Abstract
Chikungunya virus (CHIKV) often co-circulates with dengue virus (DENV). A cross-sectional surveillance study was conducted at a tertiary hospital in Manila, Philippines, to describe the prevalence and characteristics of DENV and CHIKV infections among patients seeking care for dengue-like illness. Acute blood samples from patients ≥ 6 months of age clinically diagnosed with dengue from November 2012 to December 2013 underwent reverse transcription polymerase chain reaction (RT-PCR) to detect DENV and CHIKV RNA. A total of 118 patients with clinically diagnosed dengue (age range = 1–89 years, mean = 22 years; male-to-female ratio = 1.51) were tested by DENV RT-PCR; 40 (34%) were DENV PCR-positive (age range = 1–45 years, mean = 17 years). All DENV serotypes were detected: 11 (28%) DENV-1, 6 (15%) DENV-2, 6 (15%) DENV-3, and 17 (42%) DENV-4. Of 112 patients clinically diagnosed with dengue and tested by CHIKV RT-PCR, 11 (10%) were CHIKV PCR-positive (age range = 2–47 years, mean = 20.3 years). No coinfections were detected. Presenting signs/symptoms did not differ between DENV- and CHIKV-positive cases. Sequencing of envelope 1 gene from two CHIKV PCR-positive samples showed Asian genotype. This study highlights the potential for misdiagnosis of medically attended CHIKV infections as DENV infection and the difficulty in clinically differentiating dengue and chikungunya based on presenting signs/symptoms alone. This underscores the necessity for diagnostic laboratory tests to distinguish CHIKV infections in the background of actively co-circulating DENV.
Introduction
Chikungunya virus (CHIKV) is a vector-borne alphavirus from the Togaviridae family transmitted to humans by some Aedes mosquitoes.1 CHIKV was first isolated in 1952 in Tanzania2 and was first reported in Asia in 1958.3 Since then, it has been associated with many outbreaks in Asia and Africa.4 CHIKV is usually self-limiting and resolves in 1–2 weeks but in some cases, joint pain can continue for several weeks or months.5–7 In 2005–2006, an explosive and large chikungunya epidemic caused by East/Central/South African genotype affected several Indian Ocean islands.8 It was during this unprecedented outbreak that atypical CHIKV presentations and unusual outcomes were seen,9 severe forms of the disease were recorded,10 and vertical transmission was documented.11 More recently, transcontinental movement of Asian genotype CHIKV12 into the Americas and the Pacific islands occurred on a background of other actively circulating mosquito-borne viruses such as dengue virus (DENV)13 and Zika virus.14 Co-circulation with DENV is particularly problematic since many presenting signs and symptoms of both diseases may be similar making clinical diagnosis challenging.13,15–18
In the Philippines, CHIKV has been reported since the 1950s, although previously documented CHIKV outbreaks have been limited in scale, sporadic, and geographically isolated.19–24 However, in a recent study by Capeding and others, chikungunya was the most common cause of febrile illnesses in a multicountry prospective cohort study including two sites from the Philippines in 2010–2011 with an overall incidence density of 10.8 per 100 person-years.25 In another study by Yoon and others, a high rate of subclinical and symptomatic CHIKV infections was reported in a prospective community cohort in Cebu, Philippines, from 2012 to 2013 with a total incidence density of 12.22 CHIKV infections per 100 person-years.26 These studies provide further evidence that chikungunya has re-emerged as an important disease in the Philippines as well as in Asia,17,27 and highlights the importance of distinguishing symptomatic CHIKV infection especially in the background of hyperendemic DENV transmission. Here, we describe laboratory-confirmed CHIKV infections clinically diagnosed as dengue at a tertiary care hospital in the Philippines.
Materials and Methods
Description of patients and procedures.
From November 2012 to December 2013, acute blood samples were collected from patients presenting with dengue-like illness to the outpatient and emergency departments of V. Luna General Hospital (VLGH), Armed Forces of the Philippines Medical Center (AFPMC), a tertiary care center in Quezon City, Metro Manila, Philippines. Patients ≥ 6 months old with a history of fever or measured temperature ≥ 38°C within 7 days of presentation and clinically diagnosed with dengue by the attending physician based on the 2009 World Health Organization case classification were included.28 VLGH medical personnel collected blood samples as part of a public health effort that did not require Institutional Review Board approval. A service sample case report form was used to gather coded demographic and clinical data from patients on their presentation at the outpatient department or the emergency room. Blood samples were processed into serum aliquots and tested by DENV reverse transcriptase polymerase chain reaction (RT-PCR) at the AFPMC–Armed Forces Research Institute of Medical Sciences (AFRIMS) Collaborative Molecular Laboratory located within VLGH. Serum aliquots were stored at −70°C and shipped on dry ice to AFRIMS in Bangkok, Thailand, for CHIKV RT-PCR testing.
Chikungunya virus and dengue virus RT-PCR.
Viral RNA was extracted from 140 μL of patient serum using viral RNA extraction kit (Qiagen, Valencia, CA). For CHIKV RT-PCR, a modified two-step nested PCR to amplify the capsid region of CHIKV was used as previously described in a study.29,30 DENV serotype determination using hemi-nested RT-PCR for detection of DENV RNA was performed on acute samples using methods described previously in a study.31 Laboratory confirmation of CHIKV and DENV infection was based solely on DENV and CHIKV RT-PCR testing of the acute blood sample.
CHIKV sequencing.
Two CHIKV-positive acute blood samples were selected for envelope 1 (E1) gene sequencing based on a relatively strong CHIKV RT-PCR signal. E1 gene fragments were amplified and sequenced in accordance with methods previously described by Yoon and others26. AccessQuick RT-PCR System (Promega, Madison, WI) and PCR purification kit (Qiagen) were used for gene fragment amplification and purification, respectively. Sanger sequencing of E1 gene was performed by AITbiotech (Science Park, Singapore). Sequencher software (Gene Codes Corporation, Ann Arbor, MI) was used for generating the consensus sequences of gene fragments of 1,320 base pairs (bp) in length. A phylogenetic tree of 48 E1 gene sequences including the two new sequences from this study and 46 CHIKV E1 sequences sourced from GenBank was constructed using MEGA 6 software (Tempe, AZ) and neighbor-joining (maximum composite likelihood) methods with 1,000 replicates for bootstrap testing.
Statistical analysis.
Categorical data were expressed using frequency and percentage. Continuous data were expressed as mean and standard deviation. To compare signs and symptoms between chikungunya and dengue cases, Z test of difference in proportions was used. Logistic regression was used to determine which signs and symptoms were significant predictors of laboratory-confirmed diagnosis. Validity indices in terms of specificity, sensitivity, and negative and positive predictive values of signs and symptoms in diagnosing chikungunya were computed using SAS software version 9.1 (SAS system for Microsoft Windows, copyright 2014, SAS Institute Inc., Cary, NC) and SPSS (SPSS Statistics for Windows, Version 21.0; IBM Corp., Armonk, NY). P value < 0.05 was considered statistically significant.
Results
We analyzed 118 patients clinically diagnosed with dengue (age range = 1–89 years, mean = 22 years) with a male-to-female ratio of 1.51. Of those, 107 (91%) had data on date of illness onset and medical consult. Number of days from illness onset to the date the patients were seen ranged from 0 to 7 days with a median of 3 days. Of 118 patients, 40 (34%) tested by DENV PCR were found to be positive (age range = 1–45 years, mean = 17 years). Male-to-female ratio was 1.7. All four DENV serotypes were detected: 11 (28%) DENV-1, six (15%) DENV-2, six (15%) DENV-3, and 17 (42%) DENV-4. The 5- to 9-year age group accounted for nine (22%) cases while the 20- to 24-year and ≥ 25-year age groups each had eight (20%) cases. Six of the 118 patients with clinically diagnosed dengue had insufficient serum volumes for CHIKV RT-PCR with three of the six being DENV PCR positive. Of 112 patients clinically diagnosed as dengue who underwent CHIKV RT-PCR testing, 11 (10%) were CHIKV PCR positive (age range = 2–47 years, mean = 20.3 years). Male-to-female ratio was 1.8. All 11 CHIKV PCR-positive cases were DENV PCR negative. The majority of chikungunya cases belonged to the 10- to 14-year or ≥ 25-year age groups with each group having three (27%) cases. Five (45%) of the 11 chikungunya patients were hospitalized with 4/5 (80%) ≥ 20 years of age. Among the 112 patients for which both DENV and CHIKV PCR testing had been performed, comparison of signs and symptoms was done for patients with confirmed DENV (N = 37) and CHIKV (N = 11) infections. The most common signs and symptoms on presentation for both dengue and chikungunya were measured temperature ≥ 38°C, headache, loss of appetite, chills, muscle/body pain, and malaise/fatigue (Table 1).
Table 1.
Comparison of dengue and chikungunya presenting signs and symptoms among patients seen in a tertiary hospital, Manila, Philippines, November 2012 to December 2013
| Presenting signs/symptoms | DENV PCR+ (N = 37) | CHIKV PCR+ (N = 11) | P value | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Measured temperature ≥ 38°C | 37 | 100 | 10 | 91 | 0.064 |
| Headache | 28 | 76 | 8 | 73 | 0.843 |
| Loss of appetite | 26 | 70 | 6 | 55 | 0.331 |
| Chills | 20 | 54 | 6 | 55 | 0.331 |
| Muscle/body pain | 19 | 51 | 5 | 45 | 0.731 |
| Malaise/fatigue | 19 | 51 | 5 | 45 | 0.731 |
| Cough | 18 | 49 | 3 | 27 | 0.210 |
| Vomiting | 17 | 46 | 4 | 36 | 0.574 |
| Nausea | 14 | 38 | 3 | 27 | 0.520 |
| Eye pain | 13 | 35 | 2 | 18 | 0.287 |
| Joint paint | 13 | 35 | 3 | 27 | 0.627 |
| Abdominal pain | 12 | 32 | 3 | 27 | 0.746 |
| Sore throat | 8 | 22 | 1 | 9 | 0.350 |
| Skin rash | 8 | 22 | 4 | 36 | 0.322 |
| Bleeding | 7 | 19 | 3 | 27 | 0.549 |
| Runny nose | 6 | 16 | 3 | 27 | 0.409 |
| Diarrhea | 5 | 14 | 1 | 9 | 0.697 |
| Dark urine | 5 | 14 | 0 | 0 | 0.198 |
| Shortness of breath | 4 | 11 | 0 | 0 | 0.255 |
| Seizure | 0 | 0 | 0 | 0 | 1.000 |
| Stiff neck | 0 | 0 | 0 | 0 | 1.000 |
| Abnormal movement | 0 | 0 | 0 | 0 | 1.000 |
| Jaundice | 0 | 0 | 0 | 0 | 1.000 |
| Mental status change | 0 | 0 | 0 | 0 | 1.000 |
CHIKV = chikungunya virus; DENV = dengue virus; PCR = polymerase chain reaction.
Logistic regression performed on signs and symptoms among chikungunya cases showed no variable to be a significant predictor of CHIKV infection (Table 2), whereas logistic regression among dengue cases showed skin rash (P = 0.031) to be the only significant predictor for DENV infection (Table 3). Subgroup analysis comparing chikungunya cases ≥ 18 years old and < 18 years old showed patients who were < 18 years old had significantly higher frequency of skin rash (P = 0.022) while those ≥ 18 years old had higher occurrences of bleeding (P = 0.026) and nausea (P = 0.026) (Table 4).
Table 2.
Logistic regression of signs and symptoms of CHIKV PCR+ cases (N = 11) among patients seen in a tertiary hospital, Manila, Philippines, November 2012 to December 2013
| Presenting symptoms/signs/variables | B | SE | Wald | df | P value | Exp(B) |
|---|---|---|---|---|---|---|
| Fever | −0.584 | 1.469 | 0.158 | 1 | 0.691 | 0.558 |
| Chills | 0.292 | 0.89 | 0.108 | 1 | 0.743 | 1.340 |
| Headache | −0.08 | 0.919 | 0.008 | 1 | 0.931 | 0.923 |
| Runny nose | 1.097 | 0.938 | 1.368 | 1 | 0.242 | 2.994 |
| Sore throat | −2.253 | 1.705 | 1.746 | 1 | 0.186 | 0.105 |
| Cough | −1.733 | 0.899 | 3.716 | 1 | 0.054 | 0.177 |
| Nausea | 0.814 | 1.194 | 0.465 | 1 | 0.495 | 2.257 |
| Eye pain | −0.25 | 1.116 | 0.05 | 1 | 0.823 | 0.779 |
| Vomiting | 0.175 | 0.894 | 0.038 | 1 | 0.845 | 1.191 |
| Loss of appetite | −1.11 | 1.091 | 1.035 | 1 | 0.309 | 0.330 |
| Abdominal pain | −0.938 | 0.953 | 0.969 | 1 | 0.325 | 0.391 |
| Muscle/body pain | 0.459 | 1.273 | 0.13 | 1 | 0.719 | 1.582 |
| Joint pain | −0.539 | 1.174 | 0.211 | 1 | 0.646 | 0.583 |
| Malaise/fatigue | 0.239 | 1.23 | 0.038 | 1 | 0.846 | 1.270 |
| Bleeding | 0.923 | 0.996 | 0.859 | 1 | 0.354 | 2.516 |
| Skin rash | 0.805 | 0.865 | 0.866 | 1 | 0.352 | 2.236 |
| Diarrhea | −2.34 | 1.832 | 1.632 | 1 | 0.201 | 0.096 |
| Stiff neck | −17.409 | 40,192.97 | 0 | 1 | 1.000 | 0.000 |
| Abnormal movement | −18.06 | 18,513.4 | 0 | 1 | 0.999 | 0.000 |
| Dark urine | −19.786 | 10,270.38 | 0 | 1 | 0.998 | 0.000 |
| Jaundice | 3.213 | 41,951 | 0 | 1 | 1.000 | 24.865 |
| Shortness of breath | −19.397 | 12,017.13 | 0 | 1 | 0.999 | 0.000 |
| Constant | −0.518 | 1.408 | 0.135 | 1 | 0.713 | 0.596 |
CHIKV = chikungunya virus; df = degrees of freedom; PCR = polymerase chain reaction; SE = standard error.
Table 3.
Logistic regression of signs and symptoms of DENV PCR+ cases (N = 37) among patients seen in a tertiary hospital, Manila, Philippines, November 2012 to December 2013
| Presenting symptoms/signs/variables | B | SE | Wald | df | P value | Exp(B) |
|---|---|---|---|---|---|---|
| Fever | 21.572 | 11,132.61 | 0 | 1 | 0.998 | 2.34E+09 |
| Chills | 0.745 | 0.573 | 1.688 | 1 | 0.194 | 2.11 |
| Headache | −0.25 | 0.605 | 0.171 | 1 | 0.679 | 0.78 |
| Runny nose | −0.681 | 0.607 | 1.255 | 1 | 0.263 | 0.51 |
| Sore throat | 0.448 | 0.627 | 0.51 | 1 | 0.475 | 1.57 |
| Cough | 0.257 | 0.48 | 0.287 | 1 | 0.592 | 1.29 |
| Nausea | 0.039 | 0.685 | 0.003 | 1 | 0.955 | 1.04 |
| Eye pain | 0.469 | 0.661 | 0.504 | 1 | 0.478 | 1.60 |
| Vomiting | 0.383 | 0.517 | 0.55 | 1 | 0.458 | 1.47 |
| Loss of appetite | 0.242 | 0.614 | 0.155 | 1 | 0.694 | 1.27 |
| Abdominal pain | −0.551 | 0.534 | 1.066 | 1 | 0.302 | 0.58 |
| Muscle/body pain | −0.402 | 0.725 | 0.307 | 1 | 0.579 | 0.67 |
| Joint pain | 0.177 | 0.768 | 0.053 | 1 | 0.818 | 1.19 |
| Malaise/fatigue | 0.288 | 0.666 | 0.187 | 1 | 0.666 | 1.33 |
| Bleeding | 0.134 | 0.659 | 0.041 | 1 | 0.839 | 1.14 |
| Skin rash | −1.219 | 0.564 | 4.673 | 1 | 0.031* | 0.30 |
| Diarrhea | −0.818 | 0.794 | 1.061 | 1 | 0.303 | 0.44 |
| Stiff neck | −19.954 | 40,192.97 | 0 | 1 | 1.000 | 0.00 |
| Abnormal movement | −20.614 | 17,599.41 | 0 | 1 | 0.999 | 0.00 |
| Dark urine | 1.479 | 1.002 | 2.178 | 1 | 0.140 | 4.39 |
| Jaundice | −21.276 | 40,192.97 | 0 | 1 | 1.000 | 0.00 |
| Shortness of breath | 0.313 | 1.287 | 0.059 | 1 | 0.808 | 1.37 |
| Constant | −22.242 | 11,132.61 | 0 | 1 | 0.998 | 0.00 |
DENV = dengue virus; df = degrees of freedom; PCR = polymerase chain reaction; SE = standard error.
P value is significant since the P value is less than 0.05.
Table 4.
Comparison of chikungunya signs and symptoms between ≥ 18 and < 18 years old patients seen in a tertiary hospital, Manila, Philippines, November 2012 to December 2013
| Presenting symptoms/signs | Age group (N = 11) | P value | |||
|---|---|---|---|---|---|
| < 18 years (N = 6) | ≥ 18 years (N = 5) | ||||
| n | % | n | % | ||
| Objective fever on consult | 6 | 100 | 4 | 80 | 0.251 |
| Skin rash | 4 | 67 | 0 | 0 | 0.022* |
| Loss of appetite | 4 | 67 | 2 | 40 | 0.376 |
| Headache | 4 | 67 | 4 | 80 | 0.621 |
| Muscle body pain | 2 | 33 | 3 | 60 | 0.376 |
| Malaise fatigue | 2 | 33 | 3 | 60 | 0.376 |
| Chills | 2 | 33 | 4 | 80 | 0.122 |
| Abdominal pain | 2 | 33 | 1 | 20 | 0.621 |
| Vomiting | 1 | 17 | 3 | 60 | 0.137 |
| Runny nose | 1 | 17 | 2 | 40 | 0.387 |
| Joint pain | 1 | 17 | 2 | 40 | 0.387 |
| Cough | 1 | 17 | 2 | 40 | 0.338 |
| Stiff neck | 0 | 0 | 0 | 0 | 1.000 |
| Sore throat | 0 | 0 | 1 | 20 | 0.251 |
| Shortness of breath | 0 | 0 | 0 | 0 | 1.000 |
| Seizure | 0 | 0 | 0 | 0 | 1.000 |
| Nausea | 0 | 0 | 3 | 60 | 0.026* |
| Mental status change | 0 | 0 | 0 | 0 | 1.000 |
| Jaundice | 0 | 0 | 0 | 0 | 1.000 |
| Eye pain | 0 | 0 | 2 | 40 | 0.087 |
| Diarrhea | 0 | 0 | 1 | 20 | 0.251 |
| Dark urine | 0 | 0 | 0 | 0 | 1.000 |
| Bleeding | 0 | 0 | 3 | 60 | 0.026* |
| Abnormal movement | 0 | 0 | 0 | 0 | 1.000 |
P value is significant since the P value is less than 0.05.
Sensitivity, specificity, and positive and negative predictive values for various combinations of clinical symptoms,17 namely, fever, rash, muscle/body pain, and arthralgia for diagnosing chikungunya and dengue yielded no combination of symptoms with sufficiently high sensitivity and specificity in diagnosing either chikungunya or dengue (Tables 5 and 6).
Table 5.
Sensitivity and specificity analysis of selected combinations of clinical signs/symptoms in diagnosing chikungunya among patients seen in a tertiary hospital, Manila, Philippines, November 2012 to December 2013
| Diagnostic combination | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) |
|---|---|---|---|---|
| Fever + joint pain | 30 (2–58) | 65 (55–75) | 9 (0–18) | 10 (3–18) |
| Fever + skin rash | 40 (10–70) | 70 (60–79) | 13 (1–24) | 91 (85–98) |
| Fever + muscle/body pain | 50 (19–81) | 48 (38–58) | 9 (2–17) | 90 (81–98) |
| Fever + joint pain + skin rash | 100 | 3 (0–9) | 9 (0–18) | 100 |
| Fever + joint pain + muscle/body pain | 60 (17–100) | 35 (22–49) | 9 (0–18) | 89 (76–100) |
| Fever + muscle/body pain + skin rash | 40 (0–83) | 58 (44–72) | 9 (0–21) | 90 (80–100) |
| Fever + joint pain + muscle/body pain + skin rash | 33 (0–87) | 52 (34–69) | 6 (0–18) | 89 (74–100) |
CI = confidence interval; NPV = negative predictive value; PPV = positive predictive value.
Table 6.
Sensitivity and specificity analysis of selected combinations of clinical signs and symptoms in diagnosing dengue among patients seen in a tertiary hospital, Manila, Philippines, November 2012 to December 2013
| Diagnostic combination | Sensitivity (%) (95% CI) | Specificity (%) (95% CI) | PPV (%) (95% CI) | NPV (%) (95% CI) |
|---|---|---|---|---|
| Fever + joint pain | 35 (20–51) | 66 (55–78) | 37 (21–53) | 64 (53–76) |
| Fever + skin rash | 30 (12–47) | 63 (51–75) | 25 (10–40) | 68 (57–80) |
| Fever + muscle/body pain | 51 (35–67) | 48 (36–60) | 36 (23–49) | 63 (50–77) |
| Fever + joint pain + skin rash | 31 (6–56) | 41 (20–61) | 24 (3–44) | 50 (27–73) |
| Fever + joint pain + muscle/body pain | 100 | 5 (0–13) | 38 (22–55) | 100 |
| Fever + muscle/body pain + skin rash | 32 (11–52) | 53 (36–70) | 27 (9–46) | 58 (41–75) |
| Fever + joint pain + muscle/body pain + skin rash | 31 (6–56) | 43 (22–64) | 25 (4–46) | 50 (27–73) |
CI = confidence interval; NPV = negative predictive value; PPV = positive predictive value.
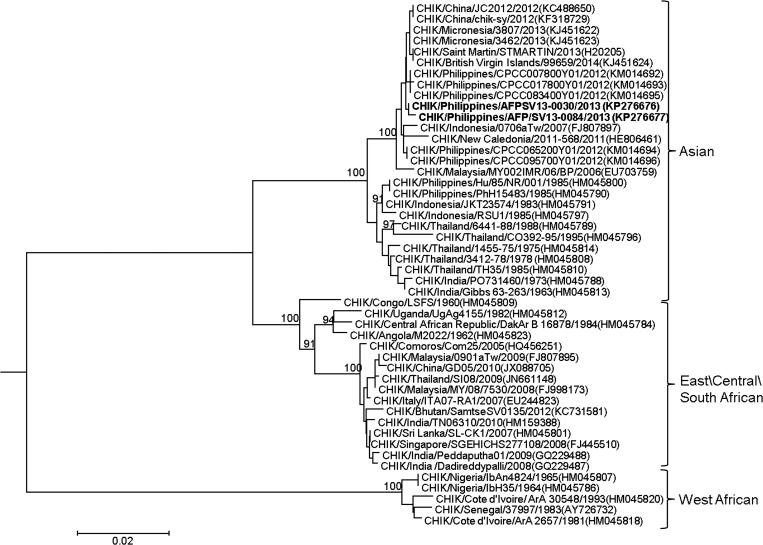
Sequencing of the E1 gene from two CHIKV PCR-positive serum samples (GenBank accession nos. KP276676 and KP276677) showed both as belonging to the Asian genotype (Figure 1 ). These two sequences were clustered together with those obtained from viruses collected in Asia from 2006 to 2012 and also with those recently reported in Micronesia (2013), St. Martin (2013), and British Virgin Islands (2014).
Figure 1.
Phylogenetic tree constructed by neighbor-joining methods (1,000 bootstrap replications) using E1 nucleotide sequences (1,320 bp) of 48 chikungunya virus (CHIKV) strains, including two newly sampled from this study designated in bold font. Bootstrap support values are shown for major nodes on the tree. Scale bar indicates nucleotide substitution per site. Genotypes are indicated on the right. The sequences were named by virus/country/strain/year of collection or isolation. GenBank accession numbers are shown in parentheses.
Discussion
Clinical differentiation of chikungunya from dengue is challenging since both diseases can share clinical signs and symptoms13,32,33 leading to potential misdiagnosis of chikungunya in areas where dengue is endemic. Furthermore, the true incidence of chikungunya is difficult to estimate since confirmatory laboratory testing for CHIKV is neither routinely available nor performed in most hospitals. Our study demonstrated that a substantial number of patients clinically diagnosed as dengue were, in fact, infected with CHIKV.
Previous studies describing co-circulation of dengue and chikungunya have included very small numbers of chikungunya cases15 or limited DENV serotype circulation.13,14,34–39 Our study is one of the few to investigate the clinical presentation of the Asian genotype of CHIKV in both children and adults in the background of a hyperendemic dengue setting with all four DENV serotypes circulating. Furthermore, a study conducted in Cebu, in 2012–2013 found most CHIKV (Asian genotype) infections to be subclinical or only mildly symptomatic.26 This suggests that the medically attended chikungunya cases in this study (which was also conducted in the Philippines and during a similar period) may represent only a small fraction of all CHIKV infections. It is also possible that the relative number of chikungunya cases misdiagnosed as dengue may be different with milder illnesses seen in primary care settings in the Philippines.
Chikungunya has been previously described as a self-limiting disease,40 but in this study, 5/11 (45%) CHIKV-confirmed patients had clinical presentations sufficient to warrant hospital admission. In 2012, the Philippines Department of Health started immunoglobulin M (IgM) enzyme-linked immunosorbent assay testing for CHIKV. In 2013, laboratory-confirmed CHIKV infections were detected in all of the 17 regions of the Philippines with approximately 9,000 clinically suspected chikungunya cases reported of which 2,232/4,745 (47%) tested positive for anti-CHIKV IgM (Philippines Department of Health data, unpublished). These data are particularly interesting considering the wide geographical dispersion and confirmation of CHIKV infections in a number of geographically distinct islands.
In our study, the most common signs and symptoms on presentation for both dengue and chikungunya were similar. This is not surprising since all the patients in our study were initially diagnosed with clinical dengue on presentation. Although other studies have found varying values for different combinations of symptoms,41,42 we did not find any combination of symptoms that had sufficiently high sensitivity and specificity in diagnosing either dengue or chikungunya (Table 5 and 6). The combination of fever, joint pain, and skin rash showed a high sensitivity but low specificity (Table 5) in diagnosing chikungunya infection, whereas the combination of fever, joint pain, and muscle/body pain had a high sensitivity but low specificity (Table 6) in diagnosing dengue. Logistic regression analysis of presenting signs and symptoms among chikungunya cases showed none of the symptoms was a significant predictor of CHIKV infection (Table 2). Skin rash (P = 0.031) was the only variable that was a significant predictor for dengue (Table 3). These results underscore the difficulty of accurately differentiating chikungunya and dengue based on clinical presentation alone.
Chikungunya outbreaks have been documented in the Philippines19–23 and in southeast Asia8,9 since the 1950–1960s. Many of these outbreaks have been described as resembling a wave-like, cyclical pattern43 characterized by an initial outbreak with high CHIKV transmission followed by a delay in CHIKV resurgence with long dormant periods44 or absences postulated to last from 3 to 4 years43 or 20 to 30 years if CHIKV prevalence rates were 60–74% or above.45,46 In our study, the CHIKV E1 gene sequences were Asian genotype, similar to those from CHIKVs collected in Cebu, in 2012 by Yoon and others.26 Phylogenetic similarity between CHIKVs from the Philippines and those found recently in the Americas indicates that these viruses probably had the same origin. This genotype has been found in the Philippines since 1985 (Figure 1) indicating that the Asian genotype has been responsible for outbreaks in the Philippines for several decades. This may indicate that chikungunya can go largely undetected because local surveillance systems are not specifically looking and testing for it, cases are being misdiagnosed as dengue, or many cases are subclinical or only mildly symptomatic.22,26,47 This emphasizes the need for sentinel, laboratory-based surveillance systems to determine the epidemiologic patterns and etiology of dengue-like illnesses such as chikungunya.
This study had several limitations. The majority of patients only had acute blood samples collected for DENV and CHIKV RT-PCR testing, so there was no serological testing of paired acute/convalescent samples. This could lead to a lower rate of laboratory-confirmed dengue and chikungunya in our study. In addition, signs and symptoms of CHIKV infections only came from clinically diagnosed dengue patients. Therefore, the full clinical spectrum of CHIKV infection was not evaluated. Moreover, detailed symptoms (e.g., location and detailed characteristics of arthralgia) and clinical laboratory data (e.g., complete blood count) were not available for analysis, and hospitalized patients were not followed during their inpatient course so data were not gathered on patient outcome/sequelae on discharge. Finally, because of the small sample size of confirmed chikungunya cases, a precise estimate of the rate of CHIKV infections clinically misdiagnosed as dengue or differences in clinical presentation of chikungunya were not possible.
In summary, this study highlights the potential for misdiagnosis of medically attended CHIKV infections as DENV infection along with the difficulty in clinically differentiating dengue and chikungunya based on presenting signs and symptoms alone. Sentinel laboratory-based surveillance systems coupled with more extensive use of diagnostic laboratory tests are needed to more accurately determine the etiology and epidemiological transmission patterns of dengue-like illness (i.e. chikungunya) and to distinguish CHIKV infections in the background of actively co-circulating DENV.
ACKNOWLEDGMENTS
We thank the medical personnel of the Armed Forces of the Philippines Medical Center, Victoriano K Luna General Hospital Department of Research and Training (Jocelyn Aquisay and Dahlziel Quintilla) and Philippines-AFRIMS Virology Research Unit Manila (Kathyleen Nogrado) for support in collection, and Jessie Batara for statistical analysis.
Disclaimer: The opinions or assertions in this article are the private views of the authors and do not necessarily reflect the official policy or position of the U.S. Army or the U.S. Department of Defense.
Footnotes
Financial support: This study was funded by the U.S. Armed Forces Health Surveillance Center-Global Emerging Infections Surveillance and Response Systems (AFHSC-GEIS).
Authors' addresses: John Mark Velasco and Maria Theresa Valderama, Philippines-AFRIMS Virology Research Unit, V. Luna General Hospital, Armed Forces of the Philippines Medical Center, Quezon City, Philippines, E-mails: john.velasco.ca@afrims.org and valderamamg.ca@afrims.org. Maria Nila Lopez, Armed Forces of the Philippines Medical Service School, Quezon City, Philippines, E-mail: manila28@yahoo.com.ph. Domingo Chua Jr. and Rene Latog II, Department of Research and Training, V. Luna General Hospital, Armed Forces of the Philippines Medical Center, Quezon City, Philippines, E-mails: dachuajr@gmail.com and latogreneii@yahoo.com. Vito Roque Jr. and June Corpuz, Epidemiology Bureau, Department of Health, Manila, Philippines, E-mails: vitogroquejr@gmail.com and jcbcorpuz@gmail.com. Chonticha Klungthong, Prinyada Rodpradit, Kittinun Hussem, Yongyuth Poolpanichupatam, Louis Macareo, Stefan Fernandez, and In-Kyu Yoon, Department of Virology, Armed Forces Research Institute of Medical Sciences, Bangkok, Thailand, E-mails: chontichak.fsn@afrims.org, prinyadar.ca@afrims.org, kittinunh.fsn@afrims.org, yongyuthp.fsn@afrims.org, louis.macareo.mil@afrims.org, stefan.fernandez.mil@afrims.org, and inkyu.yoon.mil@afrims.org.
References
- 1.Strauss JH, Strauss EG. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. I. Clinical features. Trans R Soc Trop Med Hyg. 1955;49:28–32. doi: 10.1016/0035-9203(55)90080-8. [DOI] [PubMed] [Google Scholar]
- 3.Hammon WM, Rudnick A, Sather GE. Viruses associated with epidemic hemorrhagic fevers of the Philippines and Thailand. Science. 1960;131:1102–1103. doi: 10.1126/science.131.3407.1102. [DOI] [PubMed] [Google Scholar]
- 4.Thiberville SD, Moyen N, Dupuis-Maguiraga L, Nougairede A, Gould EA, Roques P, de Lamballerie X. Chikungunya fever: epidemiology, clinical syndrome, pathogenesis and therapy. Antiviral Res. 2013;99:345–370. doi: 10.1016/j.antiviral.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Staples JE, Breiman RF, Powers AM. Chikungunya fever: an epidemiological review of a re-emerging infectious disease. Clin Infect Dis. 2009;49:942–948. doi: 10.1086/605496. [DOI] [PubMed] [Google Scholar]
- 6.Borgherini G, Poubeau P, Jossaume A, Gouix A, Cotte L, Michault A, Arvin-Berod C, Paganin F. Persistent arthralgia associated with chikungunya virus: a study of 88 adult patients on Reunion Island. Clin Infect Dis. 2008;47:469–475. doi: 10.1086/590003. [DOI] [PubMed] [Google Scholar]
- 7.Larrieu S, Pouderoux N, Pistone T, Filleul L, Receveur MC, Sissoko D, Ezzedine K, Malvy D. Factors associated with persistence of arthralgia among chikungunya virus-infected travellers: report of 42 French cases. J Clin Virol. 2010;47:85–88. doi: 10.1016/j.jcv.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Pulmanausahakul R, Roytrakul S, Auewarakul P, Smith DR. Chikungunya in southeast Asia: understanding the emergence and finding solutions. Int J Infect Dis. 2011;15:e671–e676. doi: 10.1016/j.ijid.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Powers AM, Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- 10.Renault P, Solet JL, Sissoko D, Balleydier E, Larrieu S, Filleul L, Lassalle C, Thiria J, Rachou E, de Valk H, Ilef D, Ledrans M, Quatresous I, Quenel P, Pierre V. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am J Trop Med Hyg. 2007;77:727–731. [PubMed] [Google Scholar]
- 11.Ramful D, Carbonnier M, Pasquet M, Bouhmani B, Ghazouani J, Noormahomed T, Beullier G, Attali T, Samperiz S, Fourmaintraux A, Alessandri JL. Mother-to-child transmission of chikungunya virus infection. Pediatr Infect Dis J. 2007;26:811–815. doi: 10.1097/INF.0b013e3180616d4f. [DOI] [PubMed] [Google Scholar]
- 12.Lanciotti RS, Valadere AM. Transcontinental movement of Asian genotype chikungunya virus. Emerg Infect Dis. 2014;20:1400–1402. doi: 10.3201/eid2008.140268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Omarjee R, Prat C, Flusin O, Boucau S, Tenebray B, Merle O, Huc-Anais P, Cassadou S, Leparc-Goffart I. Importance of case definition to monitor ongoing outbreak of chikungunya virus on a background of actively circulating dengue virus, St. Martin, December 2013 to January 2014. Euro Surveill. 2014;19:pii: 20753. doi: 10.2807/1560-7917.es2014.19.13.20753. [DOI] [PubMed] [Google Scholar]
- 14.Roth A, Mercier A, Lepers C, Hoy D, Duituturaga S, Benyon E, Guillaumot L, Souares Y. Concurrent outbreaks of dengue, chikungunya and Zika virus infections—an unprecedented epidemic wave of mosquito-borne viruses in the Pacific 2012. Euro Surveill. 2014;19:pii: 20929. doi: 10.2807/1560-7917.es2014.19.41.20929. [DOI] [PubMed] [Google Scholar]
- 15.Lertanekawattana S, Anantapreecha S, Jiraphongsa C, Duan-ngern P, Potjalongsin S, Wiittayabamrung W, Daroon P, Techolarn M. Prevalence and characteristics of dengue and chikungunya infections among acute febrile patients in Nong Khai Province, Thailand. Southeast Asian J Trop Med Public Health. 2013;44:780–790. [PubMed] [Google Scholar]
- 16.Rezza G, El-Sawaf G, Faggioni G, Vescio F, Al Ameri R, De Santis R, Helaly G, Pomponi A, Metwally D, Fantini M, Qadi H, Ciccozzi M, Lista F. Co-circulation of dengue and chikungunya viruses, Al Hudaydah, Yemen, 2012. Emerg Infect Dis. 2014;20:1351–1354. doi: 10.3201/eid2008.131615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laoprasopwattana K, Kaewjungwad L, Jarumanokul R, Geater A. Differential diagnosis of chikungunya, dengue viral infection and other acute febrile illnesses in children. Pediatr Infect Dis J. 2012;31:459–463. doi: 10.1097/INF.0b013e31824bb06d. [DOI] [PubMed] [Google Scholar]
- 18.Nimmannitya S, Halstead SB, Cohen SN, Margiotta MR. Dengue and chikungunya virus infection in man in Thailand, 1962–1964. I. Observations on hospitalized patients with hemorrhagic fever. Am J Trop Med Hyg. 1969;18:954–971. doi: 10.4269/ajtmh.1969.18.954. [DOI] [PubMed] [Google Scholar]
- 19.Campos LE, San Juan A, Cenabre LC, Almagro EF. Isolation of chikungunya virus in the Philippines. Acta Med Philipp. 1969;5:152–155. [Google Scholar]
- 20.Gutierrez-Rubio A, Magbitang A, Penserga E. A three-month follow up of musculoskeletal manifestations in chikungunya fever. Philippine J Int Med. 2014;52:1–5. [Google Scholar]
- 21.Retuya T, Ting D, Dacula B. Chikungunya fever outbreak in an agricultural village in Indang, Cavite, Philippines. Philipp J Microbiol Infect Dis. 1998;27:93–96. [Google Scholar]
- 22.Macasaet FF. Further observation on chikungunya fever. J Philippines Med Assoc. 1970;46:235–242. [Google Scholar]
- 23.Hayes CG, O' Rourke TF, Sarr A. Chikungunya fever among U.S. Peace Corps volunteers—Republic of the Philippines. MMWR. 1986;35:573–574. [PubMed] [Google Scholar]
- 24.Tesh RB, Gajdusek DC, Garruto RM, Cross JH, Rosen L. The distribution and prevalence of group A arbovirus neutralizing antibodies among human populations in southeast Asia and the Pacific islands. Am J Trop Med Hyg. 1975;24:664–675. doi: 10.4269/ajtmh.1975.24.664. [DOI] [PubMed] [Google Scholar]
- 25.Capeding MR, Chua MN, Hadinegoro SR, Hussain II, Nallusamy R, Pitisuttithum P, Rusmil K, Thisyakorn U, Thomas SJ, Huu Tran N, Wirawan DN, Yoon IK, Bouckenooghe A, Hutagalung Y, Laot T, Wartel TA. Dengue and other common causes of acute febrile illness in Asia: an active surveillance study in children. PLoS Negl Trop Dis. 2013;7:e2331. doi: 10.1371/journal.pntd.0002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoon IK, Alera MT, Lago CB, Tac-An IA, Villa D, Fernandez S, Thaisomboonsuk B, Klungthong C, Levy JW, Velasco JM, Roque VG, Jr, Salje H, Macareo LR, Hermann LL, Nisalak A, Srikiatkhachorn A. High rate of subclinical chikungunya virus infection and association of neutralizing antibody with protection in a prospective cohort in the Philippines. PLoS Negl Trop Dis. 2015;9:e0003764. doi: 10.1371/journal.pntd.0003764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhatia R, Narain JP. Re-emerging chikungunya fever: some lessons from Asia. Trop Med Int Health. 2009;14:940–946. doi: 10.1111/j.1365-3156.2009.02312.x. [DOI] [PubMed] [Google Scholar]
- 28.WHO . Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. Geneva, Switzerland: WHO Press; 2009. [PubMed] [Google Scholar]
- 29.Porter KR, Tan R, Istary Y, Suharyono W, Sutaryo, Widjaja S, Ma'Roef C, Listiyaningsih E, Kosasih H, Hueston L, McArdle J, Juffrie M. A serological study of Chikungunya virus transmission in Yogyakarta, Indonesia: evidence for the first outbreak since 1982. Southeast Asian J Trop Med Public Health. 2004;35:408–415. [PubMed] [Google Scholar]
- 30.Laras K, Sukri NC, Larasati RP, Bangs MJ, Kosim R, Djauzi, Wandra T, Master J, Kosasih H, Hartati S, Beckett C, Sedyaningsih ER, Beecham HJ, 3rd, Corwin AL. Tracking the re-emergence of epidemic chikungunya virus in Indonesia. Trans R Soc Trop Med Hyg. 2005;99:128–141. doi: 10.1016/j.trstmh.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 31.Klungthong C, Gibbons RV, Thaisomboonsuk B, Nisalak A, Kalayanarooj S, Thirawuth V, Nutkumhang N, Mammen MP, Jr, Jarman RG. Dengue virus detection using whole blood for reverse transcriptase PCR and virus isolation. J Clin Microbiol. 2007;45:2480–2485. doi: 10.1128/JCM.00305-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kularatne SA, Gihan MC, Weerasinghe SC, Gunasena S. Concurrent outbreaks of chikungunya and dengue fever in Kandy, Sri Lanka, 2006–07: a comparative analysis of clinical and laboratory features. Postgrad Med J. 2009;85:342–346. doi: 10.1136/pgmj.2007.066746. [DOI] [PubMed] [Google Scholar]
- 33.Nkoghe D, Kassa RF, Bisvigou U, Caron M, Grard G, Leroy EM. No clinical or biological difference between chikungunya and dengue fever during the 2010 Gabonese outbreak. Infect Dis Rep. 2012;4:e5. doi: 10.4081/idr.2012.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomasello D, Schlagenhauf P. Chikungunya and dengue autochthonous cases in Europe, 2007–2012. Travel Med Infect Dis. 2013;11:274–284. doi: 10.1016/j.tmaid.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Ratsitorahina M, Harisoa J, Ratovonjato J, Biacabe S, Reynes JM, Zeller H, Raoelina Y, Talarmin A, Richard V, Louis Soares J. Outbreak of dengue and chikungunya fevers, Toamasina, Madagascar, 2006. Emerg Infect Dis. 2008;14:1135–1137. doi: 10.3201/eid1407.071521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leroy EM, Nkoghe D, Ollomo B, Nze-Nkogue C, Becquart P, Grard G, Pourrut X, Charrel R, Moureau G, Ndjoyi-Mbiguino A, De-Lamballerie X. Concurrent chikungunya and dengue virus infections during simultaneous outbreaks, Gabon, 2007. Emerg Infect Dis. 2009;15:591–593. doi: 10.3201/eid1504.080664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chipwaza B, Mugasa JP, Selemani M, Amuri M, Mosha F, Ngatunga SD, Gwakisa PS. Dengue and chikungunya fever among viral diseases in outpatient febrile children in Kilosa District Hospital, Tanzania. PLoS Negl Trop Dis. 2014;8:e3335. doi: 10.1371/journal.pntd.0003335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee VJ, Chow A, Zheng X, Carrasco LR, Cook AR, Lye DC, Ng LC, Leo YS. Simple clinical and laboratory predictors of chikungunya versus dengue infections in adults. PLoS Negl Trop Dis. 2012;6:e1786. doi: 10.1371/journal.pntd.0001786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taraphdar D, Sarkar A, Mukhopadhyay BB, Chatterjee S. A comparative study of clinical features between monotypic and dual infection cases with chikungunya virus and dengue virus in West Bengal, India. Am J Trop Med Hyg. 2012;86:720–723. doi: 10.4269/ajtmh.2012.11-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackenzie JS, Chua KB, Daniels PW, Eaton BT, Field HE, Hall RA, Halpin K, Johansen CA, Kirkland PD, Lam SK, McMinn P, Nisbet DJ, Paru R, Pyke AT, Ritchie SA, Siba P, Smith DW, Smith GA, van den Hurk AF, Wang LF, Williams DT. Emerging viral diseases of southeast Asia and the Western Pacific. Emerg Infect Dis. 2001;7:497–504. doi: 10.3201/eid0707.017703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sissoko D, Ezzedine K, Moendandze A, Giry C, Renault P, Malvy D. Field evaluation of clinical features during chikungunya outbreak in Mayotte. Trop Med Int Health. 2005-2006;15:600–607. doi: 10.1111/j.1365-3156.2010.02485.x. [DOI] [PubMed] [Google Scholar]
- 42.Nkoghe D, Kassa RF, Caron M, Grard G, Mombo I, Bikie B, Paupy C, Becquart P, Bisvigou U, Leroy EM. Clinical forms of chikungunya in Gabon, 2010. PLoS Negl Trop Dis. 2012;6:e1517. doi: 10.1371/journal.pntd.0001517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jupp PG, McIntosh BM. Chikungunya virus disease. In: Monath TP, editor. The Arbovirus: Epidemiology and Ecology. Boca Raton, FL: CRC Press; 1988. pp. 137–157. [Google Scholar]
- 44.Chusri S, Siripaitoon P, Silpapojakul K, Hortiwakul T, Charernmak B, Chinnawirotpisan P, Nisalak A, Thaisomboonsuk B, Klungthong C, Gibbons RV, Jarman RG. Kinetics of chikungunya infections during an outbreak in southern Thailand, 2008–2009. Am J Trop Med Hyg. 2014;90:410–417. doi: 10.4269/ajtmh.12-0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7:319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- 46.Boelle PY, Thomas G, Vergu E, Renault P, Valleron AJ, Flahault A. Investigating transmission in a two-wave epidemic of chikungunya fever, Reunion Island. Vector Borne Zoonotic Dis. 2008;8:207–217. doi: 10.1089/vbz.2006.0620. [DOI] [PubMed] [Google Scholar]
- 47.Macasaet F, Nakao J, Rustia F, Beran G, Buscato N. Epidemiology of arbovirus infections in Negros Oriental: I. Clinical features of an epidemic in Amlan. J Philipp Med Assoc. 1969;45:207–215. [Google Scholar]