Abstract
Severe geographic disparities exist in liver transplantation; for patients with comparable disease severity, 90-day transplant rates range from 18%–86% and death rates range from 14%–82% across donor service areas (DSAs). Broader sharing has been proposed to resolve geographic inequity; however, we hypothesized that the efficacy of broader sharing depends on the geographic partitions used. To determine the potential impact of redistricting on geographic disparity in disease severity at transplantation, we combined existing DSAs into novel regions using mathematical redistricting optimization. Optimized maps and current maps were evaluated using the Liver Simulated Allocation Model. Primary analysis was based on 6700 deceased donors, 28,063 liver transplant candidates, and 242,727 Model of End-Stage Liver Disease (MELD) changes in 2010. Fully regional sharing within the current regional map would paradoxically worsen geographic disparity (variance in MELD at transplantation increases from 11.2 to 13.5, p=0.021), although it would decrease waitlist deaths (from 1368 to 1329, p=0.002). In contrast, regional sharing within an optimized map would significantly reduce geographic disparity (to 7.0, p=0.002) while achieving a larger decrease in waitlist deaths (to 1307, p=0.002). Redistricting optimization, but not broader sharing alone, would reduce geographic disparity in allocation of livers for transplant across the United States.
Keywords: Liver allocation, Geographic disparities, Liver Simulated Allocation Model, Broader sharing
Introduction
Severe geographic disparities in allocation of livers for transplant persist in the United States, despite recent incremental changes to geographic priority. Allografts from deceased donors are allocated to candidates in order of decreasing disease severity, as measured by the MELD (Model for End-stage Liver Disease) score,(1) yet historical precedent dictates local-first sharing within a hierarchy of donor service areas (DSAs) and regions originally designed to reflect working relationships between transplant centers. Unfortunately, historical precedent has inadvertently created DSAs and regions with vastly different supply/demand ratios, causing geographic disparities which are magnified by the growing organ shortage.
Today, for example, a patient with a MELD score of 38–39 faces a 90-day probability of waitlist death ranging from 14% in some DSAs to 82% in others, with a 90-day probability of a liver transplant ranging from 18% to 86% based on geographic location.(2) Since allocation priority is based on MELD, the MELD scores at which candidates receive transplants should be similar across geographic areas; however, average MELD at transplant varies by more than 10 points between DSAs and more than 7 points between regions.(3) Candidates in some DSAs have a 20-fold higher rate of transplantation than candidates in other DSAs.(3)
Geographic disparities violate the Code of Federal Regulations as well as ethical principles of organ allocation. The Department of Health and Human Services Final Rule (CFR 42, Chapter 1, Subchapter K, Part 121) clearly states that “neither place of residence nor place of listing shall be a major determinant of access to a transplant.”(4) And ethicists have argued since 1998 that “the United States should end policies that permit geographic inequities [in liver allocation].”(5)
In 1999, the Institute of Medicine evaluated liver allocation, recommending broader sharing of liver allografts as a solution to geographic disparities.(6) The Share 15 policy, which ranks regional candidates with MELD>15 higher than local candidates with MELD<15, began in 2005 in the hopes of narrowing the range of median MELD at transplant among DSAs.(7) Recently, tiered regional sharing plans have been proposed as extensions of Share 15 which would offer livers regionally to all candidates with MELD above a given score before offering them to local candidates with lower MELDs(8). Tiered plans can be seen as a step towards regional sharing, halfway between the current policy and the Institute of Medicine’s recommendations of a fully regional sharing system. In fully regional sharing, livers would be offered first to the candidate with the highest MELD anywhere in the region, rather than to the candidate with the highest MELD in the local DSA.
We hypothesized that broader sharing, even fully regional sharing, might not be sufficient to reduce disparities in liver availability because the current regions do not partition the country into areas that have a similar balance of transplant need and allograft supply. We used the Liver Simulated Allocation Model (LSAM) to determine the effect of fully regional sharing, within the current regional map, on the geographic disparity in median MELD at transplantation. We then applied mathematical redistricting methods to design new, optimized regions for fully regional sharing with the explicit goal of reducing disparity in MELD at transplantation. Our redistricting model preserves existing DSA boundaries, but combines DSAs into different regions that are designed to comport with the Final Rule by giving all candidates a more equitable share of organs for transplantation.
Methods
Optimal Redistricting
We designed novel regions for liver allocation by regrouping DSAs according to an integer programming model. In brief, we computed the optimal value of integer variables xdr ∈ {0,1} where xdr = 1 if DSA d should be included in region r, and 0 if not, based on an objective function (see below) and a number of constraints. Inputs included the number of incident candidates per DSA who reached a certain MELD score range during their first year on the waiting list, and the number of livers recovered per DSA. The model’s constraints were equations that perform housekeeping tasks, such as adding up the number of donors available in a region, ensuring that each DSA was assigned to exactly one region, and allocating livers in decreasing order of medical urgency (as quantified by MELD score) within the regions. The integer program was solved using the ILOG CPLEX Optimizer (IBM, New York).
Optimization Goals
The key constraint stated that the MELD level at which any region exhausted its supply of livers must be similar across regions, mandating that geographical equity be achieved. To maintain reasonably short transport delays within the new regions, the model’s objective was to minimize the sum of , the squared volume-weighted average transport times between each DSA d included in a region and a set of DSAs that we heuristically defined as the loci of the regions r:
Drive times between centers, or between centers and nearby airports, were calculated by Google driving algorithm. Flight segment lengths were estimated using scheduled departure and arrival times, per aircraft category (jet or turboprop). We estimated the transport time between every donor hospital and every transplant center according to the most likely transportation mode, based on extensive discussions and validation with Organ Procurement Organizations. These transport times were combined according to the fraction of donor volume in the source DSA attributable to that donor hospital, and according to the fraction of transplant volume in the recipient DSA attributable to that transplant center, to give a volume-weighted average transport time between any two DSAs.
Liver Simulated Allocation Model
Potential changes to liver allocation were evaluated using the Liver Simulated Allocation Model (LSAM), a validated discrete event simulator that estimates outcomes under any specified liver allocation policy.(9) The U.S. Organ Procurement and Transplantation Network and others have been using LSAM for both research and clinical policy development for over 10 years.(10, 11) Inputs to LSAM were from 2010 (the most recent year available for LSAM programming): 16,318 candidates on the waiting list at the beginning of the year, 11,745 new candidate arrivals, 6700 donor livers recovered, and 242,727 MELD status updates (including exceptions); validation inputs to LSAM were from 2006: 17,297 candidates on the waiting list at the beginning of the year, 10,661 new candidate arrivals, 7085 donor livers recovered, and 185,269 MELD status updates (including exceptions). LSAM calculates transport distances from each donor hospital to the transplant center. The following allocation scenarios were tested using LSAM: (1) current allocation policy (local-first including Share 15) in which the local DSA receives first priority for donated organs; (2) fully regional sharing (in which livers are allocated to the most medically urgent candidate anywhere in the region with no regard to local DSA boundaries) within the current regional map; and (3) fully regional sharing across two novel maps, with DSAs grouped into optimized regions according to the outcomes of our redistricting model described above.
Statistical Analysis
For each allocation scenario (local-first, fully regional sharing with current regional map, fully regional sharing with optimized maps), we calculated, by DSA and region, the median MELD of candidates who were transplanted in our simulations, as well as the variance and range of median MELD at transplant among DSAs. We also calculated the one-year rate of waitlist deaths and total deaths, the fraction of transplants to pediatric recipients, the fraction of transplants allocated locally, and other metrics. LSAM was run with 10 replications for each experiment; when comparing across allocation scenarios, the replications were performed counterfactually so that metrics across allocation scenarios could be compared using the sign test. The sign test uses paired comparisons between the replications, with statistical significance claimed at a p<0.05 level. Statistical analysis was performed using Stata 12.0 SE (Statacorp, Texas).
Results
Geographic Disparities: Current Allocation Policy
In the current (local-first) allocation policy, variance of median MELD at transplantation across DSAs was 11.2 (Table 1). In addition, clustering of disparities was noted, where some regions were composed entirely of DSAs with low median MELD at transplantation (for example, region 6 contained three DSAs, each of which had median MELD 22) while other regions were composed entirely of DSAs with high median MELD at transplantation (for example, region 5 contained five DSAs with median MELDs between 27 and 35).
Table 1. Simulation outputs from LSAM; by allocation scenario.
Four allocation scenarios are compared: the current policy of local-first allocation, fully regional sharing using the current map, and fully regional sharing using the alternative maps designed by redistricting optimization (as shown in Figure 1). Standard errors are shown in parentheses. The last row quantifies geographic disparity as the variance of the median MELD at transplant among all DSAs.
| Local-First Current Map | Fully Regional Sharing | |||
|---|---|---|---|---|
| Current Map | Optimized Map 1 | Optimized Map 2 | ||
| Total deaths per year | 2727 (10.6) | 2680 (4.7) | 2651 (9.8) | 2650 (6.6) |
| Waitlist deaths per year | 1368 (3.3) | 1329 (2.2) | 1307 (5.2) | 1310 (4.4) |
| Median MELD at transplant | 25 | 25 | 26 | 26 |
| First offers for MELD<25 (%) | 14.6% | 4.6% | 3.3% | 3.1% |
| Transplants for MELD>25, Status 1 (%) | 51.9% | 57.2% | 60.8% | 61.2% |
| Pediatric transplants (%) | 6.6% | 6.9% | 7.0% | 7.1% |
| Transplants allocated locally (%) | 74.6% | 49.8% | 49.6% | 48.5% |
| Median transport distance (miles) | 68.5 (0.3) | 132 (0.6) | 143 (0.4) | 142(0.3) |
| Variance of median MELD at transplant | 11.2 | 13.5 | 7.0 | 8.5 |
Bold font signifies that the given metric is statistically significantly different from that for fully regional sharing with the current map. Only variance of median MELD at transplantation is statistically significantly different between Map 1 and Map 2 (see text). All comparisons between local-first current map and Map 1, and between local-first current map and Map 2, are statistically significant.
Geographic Disparities: Fully Regional Sharing across Current Regions
Fully regional sharing within the current regional map did not reduce geographic disparities because of the clustering noted above (in other words, DSAs in regions with homogeneously good supply were not mapped to share their livers with DSAs in regions with poor supply). Making matters worse, in heterogeneous regions, some large DSAs with high disease severity but poor organ supply overwhelmed small DSAs with otherwise reasonable supply from the same region. As a result, the variance of median MELD at transplantation across different DSAs paradoxically increased from 11.2 to 13.5 (Table 1, p=0.021).
Geographic Disparities: Fully Regional Sharing across Optimal Regions
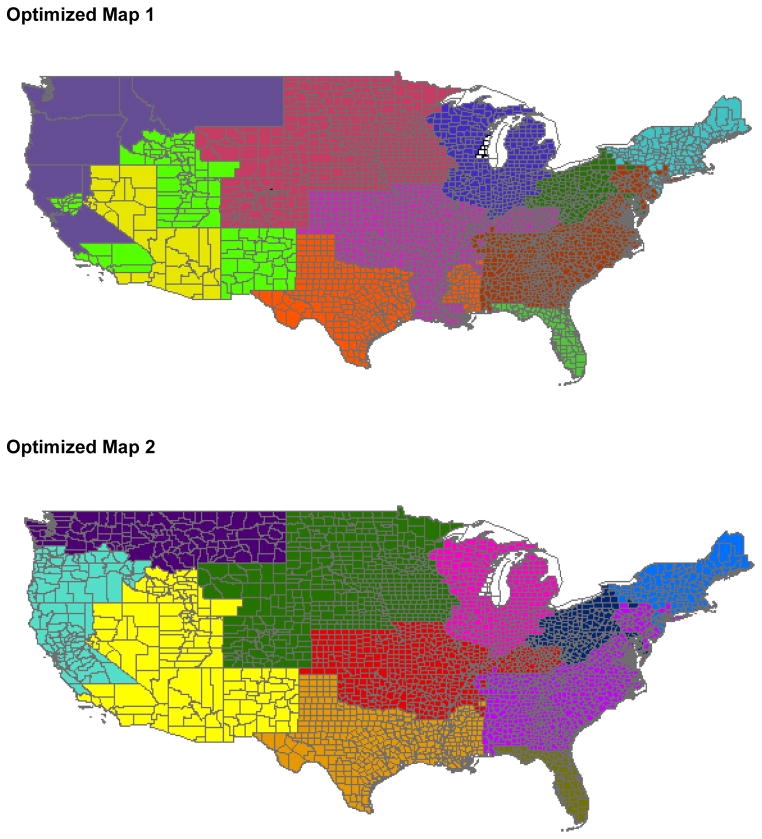
Using optimal redistricting based on most recent data, we illustrate two revised regional designs that would reduce disparity in median MELD at transplant with fully regional sharing. Optimized Map 1 partitions the country into 11 optimal regions; Optimized Map 2 additionally requires that the regions be geographically contiguous (Figure 2). As would be expected, the optimization algorithm designed these maps to join some high organ availability DSAs with lower availability DSAs. Variance of median MELD at transplant per DSA was significantly reduced using fully regional sharing with either Optimized Map 1 (7.0) or Optimized Map 2 (8.5) compared with fully regional sharing using the current map (13.5) and local-first sharing using the current map (11.2) (p=0.002 for all comparisons). These maps had the same effect when validated using older data, with variance of median MELD at transplant per DSA significantly reduced using Optimized Map 1 or Optimized Map 2 compared with fully regional sharing using the current map and local-first sharing using the current map (p=0.002 for all comparisons).
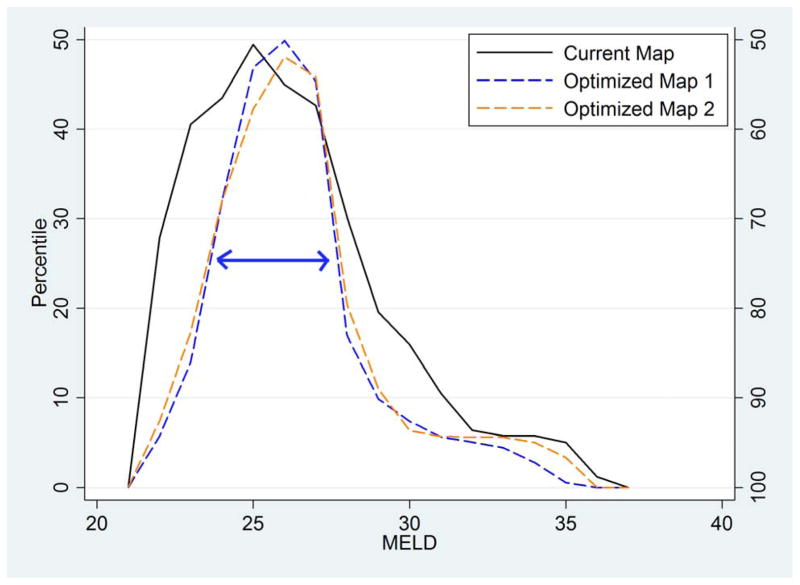
Figure 2. Distribution of median MELD at transplant across DSAs; by allocation scenario.
This mountain plot shows the cumulative distribution of median MELD at transplant across DSAs, folded at the median (i.e. the ascending part of the “mountain” indicates 0–50th percentile, and the descending part of the “mountain” indicates 50–100th percentile) to illustrate the dispersion of the distribution. The three lines show fully regional sharing over the current map (solid black line), Optimized Map 1 as in Figure 1a (dashed blue line), and Optimized Map 2 as in Figure 1b (dashed orange line). Note that Optimized Maps 1 and 2 significantly reduce geographic disparities as shown by the narrower interquartile range (indicated as a double-headed arrow on the figure).
Geographic Disparities: Illustrative Example
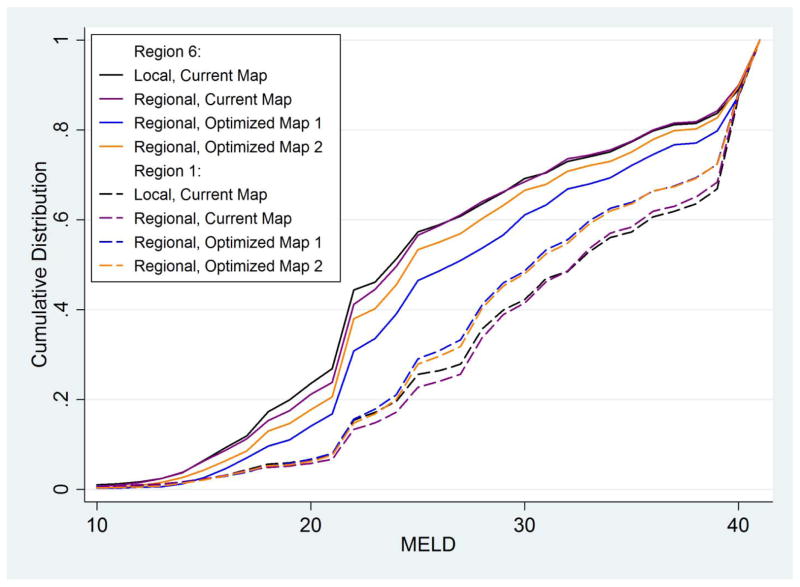
The effect of revised regional designs on the cumulative distribution of MELDs at transplant can be seen by comparing DSAs in Region 1 (CTOP and MAOB) with DSAs in Region 6 (HIOP, ORUO, and WALC) under four allocation scenarios (Figure 3). Using fully regional sharing within existing region boundaries did not significantly change the distributions of MELD at transplant: MELDs at transplant remained low in Region 6 and high in Region 1. However, fully regional sharing in optimal regions rendered the distribution of MELDs at transplant much more similar across these DSAs.
Figure 3. MELDs at transplant comparing Region 1 DSAs with Region 6 DSAs; by allocation scenario.
Cumulative distribution of MELDs at transplant, for DSAs in Region 1 (CTOP and MAOB, dashed lines) and in Region 6 (HIOP, ORUO, and WALC, solid lines) (i.e. Regions 1 and 6 as defined by the current map). Four allocation scenarios are shown for each: the current allocation system, black lines; fully regional sharing over current regions, purple lines; fully regional sharing over Optimized Map 1 as in Figure 1a, blue lines; and fully regional sharing over Optimized Map 2 as in Figure 1b, orange lines.
Summative Metrics
To confirm that reducing geographic disparities through broader sharing within optimized regions did not harm (and actually improved) summative (non-disparity) metrics of organ allocation, we examined waiting list deaths and other metrics on a national level. Fully regional sharing under Optimized Map 1 or Optimized Map 2 would reduce expected waiting list deaths by wider margins than fully regional sharing under the current map (by 61 or 58 respectively per year compared with 39, p=0.021; Table 1). Also, the optimized maps would significantly reduce the fraction of livers that are first offered to candidates with MELD less than 25 by comparable margins to fully regional sharing under the current map (from 14.6% to 4.6% under fully regional current map and 3.3% under fully regional Optimized Map 1 or 3.1% under fully regional Optimized Map 2) (Table 1). Finally, Optimized Map 1 would increase the fraction of transplants that benefit those with MELD higher than 25 (including status 1A/1B candidates) (from 51.9% to 57.2% under fully regional current map and 60.8% under fully regional Optimized Map 1 or 61.2% under fully regional Optimized Map 2) (Table 1). As expected, all fully regional sharing systems would increase the median transport distance for livers, from about 69 miles under the current system to 132 miles with fully regional sharing in the current map, or about 142 miles for either of the optimized maps.
Discussion
The impetus for broader sharing in liver transplantation is the persistent geographic disparity in access to this life-saving modality. In this simulation study based on national data and validated using two different time points, we have demonstrated that the median MELD at transplant in different geographic areas would not become more uniform if broader sharing were implemented with the current regional map. Instead, optimal redistricting techniques can be used to design new regional maps with the explicit goal of reducing geographic disparity. Broader sharing using the two novel, optimized regional maps described here is predicted not only to reduce geographic disparity, but also to reduce the number of pre-transplant deaths, to reduce the total number of deaths, and to direct more organ offers to the most medically urgent candidates.
The computational and modeling challenges of redistricting mean that no single map is the unique optimal one. For example, an optimized map designed with fewer regions would require longer transport distances, but might make allocation even more equitable; while our example maps set the number of regions to 11, it is certainly possible to use the same methods to explore the advantages and disadvantages of different numbers of regions. We have used redistricting techniques to design two different regional maps that could improve geographic equity when combined with broader sharing, one which constrains the solution to require geographically contiguous regions and one without this constraint.
Our message is not that one of these maps should be immediately adopted. Rather, we assert that innovative computational approaches should guide the process of revising allocation systems, and that disparity metrics (in addition to the commonly cited summative metrics) are critical in evaluating proposed allocation changes. Other disparity metrics, like the variance across DSAs of the waitlist death plus waitlist removal rate, or the variance across DSAs of the transplant rate for active candidates above MELD 25, could be considered. We have shown that, without optimization, the Institute of Medicine’s recommendation and the Organ Procurement and Transplantation Network (OPTN) proposal for regional sharing necessitate a tradeoff between reducing disparity and reducing waiting list death. However, with optimization, this tradeoff is obviated.
Redistricting is an established area of operations research that has elsewhere been applied to, for example, designing voting districts for political advantage(12) and school districts for racial integration.(13) Other researchers have explored integer programming approaches for redistricting liver allocation. Kong and colleagues used a set partitioning model, but this model is too large to solve exactly(14, 15) and, more importantly, does not address geographic disparity. Another analysis incorporated an equity measure similar to a raw transplant rate per region, but without considering MELD and disease severity.(16) Our work directly addressed the paramount concern for geographic equity in MELD at transplant. Also, we validated the regional designs that we proposed with the clinically detailed Liver Simulated Allocation Model, used by the OPTN to pre-test allocation proposals, to compensate for the necessarily simplified picture of allocation in a redistricting integer program.
Some limitations of this study are consequences of the structure of the Liver Simulated Allocation Model. We optimized the regions to reduce geographic disparity considering incident rates of liver candidate listings rather than existing waitlists to ensure that these regions function well over long periods of time (in other words, to optimize the new regions once they reach a steady state); indeed, our optimized regions functioned well across two versions of LSAM (2006 and 2010). However, LSAM is limited to simulating only one year of allocation, during which previously accumulated geographic disparities will play a significant role. This limitation is expected to bias our results towards the null; in other words, with multiple years of simulation, it is likely that the optimized maps would function even better than currently suggested by LSAM. Also, LSAM does not account for possible changes in acceptance patterns or listing patterns under revised allocation rules. For instance, while in current practice only livers that have been repeatedly rejected at the local level are offered regionally and thus regionally shared livers are associated with higher discard rates, discard rates for regionally shared livers will likely decrease under broader sharing. Again, this limitation is likely to bias our results towards the null, and decreased discards of regionally offered livers under a fully regional system would likely further reduce death rates and allow optimized regional maps to function even better.
Decreased local placement of donor organs might increase costs as some organs are transported over longer distances. A more fair distribution system will also likely encourage organ procurement organizations to pursue every potential donor and transplant centers to utilize all viable organs, possibly yielding more transplants(17).
In this study, we have shown that broader sharing is necessary but not sufficient to reduce geographic disparities in transplant allocation. In order to decrease geographic disparity, broader sharing needs to be organized within geographic partitions designed to address this issue. Advanced mathematical methods might point the way to reducing disparity through broader sharing with partitions that obviate the tradeoff between disparity and summative metrics.
Figure 1. Regions Designed to Reduce Geographic Disparity in Liver Allocation.
Based on redistricting integer program designed to minimize DSA-level variance in median MELD at transplantation (see methods). Upper Panel: Optimized Map 1, with 11 regions; Lower Panel: Optimized Map 2, with 11 regions constrained by geographic contiguity.
Acknowledgments
The Liver Simulated Allocation Model version 4.0 and input data files have been supplied by the Scientific Registry of Transplant Recipients, UNOS, and the Minneapolis Medical Research Foundation under contract with HHS/HRSA. The authors alone are responsible for reporting and interpreting these data; the views expressed herein are those of the authors and not necessarily those of the US Government.
The authors thank SRTR (Scientific Registry for Transplant Recipients) investigators Ajay Israni, M.D., M.S., Associate Professor of Medicine, University of Minnesota; Yi Peng, M.S., Biostatistician; Jon Snyder, Ph.D., Adjunct Assistant Professor, University of Minnesota School of Public Health; and Bert Kasiske, M.D., F.A.C.P., Professor of Medicine, University of Minnesota, for thoughtful discussions of this work during its creation. The authors thank Daniel Scharfstein, Sc.D, Professor of Biostatistics, Johns Hopkins University Bloomberg School of Public Health, for statistical advice and discussion.
This work was supported by an American Recovery and Reinvestment Act grant from the National Institute of Diabetes Digestive and Kidney Diseases, RC1 1RC1DK086450–01. This work was also supported by Health Resources Services Administration contract #HHSH250201000018C. Neither funding organization had a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Abbreviations
- MELD
Model for End Stage Liver Disease
- DSA
Donor Service Area
- LSAM
Liver Simulation Allocation Model
- OPTN
Organ Procurement and Transplantation Network
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Asrani SK, Kim WR. Model for end-stage liver disease: end of the first decade. Clin Liver Dis. 2011;15(4):685–698. doi: 10.1016/j.cld.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Massie AB, Caffo B, Gentry SE, Hall EC, Axelrod DA, Lentine KL, et al. MELD Exceptions and Rates of Waiting List Outcomes. Am J Transplant. 2011;11(11):2362–2371. doi: 10.1111/j.1600-6143.2011.03735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeh H, Smoot E, Schoenfeld DA, Markmann JF. Geographic inequity in access to livers for transplantation. Transplantation. 2011;91(4):479–486. doi: 10.1097/TP.0b013e3182066275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Organ Procurement and Transplantation Network Final Rule, CFR 42.1.K.121. In. US, 1998.
- 5.Ubel PA, Caplan AL. Geographic favoritism in liver transplantation--unfortunate or unfair? N Engl J Med. 1998;339(18):1322–1325. doi: 10.1056/NEJM199810293391811. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine Committee on Organ Procurement and Transplantation Policy. Organ procurement and transplantation: Assessing current policies and the potential impact of the DHHS Final Rule. National Academies Press; 1999. [PubMed] [Google Scholar]
- 7.Roberts JP, Dykstra DM, Goodrich NP, Rush SH, Merion RM, Port FK. Geographic differences in event rates by model for end-stage liver disease score. Am J Transplant. 2006;6(10):2470–2475. doi: 10.1111/j.1600-6143.2006.01508.x. [DOI] [PubMed] [Google Scholar]
- 8.Washburn K, Pomfret E, Roberts J. Liver allocation and distribution: possible next steps. Liver Transpl. 2011;17(9):1005–1012. doi: 10.1002/lt.22349. [DOI] [PubMed] [Google Scholar]
- 9.Thompson D, Waisanen L, Wolfe RA, Merion RM, McCullogh K, Rodgers A. Simulating the allocation of organs for transplantation. Health Care Management Science. 2004;7:331–338. doi: 10.1007/s10729-004-7541-3. [DOI] [PubMed] [Google Scholar]
- 10.Olthoff KM, Brown RS, Jr, Delmonico FL, Freeman RB, McDiarmid SV, Merion RM, et al. Summary report of a national conference: Evolving concepts in liver allocation in the MELD and PELD era. December 8, 2003, Washington, DC, USA. Liver Transpl. 2004;10(10 Suppl 2):A6–22. doi: 10.1002/lt.20247. [DOI] [PubMed] [Google Scholar]
- 11.Schaubel DE, Guidinger MK, Biggins SW, Kalbfleisch JD, Pomfret EA, Sharma P, et al. Survival benefit-based deceased-donor liver allocation. Am J Transplant. 2009;9(4 Pt 2):970–981. doi: 10.1111/j.1600-6143.2009.02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrotra A, Johnson EL, Nemhauser GL. An Optimization Based Heuristic for Political Districting. Manage Sci. 1998;44(8):1100–1114. [Google Scholar]
- 13.Belford PC, Ratliff HD. A Network-Flow Model for Racially Balancing Schools. Operations Research. 1972;20(3):619–628. [Google Scholar]
- 14.Kong N, Schaefer AJ, Hunsaker B, Roberts MS. Maximizing the Efficiency of the U.S. Liver Allocation System Through Region Design. Manage Sci. 2010;56(12):2111–2122. [Google Scholar]
- 15.Stahl JE, Kong N, Shechter SM, Schaefer AJ, Roberts MS. A methodological framework for optimally reorganizing liver transplant regions. Med Decis Making. 2005;25(1):35–46. doi: 10.1177/0272989X04273137. [DOI] [PubMed] [Google Scholar]
- 16.Demirci MC, Schaefer AJ, Romeijn HE, Roberts MS. An Exact Method for Balancing Efficiency and Equity in the Liver Allocation Hierarchy. INFORMS Journal on Computing. 2011 [Google Scholar]
- 17.Hayashi PH, Axelrod DA, Galanko J, Salvalaggio PR, Schnitzler M. Regional differences in deceased donor liver transplantation and their implications for organ utilization and allocation. Clin Transplant. 2011;25(1):156–163. doi: 10.1111/j.1399-0012.2010.01214.x. [DOI] [PubMed] [Google Scholar]