Abstract
The Dominican Republic is one of four remaining countries in the Americas with lymphatic filariasis (LF). Annual mass drug administration (MDA) with albendazole and diethylcarbamazine was conducted in La Ciénaga, an impoverished urban barrio in Santo Domingo, from 2004 to 2006. Eight years after the last MDA, a transmission assessment survey (TAS) was conducted in November–December 2014 to determine if LF transmission remains absent. Of 815 first and second grade primary school students (mean age: 6.51 years; range 5–9) tested by immunochromatographic test (ICT), zero (0.0%) were positive. This is below the TAS critical cutoff of nine, indicating that the area “passed” TAS and that transmission remains interrupted in La Ciénaga. Importantly, this also provides evidence that three rounds of effective (> 65% coverage) MDA, likely aided by environmental improvements and periodic school-based albendazole monotherapy MDA, achieved interruption of LF transmission from a relatively low-transmission setting.
The Dominican Republic (DR, population 9.5 million) is one of four remaining countries in the Americas region with lymphatic filariasis (LF)—a mosquito-transmitted parasitic disease that currently affects an estimated 67.88 million people in 73 countries.1 The island of Hispaniola, which the DR shares with Haiti, accounts for approximately 90% of cases in the Americas.2 LF in Hispaniola is caused by Wuchereria bancrofti with Culex quinquefasciatus the principal vector.3 Infection is not fatal, but 30–40% of individuals develop lymphedema, elephantiasis, and/or genital swelling (hydrocele in men) due to blockage of the lymphatic vessels by adult worms.4 Affected individuals often suffer impairment of daily activities and social isolation in addition to the pain and discomfort of severe disease.5 The Dominican Ministry of Health created the Program to Eliminate Lymphatic Filariasis (PELF) in 1998 to coordinate national LF elimination. Patterned after the global strategy, PELF targets elimination of LF through 1) annual mass drug administration (MDA) of albendazole (donated by GlaxoSmithKline) and diethylcarbamazine (DEC) to interrupt LF transmission by 2020; and 2) morbidity control to alleviate disability for those already infected. Baseline mapping, initiated in 1999, identified three focal areas of transmission in the DR: La Ciénaga, southwest, and east regions.
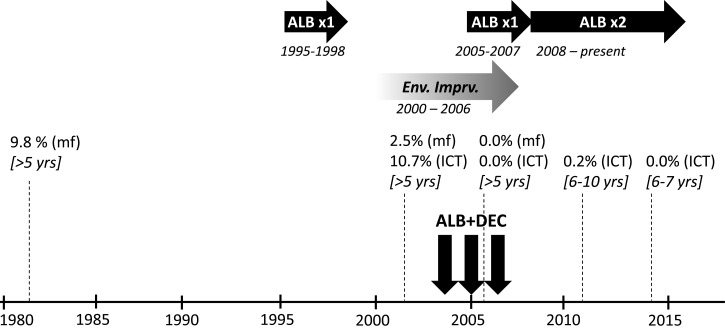
La Ciénaga, literally “swamp,” is an impoverished urban barrio on the western banks of the Ozama River in the capital Santo Domingo that contains around 50,000 inhabitants. Surveys from the early 1980s identified La Ciénaga as a hot spot of LF transmission, with microfilaremia (mf) prevalence of 9.8% among individuals older than 5 years of age.3 In 2002, before the implementation of MDA for LF, sentinel site surveys of the same age group documented mf prevalence of 2.5% and 10.7% antigen prevalence by immunochromatographic test (ICT). Annual MDA with albendazole and DEC was conducted in La Ciénaga in 2004 (May–June), 2005 (May–June), and 2006 (November–December), with population coverage rates of 67%, 92%, and 86%, respectively (Figure 1 ). In addition, distribution of albendazole monotherapy for treatment of soil-transmitted helminths (STH) in primary school children (ages 5–14 years) occurred nationally on an annual basis from 1995 to 1998 and from 2005 to 2007, and semiannually from 2008 to present. Sentinel site surveys conducted in November 2006 before the third round of LF MDA found that antigen and mf prevalence had been reduced to 0.0%. For this reason, PELF decided to stop MDA for LF after 2006. In 2011, 5 years after the final round of albendazole–DEC MDA, a community-based post-MDA survey of 539 children aged 6–10 years old found only one (0.2%) ICT-positive individual—a girl who lived in the area for less than 2 years—further indicating that LF transmission had been interrupted.
Figure 1.
Timeline of key programmatic events related to lymphatic filariasis (LF) elimination in La Ciénaga, Dominican Republic. Horizontal arrows indicate annual (albendazole [ALB] × 1) or semiannual (ALB × 2) school-based ALB mass drug administration (MDA) for soil-transmitted helminths (dark arrows), or phased environmental improvements (Env. Imprv.) that included street paving and covering of open sewers (shaded arrow). Estimates of microfilaria (mf) and/or antigen (ICT) prevalence from surveys at various time points as indicated, with the target age range for each survey shown in square brackets. Vertical arrows indicate annual community-wide LF MDA with ALB–diethylcarbamazine (DEC).
The purpose of this survey was to determine whether transmission remained absent in La Ciénaga using the World Health Organization (WHO) transmission assessment survey (TAS) protocol.6 Formalized in 2011, TAS is a lot quality assurance-type survey to determine whether LF prevalence is below thresholds under which recrudescence is unlikely to occur even in the absence of MDA (< 2% antigen prevalence in areas where W. bancrofti is transmitted by Culex or Anopheles mosquitoes). The target population for TAS is children 6–7 years old, as this population is born after the start of MDA and should be LF-free in the absence of local transmission. If the number of antigen positive individuals is less than a cutoff value corresponding to the antigen prevalence threshold for a given sample size, the survey area “passes” the TAS. In areas that have stopped MDA, two post-MDA TAS surveys are recommended 2–3 years following the last round of MDA, and 2–3 years apart to fulfill post-treatment surveillance for verification of transmission elimination.
A systematic, school-based TAS was conducted in the three sub-barrios that comprise La Ciénaga (La Ciénaga, Los Guandules and Guachupita) from November 15 to December 5, 2014, with the three areas considered as a single TAS evaluation unit (EU). This design was selected based on high (> 95%) primary-school enrollment, estimated size of target population, and the feasibility of sampling all seven schools in the area. Assuming 3.5% of the area's 50,000 inhabitants are 6–7 years old, a sample of 594 children in primary grades 1 and 2 (approximating the 6–7 year age group) was required. For this sample size, the corresponding critical cut-off of seven antigen-positive individuals provides at least a 75% chance of passing if the true antigen prevalence is 1.0% and no more than about a 5% chance of passing (incorrectly) if the true prevalence of antigenemia is ≥ 2%. Meetings were held at the local primary schools before the survey to inform parents and teachers about the survey. Written informed consent was obtained from all parents who agreed to their child's participation, and all participating children provided assent prior to sample collection. The survey protocol was reviewed by Emory University Institutional Review Board. Antigen testing from 100 μL of finger-stick blood collected in EDTA-coated microtainer tubes was performed using BinaxNOW ICT card tests (Alere, Inc., Scarborough, ME) at the Centro Nacional de Control de Enfermedades Tropicales (CENCET) laboratory in Santo Domingo on the day of sample collection.
A total of 815 primary grade 1 and 2 students from all seven area schools were tested by ICT. This represents 66.3% of the total enrolled children in these grades. The sample was 49.3% male, with mean age 6.51 years (standard deviation: 0.69; range: 5–9). Of those tested, none (0.0%) were antigen positive (Table 1). The critical cutoff value for this larger sample population is nine positives, meaning that the EU “passed” the TAS and that antigen prevalence was significantly less than 2%. This corroborates data from the first post-MDA survey conducted 3 years previously in La Ciénaga (0.2% antigen prevalence), and indicates that LF transmission remains interrupted 8 years after the final round of MDA. Two successful post-MDA surveys satisfy WHO guidelines for post-treatment surveillance.6 However, due to urbanization and domestic population movements, as well as the threat of on-going transmission in neighboring Haiti,7 PELF plans to conduct an additional TAS in all formerly endemic foci in DR once transmission has been interrupted in the third and final focus of the East. One limitation of this survey is the potential selection bias introduced by testing only children whose parent or guardian provided consent to participate. It is conceivable that those unwilling to participate may be at greater risk for infection, leading to a sample that may not completely represent the target population.
Table 1.
Results of lymphatic filariasis antigen testing by immunochromatographic test (ICT) in La Ciénaga, Dominican Republic, November–December 2014
| Area | Number of schools | Number students tested | Number (%) students ICT-positive |
|---|---|---|---|
| Guachupita | 2 | 72 | 0 (0.0%) |
| La Ciénaga | 1 | 216 | 0 (0.0%) |
| Los Guandules | 4 | 527 | 0 (0.0%) |
| Total | 7 | 815 | 0 (0.0%) |
Importantly, these results also provide empiric evidence that LF transmission can be interrupted with less than five rounds of MDA. Fewer rounds of MDA would result in programmatic cost savings and eliminate the need for unnecessary drug administration to healthy individuals. The global elimination strategy is based on provision of 4–6 “effective” (> 65% coverage6) rounds to affected areas, with current TAS guidelines, released in 2011 after MDA was stopped in La Ciénaga, indicating that five rounds should be given before TAS is implemented.6 Modeling data generally support these timelines, but also suggest that interruption can be achieved sooner depending on other factors including baseline prevalence, treatment coverage, drug selection, and vector control.8,9 La Ciénaga had a favorable combination of low baseline transmission (10.7% antigen prevalence; 2.5% mf prevalence), potent drug combination (albendazole–DEC), concurrent environmental improvements in the area, and periods of albendazole monotherapy MDA for STH. Organized vector control measures have not been implemented in the area and insecticide-treated bed nets are not widely available. Environmental improvements were gradually introduced in La Ciénaga at the start of the new millennium that included street paving and covering of open sewers from approximately 2003–2006, about the same time as LF MDA. Municipal sewer-water systems improvements are credited with eliminating LF transmission from Charleston, South Carolina in the early twentieth century by reducing breeding sites for Cx. quinquefasciatus.10 Similar improvements in La Ciénaga likely aided transmission interruption. School-based albendazole MDA for STH given annually from 1995 to 1998 in La Ciénaga also may have reduced LF transmission before the launch of LF MDA in 2004. However the efficacy of albendazole monotherapy on W. bancrofti is equivocal,11–13 and the detection of microfilaremic individuals in sentinel site surveys in 2002 prior to the launch of LF MDA suggests that albendazole monotherapy MDA in school children was insufficient to interrupt LF transmission community wide. Recent data show that 1 year of semiannual treatment with albendazole alone reduced antigen prevalence, mf prevalence, and mf density among microfilaremic individuals.14 Therefore the resumption of annual school-based albendazole MDA for STH in 2005 may have acted synergistically with concurrent community-wide albendazole–DEC to interrupt LF transmission by 2006 by providing two doses of albendazole and one dose of DEC per year to school children in 2005 and 2006. However, any direct synergy would be limited to the school age population. This raises the question of whether the school-age population is the most appropriate age group for assessing community-wide LF transmission status. Causal inferences regarding the individual contribution of these factors toward LF elimination in La Ciénaga cannot be made due to the observational nature of the available data. Nonetheless, whether due to MDA alone or in combination with other factors, national LF programs stand to benefit if guidelines were revised to permit TAS surveys to commence as soon as program indicators show that stop-MDA thresholds (< 2% antigenemia or < 1% microfilaremia in sentinel site or spot check monitoring) have been reached in implementation areas. This would follow the approach of the global trachoma elimination program, whereby WHO recommends a tiered MDA strategy (of 3 or 5 years) depending on baseline endemicity.15
ACKNOWLEDGMENTS
We thank the field staff and laboratory technicians who assisted with this survey: Licda. Angelita Méndez, Lic. Kenny Olivero, Lic. Henry Olivero. PELF would like to acknowledge the following for financial, technical, or community support for MDA campaigns in La Ciénaga: The Bill and Melinda Gates Foundation, the Lymphatic Filariasis Support Centre at the Liverpool School of Tropical Medicine, Centro de Estudios Sociales Juan Montalvo, Consejo de Desarrollo de La Ciénaga, Consejo de Desarrollo de Los Guandules, and Espacio de Coordinación de Organizaciones de Guachupita. Albendazole for MDA was generously provided by GlaxoSmithKline. We also thank Frank Richards, Jr. for useful discussions.
Footnotes
Financial support: This survey was funded in part by The Carter Center and the Pan American Health Organization.
Authors' addresses: Gregory S. Noland and Stephen Blount, The Carter Center, Atlanta, GA, E-mails: gregory.noland@cartercenter.org and stephen.blount@cartercenter.org. Manuel Gonzalez, Centro de Control de Enfermedades Tropicales, Santo Domingo, Dominican Republic, E-mail: manuelgonpe@gmail.com.
References
- 1.Ramaiah KD, Ottesen EA. Progress and impact of 13 years of the global programme to eliminate lymphatic filariasis on reducing the burden of filarial disease. PLoS Negl Trop Dis. 2014;8:e3319. doi: 10.1371/journal.pntd.0003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Meeting of the International Task Force for Disease Eradication–November 2012. Wkly Epidemiol Rec. 2013;88:75–80. [PubMed] [Google Scholar]
- 3.Vincent AL, Gonzalvo A, Cowell BC, Nayar JK, Uribe L. A survey of Bancroftian filariasis in the Dominican Republic. J Parasitol. 1987;73:839–840. [PubMed] [Google Scholar]
- 4.Nutman TB. Insights into the pathogenesis of disease in human lymphatic filariasis. Lymphat Res Biol. 2013;11:144–148. doi: 10.1089/lrb.2013.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Person B, Bartholomew LK, Addiss D, van den Borne B. Disrupted social connectedness among Dominican women with chronic filarial lymphedema. Patient Educ Couns. 2007;68:279–286. doi: 10.1016/j.pec.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . Monitoring and Epidemiological Assessment of Mass Drug Administration in the Global Programme to Eliminate Lymphatic Filariasis: A Manual for National Elimination Programmes. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 7.Oscar R, Lemoine JF, Direny AN, Desir L, Beau de Rochars VE, Poirier MJ, Varghese A, Obidegwu I, Lammie PJ, Streit TG, Milord MD. Haiti national program for the elimination of lymphatic filariasis-a model of success in the face of adversity. PLoS Negl Trop Dis. 2014;8:e2915. doi: 10.1371/journal.pntd.0002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michael E, Malecela-Lazaro MN, Simonsen PE, Pedersen EM, Barker G, Kumar A, Kazura JW. Mathematical modelling and the control of lymphatic filariasis. Lancet Infect Dis. 2004;4:223–234. doi: 10.1016/S1473-3099(04)00973-9. [DOI] [PubMed] [Google Scholar]
- 9.Stolk WA, Swaminathan S, van Oortmarssen GJ, Das PK, Habbema JD. Prospects for elimination of bancroftian filariasis by mass drug treatment in Pondicherry, India: a simulation study. J Infect Dis. 2003;188:1371–1381. doi: 10.1086/378354. [DOI] [PubMed] [Google Scholar]
- 10.Chernin E. The disappearance of bancroftian filariasis from Charleston, South Carolina. Am J Trop Med Hyg. 1987;37:111–114. doi: 10.4269/ajtmh.1987.37.111. [DOI] [PubMed] [Google Scholar]
- 11.Wamae CN, Njenga SM, Ngugi BM, Mbui J, Njaanake HK. Evaluation of effectiveness of diethylcarbamazine/albendazole combination in reduction of Wuchereria bancrofti infection using multiple infection parameters. Acta Trop. 2011;120(Suppl 1):S33–S38. doi: 10.1016/j.actatropica.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Makunde WH, Kamugisha LM, Massaga JJ, Makunde RW, Savael ZX, Akida J, Salum FM, Taylor MJ. Treatment of co-infection with bancroftian filariasis and onchocerciasis: a safety and efficacy study of albendazole with ivermectin compared to treatment of single infection with bancroftian filariasis. Filaria J. 2003;2:15. doi: 10.1186/1475-2883-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Critchley J, Addiss D, Ejere H, Gamble C, Garner P, Gelband H, Group IFR Albendazole for the control and elimination of lymphatic filariasis: systematic review. Trop Med Int Health. 2005;10:818–825. doi: 10.1111/j.1365-3156.2005.01458.x. [DOI] [PubMed] [Google Scholar]
- 14.Pion SD, Chesnais CB, Bopda J, Louya F, Fischer PU, Majewski AC, Weil GJ, Boussinesq M, Missamou F. The impact of two semiannual treatments with albendazole alone on lymphatic filariasis and soil-transmitted helminth infections: a community-based study in the Republic of Congo. Am J Trop Med Hyg. 2015;92:959–966. doi: 10.4269/ajtmh.14-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . Report of the Third Global Scientific Meeting on Trachoma Elimination. Geneva, Switzerland: WHO; 2010. [Google Scholar]