Abstract
This cross-sectional study was designed to obtain the current prevalence of deep vein thrombosis (DVT) and analyze related risk factors in patients undergoing lumbar interbody fusion.
Medical record data were collected from Department of Spinal Surgery, The Third Hospital of Hebei Medical University, between July 2014 and March 2015. Both univariate analysis and binary logistic regression analysis were performed to determine risk factors for DVT.
A total of 995 patients were admitted into this study, including 484 men and 511 women, aged from 14 to 89 years old (median 50, IQR 19). The detection rate of lower limb DVT by ultrasonography was 22.4% (223/995) in patients undergoing lumbar interbody fusion. Notably, average VAS (visual analog scale) score in the first 3 days after surgery in the DVT group was more than that in the non-DVT group (Z = −21.69, P < 0.001). The logistic regression model was established as logit P = −13.257 + 0.056∗X1 − 0.243∗X8 + 2.085∗X10 + 0.001∗X12, (X1 = age; X8 = HDL; X10 = VAS; X12 = blood transfusion; x2 = 677.763, P < 0.001).
In conclusion, advanced age, high postoperative VAS scores, and blood transfusion were risk factors for postoperative lower limb DVT. As well, the logistic regression model may contribute to an early evaluation postoperatively to ascertain the risk of lower limb DVT in patients undergoing lumbar interbody fusion surgery.
INTRODUCTION
Venous thromboembolism (VTE) consists of 2 interrelated conditions caused by blood clots, notably deep vein thrombosis (DVT) and pulmonary embolism (PE).1 Deep vein thrombosis is often overlooked, but a serious, and potentially preventable disorder that affects mostly hospitalized patients due to immobility. Deep vein thrombosis is composed of a clot of blood formed in sites of damaged vessels and areas of stagnant blood flow such as lower leg, thigh, or pelvis. This clot may remain in situ or move to the pulmonary arteries and cause PE. It can lead to severe morbidity with poor quality of life and even sudden death related to PE. Approximately half of all untreated DVT cases are complicated by PE, and conversely, 50% to 80% of all untreated PE cases are associated with DVT.2,3 The risk factors that have the strong odds for a potential VTE include, but not limited to, major general surgery, major orthopedic surgery, spinal cord injury, fracture of the pelvis, hip or long bones; multiple trauma, malignancy, congestive heart or respiratory failure, myocardial infarction, prior VTE, advancement in age, obesity, immobility, varicose veins, pregnancy and puerperium, use of oral contraceptives, antiphospholipid antibody syndrome, and such hereditary risk factors as antithrombin and protein C and S deficiencies.4–7 In spinal surgery, the factors for venous stasis are considered to be long-time horizontal ventral decubitus, lack of muscle tone, and venous compression by retractors and postoperative bed rest.8
It has been reported that venous intimal injury may occur in surgical handling.8 Consistent with this, our recent cohort study has revealed that shorter incision length during operation is more likely to contribute to lower limb DVT.9 In the same study, we have found that the patients undergoing spinal interbody fusion are the main population especially vulnerable to lower limb DVT.9 It is estimated that the DVT incidence of asymptomatic patients exceeds 15%.10 Thus, it is very necessary to detect lower limb DVT by routine use of ultrasonography to the patients undergoing spinal interbody fusion, because it may lead to potentially lethal disease such as PE, although DVT incidence of symptomatic patients is only around 0.5% to 2.5% of the procedures in the spine.10 In addition, we have noticed in clinical situations that the patients after surgery tend to do less lower limb exercise due to lower back pain. Therefore, visual analog scale (VAS) score is analyzed as a risk factor in this study to explore its relation to postoperative lower limb DVT.
Based on the above-mentioned reasons, this single-center cross-sectional study is designed with the purpose to investigate the current prevalence of lower limb DVT in the patients undergoing spinal interbody fusion surgery through the detection rate of lower limb DVT by ultrasonography, and in the meanwhile, to explore the risk factors associated with lower limb DVT. This study is expected to lead to a better understanding of the lower limb DVT after spine interbody fusion surgery, thus contributing to an early primary evaluation of postoperative lower limb DVT risk rate.
PATIENTS AND METHODS
Ethics Statement
There is no need to seek consent from patients, since in this retrospective cross-sectional study all the data were collected and analyzed anonymously without any potential harm to the patients; this is approved by Ethics Committee of The Third Hospital of Hebei Medical University.
Patients
This study included patients who underwent lumbar interbody fusion operations, admitted into Department of Spinal Surgery, The Third Hospital of Hebei Medical University in China, between July 2014 and March 2015. The inclusion criteria were complete medical records including patient number, sex, age, body weight, body height, regional distribution, hospital stay, occupation, DVT, spinal epidural hematoma, hypertension, diabetes, heart disease, surgical method, level and number of vertebrae fusion, operation duration, blood loss, blood transfusion, incision length, prothrombin time activity (PTA), fibrinogen (FIB), thrombin time (TT), D-dimer, HDL (high-density lipoprotein), LDL (low-density lipoprotein), total cholesterol (TC), total bilirubin, direct bilirubin and indirect bilirubin, as well as VAS score in the first 3 postoperative days. All of the above biochemical factors were tested before spine surgery. All the patients with lumbar interbody fusion surgery were examined by lower extremity ultrasonography pre- and postoperatively. After spine surgery, subfascial drainage was routinely used. Besides, all the patients routinely received prophylactic treatment with low-molecular-weight heparin (LMWH) at 4100 IU per day. Simultaneously, the patients were encouraged to do lower limb exercise on bed to accelerate blood circulation. The exclusion criteria included patients who did not undergo lumbar interbody fusion, had a prior DVT, or had been on anticoagulant therapy such as warfarin, aspirin, and clopidogrel prior to hospitalization.
METHODS
Data was collected from subjects after they were identified according to the inclusion and exclusion criteria. Microsoft Excel was used for the data input. Statistical analyses were performed using SPSS for Windows, version 18.0 (SPSS, Inc, Chicago, IL). All measurement data were presented as the median value (interquartile range, IQR). Chi square and Mann–Whitney U test were used for data analyses where applicable. The nonconditional binary logistic regression model was used for the exploration of the associated risk factors of lower limb DVT in postspinal surgery patients. Values for P < 0.05 were regarded as significant with 2-tailed tests.
RESULTS
General Data of Patients Included
A total of 995 patients, including 484 men and 511 women, met the criteria and were therefore incorporated into this single-center cross-sectional study. Their ages ranged from 14 to 89 years (median 50, IQR 19), and demographically, 668 patients hailed from rural areas and 327 from urban areas. The average hospital stay was 15 days (IQR, 4). Totally 223 cases (22.4%) developed postoperative DVT, which were all anonymous. One DVT site was found with 152 DVT cases, 2 DVT sites with 60 cases, and 3 DVT sites with 11 cases. With regard to DVT distribution, 208 sites were found in venous plexus of calf muscle, 52 DVT sites in posterior tibial veins, 30 in popliteal vein, 7 in calf superficial vein, and only 1 in femoral vein. In addition, no PE cases were found, and only 1 case (0.1%) developed spinal epidural hematoma.
Age, Gender, and Regional Distribution
The average age of the DVT group was 57 years (IQR = 13) whereas that of the non-DVT group was 48 years (IQR = 23). Mann–Whitney U test showed a statistically significant difference between the age of DVT patients and non-DVT group (Z = −8.692, P < 0.001). Out of the 233 postoperative DVT patients, 103 were men and 120 were women, with 166 and 57 of the cases from rural and urban areas, respectively, whereas 381 men and 391 women were also identified in the 772 non-DVT cases with 502 and 270 cases from rural and urban areas respectively. The chi square test indicated that there was no statistically significant difference in gender distribution between DVT group and non-DVT group (x2 = 0.693, P = 0.405), but regional distribution was significantly different (x2 = 6.949, P = 0.008).
Comparison of Surgical Data
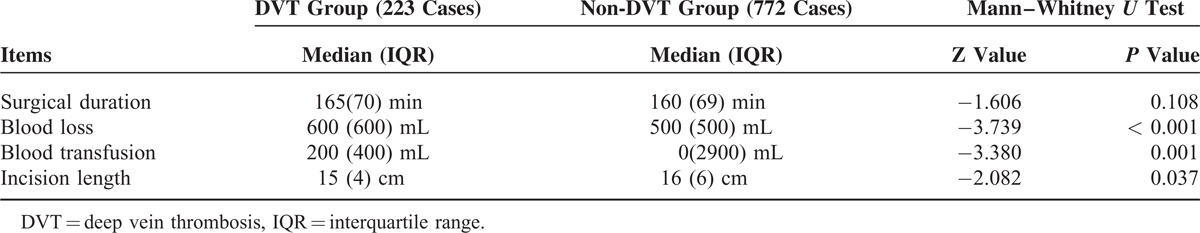
The surgical data included surgical duration, blood loss, blood transfusion, and incision length according to the patient operation note. Table 1 shows that blood loss and blood transfusion in the DVT group was significantly more than that in the non-DVT group (Mann–Whitney U test, P = 0.000 and P = 0.001, respectively). Also, incision length in the DVT group was significantly shorter than that in the non-DVT group (Mann–Whitney U test, P = 0.037). However, surgical duration between the 2 groups did not show any statistical difference (Mann–Whitney U test, P = 0.108).
TABLE 1.
Comparison of Surgical Data Associated With Postoperative DVT
Chronic Disease History
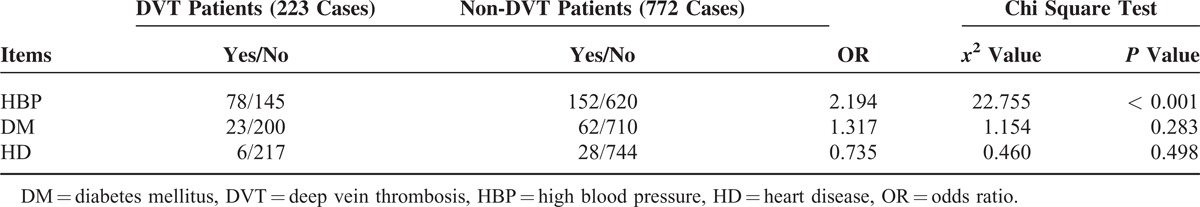
Table 2 shows comparison of chronic disease history between the DVT group and non-DVT group, including high blood pressure (HBP), diabetes mellitus (DM), and heart disease (HD). High blood pressure in the DVT group was more than that in the non-DVT group (chi square test, P < 0.001), whereas DM and HD between the 2 groups did not show any statistically significant difference.
TABLE 2.
Comparison of Chronic Disease History Associated With Postoperative DVT
Biochemical Analyses
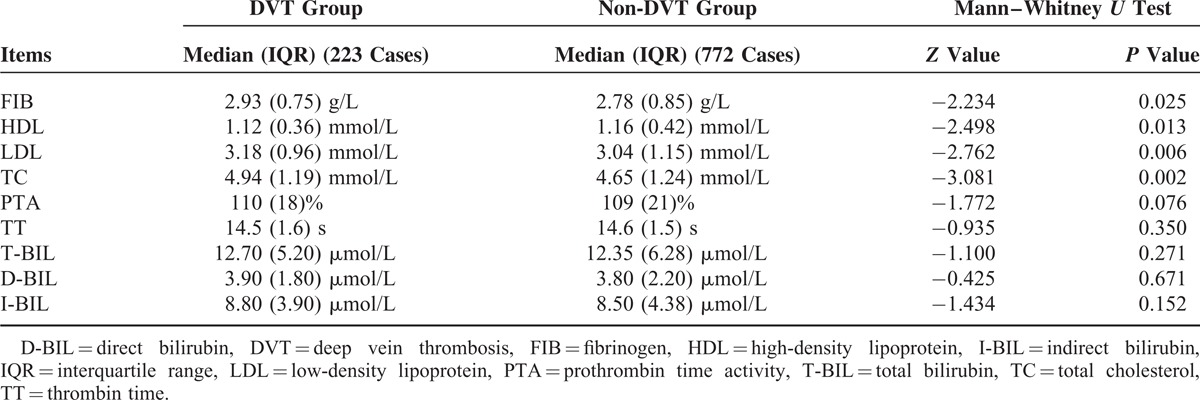
Table 3 shows the biochemical analyses associated with postoperative DVT conducted between the DVT group and the non-DVT group. There was a statistically significant difference between the 2 groups in FIB, HDL, LDL, and TC (Mann–Whitney U test, P = 0.025, P = 0.013, P = 0.006, and P = 0.002, respectively), whereas there was no statistical difference among the other analytes including PTA and TT (P = 0.163 and P = 0.518, respectively; Mann–Whitney U test).
TABLE 3.
Biochemical Analyses Associated With Postoperative DVT
DVT and D-Dimer
The cutoff D-dimer used in this study is 0.3 mg/L. In the DVT group (n = 223), there are 179 cases with D-dimer ≤ 0.3 mg/L and 44 cases with D-dimer > 0.3 mg/L. Meanwhile, in the non-DVT group (n = 772), there are 688 cases with D-dimer ≤ 0.3 mg/L and 84 cases with D-dimer > 0.3 mg/L. It was found that the proportion of patients with D-dimer > 0.3 mg/L in the DVT group was bigger than that in the non-DVT group (Pearson chi square test, χ2 = 12.09, P = 0.001). And then, we compared the data with D-dimer > 0.3 mg/L between the DVT group (n = 44) and non-DVT group (n = 84). The results showed that the level of D-dimer was 0.495 (IQR = 0.52) mg/L in the DVT group and 0.490 (IQR = 0.43) mg/L in the non-DVT group, without statistical significance (Mann–Whitney U test, Z = −1.124, P = 0.261).
Hospital Stay and BMI
The average hospital stay in the DVT group was 16(IQR = 5) days and in the non-DVT group was 15(IQR = 4) days. The difference between them was statistically significant (Mann–Whitney U test, Z = −4.339, P < 0.001). In addition, the body mass index (BMI) was calculated according to body height and weight in all 995 cases, including 223 cases in the DVT group and 772 cases in the non-DVT group. Body mass index in the DVT group was 25.81(IQR = 4.15) and in the non-DVT group 25.39 (IQR = 4.00), without significant difference (Mann–Whitney U test, Z = −1.412, P = 0.158).
DVT and Interbody Fusion Level
In this study, all 995 patients underwent different levels of interbody fusion. A total of 731 cases underwent single-level interbody fusion including 156 DVT cases, 218 cases underwent double-level interbody fusion including 51 DVT cases, and 46 cases underwent three-level and above of interbody fusion including 16 DVT cases. The chi square test applied to the analyses of this data showed no significant difference in DVT incidence in relation to fusion levels (χ2 = 4.652, P = 0.098).
DVT and VAS Score
Average VAS score in the first 3 days after surgery was 3.33 (IQR = 0.67) in the non-DVT group, and 6.00 (IQR = 1.67) in the DVT group. The difference was statistically significant between the 2 groups (Mann–Whitney U test, Z = −21.69, P < 0.001).
Logistic Regression Analysis
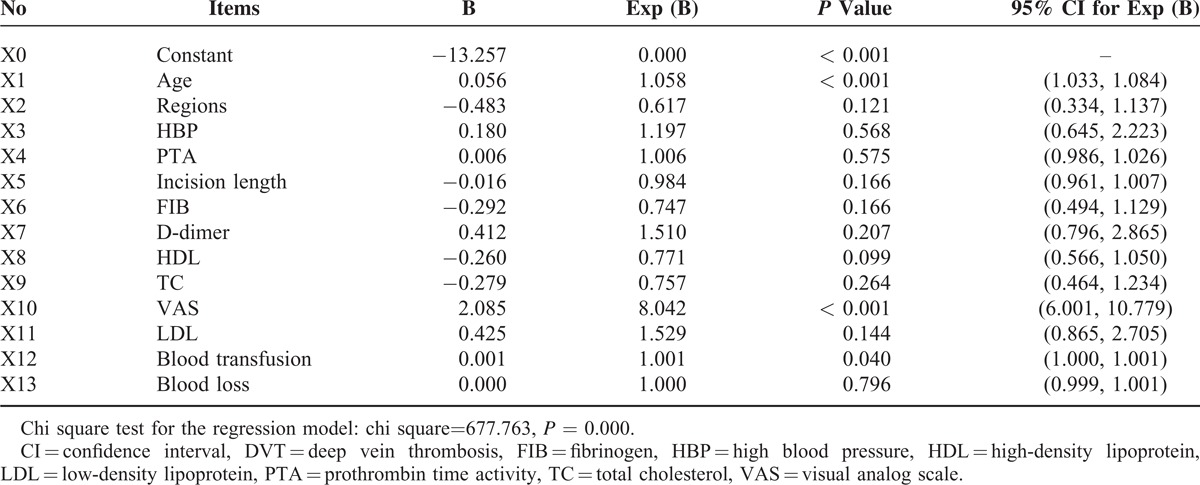
All patients were grouped into 4 categories for the purpose of analysis according to their age (∼45; 45–54; 55–64; 65). The regression analysis method was set to be Backward (LR) and probability for stepwise (Entry 0.10, Removal 0.15). As shown in Table 4, the binary logistic regression revealed advanced age, high VAS scores, and large amount of blood transfusion as the risk factors for postoperative DVT. High-density lipoprotein was however a likely protective factor for postoperative DVT. Hence, the logistic regression equation was presented as logit P = −13.257+ 0.056∗X1− 0.243∗X8+ 2.085∗X10 + 0.001∗X12, (X1 = age; X8 = HDL; X10 = VAS; X12 = blood transfusion). The regression model established in this study was statistically significant by the chi square test (χ2 = 677.763, P < 0.001).
TABLE 4.
Binary Logistic Regression Analysis of Postoperative DVT
DISCUSSION
In this study, it was found by univariate analysis that advanced age, blood loss, blood transfusion, incision length, regional distribution, hypertension, hospital stay, VAS score following surgery, FIB, HDL, LDL, and TC, were all significantly different between the patients with and without DVT. However, the regression model indicated that only advanced age, high VAS scores during the first 3 days after surgery, and large amount of blood transfusion were risk factors for lower limb DVT in patients undergoing spinal interbody fusion. On the contrary, HDL was more likely a protective factor, not a risk factor for postoperative DVT incidence.
To our knowledge, it has been widely reported that postoperative D-dimer assay can effectively predict DVT occurrence.7,11–16 However, even though high D-dimer values are of significance in the sensitivity and specificity as a risk factor for DVT, it may not be accurate to make a diagnosis, since the choice of the cutoff values usually depends on the methods used and the populations studied. In terms of the present study, only preoperative D-dimer was obtained. We have compared positive and negative DVT to positive and negative D-dimers. Taken all together, although the proportion of patients with D-dimer > 0.3 mg/L in the DVT group was bigger than that in the non-DVT group, the distributional trend of D-dimer in patients with D-dimer > 0.3 mg/L is the same between the DVT group and the non-DVT group. Thus, we cannot draw an exact conclusion from the results regarding D-dimer.
This study confirmed the anticipated advanced age as a risk factor for postoperative DVT after spinal interbody fusion, as reported in other studies as well.4,18–20 The result of this study indicated a total DVT incidence among patients with the mean age of 50 years (IQR = 19) as 22.4% after spinal surgery.4 The outcome of this study however, seemed inconsistent with a similar research by Strom et al, involving patients with significantly higher age group (mean 64), that reported only 4.3% VTE incidence (including acute/chronic DVT and PE) after spinal surgery, because lower extremity ultrasonography was performed only on patients that showed symptoms of DVT (unilateral calf pain, edema, erythema, warmth) as well as those patients who remained immobilized on the 3rd postoperative day.4 On the other hand, total DVT incidence was higher (22.4%) in this study because lower extremity ultrasonography was routinely performed on all the patients on the 7th postoperative day. The patients were routinely advised by the doctors to walk after the 7th postoperative day. Lower extremity ultrasonography was thus used to rule out the DVT-associated complications. However, DVT incidence has been reported to be ∼0.5% to 2.5% for symptomatic patients and 15% to 31% for asymptomatic patients after the spinal surgery procedures, which is in line with the 22.4% DVT incidence found in this study.10,21
Lumbar spine has been found to be more prone to defects than the other regions of the whole spine.22 Research indicates that ∼15% of patients undergoing posterior spinal surgery develop DVT if prophylactic measures are not taken, implying a need to make prophylactic measures part of spinal surgery procedures.23 Making prophylaxis part of spinal surgery procedures is vital for VTE prevention, which is more economical than treatment. Although prophylactic treatment has proven effective in preventing hospitalized patients from VTE, only one-third of them receive adequate prophylactic treatment.24 The 2 commonly identified prophylactic measures are mechanical prophylaxis involving intermittent pneumatic compression, and pharmacological prophylaxis, mainly including LMWH.25 However, the effect of LMWH prophylaxis has received bitter controversies in some studies.4,26–28
Pathological and surgical sites or surgical procedure in orthopedics has identified hip or knee arthroplasty, lower limb fracture, and spinal cord injury as high risk for VTE incidence, contrary to upper limb surgery that has comparatively lower risk.29–31 Although there have been several studies about DVT after spinal surgery, the exact incidence of PE and DVT after spinal interbody fusion has not been established. This is confirmed by the result of several studies conducted to ascertain DVT incidence in single center, same prophylaxis, and routine screening for DVT, which had VTE incidence ranging from 1% to 25.0% due to differences in the surgical procedure and surgical site.10,11,19,20,23,32 The radiological study conducted by Takahashi et al to evaluate 100 patients using contrast-enhanced CT after spine surgery reports asymptomatic PE and DVT in the patients as 18% and 19% respectively.17 A multi-institutional study by Hohl et al about patients who underwent spinal surgery, also reported 1.5%, 0.88%, and 0.66% as the prevalence rate for VTE, symptomatic PE and DVT respectively.33 Epidural hematoma is a serious complication after spinal surgery that results in spinal paresis. The result of this study showed epidural hematoma incidence of 0.1% which is consistent with that of the symptomatic postoperative epidural hematoma incidence ranging from 0.1% to 3.0% being reported.34,35
A recent prospective clinical study reported that female sex, advanced age, spinal level, and neurological deficits, were all risk factors for postoperative DVT after elective spinal surgery.20 Cervical spinal surgery in particular has an associated low risk of only 3.0%, and in patients with PE, 3 out of 4 have no DVT, indicating that screening for PE is also needed in high-risk patients since DVT is not the only cause of PE in cervical spinal surgery.20 It is an undisputable fact that PE is more critical and potentially fatal than DVT. However, since PE is rare, many researchers assume it is preceded by DVT hence their focus on DVT. Even thrombi of the calf veins have the potential of proximal propagation, ultimately resulting in PE.20 Patients with total joint arthroplasty of the lower extremities have an increased rate of fatal PE due to asymptomatic DVT.36 This therefore justifies the routine screening of patients undergoing spinal surgery for DVT.
Previous studies reported that major surgery had a high risk of VTE.32,33,37 Multilevel spinal instrumented fusion surgery also induces long operative time, prolonged bed rest, much bleeding, as well as much blood transfusion. These factors result in a high risk of VTE associated with decompression surgery and posterior lumbar interbody fusion. It is in line with the findings reported in the present study that blood transfusion is a risk factor for the postoperative DVT.
In this study, it is of note that the high VAS score following surgery was found for the first time as a risk factor of DVT in patients undergoing spinal interbody fusion surgery. We speculate that it is mainly because of the low back pain due to operative wound, since we did not routinely apply other analgesics to the patients after spine surgery, except patient controlled analgesia prescribed by an anesthetist. Fearful of pain, the patients after surgery did less lower limb exercise on bed, resulting in worse blood circulation of lower limbs, which may contribute to DVT development.
Taken all together, it may have detected several interesting correlations in this study that will help in understanding DVT development in patients with spinal interbody fusion surgery. As well, some findings have been identified as indicators of an early alert for the development of postoperative DVT. The Third Hospital of Hebei Medical University is the biggest orthopedic hospital in Hebei Province; hence, the biggest limitation of the study is the inevitable inclusion of relatively more acute and severe patients in this study. The merit of this study is notably the preoperative screening of VTE. Thus, some of patients with VTE before surgery were excluded, which has decreased 1 important confounder that may falsely increase postoperative DVT incidence.
In conclusion, in this cross-sectional study, advanced age, high VAS scores during the first 3 days after surgery, and large amount of blood transfusion were risk factors for lower limb DVT in patients undergoing spinal interbody fusion. On the contrary, HDL was more likely a protective factor, not a risk factor for postoperative DVT incidence. As well, the logistic regression model may contribute to an early evaluation postoperatively for the risk of lower limb DVT in patients undergoing lumbar interbody fusion surgery.
Acknowledgments
We would like to thank Dr. Hong-Zhi Lv for her help regarding the type of this study design. Dr. Lv is an epidemiologist from The Third Hospital of Hebei Medical University, China. We would like to thank Mr Michael Gyan and Dr Emmanuel Addipa-Adapoe for the help of language editing. Both of them come from Ghana and are now working in Hebei Medical University.
Footnotes
Abbreviations: BMI = body mass index, DM = diabetes mellitus, DVT = deep vein thrombosis, FIB = fibrinogen, HBP = high blood pressure, HD = heart disease, HDL = high-density lipoprotein, IQR = interquartile range, LDL = low-density lipoprotein, LMWH = low-molecular-weight heparin, PE = pulmonary embolism, PTA = prothrombin time activity, SD = standard deviation, TC = total cholesterol, TT = thrombin time, VAS = visual analog scale, VTE = venous thromboembolism.
S-DY and W-YD contributed equally to this study.
Authors’ contributions—conceived and designed the study: DLY; collected data: SDY, WYD, YS, and YZZ; analyzed the data: SDY, FDZ, and SQF; wrote the paper: SDY and WYD.
All authors declare that we have no conflict of interest regarding this study.
REFERENCES
- 1.Bang SM, Jang MJ, Oh D, et al. Korean guidelines for the prevention of venous thromboembolism. J Korean Med Sci 2010; 25:1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moser KM, Fedullo PF, LitteJohn JK, et al. Frequent asymptomatic pulmonary embolism in patients with deep venous thrombosis. JAMA 1994; 271:223–225. [PubMed] [Google Scholar]
- 3.Huisman MV, Buller HR, ten CJW, et al. Unexpected high prevalence of silent pulmonary embolism in patients with deep venous thrombosis. Chest 1989; 95:498–502. [DOI] [PubMed] [Google Scholar]
- 4.Strom RG, Frempong-Boadu AK. Low-molecular-weight heparin prophylaxis 24 to 36 hours after degenerative spine surgery: risk of hemorrhage and venous thromboembolism. Spine 2013; 38:E1498–E1502. [DOI] [PubMed] [Google Scholar]
- 5.Anderson FA, Jr, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med 1991; 151:933–938. [PubMed] [Google Scholar]
- 6.Geerts WH, Code KI, Jay RM, et al. A prospective study of venous thromboembolism after major trauma. N Engl J Med 1994; 331:1601–1606. [DOI] [PubMed] [Google Scholar]
- 7.Si WT, Zhang HG, Sun YB, et al. Correlation analysis on plasma D-dimer level with deep venous thrombosis after spinal surgery. Zhongguo Gu Shang 2014; 27:405–408. [PubMed] [Google Scholar]
- 8.Matsumoto MH, Rodrigues LC, Batalini LG, et al. Influence of blood coagulability after spinal surgeries. Acta Ortop Bras 2014; 22:235–239.doi:101590/1413-78522014220500930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang SD, Liu H, Sun YP, et al. Prevalence and risk factors of deep vein thrombosis in patients after spine surgery: a retrospective case-cohort study. Sci Rep 2015; 5:11834.sre 11834 doi:10 1038/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee HM, Suk KS, Moon SH, et al. Deep vein thrombosis after major spinal surgery: incidence in an East Asian population. Spine 2000; 25:1827–1830. [DOI] [PubMed] [Google Scholar]
- 11.Yoshioka K, Kitajima I, Kabata T, et al. Venous thromboembolism after spine surgery: changes of the fibrin monomer complex and D-dimer level during the perioperative period. J Neurosurg Spine 2010; 13:594–599.doi:103171/20105SPINE09883. [DOI] [PubMed] [Google Scholar]
- 12.Yukizawa Y, Inaba Y, Watanabe S, et al. Association between venous thromboembolism and plasma levels of both soluble fibrin and plasminogen-activator inhibitor 1 in 170 patients undergoing total hip arthroplasty. Acta Orthop 2012; 83:14–21.doi:103109/174536742011652886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshiiwa T, Miyazaki M, Takita C, et al. Analysis of measured D-dimer levels for detection of deep venous thrombosis and pulmonary embolism after spinal surgery. J Spinal Disord Tech 2011; 24:E35–E39.doi:101097/BSD0b013e3181f60603. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Breitenstein S, Hahnloser D, et al. Kinetics of D-dimer after general surgery. Blood Coagul Fibrinolysis 2009; 20:347–352.doi:10 1097/MBC 0b013e32832a5fe6. [DOI] [PubMed] [Google Scholar]
- 15.Sudo A, Wada H, Nobori T, et al. Cut-off values of D-dimer and soluble fibrin for prediction of deep vein thrombosis after orthopaedic surgery. Int J Hematol 2009; 89:572–576.doi:101007/s12185-009-0323-4. [DOI] [PubMed] [Google Scholar]
- 16.Hamidi S, Riazi M. Cutoff values of plasma d-dimer level in patients with diagnosis of the venous thromboembolism after elective spinal surgery. Asian Spine J 2015; 9:232–238.doi:104184/asj201592232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi H, Yokoyama Y, Iida Y, et al. Incidence of venous thromboembolism after spine surgery. J Orthop Sci 2012; 17:114–117.doi:101007/s00776-011-0188-2. [DOI] [PubMed] [Google Scholar]
- 18.Buerba RA, Giles E, Webb ML, et al. Increased risk of complications after anterior cervical discectomy and fusion in the elderly: an analysis of 6253 patients in the American College of Surgeons National Surgical Quality Improvement Program database. Spine 2014; 39:2062–2069. [DOI] [PubMed] [Google Scholar]
- 19.Tominaga H, Setoguchi T, Tanabe F, et al. Risk factors for venous thromboembolism after spine surgery. Medicine (Baltimore) 2015; 94:e466.doi:101097/MD0000000000000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoshioka K, Murakami H, Demura S, et al. Prevalence and risk factors for development of venous thromboembolism after degenerative spinal surgery. Spine 2015; 40:E301–306.doi:101097/BRS0000000000000727. [DOI] [PubMed] [Google Scholar]
- 21.McClendon J, Jr, Smith TR, O'Shaughnessy BA, et al. Time to event analysis for the development of venous thromboembolism after spinal fusion >5 levels. World Neurosurg 2015; doi:101016/jwneu201503068. [DOI] [PubMed] [Google Scholar]
- 22.Platzer P, Thalhammer G, Jaindl M, et al. Thromboembolic complications after spinal surgery in trauma patients. Acta Orthop 2006; 77:755–760. [DOI] [PubMed] [Google Scholar]
- 23.Oda T, Fuji T, Kato Y, et al. Deep venous thrombosis after posterior spinal surgery. Spine 2000; 25:2962–2967. [DOI] [PubMed] [Google Scholar]
- 24.Koo KH, Choi JS, Ahn JH, et al. Comparison of clinical and physiological efficacies of different intermittent sequential pneumatic compression devices in preventing deep vein thrombosis: a prospective randomized study. Clin Orthop Surg 2014; 6:468–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffer AK. An overview of venous thromboembolism: impact, risks, and issues in prophylaxis. Cleve Clin J Med 2008; 75 Suppl 3:S3–6. [DOI] [PubMed] [Google Scholar]
- 26.Cox JB, Weaver KJ, Neal DW, et al. Decreased incidence of venous thromboembolism after spine surgery with early multimodal prophylaxis: clinical article. J Neurosurg Spine 2014; 21:677–684.doi:103171/20146SPINE13447. [DOI] [PubMed] [Google Scholar]
- 27.Knudson MM, Morabito D, Paiement GD, et al. Use of low molecular weight heparin in preventing thromboembolism in trauma patients. J Trauma 1996; 41:446–459. [DOI] [PubMed] [Google Scholar]
- 28.Schizas C, Neumayer F, Kosmopoulos V. Incidence and management of pulmonary embolism following spinal surgery occurring while under chemical thromboprophylaxis. Eur Spine J 2008; 17:970–974.doi:101007/s00586-008-0668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brambilla S, Ruosi C, La Maida GA, et al. Prevention of venous thromboembolism in spinal surgery. Eur Spine J 2004; 13:1–8.doi:101007/s00586-003-0538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson FA, Jr, Spencer FA. Risk factors for venous thromboembolism. Circulation 2003; 107:I9–16.doi:101161/01CIR000007846907362E6. [DOI] [PubMed] [Google Scholar]
- 31.Jameson SS, James P, Howcroft DW, et al. Venous thromboembolic events are rare after shoulder surgery: analysis of a national database. J Shoulder Elbow Surg 2011; 20:764–770.doi:101016/jjse201011034. [DOI] [PubMed] [Google Scholar]
- 32.Epstein NE. Intermittent pneumatic compression stocking prophylaxis against deep venous thrombosis in anterior cervical spinal surgery: a prospective efficacy study in 200 patients and literature review. Spine 2005; 30:2538–2543. [DOI] [PubMed] [Google Scholar]
- 33.Hohl JB, Lee JY, Rayappa SP, et al. Prevalence of venous thromboembolic events after elective major thoracolumbar degenerative spine surgery. J Spinal Disord Tech 2015; 28:E310–E315.doi:10.1097/BSD.0b013e31828b7d82. [DOI] [PubMed] [Google Scholar]
- 34.Kebaish KM, Awad JN. Spinal epidural hematoma causing acute cauda equina syndrome. Neurosurg Focus 2004; 16:e1. [PubMed] [Google Scholar]
- 35.Lawton MT, Porter RW, Heiserman JE, et al. Surgical management of spinal epidural hematoma: relationship between surgical timing and neurological outcome. J Neurosurg 1995; 83:1–7.doi:103171/jns19958310001. [DOI] [PubMed] [Google Scholar]
- 36.Paiement GD, Wessinger SJ, Harris WH. Cost-effectiveness of prophylaxis in total hip replacement. Am J Surg 1991; 161:519–524. [DOI] [PubMed] [Google Scholar]
- 37.Goldhaber SZ. Risk factors for venous thromboembolism. J Am Coll Cardiol 2010; 56:1–7.doi:101016/jjacc201001057. [DOI] [PubMed] [Google Scholar]


