Abstract
Taenia solium cysticercosis is a common parasitic infection of humans and pigs. We evaluated the posttreatment evolution of circulating parasite-specific antigen titers in 693 consecutive blood samples from 50 naturally infected cysticercotic pigs, which received different regimes of antiparasitic drugs (N = 39, 7 groups), prednisone (N = 5), or controls (N = 6). Samples were collected from baseline to week 10 after treatment, when pigs were euthanized and carefully dissected at necropsy. Antigen levels decreased proportionally to the efficacy of treatment and correlated with the remaining viable cysts at necropsy (Pearson's p = 0.67, P = 0.000). A decrease of 5 times in antigen levels (logarithmic scale) compared with baseline was found in 20/26 pigs free of cysts at necropsy, compared with 1/24 of those who had persisting viable cysts (odds ratio [OR] = 76.7, 95% confidence interval [CI] = 8.1–3308.6, P < 0.001). Antigen monitoring reflects the course of infection in the pig. If a similar correlation exists in infected humans, this assay may provide a minimally invasive and easy monitoring assay to assess disease evolution and efficacy of antiparasitic treatment in human neurocysticercosis.
Introduction
Neurocysticercosis (NCC) is the infection of the central nervous system by the larval form of Taenia solium. NCC is the most important cause of late onset epilepsy worldwide and a growing problem in developed countries because of immigration from endemic zones.1,2 Humans get infected by accidental ingestion of T. solium eggs excreted with the feces of human tapeworm carriers. The oncospheres are liberated from the eggs, cross the intestinal wall, and are carried by the circulatory system to almost any organ or tissue, where they develop as the larval stage or cysticercus.
Diagnosis is based principally on neuroimaging (magnetic resonance imaging and computed tomography) and available serological tests, mainly the immunoelectrotransfer blot (EITB), which detects specific antibodies.3–5 Despite high sensitivity and specificity, the performance of antibody detection assays is suboptimal. In patients with single lesions or with calcified lesions only, the sensitivity decreases6 or some individuals have specific antibodies in the absence of established infection (exposure only or aborted infections). Also, antibodies persist long after parasite clearance.7–10 These drawbacks leave physicians dependent on imaging examinations, which are expensive and poorly available in endemic villages. In pigs, antibody detecting tests have similar limitations than in humans. They cannot distinguish between patent infection and exposure, although strong reactions may be associated with established infection and higher parasitic burden.11–13 Detection of specific circulating antigens can reflect presence and burden of patent infection.14–17 Cysticercosis antigen detection assays were reported as early as of 1989, using a monoclonal antibody-based enzyme-linked immunosorbent assay (ELISA) directed to a secretory-excretory antigen from the related cestode Taenia saginata.18,19 Brandt and others developed in 1992 an immunoglobulin M (IgM) monoclonal antibody-based assay,20 later improved by using IgG monoclonal antibodies and pretreatment of the sera by trichloroacetic acid.21–23 Antigen-ELISA has been widely used to detect cysticercosis in humans as well as in pigs.14,15,24–27
Using serial posttreatment serum circulating antigen levels in pigs, we assessed whether the changes in circulating antigen levels correlate with the response to antiparasitic treatment, and whether antigen levels at the time of necropsy correlate with the final infection burden.
Materials and Methods
Study design and animals.
This study was conducted using serum samples of 50 pigs obtained from an original study cohort of 54 naturally T. solium-infected pigs.28 These animals were bought from villages in the highly endemic Peruvian highlands and transported to veterinary facilities in Lima. The study was performed at the San Marcos School of Veterinary Medicine in Lima, Peru. Ethical approval was granted by the Animal Ethics Committee of the School of Veterinary Medicine, Universidad Nacional Mayor de San Marcos, Lima, Peru.
Pig infection was initially assessed using tongue examination (palpation of lingual viable nodules, implying the presence of active cysticercosis),29 and confirmed by serology (serum EITB). Pigs were randomly assigned to nine different groups and each group was treated with an individual scheme, including albendazole (ABZ) (Zentel®; GlaxoSmithKline, Lima, Peru), praziquantel (PZQ) (Praziquantel; IDA foundation, Mumbai, India), three schemes of combined ABZ plus PZQ, oxfendazole (OFZ) (Synanthic®; American Home Products Corporation, Madison, WI), nitazoxanide (NTZ) (Colufase®; Roemmers, Buenos Aires, Argentina), prednisone (Nisona®; LUSA, Lima, Peru), and an untreated control group. All antiparasitic drugs were administered by oral route, initiating the same day, according the following schemes28: group 1 (AbPzPd)—ABZ 15 mg/kg/day in two doses (8 am and 8 pm) for 7 days, three doses of PZQ 25 mg/kg each 2 hours starting at 8 am, and prednisone at 0.5 mg/kg/day in single doses for 5 days; group 2 (½AbPzPd)—ABZ 7.5 mg/kg/day in two doses for 7 days, three doses of PZQ 25 mg/kg each 2 hours starting at 8 am, and prednisone at 0.5 mg/kg/day in single doses for 5 days; group 3 (AbPz2Pd)—ABZ 15 mg/kg/day in two doses for 7 days, three doses of PZQ 25 mg/kg each 2 hours starting at 8 am, and prednisone at 1 mg/kg/day in single doses for 5 days; group 4 (AbPd)—ABZ 15 mg/kg/day in two doses for 7 days plus prednisone at 0.5 mg/kg/day in single doses for 5 days; group 5 (OFZ)—OFZ 30 mg/kg/day single doses; group 6 (PZQ)—three doses of PZQ 25 mg/kg each 2 hours starting at 8 am; group 7 (PDN)—prednisone at 0.5 mg/kg/day in a single daily dose for 5 days; group 8 (NTZ)—NTZ 150 mg/kg/day for 7 days (300 mg/kg on day 1, then 150 mg/kg on days 2–7), and group 9 (control group)—pigs in the control group did not receive treatment.
An extended explanation about the basis of these treatment schemes has been presented in a previous publication.28 In brief, the first three schemes were intended to evaluate the cysticidal effects of the ABZ plus PZQ as a new approach. Groups 4 ABZ and 6 PZQ simulated antiparasitic effects given in humans. The fifth group served as a positive treatment control group and received OFZ, an effective antiparasitic drug, whereas group 8 was intended to test the efficacy of NTZ. Groups 7 and 9 were non-antiparasitic treatment control groups: group 7 received PDN to rule out possible effects of steroids and group 9 did not receive any drug.
Blood sampling.
Ten milliliters of blood were obtained from each pig by venipuncture before any treatment was given. After the onset of treatment, 5 mL blood samples were collected twice a week during the initial month of follow-up, then weekly until slaughtering with the exception of pigs in the prednisone and NTZ group, which were only sampled weekly in month 1 instead of twice a week. After sampling, the blood was centrifuged at 5,000 rpm for 10 minutes, serum samples were aliquoted into 1.5-mL vials and stored at −20°C until processing.
Antigen ELISA.
The antigen-detection ELISA was performed as described previously by Dorny and others.22,23 This ELISA version is a slightly modified version from the original20,21 and has shown sensitivity and specificity values of 86.7% and 96.7%, respectively.23 All serum samples obtained from a pig during the follow-up were processed in a sole ELISA plate.
Necropsy.
Ten weeks after treatment, all pigs were anesthetized using a combination of ketamine and xylazine and then euthanized by intravenous phenobarbital injection. Standard necropsy30 was performed, and psoas and legs, the tongue, and the heart were dissected and all cysts were counted and classified by their evolution stage. Identified parasitic lesions were catalogued as viable, well defined, fluid-filled vesicles with clear fluid content; and nonviable, lesions with a preserved cystic structure but opaque or gelatinous contents, or compact, well-defined nodular lesions, or smaller, later scar stages. Brains were extracted and fixed in formalin and lesions were identified with the same criteria.
Data analysis.
The main analysis used the total parasitic load in the carcass, considering the cysts in muscles as a practical proxy for the total parasitic burden and assuming muscle to compose approximately 25% of the pig's weight.31 Since infected muscle samples and pigs had different weights, the total number of cysts per pig was estimated calculating the density of cysts per kilogram and then adjusted by the total muscle weight for each pig. In some treatment groups, there were live cysts in brain at necropsy when the effect of antiparasitc treatment in muscle, tongue, and heart was complete. To assess whether this could affect our conclusions, the analyses were repeated including brain cysts.28
The correlation between parasite burden in muscle (number of viable cysts in muscle) and circulating antigen titers at necropsy was assessed using the Pearson's test. Changes in serum antigen levels were assessed after standardization to the baseline values from each pig (ratio of the optic density [OD] value at each sampling point divided by the pretreatment OD). We modeled these relative ODs using the generalized estimating equations (GEEs) approach for panel data to account for the correlation structure of data from each pig. The relative OD was modeled in a GEE multiple regression for a Gaussian distribution, an identity link, and an exchangeable correlation structure for each animal. The direct effect of the treatment was estimated in both the rate at which the circulating antigens change (slope) as well as in the baseline level of circulating antigens (intercept). The model was also evaluated after adjusting by age and sex. A similar analysis modeled the percentage of positivity (PP), which is calculated using the OD of a known positive sample as the reference value and thus comparable across different ELISA plates. In contrast to the relative OD, the PP is not normalized.
To determine the relation between parasitic burden at necropsy and the rate of posttreatment decrease in circulating antigens, we arbitrarily classified the animals in those with no viable cysts in the carcass at necropsy (N = 26), less than 1,000 viable cysts (N = 3), with 1,000–10,000 viable cysts (N = 14), and with > 10,000 viable cysts (N = 7). The effect of the parasitic burden at necropsy and its interaction with the time since the start of treatment (in days) on the relative OD of the sample (effect of the parasitic burden at necropsy in the slope and in the intercept of the kinetic curves) were estimated in a multiple GEE regression. In the subset of pigs with no viable cysts at necropsy, we also evaluated the effect of baseline parasitic burden (grossly estimated from nonviable cysts) in the rate of antigen decrease. This multivariate model considered age, sex, and the type of treatment received as covariates.
Results
Adverse events.
From the initial 54 pigs, three pigs died during antiparasitic therapy and were excluded from the analysis. Necropsies were performed on these three pigs. One pig died at day 7 (ABZ + PZQ + PDN group) due to encephalitis caused by massive cyst destructions because this pig had approximately 140 brain cysts. Another two pigs died at day 4: one in ABZ + PDN group, from acute enteritis, and one receiving PDN alone, from pneumonia. Also the complete set of antigen results of a pig from the PZQ group was not considered because of erratic results. Finally, we had a total of 50 pigs, 26 with no viable cysts at necropsy, 3 with less than 1,000 cysts, 14 pigs with 1,000–10,000 cysts, and 7 pigs with more than 10,000 cysts. There was no evidence of Taenia hydatigena at necropsy. A total of 693 serum samples corresponding to those 50 pigs were included in this study.28
Parasiticidal efficacy in muscle cysts.
As previously published, the evaluation of the numbers of viable cysts in muscle, showed that the three combined schemes of ABZ plus PZQ, ABZ alone, and OFZ alone showed almost complete efficacy to kill cysts in pig muscles compared with control pigs. PZQ and prednisone had minor effects, 23.2% and 49.5% less cysts than did the control pigs, respectively, but without statistical significance, and NTZ did not show cysticidal effect. Nine pigs with no live muscle cysts at necropsy still had remaining live cysts in the brain.28
Correlation between parasitic burden at necropsy and values of circulating antigen levels.
At necropsy, the burden of viable parasitic cysts in pig muscles was strongly correlated with its serum antigen levels (Figure 01, Pearson's p = 0.67, P = 0.000). Correlation increased (Pearson's p = 0.78, P = 0.000) after exclusion of two outlier pigs with extremely high parasitic burdens (26,510 and 36,670 cysts). Addition of number of brain cysts did not affect the association.
Kinetics of serum antigen levels.
By the end of follow-up, serum antigen levels had decreased by five times compared with the initial values in 21 out of the 50 pigs. These were 20 of 26 pigs that were free of cysts at necropsy and 1 of 24 pigs that had persisting viable cysts (odds ratio [OR] = 76.7, 95% confidence interval [CI] = 8.1–3308.6, P < 0.001).
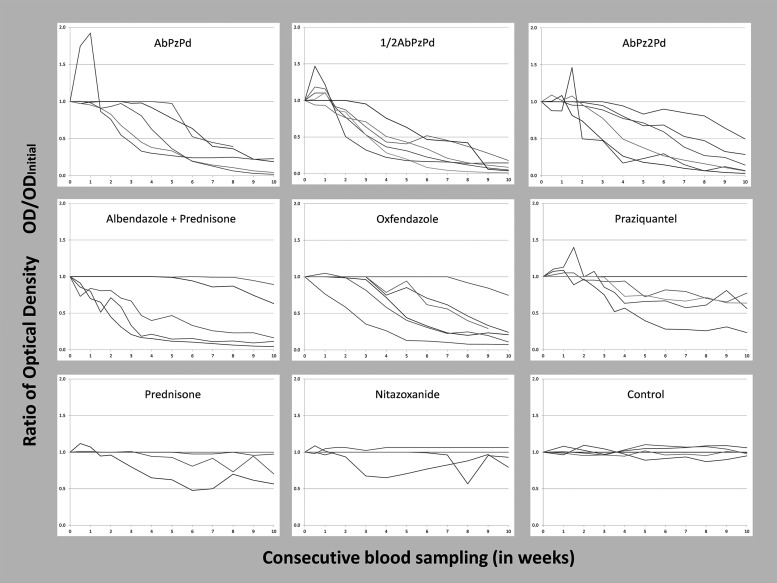
Figure 2 shows the evolution of antigen levels of each pig per treatment group. GEE regression analyses after modeling both the relative OD and the PP confirmed that the rates at which the circulating antigens decrease after treatment were significantly different (negative slope) from the control, in all treatment groups (P < 0.01) with the exception of the NTZ (P = 0.368) and prednisone (P = 0.114) groups.
Figure 2.
Evolution of antigen levels by treatment group in naturally infected pigs.28 Albendazole (Ab), praziquantel (Pz), prednisone (Pd).
Antigen dynamics were also examined in relation with the burden of cysts found at necropsy. Pigs were classified in four groups, without cysts, with 1–1,000 cysts, with 1,001–10,000 cysts, and with more than 10,000 cysts at necropsy. Unadjusted analysis showed that animals with less than 1,000 cysts (three animals with 8, 9, and 41 cysts) had no differences in the rate at which circulating antigens decreased (P = 0.17) when compared with animals with no surviving cysts. However, animals with more than 1,000 cysts showed a significantly different (larger) slope (P < 0.001), with a lower decrease rate of circulating antigens. Animals in which more than 10,000 cysts remained at necropsy did not show a significant reduction of circulating antigens during the follow-up period (P = 0.45) (Figure 3 ). Multivariate adjustment by pig's age, sex, and treatment group did not affect the kinetics of antigens among groups.
Figure 3.
(A) Evolution of antigen levels stratified by parasitic burden at necropsy. (B) Evolution of antigen level of pigs with no viable cysts in the carcass at necropsy stratified by estimated parasitic burden before treatment.
Nine out of 26 pigs with no viable cysts in the muscles still had remaining live brain cysts. GEE regression analysis including those cerebral cysts in the parasitic burden at necropsy showed that the decline rate of circulating antigens in the group with 1–1,000 live cysts (now N = 12) was significantly slower (P = 0.02) than the antigen decline in pigs free of cysts at necropsy (now N = 16, one animal was excluded from the analysis because the brain was not examined at necropsy). Antigen decay in pigs with more than 1,000 live cysts and more than 10,000 cysts at necropsy remained significantly slower (P < 0.001) than the rate of antigen decrease in pigs free of live cysts at necropsy. Age, sex, and treatment group were not significant and did not affect the results of kinetics of antigens among groups in the adjusted analysis.
An estimation of the baseline cyst burden in the subgroup of pigs free of viable cysts at necropsy was made, based on the numbers of degenerated or scarred lesions in the carcass. Inside this group, circulating antigens decreased faster in pigs with less than 1,000 cysts at baseline compared with animals with 1,000–10,000 cysts and animals with more than 10,000 cysts at baseline (Figure 3B). All pigs with less than 1,000 cysts at baseline had decreased their OD values by half by week 4 (6/6), compared with only 6/13 of those with 1,000–10,000 cysts (P = 0.45), although the mean slope was not significantly different (P = 1.08). In the group of pigs with more than 10,000 cysts at baseline, none of them reduced 50% of their OD levels by week 4 (0/7), and the slope of decrease was significantly larger (slower antigen decrease) than in pigs with less than 1,000 cysts (P = 0.021).
Discussion
Serial posttreatment follow-up of serum antigen levels in a T. solium-infected pig population demonstrated associations between antigen levels, parasite burden, and efficacy of antiparasitic treatment. As expected, there was a correlation between antigen levels and cyst burden, as previously reported in human and porcine NCC.20,25,32 Antigen levels dropped in pigs that had fewer cysts at necropsy and the decrease in antigen levels was consistent with treatment efficacy.
Antigen levels decreased rapidly in response to successful antiparasitic treatment. This finding may have implications in the management of human NCC. If serum antigen kinetic is similar in humans, more effective antiparasitic treatment should result in faster or more marked decreases in circulating antigen levels, likely measurable few weeks after treatment onset. The levels of circulating antigen and their rate of decay were also affected by the numbers of degenerated cysts or scars at necropsy, likely as a proxy for the initial cyst burden. A higher initial parasitic burden was associated with slower clearance of circulating antigens.
The antiparasitic effect was stronger in muscles than brain. Surviving brain cysts were found in nine of 26 pigs where the cysticidal effect in muscle was complete. Similar findings have been reported previously.33–35 In this cohort of heavily infected pigs, the dynamics of antigens were mostly driven by muscle cysts. The trend of decline in circulating antigen by groups, both according to estimated burden before treatment and number of viable cysts at necropsy, did not change when cerebral brain cysts were included in the analysis. The circulating antigen levels at necropsy in the nine pigs with only remaining viable cyst in the brain were not different from those of the 16 pigs in which the antiparasitic treatment cleared both muscle and brain cysts.
Interestingly, most of the pigs allocated the treatment groups receiving PZQ had an increment in antigen OD levels shortly after treatment as shown in Figure 2. This was not the case in pigs treated with benzimidazoles. This increase may result from the mode of action of PZQ that causes tegumentary damage among other effects, perhaps resulting in greater antigen exposition than that caused by benzimidazoles, which have a less aggressive mode of action (selective degeneration of parasite cytoplasmic microtubules, leading to decreased adenosine triphosphate formation and energy depletion).36,37 However, this early increment in antigen OD levels was not predictive of antiparasitic efficacy.
Naturally infected pigs differ from humans with NCC in a series of characteristics and thus its use as a model should be interpreted in this context. Given the short lifespan of pigs (usually less than a year), porcine infections are likely much more recent than brain cysts in humans. The predominance of viable muscle cysts also argues for a more recent infection. Since the large case series of NCC in British soldiers returning from India, it is well known that brain cysts may survive for several years after muscle cysts have resolved.38 Tongue-positive pigs more likely represent a heavily infected subgroup since in field settings, most infected pigs have less than 10 cysts in the entire carcass.39 As much as it is known, most humans with NCC have no evidence of viable muscle cysts by the time of diagnosis (although a significant proportion will have muscle calcifications).40 From the above reasons, it follows that antigen levels in tongue positive pigs are likely higher than those in humans with parenchymal NCC and consequently changes detected in this model may not be so easy to determine in clinical cases of parenchymal NCC. Extraparenchymal NCC, on the other hand, presents very high antigen levels, which have already been shown to reflect the evolution of the disease.14,15,27,41
Antigen detection by a monoclonal antibody-based ELISA is a reliable tool to evaluate the response to antiparasitic treatment in the porcine model and may prove useful to monitor the efficacy of antiparasitic treatment in human NCC, guiding medical decisions along patient follow-up.
ACKNOWLEDGMENTS
We are grateful to L. Sanchez and R. Manzanedo for veterinarian support. We are indebted to Humberto Zamora who performed most of the immunodiagnostic work reported here.
Footnotes
Financial support: Partial support from the Fogarty International Center/NIH (Training Grant no. TW001140), the Bill and Melinda Gates Foundation (Grant no. 23981), and NIH-1R01AI116456-01 is acknowledged. Hector H. Garcia is supported by a Wellcome Trust Senior Research Fellowship in Tropical Medicine and Public Health.
Authors' addresses: Armando E. Gonzalez, School of Veterinary Medicine, Lima, Peru, E-mail: agonza41@jhu.edu. Javier A. Bustos and Silvia Rodriguez, Department of Microbiology, School of Sciences and Center for Global Health-Tumbes, Universidad Peruana Cayetano Heredia, Lima, Peru, and Instituto Peruano de Parasitología Clínica y Experimental, Lima, Peru, E-mails: javier.bustos@jhu.edu and silvia@peruresearch.org. Hector H. Garcia, Department of Microbiology, School of Sciences and Center for Global Health-Tumbes, Universidad Peruana Cayetano Heredia, Lima, Perú, and Cysticercosis Unit, Instituto Nacional de Ciencias Neurologicas, Lima, Perú, E-mail: hgarcia@jhsph.edu. Yesenia Castillo, Department of Microbiology, School of Sciences and Center for Global Health-Tumbes, Universidad Peruana Cayetano Heredia, Lima, Peru, E-mail: yesicabe9@yahoo.es. Sarah Gabriël and Pierre Dorny, Department of Biomedical Sciences, Institute for Tropical Medicine, Antwerp, Belgium, E-mails: sgabriel@itg.be and pdorny@itg.be. Robert H. Gilman, Department of International Health, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD, E-mail: gilmanbob@gmail.com. Mirko Zimic, Unidad de Bioinformática, Laboratorios de Investigación y Desarrollo, Universidad Peruana Cayetano Heredia, Lima, Perú, E-mail: mirko.zimic@upch.pe. Nicolas Praet, Department of Biomedical Sciences, Institute of Tropical Medicine, Antwerp, Belgium, E-mail: npraet@itg.be.
References
- 1.Wallin MT, Kurtzke JF. Neurocysticercosis in the United States: review of an important emerging infection. Neurology. 2004;63:1559–1564. doi: 10.1212/01.wnl.0000142979.98182.ff. [DOI] [PubMed] [Google Scholar]
- 2.O'Keefe KA, Eberhard ML, Shafir SC, Wilkins P, Ash LR, Sorvillo FJ. Cysticercosis-related hospitalizations in the United States, 1998–2011. Am J Trop Med Hyg. 2015;92:354–359. doi: 10.4269/ajtmh.14-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Brutto OH. Neurocysticercosis: a review. Sci World J. 2012;2012:159821. doi: 10.1100/2012/159821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsang VC, Brand JA, Boyer AE. An enzyme-linked immunoelectrotransfer blot assay and glycoprotein antigens for diagnosing human cysticercosis (Taenia solium) J Infect Dis. 1989;159:50–59. doi: 10.1093/infdis/159.1.50. [DOI] [PubMed] [Google Scholar]
- 5.Garcia HH, Nash TE, Del Brutto OH. Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol. 2014;13:1202–1215. doi: 10.1016/S1474-4422(14)70094-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson M, Bryan RT, Fried JA, Ware DA, Schantz PM, Pilcher JB, Tsang VC. Clinical evaluation of the cysticercosis enzyme-linked immunoelectrotransfer blot in patients with neurocysticercosis. J Infect Dis. 1991;164:1007–1009. doi: 10.1093/infdis/164.5.1007. [DOI] [PubMed] [Google Scholar]
- 7.Garcia HH, Gonzalez AE, Gilman RH, Palacios LG, Jimenez I, Rodriguez S, Verastegui M, Wilkins P, Tsang VC, Cysticercosis Working Group in Peru Short report: transient antibody response in Taenia solium infection in field conditions-a major contributor to high seroprevalence. Am J Trop Med Hyg. 2001;65:31–32. doi: 10.4269/ajtmh.2001.65.31. [DOI] [PubMed] [Google Scholar]
- 8.Garcia HH, Del Brutto OH, Nash TE, White AC, Jr, Tsang VC, Gilman RH. New concepts in the diagnosis and management of neurocysticercosis (Taenia solium) Am J Trop Med Hyg. 2005;72:3–9. [PubMed] [Google Scholar]
- 9.Praet N, Rodriguez-Hidalgo R, Speybroeck N, Ahounou S, Benitez-Ortiz W, Berkvens D, Hul AV, Barrionuevo-Samaniego M, Saegerman C, Dorny P. Infection with versus exposure to Taenia solium: what do serological test results tell us? Am J Trop Med Hyg. 2010;83:413–415. doi: 10.4269/ajtmh.2010.10-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garcia HH, Rodriguez S, Gilman RH, Gonzalez AE, Tsang VC. Neurocysticercosis: is serology useful in the absence of brain imaging? Trop Med Int Health. 2012;17:1014–1018. doi: 10.1111/j.1365-3156.2012.03037.x. [DOI] [PubMed] [Google Scholar]
- 11.Gavidia CM, Verastegui MR, Garcia HH, Lopez-Urbina T, Tsang VC, Pan W, Gilman RH, Gonzalez AE, Cysticercosis Working Group in Peru Relationship between serum antibodies and Taenia solium larvae burden in pigs raised in field conditions. PLoS Negl Trop Dis. 2013;7:e2192. doi: 10.1371/journal.pntd.0002192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sciutto E, Hernandez M, Garcia G, de Aluja AS, Villalobos AN, Rodarte LF, Parkhouse M, Harrison L. Diagnosis of porcine cysticercosis: a comparative study of serological tests for detection of circulating antibody and viable parasites. Vet Parasitol. 1998;78:185–194. doi: 10.1016/s0304-4017(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 13.Santamaria E, Plancarte A, de Aluja AS. The experimental infection of pigs with different numbers of Taenia solium eggs: immune response and efficiency of establishment. J Parasitol. 2002;88:69–73. doi: 10.1645/0022-3395(2002)088[0069:TEIOPW]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 14.Zamora HSR, Garcia HH, Rodriguez S, Allan JC, Vasquez Y, Gilman RH, Gonzalez AE, Noh J, Patthabi S, Tsang VCW, The Cysticercosis Working Group in Perú Sensitivity and specificity of ELISA Coproantigen detection for the diagnosis of intestinal Taenia solium taeniais. Am J Trop Med Hyg. 2004;71((Suppl 4)):42. [Google Scholar]
- 15.Bobes RJ, Hernandez M, Marquez C, Fragoso G, García E, Parkhouse RM, Harrison LJ, Sciutto E, Fleury A. Subarachnoidal and intraventricular human neurocysticercosis: application of an antigen detection assay for the diagnosis and follow-up. Trop Med Int Health. 2006;11:943–950. doi: 10.1111/j.1365-3156.2006.01642.x. [DOI] [PubMed] [Google Scholar]
- 16.Fleury A, Hernandez M, Fragoso G, Parkhouse RM, Harrison LJ, Sciutto E. Detection of secreted cysticercal antigen: a useful tool in the diagnosis of inflammatory neurocysticercosis. Trans R Soc Trop Med Hyg. 2003;97:542–546. doi: 10.1016/s0035-9203(03)80019-6. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez S, Dorny P, Tsang VC, Pretell EJ, Brandt J, Lescano AG, Gonzalez AE, Gilman RH, Garcia HH, Cysticercosis Working Group in Peru Detection of Taenia solium antigens and anti-T. solium antibodies in paired serum and cerebrospinal fluid samples from patients with intraparenchymal or extraparenchymal neurocysticercosis. J Infect Dis. 2009;199:1345–1352. doi: 10.1086/597757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Correa D, Sandoval MA, Harrison LJ, Parkhouse RM, Plancarte A, Meza-Lucas A, Flisser A. Human neurocysticercosis: comparison of enzyme immunoassay capture techniques based on monoclonal and polyclonal antibodies for the detection of parasite products in cerebrospinal fluid. Trans R Soc Trop Med Hyg. 1989;83:814–816. doi: 10.1016/0035-9203(89)90340-4. [DOI] [PubMed] [Google Scholar]
- 19.Harrison LJ, Joshua GW, Wright SH, Parkhouse RM. Specific detection of circulating surface/secreted glycoproteins of viable cysticerci in Taenia saginata cysticercosis. Parasite Immunol. 1989;11:351–370. doi: 10.1111/j.1365-3024.1989.tb00673.x. [DOI] [PubMed] [Google Scholar]
- 20.Brandt JR, Geerts S, De Deken R, Kumar V, Ceulemans F, Brijs L, Falla N. A monoclonal antibody-based ELISA for the detection of circulating excretory-secretory antigens in Taenia saginata cysticercosis. Int J Parasitol. 1992;22:471–477. doi: 10.1016/0020-7519(92)90148-e. [DOI] [PubMed] [Google Scholar]
- 21.Van Kerckhoven I, Vansteenkiste W, Claes M, Geerts S, Brandt J. Improved detection of circulating antigen in cattle infected with Taenia saginata metacestodes. Vet Parasitol. 1998;76:269–274. doi: 10.1016/s0304-4017(97)00226-4. [DOI] [PubMed] [Google Scholar]
- 22.Dorny P, Vercammen F, Brandt J, Vansteenkiste W, Berkvens D, Geerts S. Sero-epidemiological study of Taenia saginata cysticercosis in Belgian cattle. Vet Parasitol. 2000;88:43–49. doi: 10.1016/s0304-4017(99)00196-x. [DOI] [PubMed] [Google Scholar]
- 23.Dorny P, Phiri IK, Vercruysse J, Gabriel S, Willingham AL, 3rd, Brandt J, Victor B, Speybroeck N, Berkvens D. A Bayesian approach for estimating values for prevalence and diagnostic test characteristics of porcine cysticercosis. Int J Parasitol. 2004;34:569–576. doi: 10.1016/j.ijpara.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Nguekam Zoli AP, Ongolo-Zogo P, Dorny P, Brandt J, Geerts S. Follow-up of neurocysticercosis patients after treatment using an antigen detection ELISA. Parasite. 2003;10:65–68. doi: 10.1051/parasite/2003101p65. [DOI] [PubMed] [Google Scholar]
- 25.Nguekam A, Zoli AP, Vondou L, Pouedet SM, Assana E, Dorny P, Brandt J, Losson B, Geerts S. Kinetics of circulating antigens in pigs experimentally infected with Taenia solium eggs. Vet Parasitol. 2003;111:323–332. doi: 10.1016/s0304-4017(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 26.Fleury A, Hernandez M, Avila M, Cárdenas G, Bobes RJ, Huerta M, Fragoso G, Uribe-Campero L, Harrison LJ, Parkhouse RM, Sciutto E. Detection of HP10 antigen in serum for diagnosis and follow-up of subarachnoidal and intraventricular human neurocysticercosis. J Neurol Neurosurg Psychiatry. 2007;78:970–974. doi: 10.1136/jnnp.2006.107243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo Y, Rodriguez S, Garcia HH, Brandt J, Van Hul A, Silva M, Rodriguez-Hidalgo R, Portocarrero M, Melendez DP, Gonzalez AE, Gilman RH, Dorny P, Cysticercosis Working Group in Perú Urine antigen detection for the diagnosis of human neurocysticercosis. Am J Trop Med Hyg. 2009;80:379–383. [PubMed] [Google Scholar]
- 28.Gonzalez AE, Bustos JA, Jimenez JA, Rodriguez ML, Ramirez MG, Gilman RH, Garcia HH, Cysticercosis Working Group in Peru Efficacy of diverse antiparasitic treatments for cysticercosis in the pig model. Am J Trop Med Hyg. 2012;87:292–296. doi: 10.4269/ajtmh.2012.11-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez AE, Cama V, Gilman RH, Tsang VC, Pilcher JB, Chavera A, Castro M, Montenegro T, Verastegui M, Miranda E, Bazalar H. Prevalence and comparison of serologic assays, necropsy, and tongue examination for the diagnosis of porcine cysticercosis in Peru. Am J Trop Med Hyg. 1990;43:194–199. doi: 10.4269/ajtmh.1990.43.194. [DOI] [PubMed] [Google Scholar]
- 30.Straw BJ, Meuten DJ. Physical examination. In: Straw BJ, Mengeling WL, D'Allaire S, Taylor DJ, editors. Diseases of Swine. 7th edition. Ames, IA: Iowa State University Press; 1992. pp. 793–807. [Google Scholar]
- 31.Flores Menendez JA, Agraz García AA. Ganado Porcino: Cria, Explotación, Enfermedades e Industrialización. 1a. ed. México: Ciencia y Técnica; 1986. [Google Scholar]
- 32.Deckers N, Kanobana K, Silva M, Gonzalez AE, Garcia HH, Gilman RH, Dorny P. Serological responses in porcine cysticercosis: a link with the parasitological outcome of infection. Int J Parasitol. 2008;38:1191–1198. doi: 10.1016/j.ijpara.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Mkupasi EM, Ngowi HA, Sikasunge CS, Leifsson PS, Johansen MV. Efficacy of ivermectin and oxfendazole against Taenia solium cysticercosis and other parasitoses in naturally infected pigs. Acta Trop. 2013;128:48–53. doi: 10.1016/j.actatropica.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 34.Peniche-Cardena A, Dominguez-Alpizar JL, Sima-Alvarez R, Argaez-Rodriguez F, Fraser A, Craig PS, Rodriguez-Canul R. Chemotherapy of porcine cysticercosis with albendazole sulphoxide. Vet Parasitol. 2002;108:63–73. doi: 10.1016/s0304-4017(02)00177-2. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez AE, Garcia HH, Gilman RH, Lopez MT, Gavidia C, McDonald J, Pilcher JB, Tsang VC. Treatment of porcine cysticercosis with albendazole. Am J Trop Med Hyg. 1995;53:571–574. doi: 10.4269/ajtmh.1995.53.571. [DOI] [PubMed] [Google Scholar]
- 36.Jung H, Gonzalez D. Pharmacology of antycisticercal therapy. In: Singh G, Prabhakar S, editors. Taenia solium Cysticercosis. From Basic to Clinical Science. Oxon, United Kingdom: CABI Publishing; 2002. pp. 363–374. [Google Scholar]
- 37.Dayan AD. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003;86:141–159. doi: 10.1016/s0001-706x(03)00031-7. [DOI] [PubMed] [Google Scholar]
- 38.Dixon HB, Lipscomb FM. Cysticercosis: An Analysis and Follow-up of 450 Cases. Vol. 299. London, United Kingdom: Medical Research Council; 1961. [Google Scholar]
- 39.Sciutto E, Martinez JJ, Villalobos NM, Hernández M, José MV, Beltrán C, Rodarte F, Flores I, Bobadilla JR, Fragoso G, Parkhouse ME, Harrison LJ, de Aluja AS. Limitations of current diagnostic procedures for the diagnosis of Taenia solium cysticercosis in rural pigs. Vet Parasitol. 1998;79:299–313. doi: 10.1016/s0304-4017(98)00180-0. [DOI] [PubMed] [Google Scholar]
- 40.Bustos JA, Garcia HH, Dorregaray R, Naranjo M, Pretell EJ, Gonzalez AE, Gilman RH, Cysticercosis Working Group in Peru Detection of muscle calcifications by thigh CT scan in neurocysticercosis patients. Trans R Soc Trop Med Hyg. 2005;99:775–779. doi: 10.1016/j.trstmh.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Fleury A, Garcia E, Hernandez M, Carrillo R, Govezensky T, Fragoso G, Sciutto E, Harrison LJ, Parkhouse RM. Neurocysticercosis: HP10 antigen detection is useful for the follow-up of the severe patients. PLoS Negl Trop Dis. 2013;7:e2096. doi: 10.1371/journal.pntd.0002096. [DOI] [PMC free article] [PubMed] [Google Scholar]