Abstract
Transition from child to adult-oriented care is widely regarded a challenging period for young people with kidney transplants and is associated with a high risk of graft failure.
We analyzed the existing transition structures in Germany and Austria using a questionnaire and retrospective data of 119 patients transferred in 2011 to 2012.
Most centers (73%) confirmed agreements on the transition procedure. Patients’ age at transfer was subject to regulation in 73% (18 years). Median age at transition was 18.3 years (16.5–36.7). Median serum creatinine increased from 123 to 132 μmol/L over the 12 month observation period before transfer (P = 0.002). A total of 25/119 patients showed increased creatinine ≥20% just before transfer. Biopsy proven rejection was found in 10/119 patients. Three patients lost their graft due to chronic graft nephropathy.
Mean coefficient of variation (CoV%) of immunosuppression levels was 0.20 ± 0.1. Increased creatinine levels ≥20% just before transfer were less frequently seen in patients with CoV < 0.20 (P = 0.007).
The majority of pediatric nephrology centers have internal agreements on transitional care. More than half of the patients had CoV of immunosuppression trough levels consistent with good adherence. Although, 20% of the patients showed increase in serum creatinine close to transfer.
INTRODUCTION
About 80% of adolescents between 15 and 18 years of age with end-stage renal disease in Germany have a functioning kidney transplant. In this age group, loss of transplant was observed to be 2% to 3% per year.1 In the USA and Canada, more transplanted kidneys are lost in patients aged 13 to 25 years than in any other age group.2 Such graft failure requires a return to dialysis, which reduces quality of life and leads to considerable additional healthcare expense. Moreover, early graft losses increase morbidity and mortality, and shorten life expectancy. Graft failure rates increase from the age of 13 and peak between 17 and 24 years of age regardless of age at transplantation.3 Two major risk factors aggravate this situation in adolescents and young adults “coming of age”: First, poor treatment adherence is considered a main cause of increased risk for graft failure. Secondly, transition from pediatric to adult care, disrupting the continuity of medical care during a difficult developmental period, results in a further increase of risk. Some studies report unexpected kidney graft failure rates within 3 years after transfer to be as high as 24% to 35%.4,5
Medication adherence and adherence to general care (eg, clinic appointments) deteriorate during adolescence and emerging adulthood.6 Increasing independence from their parents, less time under direct adult supervision and assuming more responsibility for medication and overall management of their condition may lead to poorer adherence in adolescents and young adults in comparison to children.2 Identifying and monitoring medication nonadherence in the adolescent and young adult kidney transplant population remains challenging for clinicians. Since measurement of blood medication levels is a standard practice to monitor adequacy of immunosuppression in transplanted patients, these levels can serve as surrogate parameters for patient adherence.7 Coefficient of variation (CoV%) of immunosuppressant levels can be used, as well as the percentage of subtherapeutic levels over a given time.8
In nephrology in particular, transition constitutes an important intersection in patient care.2,9 Transition can be considered successful if it promotes the patients’ health competence, supports their psychosocial rehabilitation, and improves their self-determination efficacy, including their ability to make decisions and communicate about their care. The overarching goal of transition is to enable patients to be as independent as possible and have the best possible quality of life. Historically, a lack of cooperation among the various professionals involved in treatment, and concomitant care has led to medical care issues for patients, which is particularly problematic in the transition phase. From an analysis of such deficiencies, it is possible to derive strategies for establishing a satisfactory patient-centered transition.10–12 Training programs, such as “Finally Grown Up” (“Endlich-Erwachsen”)13 and computer-based training courses,14 have been shown to produce some improvements in the transition of postkidney transplant adolescents into adult medical care.
At present, it is unclear, how transition is performed after kidney transplantation (KTX) in German and Austrian centers and which, if any, transition models are applied. Therefore, this observational study aims to evaluate transition, adherence, and outcome of adolescents after KTX in all centers for pediatric KTXs of both nations.
METHODS
A retrospective analysis of the existing structures was carried out on-site at each of 22 pediatric nephrology centers, which treat pediatric renal transplant recipients in Germany and Austria as the first phase of the TRANSNephro study. The study has been registered at ISRCTN Registry (No. 22988897), and the study protocol was published December 2014.15 The TRANSNephro-study consists of 2 parts. Part 1, the analysis of the existing mode of transition is reported in the present paper. Part 2, the examination of a new transition model for postkidney transplant adolescents, is still ongoing.
Data for this study were assessed during site visits by the same pediatric nephrologist (author MK) and a study nurse in all centers to ensure high data quality.
To assess the existing structure of patient care, transition, and rehabilitation care a standardized questionnaire was used (Table 1). Since it is difficult to determine the exact proportion of time spend only for care of transplanted patients in a pediatric nephrology unit, data on staffing are given with regard to the complete unit (including in- and outpatient care and, if applicable, dialysis). Data are provided as full-time equivalents.
TABLE 1.
Questionnaire

Anonymized information regarding each patient's medical care and treatment course were registered for all patients who were transferred out from the participating pediatric nephrology centers in the years 2011 and 2012, covering the last 12 months before transfer. Treatment course variables include serum creatinine levels, CoV% of immunosuppressive trough levels, and incidences of acute rejection and transplant loss. In addition, the type of doctor (eg, general practitioner, nephrologist) whom the patient was transferred to was registered. Baseline serum creatinine levels were defined as mean of the first 3 levels in the year.
For the definition of nonadherence we estimated the likely therapy adherence rate based on a small group of patients on immunosuppressive therapy. Data from Hannover Medical School showed that among 6 selected patients, 3 showed very good compliance, with a mean CoV% of 13 ± 3, whereas poor compliance was observed in the 3 remaining patients, with an average CoV% of 50 ± 3. We defined good (CoV% < 20%), medium ( CoV% 20%–43%), and poor compliance (CoV% >43%) using 2 standard deviations from mean, respectively. These findings correspond with those published by Hsiau et al 2011.7 The CoV% of the patients transferred at all centers from 2011 to 2012 were calculated retrospectively using at least 4 immunosuppression trough levels (with a median number of 11). Since serum mycophenolic acid levels have been proven not useful in this context,7 CoV% was calculated from tacrolimus (TAC, n = 79), ciclosporin A (n = 35), rapamycin (n = 3), and everolimus (n = 2) trough levels.
To determine adherence to scheduled appointments at the clinic we assessed the frequency of visits per year recommended at the center. Thereafter, we counted the appointments the patients kept within the year prior to transfer. The range from 80% to 120% of recommended frequency was regarded as normal adherence.
The study has been reviewed and accepted by the appropriate ethics committees of all study sites and has therefore been performed in accordance with the ethical standards laid down in an appropriate version of the 2000 Declaration of Helsinki as well as the Declaration of Istanbul 2008. Since all patients had left the pediatric centers at least 1 year before the data were obtained, it was impossible to get informed, written consent of them or their parents. The ethic committees waived the need for written consent but placed the following conditions: all data had to be anonymized at the local center and only anonymized data were provided to the study team. All data that could allow backtracking the patients’ personal information (eg, date of birth or sex) was not allowed to be obtained. Therefore, we cannot provide demographic data.
Statistical analysis was performed using SPSS 24.0 (IBM, Ehningen, Germany). The level of significance was set at P < 0.05. We used a Chi-square test for binary variables and a Kruskal–Wallis test for continuous variables that did not meet normality assumptions to compare patient characteristics. Normal distribution was tested with Kolmogorov–Smirnov test. In testing for significant differences, the t-test was used for normal distributed data. Otherwise the Mann–Whitney U test was used. We used Wilcoxons test to compare paired data that was not normally distributed. Normal distributed data are reported as means ± SD, otherwise media and rage are given.
RESULTS
Care Situation
The 22 participating centers cover over 99% of pediatric care after KTX1 in both countries. A total of 872 patients were in pediatric care after KTX in 2014. Health care provider was the KfH (Kuratorium für Dialyse und Nierentransplantation e.V.; curatorship for dialysis and kidney transplantation) in 12 centers, a university hospital in 9 centers and a community hospital in the remaining case. The KfH is a nonprofit association founded in 1969 to overcome shortage of dialysis facilities at that time in Germany. Nowadays, the KfH offers nephrologic health care (both pediatric and adult) at more than 200 kidney centers throughout Germany. In pediatric nephrology care, the KfH centers are mostly associated with university hospitals.
Eight of 22 centers held transplantation outpatient clinics everyday, and 6 centers 4 times a week. The remaining units held transplant clinic 1 to 3 times a week. All 22 centers held transplant clinics in the morning, one also in the evening. It was most commonly reasoned that measuring immunosuppression trough levels determined the timing of appointments. Median number of patients in long-term care after KTX was 30/center (range 3–120). The average staffing of the participating pediatric nephrology units is shown in Table 2.
TABLE 2.
Pediatric Nephrology Unit Staffing
In 21/22 centers (96%), adolescent patients participated in inpatient rehab programs between age 15 and 18, and in 12/22 centers (55 %) also in ambulant rehab programs. Adolescents and young adults from 18 centers (82%) attended the “Endlich Erwachsen” (Finally Grown Up) program (a specially developed coaching concept intended to assist adolescents with early childhood end-stage renal disease or kidney transplant in the transition from pediatric to adult care).13 Median 2 (range 1–4) patients participated in this schooling program per center and year – independently of the number of patients in care at the center. Vienna was the only exception with 16 patients per year sent to the schooling program.
Transfer Situation
In 2011 and 2012, a total of 119 transplanted patients were transferred from pediatric to adult care in Germany and Austria. Within the past years the number of transferred patients averaged 4/center and year (range 0.1–10). The age at transfer was subject to regulation in 16/22 centers (73%). Transfer age most commonly was regulated by “Kassenärztliche Vereinigung” (regional associations of statutory health insurance physicians) (n = 14), but also by health insurance company (n = 3), and health care provider, for example, hospital (n = 1). In all regulatory cases, transfer age was set at 18 years of age.
In Austria, transfer age was not subject to regulation. The clinicians in Vienna rated the most common age at transfer to be 19 – which corresponded well with the median transfer age at Vienna 2011 to 2012 of 19.2 years.
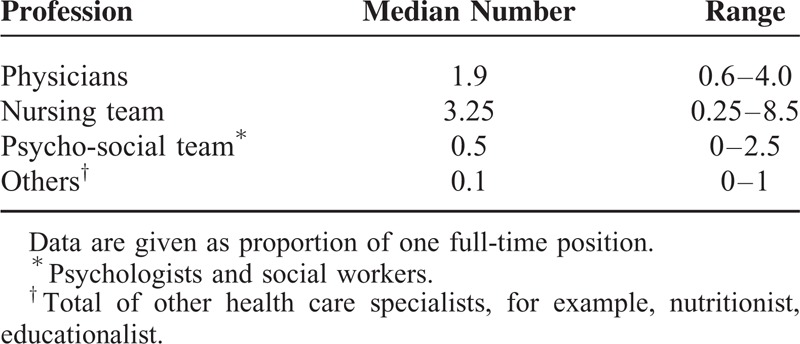
Within the remaining centers, transfer age most commonly was aimed to be 19 to 20 years of age, and in 3 centers ≥21 years. In 5 centers, the aimed age at transfer was 20 years despite the required age to transfer (as requested by the Association of SHI Physicians) being 18 years. This was achieved by individual exemptions, which had to be applied for in the single cases. Median age at transfer of the retrospective cohort was 18.3 years (range 16.5–36.7). The discrepancy in rated and actual transfer age is displayed in Figure 1. A total of 22% of patients were transferred below the age of 18.
FIGURE 1.
Comparison of frequency of actual transfer age of 119 patients from 2011 to 2012 and rated age by pediatric nephrologist in the questionnaire.
In 10/22 centers (46%), the transition process was aimed to start at age 14 to 16, in 8 centers at age 16 to 18, and in 4 centers below an age of 14 years. A total of 16/22 centers (73%) confirmed existence and application of an at least unwritten transition procedure for adolescents. At all centers the pediatric nephrologist and the adult nephrologist were involved in the transition process. In addition, in the majority of centers (19/22) a member of the pediatric psycho-social care team and the nursing staff (14/22) were also involved in the transition process. In all centers, transferred patients were still allowed to (informally) contact the pediatric center in case of problems.
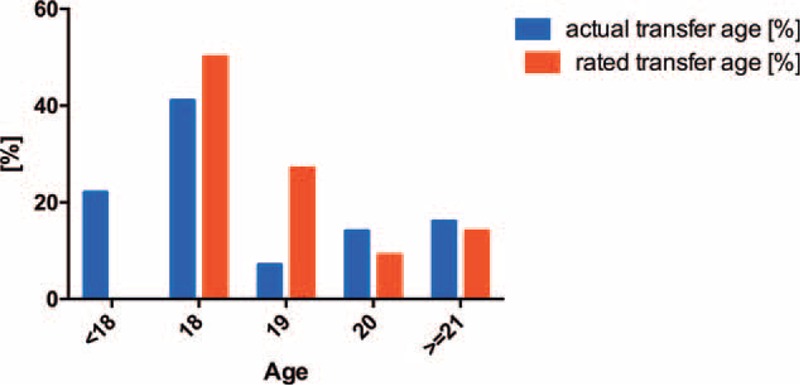
In 2011 and 2012, most of the 119 patients (38%) were primarily transferred to a local nephrologist office (without affiliation to a hospital or university). A quarter of the patients were transferred to an adult transplant clinic at the local university hospital. Twenty-two percent of patients were transferred to a KfH center of nephrology, which is comparable to a nephrologist office but has the KfH (organization) as super-regional health care provider. The distribution of adult nephrology care provider after transition is given in Figure 2.
FIGURE 2.
Distribution of health care providers transferred to in 2011 to 2012.
Graft Function at Transfer
In 2011 and 2012, 116/119 patients were transferred with a functioning kidney transplant. Percutaneous needle biopsy of the graft was performed in 10 patients due to an elevated serum creatinine level compared to baseline. Biopsy proven rejection was found in 2/119 patients within the year before transfer. Additionally, 8 patients had a “borderline” finding (Banff classification16) when biopsied. No biopsy showed chronic humoral rejection.
Three patients lost their graft within the year prior to transfer due to progressive graft nephropathy. They were not biopsied. There was no graft loss related to acute rejection within the year prior to transfer.
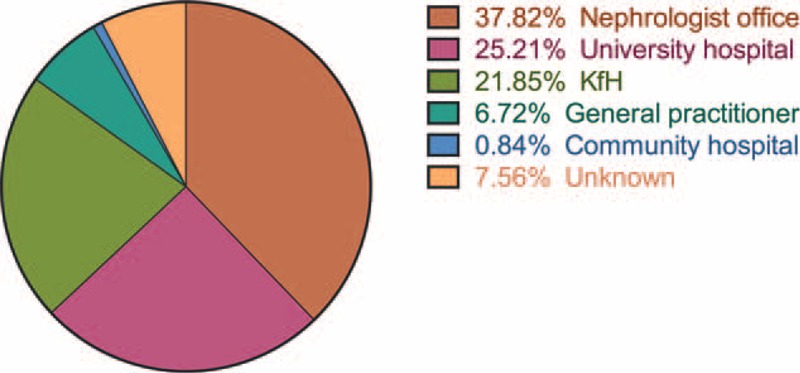
Median serum creatinine 1 year prior to transfer was 123 μmol/L. An increase of median creatinine levels to 132 was observed at the final pediatric visit (P = 0.0015). Furthermore, we found an increase of serum creatinine ≥20% as compared to the baseline levels in 25/119 patients (21%) at the final pediatric visit. This group of patients originated from 13 centers and showed no statistically significant difference in median transfer age (18.4 vs 18.1 years), or frequency of rejections as compared to the other patients. We found a statistically significant difference only in median CoV% of immunosuppressive trough levels (0.22 vs 0.18; P = 0.0356). Six of these patients had progressive graft failure; 3 of them restarted dialysis prior to transfer and 1 underwent surgery for a cimino fistula (restart of dialysis planned for just after transfer). The course of serum creatinine levels of all 25 patients is given in Figure 3.
FIGURE 3.
A total of 25 patients with increased creatinine levels ≥20% as compared to baseline: course of serum creatinine levels within the final year under pediatric health care. Dotted lines indicate the 3 patients who restarted dialysis due to progressive graft failure. ∗On dialysis at final pediatric visit, ∗∗Working cimino fistula/dialysis planned at final pediatric visit.
Adherence
Mean CoV% of immunosuppression levels was 20% ± 10%. An overview of the immunosuppressive regimen is given in Table 3. According to the classification 75/119 patients (63%) showed CoV% consistent with good compliance (CoV% < 20), 39 showed CoV% consistent with medium compliance, and only 5/119 patients showed poor compliance with CoV% >43 (Fig. 4). Patients with transfer age ≤18 years showed CoV% consistent with good adherence in 55% of cases, whereas this proportion in patients older age 21 at transfer was 68%. However, this difference was not statistically significant (P = 0.33).
TABLE 3.
Immunosuppressive Regimen of 119 Patients Transferred to Adult Health Care 2011 to 2012
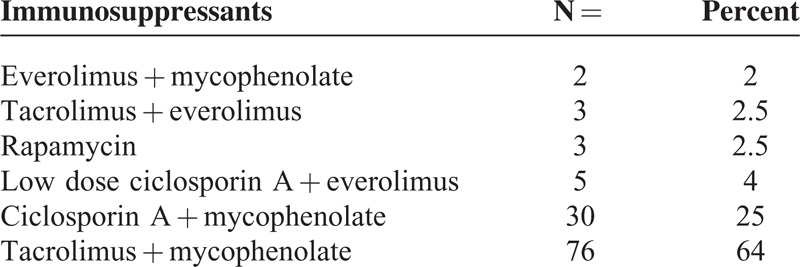
FIGURE 4.
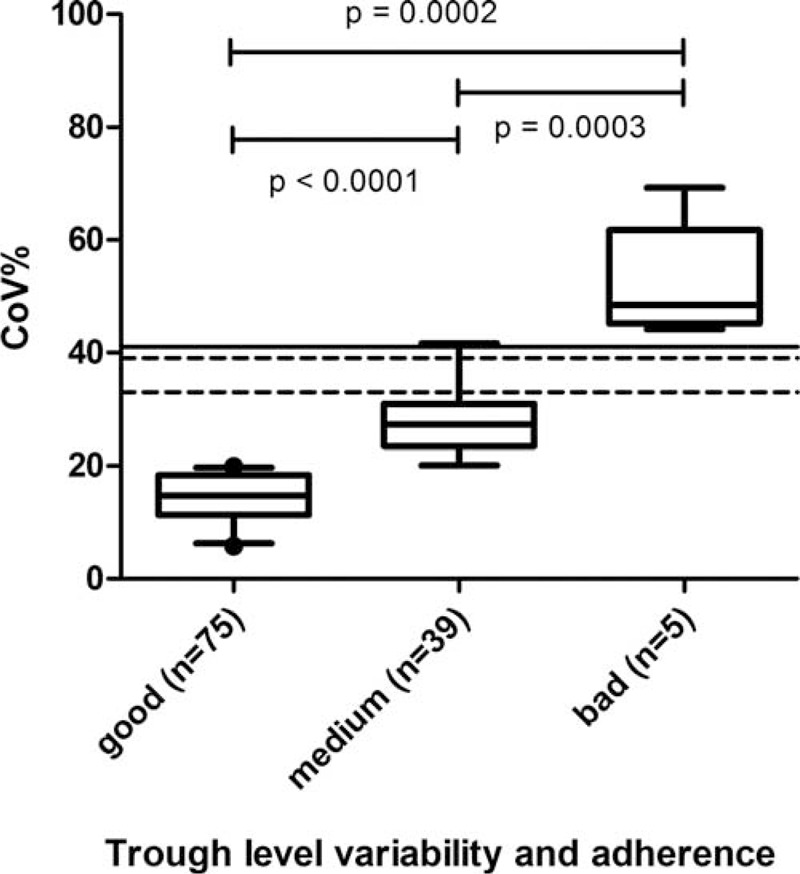
Coefficient of variation (CoV%) of immunosuppressive trough levels as surrogate parameter for medication adherence: distribution in 119 patients. The lines mark the CoV% above which it was statistically significantly associated with an increased risk for acute rejections in previous studies: dotted line at 33%,17 and at 39%,18,19 line at 41%.7.
We found a statistically significant difference between CoV% in the 2 most frequent used immunosuppressants: CoV% in the group on ciclosporin A (n = 39) was lower as compared with the patients on TAC, 16% ± 8% versus 22% ± 11%, respectively (P = 0.001). However, the number of rejections within the final year did not differ between both groups (8.6% vs 8.9%, P = 0.81). There was no difference in serum creatinine at transfer (CSA 149 ± 59 μmol/L vs TAC 150 ± 76 μmol/L, P = 0.95).
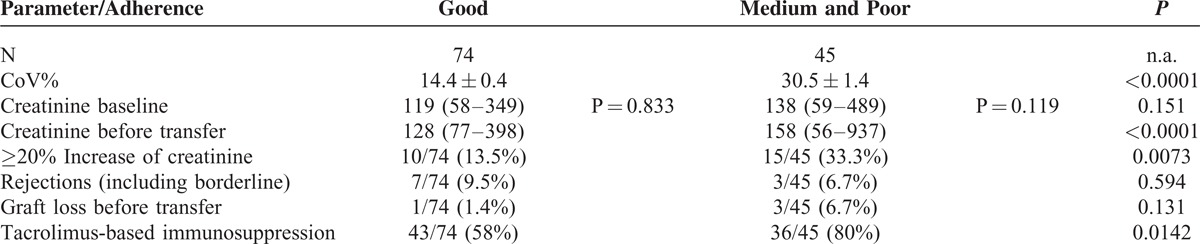
In the group of patients with CoV% associated with medium or poor adherence, we found a serum creatinine increase ≥20% at the final pediatric visit in 15/44 patients (34%) as compared to 10/75 patients (13%) in the group with good adherence (P = 0.0073). For a comparison of the group with good adherence with the others see Table 4.
TABLE 4.
Comparison of Patients With Good Coefficient of Variation (CoV%) With Combined Medium and Poor CoV%
Six of 8 patients with borderline finding in kidney biopsy had CoV% consistent with good medication adherence, and one each with medium and poor adherence, respectively. Of the 2 patients with biopsy proven rejection one had CoV% consistent with good, and the other one with poor adherence.
Clearly subtherapeutical trough levels were rarely observed and found only in 2 patients on TAC + mycophenolate regimen. Both patients had TAC levels <1.0 ng/mL twice within the period observed.
The majority of patients kept the appointments as scheduled by their nephrologist. Eight of 119 patients kept less than 80% of recommended appointments.
DISCUSSION
This is the first comprehensive multicenter, nationwide (even binational) report on transitional care in a special entity (KTX) covering more than 99% of Germany and Austria pediatric kidney transplant population. Furthermore, it is the first multicenter analysis of variation of immunosuppressive trough levels as surrogate parameter for medication adherence. The report highlights 3 major findings: First, although highly specialized care is broadly available in both Germany and Austria, the transition situation is remarkably heterogeneous and differs significantly between centers. Second, we found a high prevalence of serum creatinine increase ≥20% as compared to baseline at the final pediatric appointment – a worrying finding. Third, variation of immunosuppressive trough levels was not as bad in our retrospective cohort as compared to data of the United States6,7 or Canada.17,18 However, patients with higher variation of immunosuppressive trough levels (associated with medium or poor medication adherence) showed significantly more frequently an increase of serum creatinine and impairment of graft function over the time observed. Their loss of transplant rate within the final year at pediatrics was 5-times higher as compared to the other patients – but not statistically significant.
Pediatric Nephrology Care situation in Germany and Austria
In Germany and Austria, health-care for over 800 children and adolescents with kidney transplant is centralized at 22 specialized pediatric nephrology centers. One specific feature in Germany is health-care provided by the KfH. The KfH was founded in 1969 as nonprofit-making association with the goal to provide nationwide renal replacement therapy (as a result of shortage of dialysis sites). Nowadays, 16 KfH centers for pediatric nephrology care for pediatric renal replacement therapy in association with university hospitals and large community hospitals.
Overall, we found satisfying access of adolescents and young adults to rehab facilities and schooling program.9–13 However, the number of participating patients in schooling programs could be improved in Germany–Austria being commendable in this matter.
In both, Germany and Austria, health insurance is statutory and covers expense for medical treatment and medication needed.
Current Transition Situation
Transfer age was subject to regulation in nearly three quarter of the centers, and 64% of patients were transferred before their 19th birthday. Although, a transfer age of 18 to 19 years is commonly found in the literature, and in various countries4,5,20–22 a rigid regulation is a dissatisfying situation in Germany. It does no justice to the transition process and the special needs of the individual transplanted adolescent at all. The essence of timing in all aspects of transition is that of flexibility.9 Timing of events within the transitional process depends on many variables and must be individualized for each patient. Furthermore, various transfer readiness tools have been developed within the past decade and most of them are easily applied.9
The vast majority of clinicians set the start of the transition process to the age of 14 to 16 – which most specialists on transitional medicine would judge to be too late.9,23 There is no data on children with kidney transplant, but data from other chronically ill patients show that independent visits are a determinant of effective transition.24,25 Young people with cystic fibrosis have reported to feel that the age of 13 to 16 was the best time for them to be seen independently.25 Some authors recommend introducing the concept of independent visits in early adolescence (11+ year).9
The largest group of patients was transferred to a local nephrologist practice. Medical specialists working freelance without association with a hospital have been a cornerstone of German health policy for some time. So, a medical professional has to work like a businessman and economic aspects may become more important. Unfortunately, this is the point where barriers for successful transition occur – since time for the patient is limited in adult care. The second largest group of patients was transferred to a university clinic, which has some benefits, but also some drawbacks. Fluctuation of physicians at the university outpatient service is high and, therefore, the transplant clinician does not necessarily know the patient very well.26 Nephrology transition clinics are rare in Germany.27 An interdisciplinary young adult clinic, as introduced in nephrology by Paul Harden in Great Britain,22 would be some sort of gold standard and should be considered as future prospect in Germany and Austria. With about 60 transplanted patients leaving pediatric care per year this should be feasible at (at least) 10 locations.
Graft Function and Medication Adherence in Transition
Nonadherence remains to be a major cause of late allograft loss.2,7 Despite variability in definition and measurement technique one can agree that about 30% of pediatric kidney transplant patients have difficulties adhering to the immunosuppressive regimen.16 The rate of poor adherence in adolescent and young adult solid organ transplant patients is reported 2 to 3 times higher as compared to younger children28,29 or adults (aged 24 to 44 years).30 The factors causing medication nonadherence in adolescents and young adults are multifactorial.2,7 They include neurocognitive immaturity, identity and autonomy conflicts, inadequate social adjustment, lack of social support, and sometimes even psychological disorders such as posttraumatic stress disorder or depression.2,7
Several studies have shown that a high variability in TAC blood levels predict a high risk for late rejection and graft failure in kidney18 and other solid organ transplanted children.17,19,31 Hsiau et al7 demonstrated that higher variation in serum TAC levels (as measured by CoV%) is associated with more frequent late rejection episodes in pediatric renal transplantation patients. They found a significantly higher rejection rate in patients with the TAC CoV% exceeding 41%. We calculated the mean CoV% from the patient groups with rejection from Pollock-BarZiv et al17 and Sapir-Pichhadze et al18 using mean and the statistically significant standard deviation of TAC trough levels. TAC CoV% in this group was 33% and 39%, respectively. Thus, we conclude that a TAC CoV% considerably below 40% is not associated with an increased risk of rejection – which is supported by our data. Within the pediatric setting the majority of adolescents and young adults (63%) in Germany and Austria showed CoV% below 20%, which is compatible with a good medication adherence. This is supported by the low rejection and graft loss rate in our cohort. In our retrospective cohort, CoV% had no statistically significant predictive value for rejection or graft loss. However, our study is limited by its retrospective nature and a very short observation period of 1 year. Interestingly, the CoV% in our binational cohort is identical to the one found in adults by our colleagues at the department of nephrology at Hannover Medical School.26
Keeping scheduled appointments at the transplant clinic seemed not to be an issue in the pediatric setting in both countries.
Three transplants were lost within the final year before transfer due to progressive graft nephropathy and one shortly after. This equals an overall graft loss rate of 3 in 100 patients/year. This data are compatible with the registry data of QuaSi Niere (a quality assurance system for renal replacement therapy in Germany, which was terminated in 2007)1 and is considerably lower as compared to data from the Organ Procurement an Transplantation Network registry (1988–2009) with a graft loss rate of 4 to 6 in the same age group.2
In general TAC is regarded a more potent immunosuppressive agent as compared to ciclosporin A (CSA). Patients are often switched to TAC after acute or due to chronic rejection – which is also associated with bad medication adherence. Consequently, there might be a selection for more nonadherent children in the TAC group in Germany – which can be an explanation for the statistically significant difference in CoV% of trough levels we found in the 2 groups. However, in our analysis no prognostic difference (number of rejections, serum creatinine) between patients with TAC and those with CSA could be found.
There is one major and worrying finding in our analysis: 25 of 119 patients (21%) presented with markedly increased serum creatinine levels at their final appointment in the pediatric setting. In the minority (6 patients), this was owed to progression of allograft nephropathy. Patients entering the final year at the pediatric setting with high serum creatinine levels seem to have an increased risk of further impairment of graft function within this period of time. In the other 19 patients, one can observe a stable creatinine level over the year and an increase at the last 1 to 2 (3) visits. Since the retrospective data are limited one can only speculate about the cause, eventually, this might be some subconscious plead of some of the adolescents to remain in their conversant pediatric setting. A rigid regulation of transfer age in these circumstances can be catastrophic both for graft and patient.
Future Prospects
Taking into account the results of our study, there is an urgent need for improvement in transition and patient education, as well as psychosocial care in Germany in order to harmonize transition and to improve adherence to medication. Kidney graft survival would profit from an individualized transition age and more patient education and psychological interventions in case of bad adherence as, for example, determined by CoV%.
This retrospective study was performed as the first part of the TRANSNephro project to assess the current transition situation in Germany and Austria and provide data on graft function, graft loss rates, and the variation of immunosuppression trough levels. A prospective, randomized, interventional binational multicenter study is the second part of the TRANSNephro project,15 and patient recruitment has started at the pilot center Hannover in October 2014 and from January to June at the other participating centers. This study will not only provide additional information about allograft nephropathy, acute and chronic rejection, formation of donor specific antibodies and clinical course in the pediatric, and adult nephrology setting, but is the first interventional, prospective, randomized trial in transition with the possibility to prove to which extent a transition program can really improve health.
Acknowledgments
The TRANSNephro team wishes to cordially thank all physicians, members of the psycho-social, and nursing team for the warm welcome given at all 22 participating centers. We thank for the time people spent with the team and for all the input, ideas, information, and personal experience they shared.
Footnotes
Abbreviations: CoV% = coefficient of variation, KfH = Curatorship for dialysis and kidney transplantation (Kuratorium für Dialyse und Nierentransplantation), KTX = kidney transplantation, TAC = tacrolimus.
MK and JP contributed equally to this work.
LP conceived the study. LP, RB, MO, DB, SM, MK, JP, and MLD designed the study. MK and LP drafted the study protocol. MK, LP, and JP performed the site visits. MK evaluated the data and performed the statistics. MK, JP, and LP wrote the first draft of the paper. JT, BH, AB, WR, MH, MP, MK, JD, SR, UJ, CT, KD, SH, GK, HF, BK, CM, BLS, BR, HB, HS, and KHR provided data for the questionnaire and patient data, read, revised, and approved the manuscript.
This study was supported by a research grant of the KfH-Stiftung Präventivmedizin (KfH foundation for preventive medicine).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.U Frei. Schober-Halstenberg HJ. Nierenersatztherapie in Deutschland. Bericht über Dialysebehandlung und Nierentransplantation in Deutschland 2007 QuaSiNiere (Renal replacement therapy in Germany. Report on dialysis and kidney transplantation in Germany 2007 from the QuaSi-Niere registry.) Berlin, Germany: QuaSi-Niere gGmbH; 2008. ISBN 3-9809996-3-7. [Google Scholar]
- 2.Foster BJ. Heightened graft failure risk during emerging adulthood and transition to adult care. Pediatr Nephrol 2015; 30:567–576. [DOI] [PubMed] [Google Scholar]
- 3.Foster BJ, Dahhou M, Zhang X, et al. Association between age and graft failure rates in young kidney transplant recipients. Transplantation 2011; 92:1237–1243. [DOI] [PubMed] [Google Scholar]
- 4.Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol 2000; 14:469–472. [DOI] [PubMed] [Google Scholar]
- 5.Prestidge C, Romann A, Djurdjev O, et al. Utility and cost of a renal transplant transition clinic. Pediatr Nephrol 2012; 27:295–302. [DOI] [PubMed] [Google Scholar]
- 6.Pai AL, Ingerski LM, Perazzo L, et al. Preparing for transition? The allocation of oral medication regimen tasks in adolescents with renal transplants. Pediatr Transplant 2011; 15:9–16. [DOI] [PubMed] [Google Scholar]
- 7.Hsiau M, Fernandez HE, Gjertson D, et al. Monitoring nonadherence and acute rejection with variation in blood immunosuppressant levels in pediatric renal transplantation. Transplantation 2011; 92:918–922. [DOI] [PubMed] [Google Scholar]
- 8.Akchurin OM, Melamed ML, Hashim BL, et al. Medication adherence in the transition of adolescent kidney transplant recipients to the adult care. Pediatr Transplant 2014; 18:538–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonagh JE. Growing up and moving on: transition from pediatric to adult care. Pediatr Transplant 2005; 9:364–372. [DOI] [PubMed] [Google Scholar]
- 10.AAP (American Academy of Pediatrics), AAFP (American Academy of Family Physicians) and ASIM (American College of Physicians-American Society of Internal Medicine. A consensus statement on health care transitions from young adults with special health care needs. Pediatrics 2002; 110:1304–1306. [PubMed] [Google Scholar]
- 11.Por J, Golberg B, Lennox V, et al. Transition of care: health care professionals’ view. J Nurs Manag 2004; 12:354–361. [DOI] [PubMed] [Google Scholar]
- 12.Scal P. Transition for youth with chronic conditions: primary care physicians’approaches. Pediatrics 2002; 110:1315–1321. [PubMed] [Google Scholar]
- 13.John U, Offner G, Breuch K, et al. Concept to improve adherence in adolescents following renal transplantation: vision or reality? Urologe 2009; 48:1468–1472. [DOI] [PubMed] [Google Scholar]
- 14.Freier C, Oldhafer M, Offner G, et al. Impact of computer-based patient education on illness-specific knowledge and renal function in adolescents after renal transplantation. Pediatr Transplant 2010; 14:596–602. [DOI] [PubMed] [Google Scholar]
- 15.Kreuzer M, Prüfe J, Bethe D, et al. The TRANSNephro-study examining a new transition model for post-kidney transplant adolescents and an analysis of the present health care: study protocol for a randomized controlled trial. Trials 2014; 15:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hara S. Banff 2013 update: pearls and pitfalls in transplant renal pathology. Nephrology (Carlton) 2015; 20 Suppl 2:2–8. [DOI] [PubMed] [Google Scholar]
- 17.Pollock-Barziv SM, Finkelstein Y, Manlhiot C, et al. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant 2010; 14:968–975. [DOI] [PubMed] [Google Scholar]
- 18.Sapir-Pichhadze R, Wang Y, Famure O, et al. Time-dependent variability in tacrolimus trough blood levels is a risk factor for late kidney transplant failure. Kidney Int 2014; 85:1404–1411. [DOI] [PubMed] [Google Scholar]
- 19.Stuber ML, Shemesh E, Seacord D, et al. Evaluating non-adherence to immunosuppressant medications in pediatric liver transplant recipients. Pediatr Transplant 2008; 12:284–288. [DOI] [PubMed] [Google Scholar]
- 20.Samuel SM, Nettel-Aguirre A, Hemmelgarn BR, et al. Graft failure and adaptation period to adult healthcare centers in pediatric renal transplant patients. Transplantation 2011; 91:1380–1385. [DOI] [PubMed] [Google Scholar]
- 21.Van den Heuvel ME, Van der Lee JH, Cornelissen EA, et al. Transition to the adult nephrologist does not induce acute renal transplant rejection. Nephrol Dial Transplant 2010; 25:1662–1667. [DOI] [PubMed] [Google Scholar]
- 22.Harden PN, Walsh G, Bandler N, et al. Bridging the gap: an integrated paediatric to adult clinical service for young adults with kidney failure. BMJ 2012; 344:e3718. [DOI] [PubMed] [Google Scholar]
- 23.McManus M, White P, Barbour A, et al. Pediatric to adult transition: a quality improvement model for primary care. J Adolesc Health 2015; 56:73–78. [DOI] [PubMed] [Google Scholar]
- 24.Reid GJ, Irvine MJ, McCrindle BW, et al. Prevalence and correlates of successful transfer from pediatric to adult health care among a cohort of young adults with complex ongenital heart defects. Pediatrics 2004; 113e:e197–e205. [DOI] [PubMed] [Google Scholar]
- 25.Zack J, Jacobs CP, Keenan PM, et al. Perspectives of patients with cystic fibrosis on preventive counselling and transition to adult care. Pediatr Pulmonol 2003; 36:376–383. [DOI] [PubMed] [Google Scholar]
- 26.Pabst S, Bertram A, Zimmermann T, et al. Physician reported adherence to immunosuppressants in renal transplant patients: prevalence, agreement, and correlates. J Psychosom Res 2015; 79:364–371. [DOI] [PubMed] [Google Scholar]
- 27.Pape L, Lämmermühle J, Oldhafer M, et al. Different models of transition to adult care after pediatric kidney transplantation: a comparative study. Pediatr Transplant 2013; 17:518–524. [DOI] [PubMed] [Google Scholar]
- 28.Dobbels F, Ruppar T, De Geest S, et al. Adherence to the immunosuppressive regimen in pediatric kidney transplant recipients: A systematic review. Pediatr Transplant 2010; 14:603–613. [DOI] [PubMed] [Google Scholar]
- 29.Dew MA, Dabbs AD, Myaskovsky L, et al. Meta-analysis of medical regimen adherence outcomes in pediatric solid organ transplantation. Transplantation 2009; 88:736–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinsky BW, Takemoto SK, Lentine KL, et al. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Transplant 2009; 9:2597–2606. [DOI] [PubMed] [Google Scholar]
- 31.Shemesh E, Shneider BL, Savitzky JK, et al. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics 2004; 113:825–832. [DOI] [PubMed] [Google Scholar]


