patients (96.1%) recovered, 9 patients (3.5%) progressed, and 1 patient (0.4%) died. For moderate AP patients classified by DBCAPS, 167 patients (96.0%) recovered, 6 patients (3.4%) progressed, and 1 patient (0.6%) died. For severe AP patients, classified by RACAP, 192 patients (88.5%) recovered, 22 patients (10.1%) progressed, and 3 patients (1.4%) died. For severe AP patients, classified by DBCAPS, 191 patients (89.3%) recovered, 21 patients (9.8%) progressed, and 2 patients (0.9%) died. For critical AP patients introduced by DBCAPS, 2 patients recovered, 1 patient progressed, and 1 patient died (Figs. 1 and 2).
FIGURE 1.
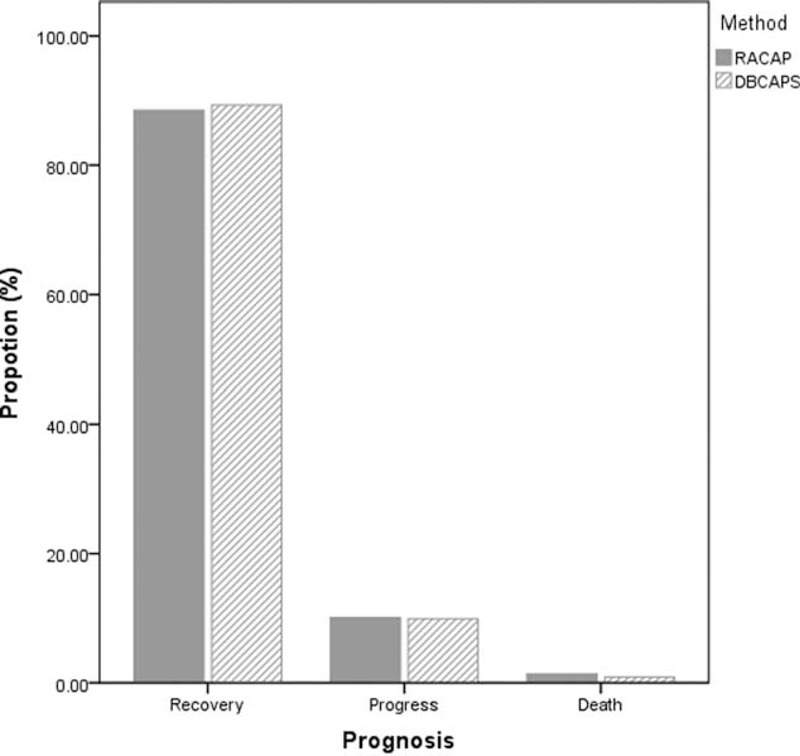
Prognosis of severe acute pancreatitis according to revised Atlanta classification and determinant-based classification of acute pancreatitis severity.
FIGURE 2.
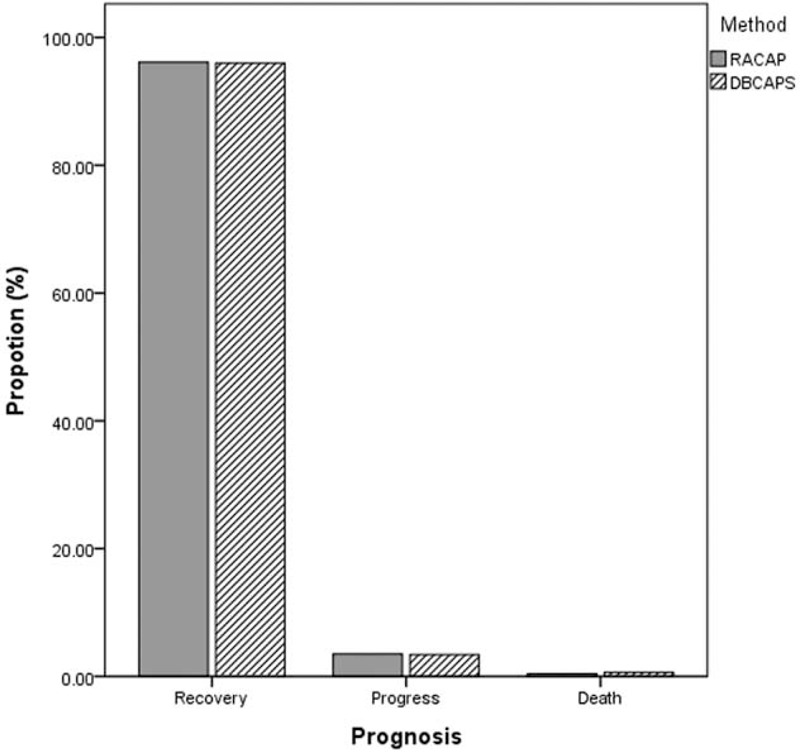
Prognosis of moderate acute pancreatitis according to revised Atlanta classification and determinant-based classification of acute pancreatitis severity.
DISCUSSION
The RACAP classification system is based on 2 types of pancreatitis (interstitial edematous pancreatitis and necrotizing pancreatitis), 2 overlapping phases, 3 categories, and 4 types of local complications.4,7 The 2 overlapping phases in a patient's dynamic condition with 2 peaks of mortality are the early and late phases. The early phase generally concludes by the end of the first week, although a small minority of patients may experience an extension into the second week. The late phase is characterized by persistence of systemic signs or by the presence of local complications. This is a dynamic phase with respect to individual progression of disease, rather than an arbitrary time point (such as, for instance, 1 week after onset of symptoms). The 3 categories of severity are classified as mild, moderate, and severe. Mild AP is the most common type and is not accompanied by organ failure or local or systemic complications. Mild AP generally resolves within the first week. Moderate AP is defined by the presence of transient organ failure, as well as local complications or exacerbation of comorbidities. Severe AP is defined by persistent organ failure for a period longer than 48 hours.8 The 4 local complications are APFC, pancreatic pseudocysts, ANC, and WON.
Determinant-based classification of acute pancreatitis severity is based on actual local and systemic determinants of severity, rather than descriptions of events correlated with severity.5,9 The local determinant relates to the presence of sterile or infected (peri-) pancreatic necrosis, whereas the systemic determinant relates to transient or persistent organ failure. The presence of both infected (peri-) pancreatic necrosis and persistent organ failure has a greater effect on severity than either determinant alone. The derivation of a classification based on the above principles results in 4 categories of severity: mild, moderate, severe, and critical.
From the data presented in Table 1, it is clear is that there is either a common basis or a fine distinction between the 2 classification systems. Firstly, the description of mild AP is the same in both systems. Confusingly, there, however, are duplicated terms for describing AP in RACAP-defined interstitial edematous pancreatitis with morphologic features and mild AP with severity. The complication APFC is included in the classification of interstitial edematous pancreatitis. Interestingly, there is little mention of the role APFC plays in mild acute pancreatitis.10 It is not clear as to whether or not all local complications are included in RACAP-defined moderate AP. Another common opinion is that both working groups proposed a “moderate” category for classifying severity, characterized by the presence of local complications in sterile (peri-) pancreatic necrosis in DBCAPS and/or transient organ failure.11 Physicians know that local complications are not in fact equivalent with regard to disease severity. The onset of infected pancreatic necrosis often indicates severe disease and is quite different in significance to the onset of an acute peripancreatic collection without infection.12 As demonstrated in the current study, moderate AP accounted for a large proportion of patients, generally as a result of local complications such as APFC. In addition, the primary prognosis was recovery as opposed to disease progression.
The major differences observed when comparing RACAP and DBCAPS are derived from the role of infection in severe AP. The definition of severe AP in RACAP is characterized only by the presence of persistent organ failure.13 Determinant-based classification of acute pancreatitis severity, however, introduced a new category of severe AP known as critical AP, which is classified according to the presence of both infected (peri-) pancreatic necrosis and/or persistent organ failure.14 The severe AP classification focused on patients with a higher risk of mortality because of: persistent organ failure; infected (peri-) pancreatic necrosis, as the onset of infected pancreatic necrosis is most often indicative of severe disease; and prohibitive mortality where both infected pancreatic necrosis and persistent organ failure are present, defined as critical AP.15,16 Our study showed that there is a higher risk of progress for critical AP in DBCAPS when compared with RACAP. Indeed, RACAP should be of limited predictive value in severe cases of infected (peri-) pancreatic necrosis.
Both RACAP and DBCAPS emphasize clinical determinants in their severity estimations, while pathophysiological significance of risk is downplayed, especially with regard to systemic inflammatory response syndrome (SIRS).17 Cytokine cascades are activated by pancreatic inflammation that clinically manifests as SIRS. The sheer complexity of concurrent proinflammatory and anti-inflammatory responses belies a clear understanding of the pathophysiology of the biochemical mechanisms responsible for initiating local and systemic manifestations of the underlying inflammatory process.18 Although SIRS is considered a significant risk for persistent organ failure in severe AP, its role in the classification of AP severity is ambiguous. It is well known that AP is an inflammatory process; thus, a more sophisticated understanding of the dynamic condition should take into account the balance of pro- and anti-inflammatory responses leading to local and systemic complications. Unfortunately, our study did not include pathologic diagnoses and future studies should focus on this aspect.
As presented in Table 2, there is no essential difference in terms of the definitions for (peri-) pancreatic tissue infection, with the exception of the level of detail described. Different definitions of local complications, however, have been proposed by RACAP and DBCAPS. The most important contribution of RACAP is the redefinition of local complications of AP, which is derived from their content, wall, site, and evolution. Through the use of high-resolution computed tomography (CT) scans and an improved understanding of the natural history of local complications, a series of morphologic descriptions have been defined that ensure more consistent and accurate radiologic reports of CT scan results.10 Resolution CT is further able to identify APFC, pancreatic pseudocysts, ANC, and WON.19 It is understood that while RACAP has proposed that infections may develop in the ANC and WON, infection can occur in all 4 lesion types. On the contrary, the most significant contribution of DBCAPS is the distinction between the definitions of sterile peripancreatic necrosis, sterile pancreatic necrosis, infected peripancreatic necrosis, and infected pancreatic necrosis.
The only accurate measure for confirming infected (peri-) pancreatic necrosis, however, is a positive culture and current management of local complications in acute pancreatitis does not recommend early intervention.20 As a result, application of DBCAPS relies heavily on the diagnosis of (peri-) pancreatic necrosis, defined as necrosis in either the pancreatic parenchyma or the peripancreatic fat, or both. Necrosis is identified using contrast-enhanced imaging to visualize areas of nonenhancement.3 Using CT scans alone, differentiation between peripancreatic fat necrosis (less common) and acute peripancreatic fluid collections (quite common) is unreliable, and could lead to overreading.3
This study had several limitations. These include: the restrospective study type, possible bias as a result of admission rate in our hospital, minimal pathophysiological evidence, and additional aspects, such as follow-up data, that were somewhat insufficient. Although none of the tools currently available meet all criteria, every step forward in the classification of acute pancreatitis will improve the development of diagnostic techniques and therapies. It is not surprising that different approaches have yielded such varying results. Clinicians and researchers may need to decide which classification of severity will better meet their needs. Indeed, the comparison and possible combination of multiple tools is one of the best ways to find a new path forward in science. If RACAP and DBCAPS could be combined, a new classification for AP may emerge.
CONCLUSIONS
Both the RACAP and DBCAPS classification systems include organ failure and complications as determinants of severity. Revised Atlanta classification refines local complications whereas DBCAPS has modified severity and added a critical AP classification. In accordance with the demands of precision medicine, a combination of the 2 could prove to be significant for both clinical practice and scientific research. Furthermore, a clear demonstration of the pathophysiology of the biochemical mechanisms responsible for initiating local and systemic manifestations will be key to elucidating the morbidity and mortality of AP in the future.
Footnotes
Abbreviations: ANC = acute necrotic collection, AP = acute pancreatitis, APFC = peripancreatic fluid collection, DBCAPS = determinant-based classification of acute pancreatitis severity, RACAP = revised Atlanta classification, SIRS = systemic inflammatory response syndrome, WON = walled-off necrosis.
This study was supported by the Gansu Province Natural Science Foundation (No. 145RJZA177).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Bradley EL. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg 1993; 128:586–590.3rd. [DOI] [PubMed] [Google Scholar]
- 2.Uomo G, Patchen Dellinger E, Forsmark CE, et al. Multidisciplinary international classification of the severity of acute pancreatitis: Italian version 2013. Minerva Med 2013; 104:649–657. [PubMed] [Google Scholar]
- 3.Bradley EL. Atlanta redux revisiting the severity stratification system for acute pancreatitis. Ann Surg 2012; 256:881–882. [DOI] [PubMed] [Google Scholar]
- 4.Sarr MG, Banks PA, Bollen TL, et al. The new revised classification of acute pancreatitis 2012. Surg Clin North Am 2013; 93:549–562. [DOI] [PubMed] [Google Scholar]
- 5.Dellinger EP, Forsmark CE, Layer P, et al. Determinant-based classification of acute pancreatitis severity: an international multidisciplinary consultation. Ann Surg 2012; 256:875–880. [DOI] [PubMed] [Google Scholar]
- 6.Wang YT, Chen YX. Further understanding the Chinese guide of acute pancreatitis management (in Chinese). Chin J Pract Int Med 2009; 29:317–319. [Google Scholar]
- 7.Petrov MS, Windsor JA. Classification of the severity of acute pancreatitis: how many categories make sense? Am J Gastroenterol 2010; 105:74–76. [DOI] [PubMed] [Google Scholar]
- 8.Matas-Cobos AM, Redondo-Cerezo E, Alegría-Motte C, et al. The role of Toll-like receptor polymorphisms in acute pancreatitis occurrence and severity. Pancreas 2015; 44:429–433. [DOI] [PubMed] [Google Scholar]
- 9.Choi JH, Kim MH, Oh D, et al. Clinical relevance of the revised Atlanta classification focusing on severity stratification system. Pancreatology 2014; 14:324–329. [DOI] [PubMed] [Google Scholar]
- 10.Windsor JA, Petrov MS. Acute pancreatitis reclassified. Gut 2013; 62:4–5. [DOI] [PubMed] [Google Scholar]
- 11.Jin T, Huang W, Yang XN, et al. Validation of the moderate severity category of acute pancreatitis defined by determinant-based classification. Hepatobiliary Pancreat Dis Int 2014; 13:323–327. [DOI] [PubMed] [Google Scholar]
- 12.Petrov MS, Shanbhag S, Chakraborty M, et al. Organ failure and infection of pancreatic necrosis as determinants of mortality in patients with acute pancreatitis. Gastroenterology 2010; 139:813–820. [DOI] [PubMed] [Google Scholar]
- 13.Wig JD, Bharathy KG, Kochhar R, et al. Correlates of organ failure in severe acute pancreatitis. JOP 2009; 10:271–275. [PubMed] [Google Scholar]
- 14.Lytras D, Manes K, Triantopoulou C, et al. Persistent early organ failure: defining the high-risk group of patients with severe acute pancreatitis? Pancreas 2008; 36:249–254. [DOI] [PubMed] [Google Scholar]
- 15.Petrov MS, Windsor JA, Levy P, et al. New international classification of acute pancreatitis more than just 4 categories of severity. Pancreas 2013; 42:389–391. [DOI] [PubMed] [Google Scholar]
- 16.Talukdar R, Rau BM. Determinant-based classification of severity of acute pancreatitis: have we really reached consensus? Ann Surg 2014; 261:e22. [DOI] [PubMed] [Google Scholar]
- 17.Kylanpaa ML, Repo H, Puolakkainen PA. Inflammation and immunosuppression in severe acute pancreatitis. World J Gastroenterol 2010; 16:2867–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayerle J, Dummer A, Sendler M, et al. Differential roles of inflammatory cells in pancreatitis. J Gastroenterol Hepatol 2012; 27:47–51. [DOI] [PubMed] [Google Scholar]
- 19.Brun A, Agarwal N, Pitchumoni CS. Fluid collections in and around the pancreas in acute pancreatitis. J Clin Gastroenterol 2011; 45:614–625. [DOI] [PubMed] [Google Scholar]
- 20.Amano H, Takada T, Isaji S, et al. Therapeutic intervention and surgery of acute pancreatitis. J Hepatobiliary Pancreat Sci 2010; 17:53–59. [DOI] [PubMed] [Google Scholar]


