Abstract
We report the elimination of Onchocerca volvulus transmission from the Central Endemic Zone (CEZ) of onchocerciasis in Guatemala, the largest focus of this disease in the Americas and the first to be discovered in this hemisphere by Rodolfo Robles Valverde in 1915. Mass drug administration (MDA) with ivermectin was launched in 1988, with semiannual MDA coverage reaching at least 85% of the eligible population in > 95% of treatment rounds during the 12-year period, 2000–2011. Serial parasitological testing to monitor MDA impact in sentinel villages showed a decrease in microfilaria skin prevalence from 70% to 0%, and polymerase chain reaction (PCR)-based entomological assessments of the principal vector Simulium ochraceum s.l. showed transmission interruption by 2007. These assessments, together with a 2010 serological survey in children 9–69 months of age that showed Ov16 IgG4 antibody prevalence to be < 0.1%, meeting World Health Organization (WHO) guidelines for stopping MDA, and treatment was halted after 2011. After 3 years an entomological assessment showed no evidence of vector infection or recrudescence of transmission. In 2015, 100 years after the discovery of its presence, the Ministry of Health of Guatemala declared onchocerciasis transmission as having been eliminated from the CEZ.
Introduction
Human onchocerciasis (river blindness) is caused by Onchocerca volvulus, a tissue-dwelling filarial nematode transmitted by certain species of the genus Simulium.1 Adult male and female worms form fibrous, often palpable, subcutaneous onchocercomas (“nodules”), in which fertilized female worms produce microfilariae (mf). The mf leave the nodule and reside in the dermis. They may also enter the eyes. Human disease results largely from death of the mf, which results in inflammation, itching, visual impairment, and blindness. The Simulium (“black fly”) vectors breed in rapidly flowing rivers and streams and become infected when they ingest mf during a blood meal; in competent black fly species, mf develop into third stage larvae that can infect humans when the vector takes a subsequent blood meal. There are no important animal reservoirs of O. volvulus to maintain the transmission cycle independent of the human population itself.2
Human onchocerciasis is thought to have originated in Africa when Onchocerca species in ungulates adapted to man; the parasite is believed to have been brought to the Americas through the Atlantic slave trade.3 Transmission was only focally established in the Americas, in contrast to the extensive transmission zones that exist in Africa. In the Americas, due to a limited distribution of competent vectors, transmission zones (“foci”) are small and sharply delimited geographically, and are generally maintained by high vector biting rates. Before control measures were implemented, there were 13 foci in six countries in Latin America (Brazil, Colombia, Ecuador, Guatemala, Mexico, and Venezuela), with an overall human population at risk in 2013 of just over 500,000 persons.4
Guatemala accounts for the largest at risk population for onchocerciasis in the Americas (231,467, representing about 41% of the total at risk regional population). The infection is transmitted by Simulium ochraceum s.l., which breeds at an altitude between 500 and 1,500 m in small streams and their tributaries.5 Guatemala has four foci: Santa Rosa (in the province of Santa Rosa), Huehuetenango (in the province of Huehuetenango), Escuintla-Guatemala (parts of the provinces of Escuintla and Guatemala), and the Central Endemic Zone (CEZ) (parts of the provinces of Suchitepéquez, Sololá, and Chimaltenango).6 The CEZ (Figure 1 ) is the largest of the four foci in terms of population (126,430, or 55% of Guatemala's population at risk), and intensity of transmission. Of historical interest, the CEZ was the first onchocerciasis endemic area to be described in the Americas, discovered 100 years ago (1915) by the famed Guatemalan researcher, Rodolfo Robles Valverde. It was Robles' work in the CEZ that first described the relationship between the O. volvulus infection, nodule rates, and ocular disease,7,8 and gave rise to human onchocerciasis in this hemisphere being referred to as “Robles' Disease.”
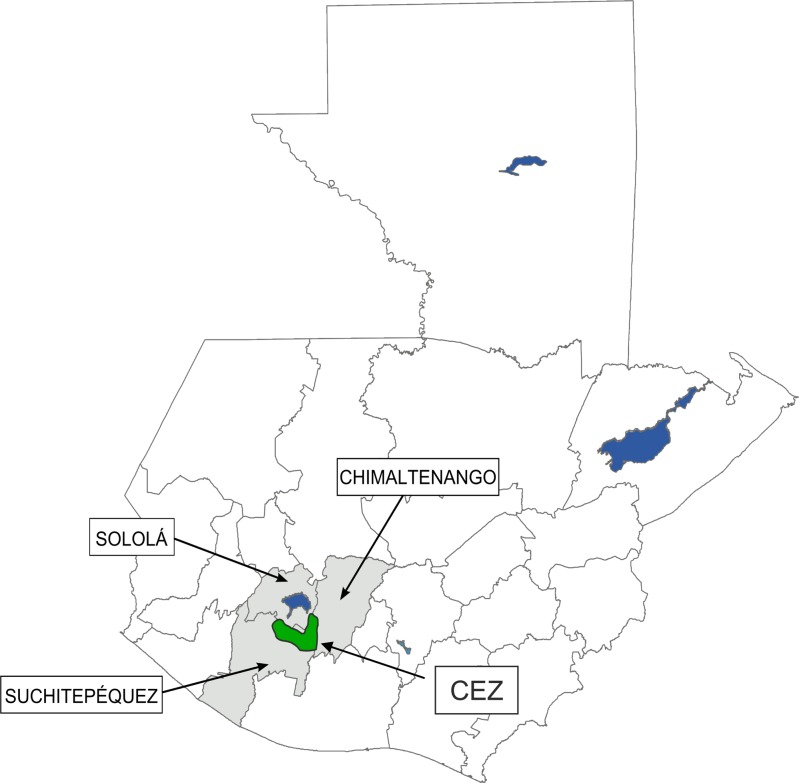
Figure 1.
This map of Guatemala shows the three provinces (Sololá, Suchitepéquez, and Chimaltenango) that contribute to the Central Endemic Zone (CEZ) for onchocerciasis in Guatemala. The CEZ (dark polygon) indicates the contiguous parts of the provinces where onchocerciasis transmission took place, just south of Lake Atítlan, in the highlands at elevations from 500 to 1500 m.
To control onchocerciasis, the Guatemalan Ministry of Public Health and Social Welfare (MSPAS, in its Spanish acronym) established a “Department of Robles' Disease” in the mid-1930s (the current name is “Subprograma de Oncocercosis”). Although vector control was done in Escuintla-Guatemala for a short time,9 the primary activity of the MSPAS against onchocerciasis consisted of sending “nodulectomy brigades” to the four endemic areas to provide outreach surgical services. The most systematic and sustained nodulectomy activities were in the CEZ.6 Within 1 year of the 1987 Merck donation of the oral microfilaricidal medicine ivermectin (Mectizan®,Merck & Co., Whitehouse Station, NJ) to treat onchocerciasis, these brigades initiated mass drug administration (MDA) in parts of the CEZ; in 1990, the MSPAS adopted MDA, with related health education, as the primary intervention for all onchocerciasis-endemic areas of the country.10
In this report, we review the history of the CEZ MDA program and the monitoring and evaluation activities that led to a 2015 declaration that transmission of O. volvulus had been eliminated, based on fulfillment of the 2001 World Health Organization (WHO) guidelines for elimination of onchocerciasis.11
Methods
The central endemic zone.
The CEZ is found in the highlands south of Lake Atitlán in contiguous parts of three provinces: Sololá, Suchitepéquez, and Chimaltenango (Figure 1). The area is largely populated by people of Mayan Indian descent who make their living by working on large coffee or tea estates (fincas).6,10 There are 321 endemic communities in the CEZ, with a total population at risk of onchocerciasis during the last year of MDA (in 2011) calculated to be 124,498, and a treatment eligible population (which excludes pregnant women and children under 5 years of age) of 112,338 persons. Over 90% of the population and communities are in two provinces: Suchitepéquez and Chimaltenango (Table 1).
Table 1.
Number of communities, population at risk, and treatment eligible population at risk in 2011, CEZ, Guatemala
| Province | Number of communities (%) | Population at risk (%) | Eligible population (%) |
|---|---|---|---|
| Suchitepéquez | 153 (47) | 71,445 (57) | 64,559 (57) |
| Chimaltenango | 137 (43) | 42,846 (34) | 38,658 (34) |
| Sololá | 31 (10) | 10,207 (9) | 9,171 (9) |
| Total | 321 (100) | 124,498 (100) | 112,388 (100) |
CEZ = Central Endemic Zone.
History of MDA in the CEZ.
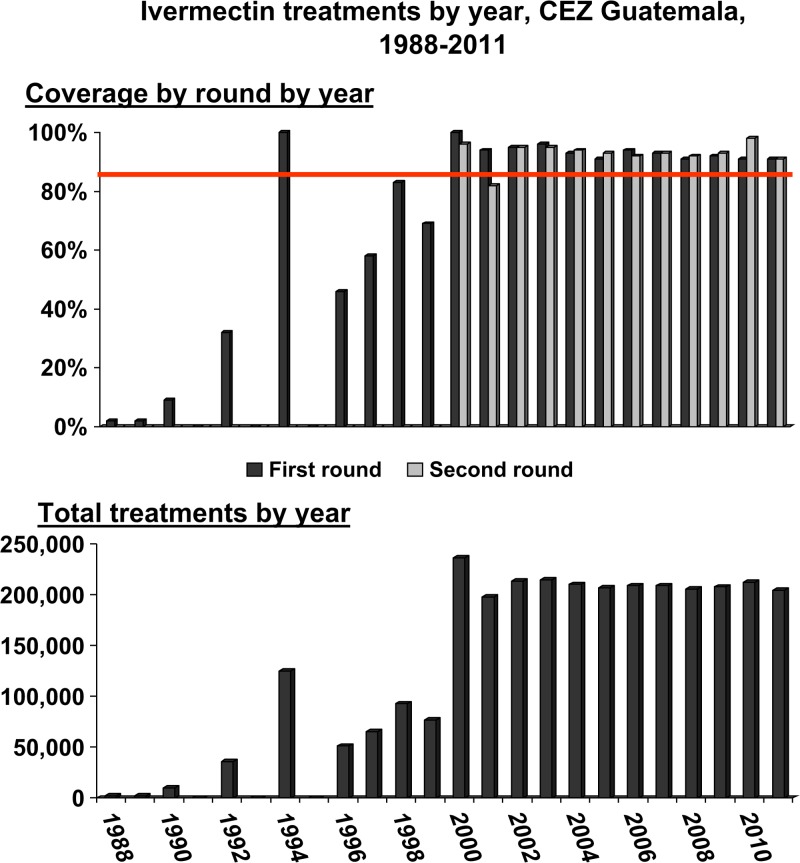
A cumulative total of 2.9 million directly observed ivermectin treatments were delivered by the MSPAS's CEZ MDA program during the period 1988–2011. Figure 2 shows the CEZ treatment coverage (top panel) of the eligible population by treatment round (top panel) and total treatments by year (bottom panel). The MDA program began in 1988 as a pilot study in some of the most affected CEZ communities.12,13 A MSPAS “decentralization” policy that moved onchocerciasis operations from the central to provincial levels in the 1990s resulted in major challenges sustaining the MDA strategy; MDA was intermittent, and data reporting was spotty, with no treatments reported for 1991, 1993, and 1995.
Figure 2.
The top panel shows the Central Endemic Zone (CEZ) treatment percent coverage of the eligible population during the years 1988–2011, by treatment round (dark bars first round, light bars second round). The horizontal line indicates the coverage goal of ≥ 85% per round. The bottom panel shows single bars representing the total treatments provided by year (the bars represent the sum of treatments given during the year in those years when two rounds were given). Mass drug administration (MDA) was halted in 2012 after over 2.9 million cumulative treatments had been delivered over the period.
The regional Onchocerciasis Elimination Program for the Americas (OEPA) was established with support from the River Blindness Foundation in 1993 in response to the 1991 Resolution 35:14 of the Directing Council of the Pan American Health Organization (PAHO/WHO) that called for the elimination of onchocerciasis morbidity from the Americas by 2007.14 OEPA (with its headquarters in Guatemala City) together with other partners, began to provide sustained technical and financial support for the MSPAS MDA program in 1994,15 and by 1996 annual MDA rounds were established in the CEZ. In 2000, the MSPAS made its policy to provide semiannual (every 6 months) MDA in all endemic communities in the CEZ, and to stop routine nodulectomy campaigns. The treatment program provided “effective” coverage (e.g., directly observed treatment of ≥ 85% of the eligible population13) from 2000 to 2011 (Figure 2, top panel) in 23 (96%) of the 24 MDA rounds. A 2008 unpublished interview survey conducted by the U.S. Centers for Disease Control and Prevention (CDC) (Kim Lindblade, CDC, unpublished data) confirmed the veracity of the program's reported coverage statistics. Based on the assessments conducted in 2009–2011 reported in this article, the MDA was halted in 2012 and a 3-year posttreatment surveillance (PTS) period was launched. PTS ended in 2014 with the successful completion of an entomological survey, following OEPA recommendations.16
Parasitological, ophthalmological, and entomological evaluations in sentinel villages.
The impact of the program on onchocerciasis in the CEZ was monitored over time by epidemiological and entomological assessments conducted in nine sentinel villages (SVs). SVs were selected from among the most highly endemic communities for onchocerciasis; the strategy was to monitor the program in communities that had the greatest force of transmission, as these represented the “worst case scenario” for achieving transmission interruption. Five SVs were in Suchitepéquez, three in Chimaltenango, and one in Sololá (Table 2). The earliest (1981) pre-MDA SV baseline mf and ophthalmology data used in this report were for the SVs Santa Isabel, Los Tarrales, and Vesubio, from a publication by Brandling-Bennett and others.17
Table 2.
Baseline and final microfilaria prevalences in skin and anterior chamber of the eye, CEZ, Guatemala
| Province | Municipality | SV | Microfilaria in skin | Microfilaria in the eye | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final assessment | Baseline | Final assessment | |||||||
| Prevalence | Year | Prevalence | Year | Prevalence | Year | Prevalence | Year | |||
| Solola | Santiago Atitlan | El Brote | 80 | 1994 | 0 | 2010 | 0 | 2003 | 0 | 2007 |
| Suchitepequez | Chicacao | Monte Carlo | 68 | 1988 | 0 | 2010 | 39 | 1988 | 0 | 2007 |
| Santa Barbara | Los Andes | 74 | 1988 | 0 | 2007 | 0 | 2003 | 0 | 2007 | |
| Patulul | Vesubio | 82 | 1981 | 0 | 2010 | 21.9 | 1981 | 0 | 2009 | |
| ″ | Santa Isabel | 90 | 1981 | 0 | 2010 | 31.6 | 1981 | 0 | 2009 | |
| ″ | Tarrales | 65 | 1981 | 0 | 2010 | 10.7 | 1981 | 0.5 | 2009 | |
| Chimaltenango | San Miguel Pochuta | Costa Rica | 36 | 1994 | 0 | 2007 | 0.6 | 2003 | 0 | 2007 |
| Acatenango | Buena Vista | 80 | 1994 | 0 | 2007 | 0 | 2003 | 0 | 2007 | |
| San Pedro Yepocapa | La Estrellita | 56 | 1994 | 0 | 2010 | 4.8 | 2003 | 0 | 2007 | |
| Mean | 70 | 0.0 | 12.1 | 0.1 | ||||||
CEZ = central endemic zone; SV = sentinel village.
The latitude and longitude locations of the SVs were El Brote (14°33′26″, −91°16′34″), Monte Carlo (14°33′0″,−91°16′49″), Los Andes (14°31′37″, −91°11′25″), Costa Rica (14°30′35″, −91°5′24″), Buena Vista (14°31′20″, −91°1′53″), La Estrellita (14°28′51″, −91°2′52″), Vesubio (14°32′46″, −91°9′41″), Santa Isabel (14°32′44″, −91°9′14″), Tarrales (14°31′18″, −91°8′14″). Shown are the results of baseline and final assessment parasitological evaluations (superficial skin biopsies (“snips”) for mf prevalence and slit lamp ophthalmological examinations in residents by an experienced ophthalmologist for presence of microfilariae (mf) in the anterior chamber (MfAC).
Parasitological evaluations.
Superficial skin biopsies (“snips”) were obtained from those who were ≥ 5 years old and who had resided in the community for at least the last 5 years. Using a 2.0-mm corneoscleral biopsy punch, a snip was taken from each scapular region, as described by Brandling-Bennett and others.17 The snips were incubated overnight in a saline solution and the fluid then examined microscopically for mf. Results are reported as number of persons positive for mf divided by number of persons examined.
Ophthalmological evaluations.
Slit lamp examinations were conducted by an experienced ophthalmologist in residents ≥ 7 years old and who had resided in the community for at least the last 5 years. The indicator used was the presence of mf in the anterior chamber (MfAC).18,19 Examinations were conducted in a darkened area after the patients were asked to sit with their head between their legs for 5 minutes. Results are reported as number of persons positive for MfAC divided by number of persons examined.
Entomological evaluations.
Polymerase chain reaction (PCR)-based entomological monitoring was not launched until 2002, 2 years after the MDA program achieved full geographic and effective treatment coverage with the semiannual MDA strategy. Vector collections took place from November to April (the peak S. ochraceum s.l. biting season in the CEZ)5 using the method described by Lindblade and others19 in 2001–2002, 2006–2007, 2009–2010, 2010–2011, and 2012–2013. In this report, we will use the convention of naming the transmission season by the last year of vector collections (i.e., 2001–2002 will be called the 2002 transmission season).
Simulium ochraceum s.l. seeking a blood meal were collected in the SV being studied on at least 2 days/month. Two teams rotated between two collection sites (one in the fields and the other near houses) in each SV. Each team consisted of a collector and paid attractant, the latter being a resident of the SV and ≥ 18 years of age. The task of the collector was to capture the vectors from the attractant's exposed back using an aspirator. Black flies were collected just after landing and before taking a blood meal. The attractants provided informed consent to participate and were given ivermectin 1 month before starting collections and 1 month after their completion. They also had Ov16 antibody blood tests (see below) prior to and again after completion of the collections. Collections started between 8:00 and 11:00 am and ended at 5:00 pm. They took place for 50 minutes each hour, with workers taking 10-minute breaks at the end of every hour and a 1 hour break at noon.
The final entomological evaluations during the MDA phase in the CEZ were conducted over two transmission seasons (2010 and 2011) and the data from these two seasons were combined in the analysis. Based on the negative results from these evaluations, and the serological studies, (described below) MDA was halted at the end of 2011. PTS entomological collections took place from November 2013 to April 2014.
At the laboratory, S. ochraceum s.l. heads were separated from thoraces/abdomen (“bodies”). Head and body “pools,” having up to 50 heads or 50 bodies per tube, were kept separated by SV, site (field or house), hour, day, and month of collection. The bodies were analyzed first, using the standard O-150 PCR assay to detect O. volvulus deoxyribonucleic acid (DNA).19,20 Pools that were PCR positive were confirmed by a second PCR. If a positive body pool was confirmed, then all the head pools from that community for that transmission season were tested. However, if all body pools were negative, then the head pools from that community were not tested, based on the fact that bodies (containing first stage larvae [L1] and second stage larvae [L2]) are 4–5 times more likely than heads (containing third stage larvae [L3]) to have parasite stages.21
Serological evaluation.
A serosurvey was conducted from a representative sample of young children to determine the CEZ prevalence of IgG4 antibodies to the O. volvulus recombinant antigen Ov-16.22 WHO guidelines for elimination require an infection rate of < 0.1% in children under 5 years of age, and to satisfy this requirement, a sample of at least 3,000 was needed to determine a one-sided 95% confidence interval (CI) that excluded 0.1%. Assuming a 20% refusal rate to participate, we determined a target sample of 3,800. Study communities were selected from among the 321 communities under the MDA program. Independent sampling was performed in each of the three provinces (Sololá, Suchitepéquez, and Chimaltenango; Table 1) in accord with the different sizes of the populations at risk in each province, with 89% of the children sampled being from Suchitepéquez and Chimaltenango. Communities in each province were ordered at random and the number of young children under 5 years of age likely to be in those communities estimated based on the latest census data and standard population pyramids for rural Guatemala. A skip interval was calculated, and a random number was chosen to determine in which community on the list the skip interval calculations would begin to be applied. The community selections continued along the skip interval until the necessary number of children for sample for the given province was reached.
The sample communities were visited and the purpose of the blood study was explained in community meetings. In those meetings it was stressed that it was the right of each individual and their parents to decide whether to participate and that the results of the tests would be provided on request. Written, informed consent/assent was obtained from the parents or guardians of all participant children. Blood was obtained from children reported to be 9 months of age up to their reported fifth birthday. Using standard sterile finger prick, 80–120 μL blood was placed directly on Whatman filter paper No. 2 and allowed to dry. The dried blood spots (DBSs) were placed in plastic bags with a desiccant, and stored at −20°C until analyzed within 2 months of collection using a standard enzyme-linked immunosorbent assay (ELISA) at the Universidad del Valle de Guatemala (UVG) laboratory in Guatemala City. Two 6-mm punches from the DBS were eluted overnight in a phosphate-buffered saline-Tween/bovine serum albumin solution. The eluted solution was run in duplicate in a standard Ov-16 ELISA as previously described.19,23 Any positive results were repeated before being reported as positive. The IgG4 based ELISA as configured in the Guatemala laboratory has a sensitivity of 67% and a specificity of 100% and performs equally well on sera and filter paper DBSs (Vitaliano Cama, CDC, personal communication).
Archival review.
After the survey and laboratory work was completed, the communities selected for the serosurvey were compared with available MSPAS records from community level nodulectomy brigade visits over the 11-year pre-MDA period (1980–1990) before ivermectin MDA was widely introduced.24 The data consisted of convenience samples of community residents who presented themselves to MSPAS staff for a palpation examination for onchocercomas. Many of these persons were undoubtedly “self-selected” (as were their children) for examination, believing that they had nodules that needed to be removed by the brigadistas. Data included the date of the visit, the number of persons examined, the number of persons with nodules, and numbers of children under 5 years of age with nodules (as an indicator of recent incidence). Data from all visits to a sample community over the period were summed to give a single value for pre-MDA “convenience sample/self-section” community nodule rate (number of persons with nodules divided by number of persons examined), as well as the number of children under 5 years of age with nodules. Unfortunately, the total number of children under 5 years who were examined was not available, so a nodule rate among children who presented for examination could not be calculated.
Analysis.
Critical thresholds were based on the 2001 WHO Guidelines for Elimination of Onchocerciasis, as adapted by OEPA: 1) interruption of transmission—infection in vectors; an upper bound of the 95% CI of the prevalence of flies carrying infective larvae of < 1 infective fly/2,000, upper bound of the 95% CI of the seasonal transmission potential (STP) of < 20 L3/person/transmission season, and upper bound of the 95% CI of infection rates in young children of < 0.1%; 2) elimination of morbidity—MfAC of the eye at a level where the upper bound of the 95% CI of < 1%; and 3) elimination of transmission—3 years after stopping MDA (the PTS phase) infection in vectors remains at a level where the upper bound of the 95% CI is < 1 infective fly/2,000 and the upper bound of the 95% CI of the STP remains < 20 L3/person/transmission season.11,16,18,19
The one-sided 95% CIs for the prevalence were calculated using the SAS (version 9.0; SAS Institute, Cary, NC) FREQ procedure with the EXACT statement, BINOMIAL option, and an alpha level of 0.10. Entomological data were analyzed using the Poolscreen 2.0 program, which was used to calculate the proportion of infective flies based on the number of positive pools and the associated 95% CIs.20,25,26 Biting rates STPs were calculated as described by Lindblade and others.19 Overall means reported for SVs were an average of the mean infection rate for each SV, in a given survey year.
Ethics.
Before their execution, the surveys reported herein received appropriate review by the MSPAS, the UVG, the CDC, and Emory University, and were considered as program evaluation necessary in the monitoring of a public health program. Written, informed consent was obtained from all participants over 18 years of age, and from the parents or guardians of all participant children between 12 and 18 years of age. Children under 12 required parental assent. Fly attractants, who were residents of the SVs and so routinely exposed to vector bites in their daily activities, were paid for their time: they were read or had read to them a consent form and indicated their willingness to participate as attractants with their signature or fingerprint.
Results
Parasitological evaluations.
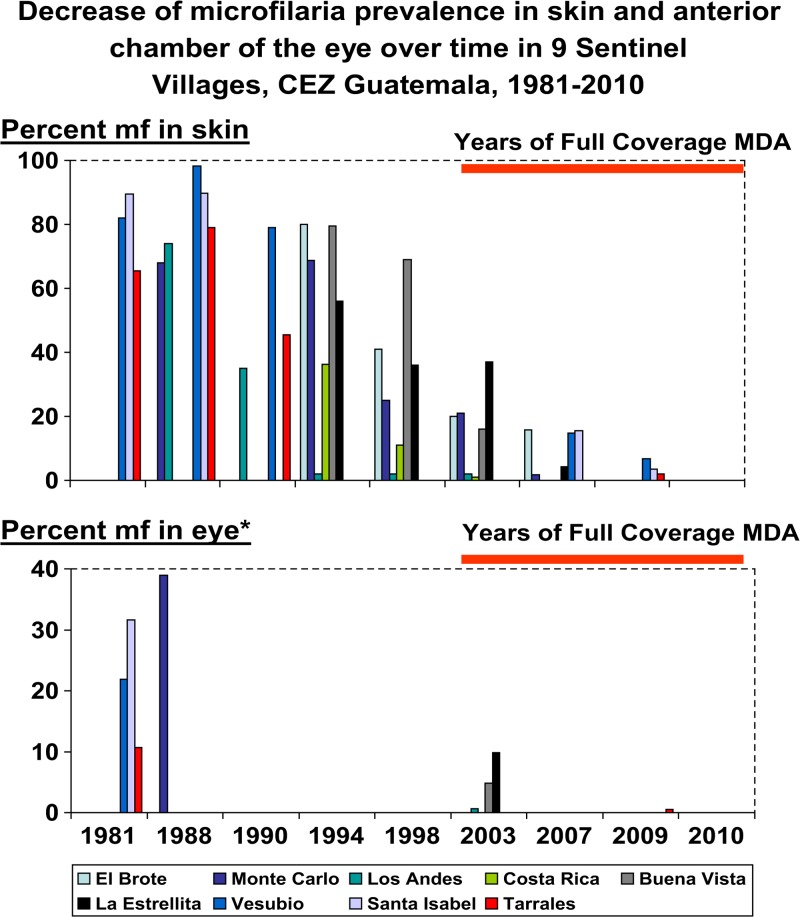
Between 1981 and 2010, there were 47 community visits for skin snip evaluations to determine mf prevalence in the nine SVs of the CEZ (mean: 5.2 assessments per SV, range: 4–6). Figure 3 (top panel) depicts the decrease over time of skin snip prevalence that was first observed after the MDA program reached sustained and effective coverage. Table 2 shows the results of initial baseline (1981–1994) and final (2007–2010) skin snip assessments, by SV. The mean SV baseline mf prevalence (obtained prior to the launching of MDA) was 70% (range 36–90%) for the nine SVs. In the final SV skin snip assessments (based on the examination of 1,032 individuals) no mf positives were found.
Figure 3.
The top panel shows the results from 47 sentinel village (SV) visits between 1981 and 2010 for skin snip evaluations to determine microfilariae (mf) prevalence in the nine SVs of the Central Endemic Zone (CEZ). Each village was visited between four and six times (mean: 5.2 assessments per SV). The graphic only shows positive results. Zero skin mf prevalence was first observed in 2007 and occurred in Buena Vista, Costa Rica, Los Andes, and Tarrales. In 2010, mf prevalence was zero in El Brote, Buena Vista, La Estrellita, Monte Carlo, Santa Isabel, Tarrales, and Vesubio. The bottom panel shows similar data for fewer (19) SV visits for ophthalmological evaluations to determine mf prevalence in the anterior chamber (MfAC); note that Monte Carlo and El Brote baseline are mf in cornea, not MfAC. Each of the nine SVs of the CEZ was visited at least twice (range 2–3). Zero values MfAC was recorded in 2007 in El Brote, Buena Vista, Costa Rica, La Estrellita, Los Andes, and Monte Carlo. In 2009, MfAC was zero in Santa Isabel and Vesubio. One individual (0.5%) was positive in Tarrales in 2009. See text and Table 2 for additional SV information.
Ophthalmological evaluations.
Between 1981 and 2010, there were 19 community visits for ophthalmological evaluations for onchocerciasis in the nine SVs of the CEZ (mean: 2.1 assessments per SV, range: 2–3). Figure 3 (bottom panel) shows the decrease over time of MfAC prevalence. Table 2 shows the results of baseline (1981–2003) and final (2007–2008) MfAC assessments in the nine SVs of the CEZ, by SV. The mean SV baseline MfAC prevalence was 12.1% (range 0–39%). In the final slit lamp assessments in the SVs, a total of 857 persons were examined; a single person with MfAC was found in Los Tarrales, resulting in a 0.5% prevalence for that community, and a 0.1% overall MfAC prevalence (with a one-sided 95% CI of 0–0.6%).
Entomological evaluations.
A total of four entomological field exercises were conducted during transmission seasons 2002, 2007, 2010–2011 (years combined), and the 2014 PTS period. These exercises consisted of a total of 6,023 person-collecting hours over 351 days during the peak S. ochraceum s.l. biting season of those collection years.5 Table 3 and its footnote summarize the number of SVs, collection sites, and months of vector collection activities.
Table 3.
Entomological sampling activities (2002–2014) in SVs in the CEZ, Guatemala
| Year of collection | Number of SVs sampled | Number of collection sites per SVs | Number of months of collections | Months collections took place | Number of collection days | Daily periods of collection (hours) | Estimated number of collection hours |
|---|---|---|---|---|---|---|---|
| 2002 | 6 | 2 | 4 | February–May | 86 | 6 | 860 |
| 2007 | 6 | 2 | 5 | January–May | 97 | 6 | 970 |
| 2010–2011 | 6 | 4 | 7 | December 2009 to February 2010 and February–May 2011 | 108 | 6 (2010) and 8 (2011) | 2,593 |
| 2014 | 5 (plus one spot check village) | 4 | 6 | November–December 2013 and January–April 2014 | 60 | 8 | 1,600 |
CEZ = central endemic zone; SV = sentinel village; PTS = posttreatment surveillance.
Daily collection periods were 50 minutes/hour (see Methods). The six SVs visited in 2002 and 2007 were El Brote, Buena Vista, Costa Rica, La Estrellita, Los Andes, and Montecarlo; in the combined 2010–2011 analysis unit, the six SVs were El Carmen Metzabal, Santa Isabel, Tarrales, and Vesubio in 2010 and La Estrellita, Montecarlo, Santa Isabel, and Vesubio in 2011. The 2014 PTS assessments took place in five SVs (El Brote, La Estrellita, Montecarlo, Santa Isabel, and Vesubio) and one-spot check village (Nueva Providencia).
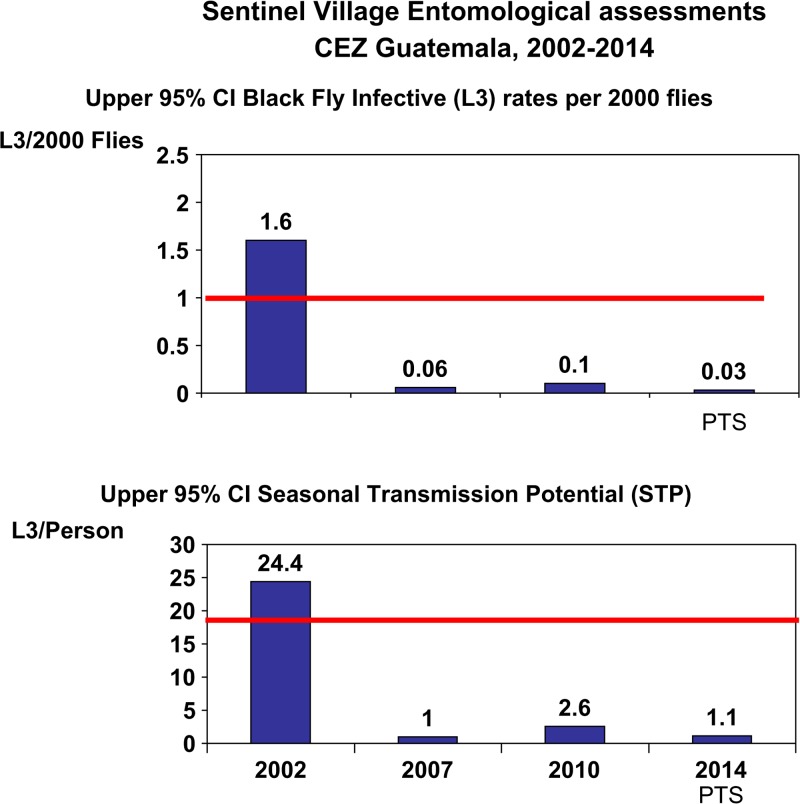
A total of 328,575 vectors were collected and tested by PCR for O. volvulus DNA. Table 4 shows a summary of PCR PoolScreen results. The trend of the 95% upper CIs of key indices (< 1 infective fly/2,000 vectors and STP < 20 L3/person/transmission season) over the entomological monitoring period in the CEZ is shown in Figure 4 . In 2002, 2 years into the full coverage MDA program, the point estimate for the prevalence of flies carrying infective larvae was 1.02/2,000 (95% CI: 0.8–1.60/2,000; Table 4 and Figure 4 top panel), and the upper 95% CI for the STP was 24.4 (mean 15.6, 95% CI: 12.2–24.4; Table 4 and Figure 4, bottom panel), both indicative of being right at the threshold of interrupting transmission by WHO/OEPA criteria. By 2007 (5 years and 10 MDA treatment rounds later), the upper 95% CIs for both the infective rate per 2,000 flies (0.06) and STP (1.0) were below the transmission interruption thresholds. The final entomological evaluations for the CEZ MDA phase in 2010–2011 were similarly below the thresholds, with the upper bound of the 95% upper CI of the vector infective rate of 0.1/2,000 flies and the STP was 0 with an upper bound of the 95% upper CI of 2.6. These results, taken together with results from the serological evaluations (described below) led to a MSPAS decision to stop MDA. During PTS entomological surveillance, PCR results from testing over 119,000 flies collected from November 2013 to April 2014 showed no evidence of recrudescence of transmission: 0 infection in the vectors (95% upper CI of 0.03/2,000 flies) and 0 STP (95% upper confidence of 1.1 L3/person/transmission season). The five SVs involved in the PTS evaluation included La Estrellita, Montecarlo, El Brote, Santa Isabel, and Vesubio. An additional non-sentinel village (Nueva Providencia, located in San Lucas Toliman, Solola) that was known to have been hyperendemic prior to MDA was included in the entomological assessment, as a “spot check.”
Table 4.
Entomological indices (2002–2014) for the CEZ, Guatemala
| Year of collection | Number of flies tested by PCR | Seasonal biting rate (range per community) | PCR infection rate per 2,000 flies (95% CI) | Seasonal transmission potential (95% CI) |
|---|---|---|---|---|
| 2002 | 46,160 | 30,530 (26,029–35,769) | 1.02 (0.80–1.60) | 15.57 (12.2–24.4) |
| 2007 | 67,808 | 33,485 (30,868–36,313) | 0 (0–0.06) | 0 (0–1.0) |
| 2010–2011 | 95,306 | 51,704 (23,323–82,863) | 0 (0–0.10) | 0 (0–2.6) |
| 2014 | 119,301 | 71,295 (66,243–76,720) | 0 (0–0.03) | 0 (0–1.1) |
PCR = polymerase chain reaction; CEZ = Central Endemic Zone; CI = confidence interval.
Figure 4.
The two graphs show the upper 95% confidence intervals for vector infectivity and for annual transmission potential in sentinel villages as calculated by PoolScreen. Horizontal lines indicate the breakpoint thresholds (< 1/2,000 cutoff for vector infectivity and < 20 for seasonal transmission potential [STP]) below which onchocerciasis transmission is not possible. Data are based on the examination of 328,575 vectors in 4,616 polymerase chain reaction (PCR) reactions (pools): 46,160 vectors in 835 pools in 2002, 67,808 flies in 1,260 pools in 2007, 95,306 flies in 1,836 pools in 2010–2011, and 119,301 flies in 685 pools in 2014.
2010 Serological evaluation.
Our study design aimed to select 3,800 children with an estimate that we would have a 20% refusal rate; we sampled 3,417 (90% of our target) in 29 communities: 2,171 (63% of sampled children) were from 15 (52% of the sampled) communities in Suchitepequez; 878 (26%) children in 12 (41%) communities in Chimaltenango (including children from La Estrellita, the only SV randomly selected in the sample); and 368 (11%) children in 2 (7%) communities in Sololá (Table 5). All 3,417 children were negative in ELISA testing for Ov16 IgG4 (0% prevalence, upper 95% CI: 0.08%), thus satisfying the WHO guideline that 5-year cumulative prevalence rates in young children should be < 0.1% to demonstrate interruption of O. volvulus transmission. The median age of the children tested was 36 months (range 9–69 months).
Table 5.
Results of Ov16 IgG4 serological testing (2010) of young children, CEZ, Guatemala, together with historical (pre-MDA) nodule rates in the sampled communities
| Province | Municipality | Community | 1980–1990 (Pre-MDA) nodule prevalence* | Number of children tested | OV16 Pct positive |
|---|---|---|---|---|---|
| Suchitepéquez | Santa Barbara | Santa Adelaida | 28† | 87 | 0 |
| Guayabal | NA | 296 | 0 | ||
| Patulul | Santa Luisa | NA | 77 | 0 | |
| San Juan Bautista | Veracruz | 9 | 53 | 0 | |
| San Miguel Panan | Finca La Concha | 0 | 145 | 0 | |
| San José Panan | 0 | 88 | 0 | ||
| Chicacao | Concepción Chinan | 12 | 399 | 0 | |
| El Recuerdo | 2 | 267 | 0 | ||
| Finca Chinan | 1 | 138 | 0 | ||
| Labor Los Mangales | NA | 74 | 0 | ||
| Cantón La Libertad | 0 | 164 | 0 | ||
| Alejandría | 0 | 49 | 0 | ||
| Santa Lucía Pamaxan | NA | 155 | 0 | ||
| Finca Baja Vista | 0 | 15 | 0 | ||
| El Pito | 1 | 164 | 0 | ||
| Subtotal | 2,171 (63%) | 0 | |||
| Sololá | San Lucas Tolimán | Pampojilá | 3 | 173 | 0 |
| Xejuyú | NA | 195 | 0 | ||
| Subtotal | 368 (11%) | 0 | |||
| Chimaltenango | San Miguel Pochuta | Finca El Recuerdo | 3 | 23 | 0 |
| Santa Ana | NA | 52 | 0 | ||
| Finca Mirandilla | 14 | 15 | 0 | ||
| Acatenango | Finca Rafael Pacún | 9 | 8 | 0 | |
| San Pedro Yepocapa | Finca Peña Plata | 12 | 10 | 0 | |
| Finca Santa Teresa | 5 | 17 | 0 | ||
| Morelia | 0 | 295 | 0 | ||
| La Estrellita | 78† | 38 | 0 | ||
| Nueva Victoria | NA | 34 | 0 | ||
| San Francisco | 16 | 201 | 0 | ||
| El Porvenir | 5 | 48 | 0 | ||
| Aldea La Cruz | 8 | 137 | 0 | ||
| Subtotal | 878 (26%) | 0 | |||
| Total | 3,417 | 0 (0–0.08)‡ |
CEZ = Central Endemic Zone; MDA = mass drug administration; NA = data not available.
1980–1990 (Pre-MDA) nodule rates are based on convenience samples, often among individuals who were self-selected based on their belief that they had a nodule, and are therefore not based on a statistically representative sample.
Nodules found in children during the pre-MDA period.
Number in parenthesis, 95% confidence interval.
Archival review.
Past (pre-MDA) MSPAS convenience sample/self-selection nodule data were available for 22 (76%) of the 29 sample communities (Table 5). Over the period 1980–1990, MSASP brigades visited these 22 communities a total of 84 times (range 1–14 visits/community). Out of 8,456 palpation examinations, 1,009 (12%) were positive for onchocercomas. The range of pre-MDA community nodule rates was 0–78%, with the SV La Estrellita having the highest prevalence, followed by Santa Adelaida (28%). Also in the pre-MDA surveys conducted between 1980 and 1990, 124 children under 5 years of age were found to have nodules; 99 of these were from Santa Adelaida and 13 from La Estrellita. In contrast, over 20 years later, in a new (2010) generation of 125 children living in those same two communities, none had detectable Ov16 IgG4 antibodies, evidence of an absence of transmission there for many years.
Discussion
In this report, we review the history of the 24 years of MDA with ivermectin in the CEZ of Guatemala. The MDA program struggled in its first 12 years (1988–1999) to scale up and reach sustained annual treatment coverage. It was not until the year 2000, when, with better financial, administrative, and political support, the CEZ MDA program began delivering effective MDA rounds. This second 12-year phase of programmatic maturity was when, based on results from serial SV monitoring and evaluation activities, O. volvulus transmission interruption was actually accomplished. MDA was suspended in 2012, and after a 3-year (2012–2014) period of PTS, an entomological PCR assessment of over 119,000 flies showed no evidence of vector infection. Transmission was declared eliminated by the Guatemalan Ministry of Health in 2015. This declaration occurred 100 years after the discovery of onchocerciasis in the Americas in 1915 by Rodolfo Robles Valverde, whose work was conducted in the CEZ.
Robles (1878–1932) was born in Quetzaltenango and at the age of 17 went to France for his education. He was granted his medical degree from The Sorbonne in Paris in 1904, where he specialized in “colonial medicine,” a field focused on malaria, public health, and microbiology. After his return to Guatemala, he maintained a keen research interest in tropical pathology, and in 1915 encountered an 8-year-old patient with a subcutaneous nodule in which Robles discovered the adult worms of O. volvulus. He subsequently dedicated much of his research to the study of the transmission, clinical pathology, and epidemiology of this parasite, and most importantly established for the first time that onchocerciasis was linked to visual loss.7,8 France later awarded him the Legion of Honor. The highest medical award of Guatemala is named in his honor, as is an ophthalmological hospital in Guatemala City.27
The CEZ is the largest transmission zone (focus) of onchocerciasis in Guatemala, and with a population at risk of 124,498, is larger than the other three Guatemalan foci combined. The CEZ also has the largest at risk population of the 13 onchocerciasis foci in the Americas, comprising 22% of the 565,232 persons originally at risk for contracting the disease. It is followed in size by the south Chiapas focus in Mexico (with 117,825 persons at risk, or 21% of the regional total) and the northeast focus of Venezuela (95,567 persons at risk, 17%).4 Together these three foci account for 60% of the population at risk in the Americas. MDA has been halted in all three of these foci,28,29 but only south Chiapas and the CEZ have completed PTS. Overall, MDA has been stopped in 11 of the 13 American foci; the only active MDA program for onchocerciasis in the Americas at this time is in a cross-border focus (shared by Brazil and Venezuela) in the Amazon jungle. The difficult to access indigenous Amerindian population (the Yanomami) who are targeted for treatment in this area comprise only 5% of the population in the Americas originally targeted for MDA.4
The roadmap for establishing the elimination of onchocerciasis in Guatemala used the WHO guidelines published in 2001, as modified for field operations and observations and conditions reported by Lindblade19 and OEPA.16 The modified guidelines have been successfully used by independent verification teams (IVTs), sent under the auspices of the WHO Neglected Tropical Disease Department to verify the elimination of onchocerciasis in Colombia in 201330and Ecuador in 2014.31 In this process, an IVT country visit is undertaken after the submission by a ministry of health of a detailed dossier that provides evidence that all foci in the country have eliminated O. volvulus transmission. In Guatemala, the elimination process has already been completed and published in peer-reviewed journals for three of the four of its onchocerciasis foci: Santa Rosa,19 Escuintla-Guatemala,32 and Huehuetenango.33 Based on the completion of elimination evaluations in the CEZ, as reported herein, Guatemala submitted its dossier to WHO in 2015, along with a request for an IVT visit.
In the 1990s, several key studies seeking to determine how to best use ivermectin MDA to break O. volvulus transmission were conducted in the CEZ by MSPAS, CDC, the UVG, the University of Arizona, and other partners.10,12,13,34 The OEPA strategy of providing at least semiannual (every 6 month) treatment rounds in all affected communities, each achieving “effective” (≥ 85% directly observed treatment of eligibles) coverage, was based largely on the findings from these studies. An important challenge was that ivermectin, a potent oral microfilaricidal drug, is not immediately lethal to the adult O. volvulus worms. The elimination strategy in the Americas was therefore based on using ivermectin to keep levels of mf in the human population low enough to prevent them from infecting the vector. With the transmission cycle so suppressed, MDA under this strategy would then need to be given for the duration of the reproductive life of remaining adult worms being exposed to the twice per year doses of ivermectin. Cupp and Cupp estimated the reproductive adult female worm life span under such intense ivermectin exposure to be about 6.5 years.35 Adult male worms are more sensitive to ivermectin than female worms, and perish even more rapidly. Histological studies of nodules showed fewer than 20% of nodules contained male worms and more than 80% of female worms were uninseminated and producing unfertilized oocytes in populations where transmission was broken and twice per year MDA was being provided.36
The entomological results from this study are worthy of note. The first PCR-based entomological measurements did not begin until 2002, 2 years into effective MDA delivery. These early PCR results showed the infective rate and the STP to be still above the breakpoint thresholds of an upper bound of the 95% CI of < 1/2,000 and < 20 L3/person/transmission season (Figure 4). We can estimate the pre-MDA baseline using dissection data from two SVs. Collins and others, working in the SV Los Tarrales from 1976 to 1977 found an S. ochraceum s.l. infective rate of 61/2,000, and an STP of 174 L3/person.21 Cupp and others, working in the SV Los Andes in 1988, reported a pre-MDA STP of 108 L3/person.13 If the pre-MDA baseline STP was assumed to be the mean value from these two studies (141 L3/person/season), then by 2 years into the effective MDA program, transmission had been reduced by approximately 82% (from 141 to 24). By 2007, after 7 years and 14 MDA treatment rounds, the entomological breakpoint thresholds had been achieved (with an STP reduction from pre-MDA baseline of > 99%). Effective MDA was continued for another 5 years (10 rounds) past this point, at which time a total absence of Ov16 IgG4 antibody reactivity was demonstrated in > 3,400 children under 5 years of age. These children were sampled from randomly selected communities within the CEZ that included known and unknown endemic villages; in two of the known endemic villages, children under 5 had had nodules noted by the MSPAS brigades in the pre-MDA era. The serological results confirmed the 2007 and 2011 entomological data that there had been no parasite transmission for at least 5 years. During the PTS entomological surveillance, PCR results from testing over 119,000 flies in sentinel, as well as an extra sentinel village, showed no evidence of recrudescence of transmission or of human–parasite vector contact. These PTS entomological results are strong evidence that the adult O. volvulus worms in the transmission zone were either absent, or at such a low reproductive levels that the population could not recover.16
A decline in human onchocerciasis prevalence and intensity of infection in the CEZ likely began before full coverage with MDA was achieved in 2000. Any such decline in the human population would have been accompanied by a decrease in the force of transmission, although probably insufficient to break transmission, especially in hyperendemic areas. As noted above, the 2002 entomological assessment conducted after only 2 years of full coverage twice per year CEZ MDA showed both the prevalence in flies (with an upper 95% CI of 1.60/2,000) and the STP (with an upper 95% CI: 24.4 L3/person/season) were just above the transmission breakpoint as defined by the current WHO/OEPA guidelines. The 2007 entomological evaluation, conducted 5 years later, was the first time that the breakpoint was documented to have been reached. However, it is highly likely that transmission was broken much earlier than 2007, probably within 2 years of the 2002 survey. Thus we believe the MDA program provided sufficient treatment (2004–2011) for the 6.5 year expected reproductive life span of adult O. volvulus female worms projected under twice per year ivermectin treatment pressure.35 Although there might have been a small risk with the halting MDA in 2012 (after only 5 years after documenting transmission interruption in 2007) that some adult female worms remained, we could find no evidence that there were sufficient reproductively active parasites to result in the resumption of transmission in the SVs where PTS was conducted. This could also have been due to the impact of MDA on the adult male worm population that appears more sensitive than O. volvulus females to recurrent twice yearly treatments.36
The success against onchocerciasis in the Americas is in large part due to a durable public–private partnership, embodied in OEPA, that includes the six endemic countries (Brazil, Colombia, Ecuador, Guatemala, Mexico, and Venezuela), PAHO/WHO, The Carter Center, Merck and the Mectizan Donation Program, and many other partners. Annual meetings (the Inter-American Conferences on Onchocerciasis) of all partners have been held since 1991. A small secretariat for the OEPA, partnership, based in Guatemala City, provides technical, administrative, advocacy, and some financial support to national programs.15 The Carter Center has shouldered the administrative responsibilities for the secretariat for the last 20 years. A Program Coordinating Committee meets twice per year to act as the technical steering committee for OEPA. It reviews and approves the annual Weekly Epidemiological Record progress reports of the regional initiative that have been published by WHO since 1996. PAHO/WHO provided the initial 1991 and subsequent key regional resolutions to provide the political mandate behind the effort. In 2008, PAHO's Directing Council renewed the call to eliminate onchocerciasis throughout the region in Resolution CD48.R12. The following year the Directing Council issued CD49.R19 that called for the elimination or control of 12 neglected infectious diseases of poverty in the Americas, which includes onchocerciasis elimination as one of its 2015 targets.4,14
In conclusion, we report the elimination of O. volvulus transmission from the CEZ in Guatemala, the largest focus of onchocerciasis in the Americas and the first to be discovered in this hemisphere by Rodolfo Robles in 1915. Elimination was achieved by a national program that delivered semiannual mass ivermectin administration reaching at least 85% of the eligible population over a 12-year period (2000–2011). Progress toward elimination was demonstrated through serial ocular, parasitological, and entomological surveys conducted in nine sentinel villages, as well as in a broad sampling of young children for Ov16 IgG4 antibody reactivity throughout the CEZ prior to stopping the MDA campaign. Three years after halting mass treatment an entomological assessment showed no evidence of vector infection. The Ministry of Health of Guatemala declared onchocerciasis transmission as having been eliminated from the CEZ 100 years after its discovery there by Robles.
ACKNOWLEDGMENTS
We thank colleagues at the Centro de Estudios en Salud of the Universidad del Valle de Guatemala (Nazario Lopez, Julio Illescas, Aura Paniagua, Lisbeth Paniagua, Nancy Zamora, Soledad Rodas, Rodrigo González, Lucía García, Nancy Say, Mynor López, José Luis Boteo, Jorge Sincal, Marvin Xiquitá, Efraín Gramajo Mazariegos, Adriana Santis, Byron Arana, Ricardo Luján, and the late Nancy Cruz-Ortiz); Ministerio de Salud Pública y Asistencia Social (Julio Castro, Eduardo Catú, Arturo Sánchez, Sayra Chanquín, Carlos Blanco, Brenda Villatoro, Juan Carlos Solares, the late Reginaldo Pichillá, Eusebio Efraín Montejo, Luis Blanco, Luis Posada Colindres, Victor Barrios, Pedro Yax and the late Onofre Ochoa); Onchocerciasis Elimination Program for the Americas (OEPA) (Silvia Sagastume, Luis Erchila, Jack Blanks, Edmundo Alvarez, and Oswaldo Mejía); Pan American Health Organization (Steve Ault, Fernando Beltran, John Ehrenberg, Luis Gerardo Castellanos); Division of Parasitic Diseases and Malaria, Centers for Disease Control and Prevention, Atlanta, GA (Kim Lindblade, Charles Porter, Vita Cama, Joseph Amann, Patricia Juliao, Monica Parise, George Punkosdy, James MaGuire); Lions Clubs (Alfonso Barahona, Carlos Samuel Arévalo); The Bill and Melinda Gates Foundation (David Brandling-Bennett, Julie Jacobson); The River Blindness Foundation (the late BOL Duke, the late William Baldwin, John Moores), The Carter Center (Don Hopkins, Craig Withers, Nicole Kruse, Emily Howard, Madelle Hatch, Lindsay Rakers, Emily Griswold, Lauri Hudson-Davis, Jennifer Lee and the late Andrew Agle), the International Eye Foundation (Victoria Sheffield, John Barrows), Merck and Co. and the Mectizan Donation Program (Joni Lawrence, Stefanie Meredith, Adrian Hopkins, Ken Gustavsen, Brenda Colatrella) and Richard Collins, Tom Nutman, Kevin Winthrop, Jill Heeringa, John Davies, Rodolfo Zea Flores, Edgar Lobos, Carlos Gonzales-Peralta, and Orlando Oliva. The Onchocerciasis Elimination Program for the Americas (OEPA) coalition includes The Carter Center (as administrative lead), the six endemic countries, PAHO/WHO, the Lions Clubs International Foundation and local Lions Clubs, the Bill and Melinda Gates Foundation, Merck and the Mectizan Donation Program, CDC, USAID, the Carlos Slim Foundation, and multiple regional universities.
Footnotes
Financial support: The Guatemalan program received financial support from the Guatemalan Government and the Pan American Health Organization, and from 1993 to 2014 through OEPA/The Carter Center that included grants from the Lions Clubs International Foundation, the Bill and Melinda Gates Foundation, Merck and the Mectizan Donation Program, the U.S. Centers for Disease Control and Prevention (CDC), the United States Agency for International Development (USAID), The Carlos Slim Foundation, The Inter-American Development Bank, The River Blindness Foundation, the OPEC Fund for International Development (OFID), The Alwaleed Bin Talal Foundation, The Starr Foundation, Falconer Charitable Remainder Trust, The Carter Center, The Cartre UK Foundation, The Baxter International Foundation, Alcon Laboratories, John C. and Karyl Kay Hughes Foundation, The Osprey Foundation of Maryland, The UPS Foundation and many private individuals. Prior to OEPA, in the late 1980s and 1990s, key support to Guatemala for ivermectin distribution, in addition to that of the Government of Guatemala, was provided by the River Blindness Foundation, the Pan American Health Organization, the International Eye Foundation, the Japan International Cooperation Agency (JICA), and the Public Welfare Foundation. Ivermectin (Mectizan®) was provided by Merck.
Authors' addresses: Frank Richards Jr., River Blindness Program, The Carter Center, Atlanta, GA, E-mail: frank.richards@emory.edu. Nidia Rizzo, Renata Mendizabal de Cabrera, and Oscar de Leon, Centro de Estudios en Salud, Universidad del Valle de Guatemala, Guatemala, Guatemala, E-mails: nrizzo@ces.uvg.edu.gt, rmendizabal@ces.uvg.edu.gt, and odeleon@ces.uvg.edu.gt. Carlos Enrique Diaz Espinoza, Ministerio de Salud Pública y Asistencia Social, Guatemala, Guatemala, E-mail: carlosenriquediazespinoza@gmail.com. Zoraida Morales Monroy, Programa Nacional de Enfermedades Transmitidas por Vectores, Ministerio de Salud Pública y Asistencia Social de Guatemala, Ciudad de Guatemala, Guatemala, E-mail: zoraidamoralesmonroy@gmail.com. Carol Guillermina Crovella Valdez, Ministerio de Salud Pública y Asistencia Social de Guatemala, Ciudad de Guatemala, Guatemala, E-mail: ccrovellav@gmail.com. Guillermo Zea-Flores, Guatemala, E-mail: gzeaflores@hotmail.com. Mauricio Sauerbrey, Alba Lucia Morales, Dalila Rios, and Alfredo Domínguez, Onchocerciasis Elimination Program for the Americas, Edificio Murano Center, Guatemala City, Guatemala, E-mails: msauercar@gmail.com, almorales@oepa.net, drios@oepa.net, and adominguez59@hotmail.com. Thomas R. Unnasch and Hassan K. Hassan, Global Health Infectious Disease Program, Department of Global Health, University of South Florida, Tampa, FL, E-mails: tunnasch@health.usf.edu and hhassan@health.usf.edu. Robert Klein, Medical Entomology Research and Training Unit/Guatemala Miami, FL, E-mail: roeklein64@gmail.com. Mark Eberhard, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: mle1@cdc.gov. Ed Cupp, Auburn University, Department of Entomology, Owensboro, KY, E-mail: cuppedd@auburn.edu.
References
- 1.Cupp EW, Sauerbrey M, Richards FO. Elimination of human onchocerciasis: history of progress and current feasibility using ivermectin (Mectizan®) monotherapy. Acta Trop Suppl. 2011;120(Suppl 1):S100–S108. doi: 10.1016/j.actatropica.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Richards FO, Miri E, Meredith S, Guderian R, Sauerbrey M, Remme H, Packard R, Ndiaye JM. Onchocerciasis. Bull World Health Organ. 1998;76(Suppl 2):147–149. [PMC free article] [PubMed] [Google Scholar]
- 3.Zimmerman PA, Katholi CR, Wooten MC, Lang-Unnasch N, Unnasch TR. Recent evolutionary history of American Onchocerca volvulus, based on analysis of a tandemly repeated DNA sequence family. Mol Biol Evol. 1994;11:384–392. doi: 10.1093/oxfordjournals.molbev.a040114. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous Progress toward elimination of onchocerciasis in the Americas. MMWR. 2013;87:404–407. [Google Scholar]
- 5.Porter CH, Collins RC. Seasonality of adult black flies and Onchocerca volvulus transmission in Guatemala. Am J Trop Med Hyg. 1988;38:153–167. doi: 10.4269/ajtmh.1988.38.153. [DOI] [PubMed] [Google Scholar]
- 6.Yamagata Y, Suzuki T, Garcia Manzo GA. Geographical distribution of the prevalence of nodules of Onchocerca volvulus in Guatemala over the last four decades. Trop Med Parasitol. 1986;37:28–34. [PubMed] [Google Scholar]
- 7.Robles R, Calderón V. Enfermedad nueva en Guatemala. Vol. 8. Guatemala: 1917. pp. 9–116. Revista de la Juventud Médica, año XVIII, tomo XVII. 177. [Google Scholar]
- 8.Robles R. Onchocercose humaine au Guatemala produisant la cecite´ et “l'erysipe´ le du littoral” (Erisipela de la costa) Bull Soc Pathol Exot. 1919;12:442–460. [Google Scholar]
- 9.Ochoa JO, Castro JC, Barrios VM, Juarez EL, Tada I. Successful control of onchocerciasis vectors in San Vicente Pacaya, Guatemala, 1984–1989. Ann Trop Med Parasitol. 1997;91:471–479. doi: 10.1080/00034989760833. [DOI] [PubMed] [Google Scholar]
- 10.Richards FO, Klein R, Zea-Flores R, Gonzáles-Peralta C, Castro J, Zea-Flores G. Knowledge, attitudes, and perceptions during a community-level ivermectin distribution campaign in Guatemala. Health Policy Plan. 1995;10:404–414. doi: 10.1093/heapol/10.4.404. [DOI] [PubMed] [Google Scholar]
- 11.Anonymous . Certification of Elimination of Human Onchocerciasis: Criteria and Procedures, Guidelines. Geneva, Switzerland: WHO WHO/CDS/CPE/CEE.18.b; 2001. [Google Scholar]
- 12.Collins RC, Gonzales-Peralta C, Castro J, Zea-Flores G, Cupp M, Richards FO, Cupp EW. Ivermectin: reduction in prevalence and infection intensity with Onchocerca volvulus following biannual treatments in five Guatemalan communities. Am J Trop Med Hyg. 1992;47:156–169. doi: 10.4269/ajtmh.1992.47.156. [DOI] [PubMed] [Google Scholar]
- 13.Cupp EW, Ochoa JO, Collins RC, Cupp MS, Gonzales-Peralta C, Castro J, Zea-Flores G. The effects of repetitive community-wide ivermectin treatment on transmission of Onchocerca volvulus in Guatemala. Am J Trop Med Hyg. 1992;47:170–180. doi: 10.4269/ajtmh.1992.47.170. [DOI] [PubMed] [Google Scholar]
- 14.Sauerbrey MS. The Onchocerciasis Program for the Americas (OEPA) Ann Trop Med Parasitol. 2008;102(Suppl 1):S25–S29. doi: 10.1179/136485908X337454. [DOI] [PubMed] [Google Scholar]
- 15.Blanks J, Richards FO, Beltran F, Collins RC, Alvarez E, Zea-Flores G, Bauler B, Cedillos R, Heisler M, Brandling-Bennett D, Baldwin W, Bayona M, Klein R, Jacox M. The Onchocerciasis Elimination Program of the Americas: a history of partnership. Rev Panam Salud Publica. 1998;3:367–374. doi: 10.1590/s1020-49891998000600002. [DOI] [PubMed] [Google Scholar]
- 16.Program Coordinating Committee, OEPA Staff Guide to detecting a potential recrudescence of onchocerciasis during the post treatment surveillance period: the American paradigm. Res Rep Trop Med. 2012;3:21–33. doi: 10.2147/RRTM.S30482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandling-Bennett AD, Anderson J, Fuglsang H, Collins RC. Onchocerciasis in Guatemala. Epidemiology in fincas with various intensities of infection. Am J Trop Med Hyg. 1981;30:970–981. [PubMed] [Google Scholar]
- 18.Winthrop K, Proaño R, Oliva O, Arana B, Mendoza C, Dominguez A, Amann J, Punkosdy G, Blanco C, Klein R, Sauerbrey M, Richards FO. The reliability of anterior segment lesions as indicators of onchocercal eye disease in Guatemala. Am J Trop Med Hyg. 2006;75:1058–1062. [PubMed] [Google Scholar]
- 19.Lindblade KA, Arana B, Zea-Flores G, Rizzo N, Porter CH, Dominguez A, Cruz-Ortiz N, Unnasch TR, Punkosdy GA, Richards J, Sauerbrey M, Castro J, Catú E, Oliva O, Richards FO. Elimination of Onchocerca volvulus transmission in the Santa Rosa Focus of Guatemala. Am J Trop Med Hyg. 2007;77:334–341. [PubMed] [Google Scholar]
- 20.Rodr𝚤guez-Perez MA, Katholi CR, Hassan HK, Unnasch TR. Large scale entomologic assessment of Onchocerca volvulus transmission by poolscreen PCR in Mexico. Am J Trop Med Hyg. 2006;74:1026–1033. [PubMed] [Google Scholar]
- 21.Collins RC. Onchocerciasis transmission potentials of four species of Guatemalan Simuliidae. Am J Trop Med Hyg. 1979;28:72–75. doi: 10.4269/ajtmh.1979.28.72. [DOI] [PubMed] [Google Scholar]
- 22.Lobos E, Weiss N, Karam M, Taylor HR, Ottesen EA, Nutman TB. An immunogenic Onchocerca volvulus antigen: a specific and early marker of infection. Science. 1991;251:1603–1605. doi: 10.1126/science.2011741. [DOI] [PubMed] [Google Scholar]
- 23.Oguttu D, Byamukama E, Katholi CR, Habomugisha P, Nahabwe C, Ngabirano M, Hassan HK, Lakwo T, Katabarwa M, Richards FO, Unnasch TR. Serosurveillance to monitor onchocerciasis elimination: the Ugandan experience. Am J Trop Med Hyg. 2014;90:339–345. doi: 10.4269/ajtmh.13-0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richards FO. Using GIS to target onchocerciasis control activities in Guatemala. In: David B, Hippolyte F, editors. Geographical Targeting for Poverty Alleviation: Methodology and Applications. The World Bank Regional and Sectoral Studies. Washington, DC: 2000. pp. 235–257. [Google Scholar]
- 25.Katholi CR, Toe L, Merriweather A, Unnasch TR. Determining the prevalence of Onchocerca volvulus infection in vector populations by polymerase chain reaction screening of pools of black flies. J Infect Dis. 1995;172:1414–1417. doi: 10.1093/infdis/172.5.1414. [DOI] [PubMed] [Google Scholar]
- 26.Unnasch TR, Meredith S. The use of degenerate primers in conjunction with strain and species oligonucleotides to classify Onchocerca volvulus. Methods Mol Biol. 1996;50:293–303. doi: 10.1385/0-89603-323-6:293. [DOI] [PubMed] [Google Scholar]
- 27.Wikipedia Rodolfo Robles. 2015. http://wikiguate.com.gt/rodolfo-robles/ Available at. Accessed March 13, 2015.
- 28.Rodr𝚤guez-Perez MA, Dom𝚤nguez-Vazquez A, Unnasch TR, Hassan HK, Arredondo-Jimenez JI, Orozco-Algarra ME, Rodríguez-Morales KB, Rodríguez-Luna IC, Prado-Velasco FG. Interruption of transmission of Onchocerca volvulus in the southern Chiapas Focus, Mexico. PLoS Negl Trop Dis. 2013;7:e2133. doi: 10.1371/journal.pntd.0002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Convit J, Schuler H, Borges R, Olivero V, Domínguez-Vázquez A, Frontado H, Grillet ME. Interruption of Onchocerca volvulus transmission in northern Venezuela. Parasit Vectors. 2013;6:289. doi: 10.1186/1756-3305-6-289. http://www.parasitesandvectors.com/content/6/1/289 Available at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anonymous Progress towards eliminating onchocerciasis in the WHO Region of the Americas: verification by WHO of elimination of transmission in Colombia. Wkly Epidemiol Rec. 2013;88:381–383. [PubMed] [Google Scholar]
- 31.Anonymous Progress towards eliminating onchocerciasis in the WHO Region of the Americas: Ecuador's progress towards verification of elimination. Wkly Epidemiol Rec. 2014;89:401–408. [Google Scholar]
- 32.Gonzales RJ, Cruz-Ortiz N, Rizzo N, Richards J, Zea-Flores G, Domínguez A, Sauerbrey M, Catú E, Orlando O, Richards FO, Lindblade KA. Successful interruption of transmission of Onchocerca volvulus in the Escuintla-Guatemala focus, Guatemala. PLoS Negl Trop Dis. 2009;3:e404. doi: 10.1371/journal.pntd.0000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cruz-Ortiz N, Gonzalez RJ, Lindblade KA, Richards F, Sauerbrey M, Dominguez A, Zea-Flores G, Olivia O, Catú E, Rizzo N. Elimination of Onchocerca volvulus transmission in the Huehuetenango Focus of Guatemala. J Parasitol Res. 2012;2012:638429. doi: 10.1155/2012/638429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cupp EW, Ochoa O, Collins RC, Ramberg F, Zea-Flores G. The effect of multiple ivermectin treatments on infection of Simulium ochraceum with Onchocerca volvulus. Am J Trop Med Hyg. 1989;40:501–506. doi: 10.4269/ajtmh.1989.40.501. [DOI] [PubMed] [Google Scholar]
- 35.Cupp EW, Cupp MS. Impact of ivermectin community-level treatments on elimination of adult Onchocerca volvulus when individuals receive multiple treatments per year. Am J Trop Med Hyg. 2005;73:1159–1161. [PubMed] [Google Scholar]
- 36.Cupp EW, Duke BO, Mackenzie CD, Guzmán JR, Vieira JC, Mendez-Galvan J, Castro J, Richards FO, Sauerbrey M, Dominguez A, Eversole RR, Cupp MS. The effects of long-term community level treatment with ivermectin on adult Onchocerca volvulus in Latin America. Am J Trop Med Hyg. 2004;71:602–607. [PubMed] [Google Scholar]