Abstract
This study aimed to investigate the pharmacokinetic interactions between quinine and lopinavir boosted with ritonavir (LPV/r) in healthy Thai adults (8 males and 12 females). Period 1 (day 1): subjects received a single oral dose of 600 mg quinine sulfate. Period 2: subjects received LPV/r (400/100 mg) twice daily. Period 3: subjects received a single quinine sulfate dose plus LPV/r twice a day. Intensive blood sampling was performed during each phase. Quinine AUC0–48h (area under the plasma concentration–time curve from time 0 to 48 hours), AUC0–∞ (area under the plasma concentration–time curve from time 0 to infinity), and Cmax (maximum concentration over the time-span specified), were 56%, 57%, and 47% lower, respectively, in the presence of LPV/r. 3-Hydroxyquinine AUC0–48h, AUC0–∞, and Cmax were significantly lower and the metabolite-to-parent ratio was significantly reduced. Lopinavir and ritonavir exposures were not significantly reduced with quinine coadministration, but Cmax of both drugs were significantly lower. The geometric mean ratio (GMR) and 90% CI of AUC0–48h, AUC0–∞, and Cmax for quinine, 3-hydroxyquinine, lopinavir, and ritonavir lay outside the bioequivalent range of 0.8–1.25. Drug treatments during all periods were generally well tolerated. The reduction in systemic exposure of quinine and 3-hydroxyquinine with concomitant LPV/r use raises concerns of suboptimal exposure. Studies in HIV/malaria coinfection patients are needed to determine the clinical impact to decide if any change to the quinine dose is warranted.
Introduction
Malaria and human immunodeficiency virus (HIV)/acquired immunodeficiency syndrome (AIDS) are among the top 10 leading causes of death in low-income countries.1 The geographical areas most affected by these epidemics overlap, particularly, in sub-Saharan Africa, southeast Asia, and Latin America. The incidence of malaria–HIV coinfection is approximately 10% in Africa and southern India.2 In Thailand, a recent retrospective analysis in 867 patients with malaria between 2005 and 2013 in Tak province, an area bordering Myanmar, showed an incidence of 1.85% malaria (Plasmodium falciparum and Plasmodium vivax) and HIV coinfection. Concomitant use of antimalarial and antiretroviral drugs is therefore becoming increasingly frequent in areas where malaria and HIV coexist, and significant interactions between the two diseases and classes of chemotherapeutic drugs have been reported. Malaria infection stimulates immune mechanisms that activate HIV replication causing a transient increase in HIV viral load.3–5 HIV infection also increases the risk of malaria frequency and severity.6,7 There is accumulating evidence suggesting clinically important drug–drug interactions occur between antimalarial and antiretroviral drugs, which could potentially affect treatment efficacy and/or tolerability.8–11
Quinine is the first-line antimalarial drug recommended by the World Health Organization (WHO) for treatment of pregnant women with uncomplicated P. falciparum malaria during the first trimester as 7-day quinine–clindamycin combination. In addition, it is the second-line treatment of severe P. falciparum malaria in most endemic areas.12 Drug-related cardiotoxicity, however, remains a safety concern with quinine use.12,13 For HIV therapy, ritonavir-boosted protease inhibitors (PIs) are currently recommended by the WHO as part of second-line antiretroviral therapy for adults. Globally, lopinavir boosted with ritonavir (LPV/r) remains the most commonly used PI due to its availability as a fixed-dose combination and high genetic barrier to resistance.14,15 Both quinine and LPV/r are extensively metabolized by the hepatic cytochrome P450 (CYP) 3A4 enzyme.16–21 Elimination half-lives of quinine, lopinavir, and ritonavir are in the ranges of 9–15, 6–14, and 3–8 hours, respectively.8–11 Ritonavir is a potent inhibitor and/or inducer of CYP3A4 and several membrane transporter proteins.16–18,22–24 CYP3A4 inhibition by LPV/r results in a higher concentration of the antimalarial lumefantrine (2- to 3-fold increase in systemic exposure) in healthy subjects,25 and was associated with lower incidence of malaria and longer posttreatment prophylaxis.26 Inhibition of CYP3A4-mediated metabolism of quinine may result in toxic quinine plasma concentrations, leading to risk of toxicity or untoward side effects.
Reports on the pharmacokinetic interaction between quinine and ritonavir when given alone or as lopinavir-boosted dose remain controversial. A significant increase in systemic exposure of quinine was reported when quinine was co-administered with ritonavir in healthy subjects,8 while a decrease in systemic exposure was found when it was co-administered with LPV/r.11 Our objective was to investigate the pharmacokinetic interactions between quinine and LPV/r at steady state in healthy Thai adults.
Materials and Methods
Subjects and study design.
This was an open-label, three-way, sequential cross-over pharmacokinetic study in healthy Thai subjects. Inclusion criteria included 1) males and nonpregnant females, 2) aged 15–55 years, 3) body weight 40–65 kg, 4) nonsmokers and non-alcohol drinkers, and 5) residents of Mae Sot District, Tak Province. Exclusion criteria included those with 1) hepatic or renal diseases; 2) using any drug or herbal medicine within the past 14 days, except antipyretic or antiemetic drugs; or 3) history of intolerance to quinine, lopinavir, and ritonavir. The minimum requirement of the sample size for the study was 19 subjects based on α = 0.05, target power = 80% (β = 0.02), and coefficients of variation (CV) of clearance = 20%. Consenting adults were screened for eligibility according to the inclusion/exclusion criteria. A physical examination, electrocardiogram, and laboratory safety tests (hematology, biochemistry, urinalysis, and pregnancy status) were performed.
Drug administration.
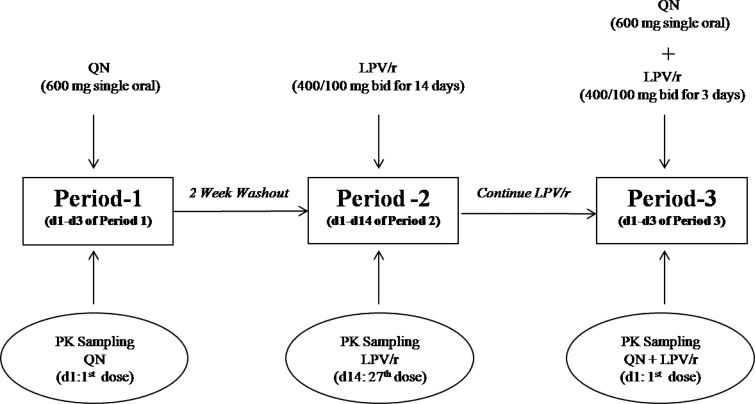
Figure 1 summarizes the study design. The pharmacokinetic investigation was performed sequentially on three occasions (periods 1, 2, and 3). Period 1: starting on day 1, subjects received a single oral dose of 600 mg quinine sulfate (two tablets: 300-mg quinine base per tablet, manufactured by the Government Pharmaceutical Organization, Bangkok, Thailand). There was a 2-week washout period between periods 1 and 2. Period 2: subjects received oral doses of LPV/r (two tablets: 400/100 mg of LPV/r, manufactured by Matrix Laboratories Co. Ltd., India) twice daily for 14 days (27 doses). There was no washout between periods 2 and 3. Period 3: subjects received an oral dose of 600 mg quinine sulfate and LPV/r (400/100 mg) twice daily for 3 days.
Figure 1.
Schematic diagram depicting the study design for investigation of pharmacokinetic interaction between quinine (QN) and lopinavir boosted with ritonavir (LPV/r) in healthy Thai subjects.
All subjects were admitted to Mae Sot General Hospital for observation during the pharmacokinetic sampling period, and drug dosage was taken at least 2 hours before meal with water (standard volume 150 mL). Only analgesic/antipyretic (paracetamol) and antiemetic (dimenhydrinate) were allowed in cases of fever and nausea. Drugs with potential interactions with the study drugs (i.e., CYP3A4 inhibitors such as antiretroviral protease inhibitors, erythromycin, clarithromycin, cyclosporine, verapamil, ketoconazole, itraconazole, and voriconazole and CYP3A4 inducers such as carbamazepine, dexamethasone, efavirenz, nevirapine, phenobarbital, phenytoin, primidone, rifampicin, and St. John's wort) were disallowed during the study period.27
Assessments of safety and tolerability.
Safety and tolerability of the three-drug regimens were assessed based on clinical and laboratory assessments during follow-up according to National Institute of Health/National Cancer Institute (NIH/NCI) Common Toxicity Criteria Grading System for Adverse Events.28 Clinical assessments included physical examination and monitoring of vital signs and adverse events. Safety laboratory assessments (hematology, biochemistry, and urinalysis) were performed during each period. All female subjects had a pregnancy test (β-human chorionic gonadotropin test) performed during each period. Any abnormal laboratory result was followed up with repeat checks every week until it returned to normal.
Blood sample collection for pharmacokinetic assessment.
During periods 1 (quinine alone) and 3 (quinine plus LPV/r), blood samples were drawn before the first dose and at 1, 2, 4, 6, 8, 12, 24, 36, and 48 hours after the first dose. During period 2 (LPV/r), blood sample were drawn before the 27th dose and at 1, 2, 4, 6, 8, and 12 hours after the first dose. Immediately after collection, blood samples were centrifuged (1,200 × g, 10 minutes), and the plasma was stored at −20°C until analysis.
Measurement of drug concentrations.
Measurement of plasma concentrations of quinine (both free and bound forms) and its metabolite, 3-hydroxyquinine, was performed using high-performance liquid chromatography (HPLC) with fluorescence detection, according to the methods of Karbwang and others27 at the Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma, Thammasat University. Unbound concentration of quinine in plasma at 2 hours after dosing was measured in 0.5-mL plasma samples after ultrafiltration at 25°C using an Amicon YMT system (Amicon Corporation, Bedford, MA). The assay limit of quantification (LOQ) for quinine and 3-hydroxyquinine was 25 and 50 ng/mL, respectively. Recoveries for both compounds were between 96% and 112% and between 94% and 101%, respectively. The intra- and inter-day %CV of quinine and 3-hydroxyquinine ranged from 0.9% to 2.1% and 0.2% to 10.4% and from 1.1% to 2.4% and 0.7% to 9.2%, respectively.
Lopinavir and ritonavir plasma drug concentrations were measured using a validated HPLC ultraviolet assay at the Faculty of Associated Medical Sciences, Chiang Mai University.29 The Program for HIV Prevention and Treatment Laboratory participates in the U.S. AIDS Clinical Trial Group Pharmacology Quality Control (precision testing) program.31 The assay LOQ was 100 ng/mL for lopinavir and 50 ng/mL for ritonavir. The recoveries of lopinavir and ritonavir were between 96% and 112% and between 90% and 94%, respectively. Intra- and inter-assay precisions were less than 4% of the CV.
Quality control (QC) samples were run in duplicate in each analytical batch at low, medium, and high concentrations. Criteria for acceptability were four out of six of the QC analyses to lie inside 100 ± 15% of the nominal values.
Pharmacokinetic analysis and criteria for pharmacokinetic drug interaction.
Pharmacokinetic parameters were determined by a non-compartmental analysis31 using WinNonLin software (version 6.3, Pharsight, Certara, St. Louis, MO). Concentrations of drugs lower than the LOQ levels were expressed as zero (undetectable). The Cmax (maximum concentration over the time-span specified) and tmax (time of maximum concentration) were determined by direct inspection of the plasma concentration–time data. AUC0–48h (area under the plasma concentration–time curve from time 0 to 48 hours) and AUC0–∞ (area under the plasma concentration–time curve from time 0 to infinity) were calculated using the trapezoidal rule. Other pharmacokinetic parameters analyzed included elimination rate constant (λz) calculated from at least five concentration–time points of elimination phase, apparent oral clearance (CL/F) calculated as dose/AUC0–∞, volume of distribution (Vz/F) calculated as CL/F/λz, and the terminal half-life (t1/2z) calculated as 0.693/λz. Metabolic ratio (MR) was defined as the ratio between AUC0–∞ of 3-hydroxyquinine and quinine. The geometric mean ratio (GMR: the ratio of the value of the parameter for the drug when used in combination versus the corresponding value for the drug used alone) and its 90% confidence interval (CI) were determined for each parameter. A clinically significant pharmacokinetic drug interaction occurred whenever the 90% CI for systemic exposure ratio fell entirely outside the equivalence range of 0.8–1.25.32
Statistical analysis.
Statistical analysis of the data was performed using SPSS version 16.0 (SPSS Inc., Gorinchem, The Netherlands). Pharmacokinetic parameters are presented as median and 95% CI. Comparison of all pharmacokinetic parameters of quinine, 3-hydroxyquinine, lopinavir, and ritonavir obtained during the two periods (period 1 versus period 3; period 2 versus period 3) were performed using Wilcoxon signed-rank test. Comparison of the frequency of subjects with adverse events between the two groups was performed using a χ2 test. Statistical significance level was set at α = 0.05 for all tests.
Ethical considerations.
Ethical approval was obtained for all of the studies from which data were obtained in this analysis, and the investigators adhered to the Declaration of Helsinki and Good Clinical Practice. The study protocol was approved by the Institute for Development of Human Research Protection at the Ministry of Public Health in Thailand. Approval was also obtained from each independent ethics committee and local institutional review board. All participants provided written informed consents for study participation.
Results
Subject characteristics.
Twenty healthy subjects (8 males and 12 females) were enrolled in the study. The demographic, clinical, and laboratory information at baseline are summarized in Table 1. All were healthy as verified by results of clinical and laboratory investigations. One female subject discontinued from the study on day 11 because of skin rash during period 2 (LPV/r alone).
Table 1.
Demographic, clinical, and laboratory data of 19 healthy Thai subjects (8 males and 11 females) at baseline
| Median (interquartile range) | |
|---|---|
| Age (years) | |
| Male | 32 (22–41) |
| Female | 29 (21–38) |
| Body weight (kg) | |
| Male | 59 (55–62) |
| Female | 50 (47–55) |
| White blood cell count (×10−3/μL) | 7.8 (7.1–9.5) |
| Red blood cell count (×10−6/μL) | 5.1 (4.6–5.5) |
| Hematocrit (%) | 39.4 (36.6–41.2) |
| Hemoglobin (g/dL) | 13.2 (12–14.4) |
| Platelet count (×10−3/μL) | 2.63 (2.21–3.12) |
| BUN (mg/dL) | 8.8 (7.4–10.4) |
| Creatinine (mg/dL) | 0.80 (0.70–1.00) |
| AST (U/L) | 24.00 (18.00–28.00) |
| ALT (U/L) | 23 (18.00–33.00) |
| Total protein (g/dL) | 7.00 (6.80–7.20) |
| Albumin (g/dL) | 4.40 (4.20–4.50) |
| Triglyceride (mg/dL) | 87 (77–118) |
| Fasted blood sugar (mg/dL) | 84 (76–94) |
| Blood pressure (mmHg) | 121 (114–138), 76 (65–95) |
| PR interval (ms) | 150 (134–158) |
| QRS duration (ms) | 88 (86–96) |
| QT interval (ms) | 382 (374–398) |
| The corrected QT interval (msec) | 407 (400–427) |
ALT = alanine transaminase; AST = aspartate transaminase; BUN = blood urea nitrogen.
Data are presented as median (interquartile range).
Pharmacokinetics of quinine and 3-hydroxyquinine.
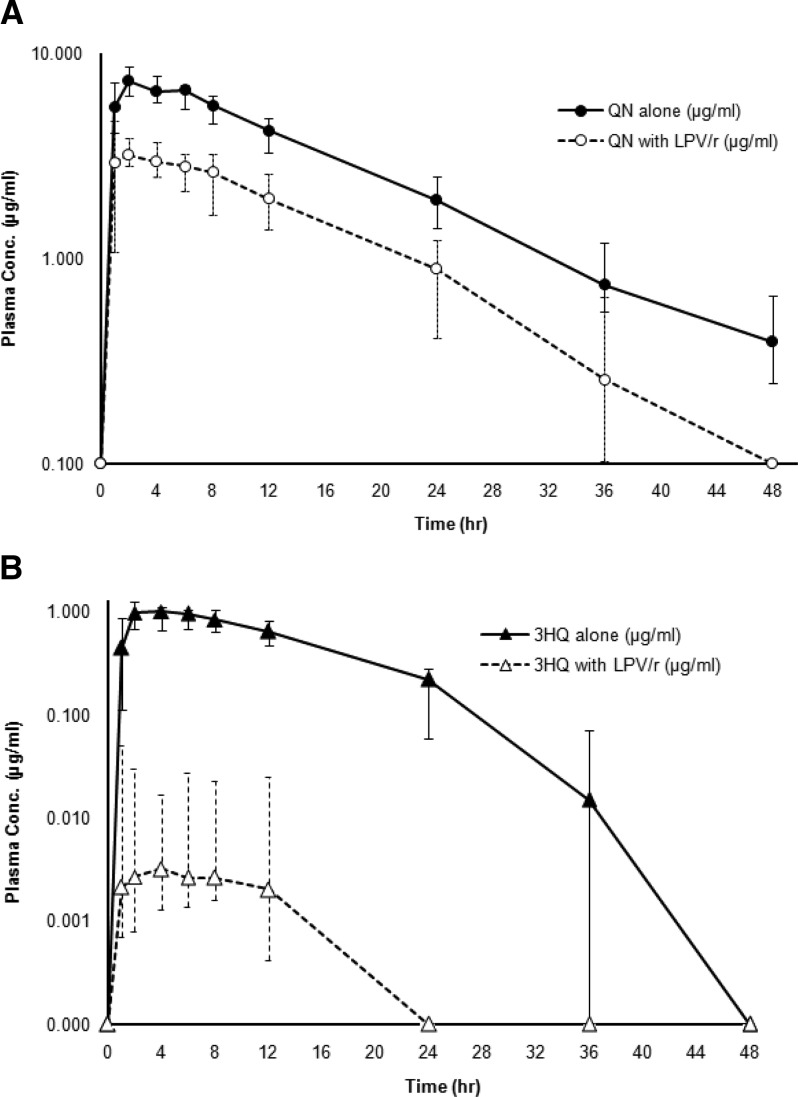
Pharmacokinetic analysis of plasma concentration–time profiles of quinine and 3-hydroxyquinine was performed using data from 190 samples collected from subjects during each period. For quinine, plasma concentrations in 21 (11.05%) and 29 (16.20%) samples were below the LOQ levels of samples collected during phases 1 and 3, respectively. The corresponding values for 3-hydroxyquinine were 46 (24.21%) and 77 (40.52%), respectively. The median plasma concentration–time profiles of quinine and 3-hydroxyquinine after administration of 600 mg quinine sulfate alone (period 1) and 600 mg quinine sulfate in combination with LPV/r (period 3) are shown in Figure 2 . The pharmacokinetic parameters of quinine and 3-hydroxyquinine are summarized in Table 2. Relatively high interindividual variation was observed for most pharmacokinetic parameters of quinine (31% CV) and 3-hydroxyquinine (45% CV). In the presence of steady-state LPV/r, statistically significant differences in quinine pharmacokinetic parameters were observed. Cmax, AUC0–48h, and AUC0–∞ of quinine including free quinine concentrations at 2 hours were significantly decreased (P < 0.005 for all), while Vz/F and CL/F were significantly increased (P < 0.005 for both). The t1/2z of quinine was also significantly shorter (P < 0.01). The Cmax, AUC0–48h, and AUC0–∞ of 3-hydroxyquinine were also significantly decreased (P < 0.005 for all). The MR of 3-hydroxyquinine to quinine was significantly reduced from 0.12 to 0.0016 (P < 0.005).
Figure 2.
Plasma concentration–time profiles of (A) quinine (QN) and (B) 3-hydroxyquinine after administrations of a single 600-mg oral dose of quinine sulfate with (QN with lopinavir boosted with ritonavir [LPV/r]) or without (QN alone) steady-state oral doses of LPV/r.
Table 2.
Pharmacokinetic parameters of quinine and 3-hydroxyquinine when quinine was administered alone and in combination with LPV/r (n = 19)
| Pharmacokinetic parameter | Quinine | 3-Hydroxyquinine | ||||
|---|---|---|---|---|---|---|
| Alone | With LPV/r | GMR (90% CI) | Alone | With LPV/r | GMR (90% CI) | |
| AUC0–48h (μg⋅h/mL) | 132.04 (108.96–155.12) | 57.04 (40.70–73.38)† | 0.44 (0.33–0.59) | 15.56 (11.57–19.55) | 0.07 (0.04–0.53)† | 0.01 (0.00–0.02) |
| AUC0–∞ (μg⋅h/mL) | 136.83 (112.71–160.95) | 57.06 (38.17–75.96)† | 0.43 (0.32–0.59) | 16.95 (13.02–20.87) | 0.09 (−0.80 to 0.97)† | 0.02 (0.01–0.04) |
| Cmax (μg/mL) | 7.45 (6.50–8.39) | 3.78 (2.86–4.70)† | 0.53 (0.41–0.68) | 1.05 (0.76–1.34) | 0.0 (−0.03 to 0.03)† | 0.15 (0.01–0.04) |
| Cmax free (μg/mL) | 0.11 (0.05–0.82) | 0.37 (0.23–0.50)† | NA | NA | NA | NA |
| tmax (hour) | 2 (2–2) | 2 (1.47–2.53) | 0.85 (0.64–1.20) | 2 (0.94–3.06) | 2 (−1.18 to 5.18) | 1.10 (0.67–1.80) |
| t1/2z (hour) | 10.95 (9.26–12.64) | 8.44 (5.72–11.16)* | 0.79 (0.63–0.98) | 5.49 (3.96–7.01) | 13.26 (7.51–19.00) | 1.95 (1.17–3.25) |
| CL/F (L/h) | 4.39 (3.58–5.20) | 10.51 (6.92–14.10)† | 2.31 (1.70–3.15) | NA | NA | NA |
| Vz/F (L/kg) | 1.06 (0.86–1.26) | 2.18 (1.75–2.61)† | 1.82 (1.40–2.37) | NA | NA | NA |
AUC = area under the plasma concentration–time curve; CI = confidence interval; CL = oral clearance; GMR = geometric mean ratio; LPV/r = lopinavir boosted with ritonavir; NA = not applicable (no data).
Significantly different from quinine alone with P value *< 0.01 and †< 0.005, respectively.
Pharmacokinetics of LPV/r.
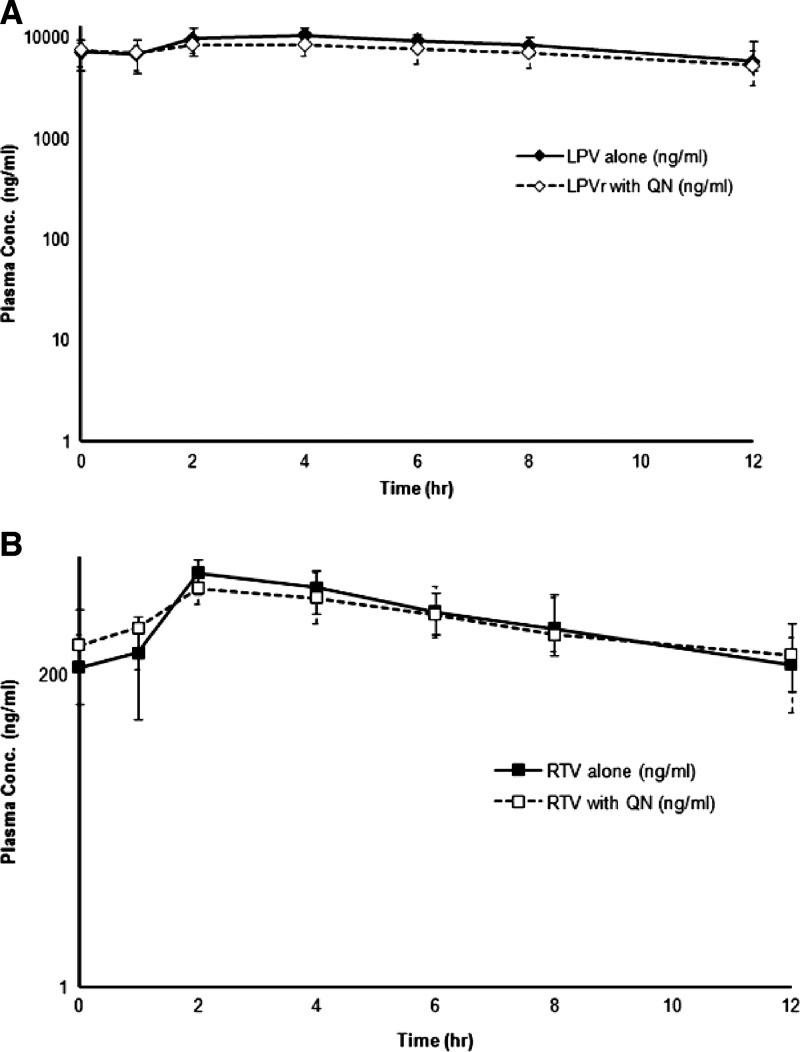
Pharmacokinetic analysis of plasma concentration–time profiles of lopinavir and ritonavir was performed using data from 133 samples collected from subjects during each period. For lopinavir, plasma concentrations in 2 (1.51%) and 0 (0%) samples were below the LOQ levels of samples collected during phases 2 and 3, respectively. The corresponding numbers for ritonavir were 1 (0.77%) and 0 (0%), respectively. The median plasma concentration–time profiles of LPV/r after the administration during periods 2 and 3 are shown in Figure 3 . One subject had undetectable plasma lopinavir and ritonavir concentrations until 1 hour after the first dose in period 3. The pharmacokinetics of lopinavir (400 mg) given as ritonavir-boosted dose alone (period 2) and in combination with quinine (period 3) are summarized in Table 3. In the presence of quinine, Cmax of both lopinavir and ritonavir were significantly decreased (P < 0.01 for both).
Figure 3.
Plasma concentration–time profiles of (A) lopinavir (LPV) and (B) ritonavir (RTV) following oral doses of 400 mg LPV plus 100 mg RTV twice a day with (RTV with quinine [QN]) or without (RTV alone) a single oral dose of 600 mg quinine sulfate.
Table 3.
Pharmacokinetic parameters of LPV/r when administered alone and in combination with quinine (n = 19)
| Pharmacokinetic parameter | Lopinavir | Ritonavir | ||||
|---|---|---|---|---|---|---|
| Alone | With quinine | GMR (90% CI) | Alone | With quinine | GMR (90% CI) | |
| AUC0–12h (μg⋅h/mL) | 100.51 (80.16–120.86) | 78.68 (58.41–98.95) | 0.94 (0.75–1.18) | 6.85 (4.21–9.49) | 5.97 (3.64–8.30) | 0.93 (0.67–1.29) |
| AUC0–∞ (μg⋅h/mL) | 181.44 (92.42–270.46) | 127.96 (59.83–196.09) | 0.78 (0.53–1.16) | 8.58 (5.20–11.96) | 7.11 (2.70–11.51) | 0.81 (0.55–1.19) |
| Cmax (μg/mL) | 10.72 (8.84–12.60) | 8.54 (6.86–10.22)* | 0.87 (0.71–1.05) | 1.23 (0.61–1.85) | 0.99 (0.65–1.33)* | 0.78 (0.54–1.11) |
| tmax (hour) | 2 (0.94–3.01) | 2 (0.94–3.06) | 1.20 (0.90–1.60) | 2 (0.94–3.06) | 2 (0.94–3.06) | 1.36 (0.97–1.90) |
| t1/2z (hour) | 11.04 (8.17–13.91) | 8.40 (5.33–11.47) | 0.89 (0.65–1.22) | 4.42 (3.70–5.13) | 4.14 (2.53–5.74) | 0.99 (0.77–1.28) |
| CL/F (L/h) | 2.21 (1.39–3.02) | 3.13 (1.94–4.32) | 1.28 (0.86–1.89) | 11.83 (6.99–16.66) | 14.07 (7.18–20.96) | 1.24 (0.84–1.82) |
| Vz/F (L/kg) | 0.48 (0.37–0.59) | 0.64 (0.49–0.79) | 1.14 (0.92–1.42) | 1.07 (0.66–1.47) | 1.52 (1.04–1.99) | 1.24 (0.86–1.79) |
AUC = area under the plasma concentration–time curve; CI = confidence interval; CL = oral clearance; GMR = geometric mean ratio; LPV/r = lopinavir boosted with ritonavir.
Significantly different from quinine alone with P value < 0.01.
Pharmacokinetic interaction between quinine and LPV/r.
On the basis of the criteria set,32 clinically relevant drug interaction is likely to occur when quinine and LPV/r were co-administered. The 90% CI of the GMR of AUC0–48h, AUC0–∞, and Cmax for quinine, 3-hydroxyquinine, lopinavir, and ritonavir were all outside the acceptable bioequivalent range of 0.8–1.25 (Tables 2 and 3).
Safety and tolerability.
Drug treatments during all investigation periods were generally well tolerated. Only mild-to-moderate (NIH/NCI Grade 1 and 2) severity grade adverse events possibly related to the study drugs were observed. The frequency of adverse events occurred during the three periods were similar. The adverse events during period 1 (quinine alone) included vertigo (five cases, 26.3%), tinnitus (three cases, 21.1%), and nausea/vomiting (two cases, 10.5%). The adverse events observed during period 2 (LPV/r) were diarrhea (five cases, 26.3%) and skin rash, stomach ache, and vertigo (one case 5.3%). One case (5.3%) reported nausea/vomiting and syncope during drug administration during period 3 (quinine plus LPV/r). One female subject discontinued from the study on day 11 because of skin rash during period 2 (LPV/r alone). QTc prolongation (from 460 to 481 ms) was observed in one female during period 3 without any sign and symptom of cardiac dysrhythmia. A markedly high proportion of subjects (16/19) with increased serum triglyceride (about 2–7.7 times of baseline) was observed during period 2 after 34 doses of LPV/r. However, the levels in almost all subjects returned to normal within 8 weeks after the first dose, except in one male subject, whose level returned to normal at 12 weeks of the first dose.
Discussion
This study is the first reporting the pharmacokinetic interactions between quinine and LPV/r in Asian subjects. We found marked changes in the pharmacokinetics of quinine and its active plasma metabolite, 3-hydroxyquinne. The impact of quinine on LPV/r pharmacokinetics was less pronounced. A greater systemic exposure of both quinine and 3-hydroxyquinine was observed in Thai compared with Caucasian subjects, while the pharmacokinetics of lopinavir and ritonavir were similar with those previously reported.11 This could be explained by the higher dose per kilogram body weight in Thai population. A relatively lower frequency of adverse events was found in this study compared with previous studies using a similar dose regimen and design.11,33,34 The adverse events after quinine and LPV/r administration included gastrointestinal-related symptoms, that is, vertigo, nausea/vomiting, and diarrhea. These symptoms are the most common adverse effects of lopinavir and quinine.35,36 Skin rash and stomach ache were found only after LPV/r dosing. Increase in serum triglyceride level was commonly observed after LPV/r treatment.37
Almost all of the key pharmacokinetic parameters of quinine were modified when quinine was administered in combination with LPV/r. The systemic exposure of quinine represented by Cmax, AUC0–48h, and AUC0–∞ was significantly reduced (49.3%, 56.8%, and 58.0%, respectively). The decrease in systemic exposure is unlikely to be due to impaired drug absorption since there was no delay in tmax of either quinine or 3-hydroxyquinine. A significant increase (13%) in free (unbound) quinine concentration at 2 hours (Cmax) resulted in an expansion of apparent volume of distribution (106%). A parallel significant reduction in the systemic exposure of its active plasma metabolite, 3-hydroxyquinine, as well as the metabolic ratio was unexpected. The 3-hydroxyquinine metabolite exhibits about 10–15% antimalarial activity of quinine. If the mechanism by which the reduced systemic exposure and increased clearance of quinine was due to induction of CYP3A4-mediated hepatic metabolism of quinine, the systemic exposure of 3-hydroxyquinine should have increased. Our observation is in agreement with the report by Nyunt and others11 who assessed the effect of LPV/r on quinine/3-hydroxyquinine pharmacokinetics in healthy Caucasians, where the exposure of both quinine and 3-hydroxyquinine were significantly reduced in the presence of steady-state LPV/r concentrations. One difference we observed was an increase instead of a decrease in free quinine concentration at 2 hours post-dose. The total oral clearance of quinine in healthy Thai subjects was about half of that observed in Caucasian subjects, resulting in about 2- to 3-fold higher quinine and 3-hydroxyquinine concentrations. Another study conducted in healthy Nigerian subjects8 found a 4-fold increase in systemic exposure (Cmax and AUC0–∞) and a 20% increase in t1/2z when quinine (600 mg) was coadministered with ritonavir (200 mg every 12 hours for nine doses), although the metabolic ratio was shown to be significantly reduced. The discrepancy of results obtained in this study and that reported by Soyinka and others8 could be explained by the difference in ethnicity and study design. In the study in Nigerian subjects,8 only ritonavir was given at the dose of 200 mg every 12 hours for 13 doses alone, and 200 mg every 12 hours for five doses together with quinine. In addition, quinine dose was given on day 8 during the 15th ritonavir dose. The systemic exposure and half-life of ritonavir was significantly increased, and the observed reduction of systemic exposure of 3-hydroxyquinine was similar. The authors concluded that the mechanism underlying this change was likely to be due to inhibition of hepatic metabolism of quinine, and a reduction in quinine dosing was recommended. Although the study design was similar to our study, a discrepancy between the two studies could be due difference in dose administration (ritonavir was given alone) and possibly, ethnic difference in hepatic metabolism and disposition of quinine.
Altogether, results from this study support the supposition of a complex pharmacokinetic interaction between quinine and LPV/r involving hepatic/gastrointestinal metabolism, drug transportation across membranes, and plasma protein binding. Quinine is primarily eliminated through hepatic metabolism.18,20 Lopinavir and ritonavir are substrates or potent inhibitors of CYP3A4, CYP2B6, and CYP2D6, as well as inducers of CYP1A2, 2B6, 2C9, 2C19 and uridine 5'-diphospho-glucuronosyltransferase (UGTs).38–43 All protease inhibitors are both substrates and inhibitors of P-glycoprotein (P-gp), with ritonavir being the most potent inhibitor,44–47 while quinine is a substrate for P-gp.48,49 The significant changes in the pharmacokinetics of quinine and 3-hydroxyquinine observed with LPV/r coadministration suggests the involvement of multiple drug metabolizing enzymes and drug transporters in the interactions. The relatively high magnitude of the decrease in systemic exposure of 3-hydroxyquinine compared with the parent drug quinine suggests sequential inhibition of CYP3A4-mediated quinine metabolism, followed by the induction of UGT-mediated 3-hydroxyquinine metabolism. The reduction of systemic exposure of quinine could also be due to decrease in its oral bioavailability as a result of induction of pre-systemic metabolism of quinine by ritonavir. The decrease in metabolic ratio was a consequence of the more pronounced reduction of 3-hydroxyquinine exposure compared with quinine. Apart from the metabolic and transporter interactions and plasma protein binding displacement of quinine by ritonavir are also possible. Ritonavir binds extensively to α1-acidglycoprotein, while the extent of the binding of quinine is moderate.49,50 This assumption is supported by the increase in free quinine concentrations observed when quinine was coadministered with LPV/r. However, since quinine was administered orally and has a low hepatic extraction ratio 54, the contribution of a change in plasma protein binding would be likely minimal.
The large reduction in systemic exposure of quinine and its active plasma metabolite, 3-hydroxyquinine, raises concern regarding the higher risk of treatment failure rate when quinine is prescribed to treat malaria in patients with HIV coinfection receiving LPV/r. However, this effect could be counterbalanced by a 3-fold increase in the pharmacologically active free quinine when given with LPV/r. Moreover, the pharmacokinetics of the quinine and LPV/r interaction would be even more complex in patients with malaria considering the increase in α1-acid glycoprotein with disease severity.49,50 Furthermore, multiple doses of quinine are used to treat malaria (600 mg three times a day for 7 days),12 and not a single dose was used in this study.
In vitro studies suggest that antiretroviral protease inhibitors may have activity against Plasmodium parasite,51 and could potentiate the efficacy of antimalarial drugs.52 A recent randomized study comparing treatment with nevirapine versus LPV/r in HIV-infected children in sub-Saharan Africa found that patients in the nevirapine arm had a significantly higher risk of developing malaria.24 High plasma protein binding of both lopinavir and ritonavir and lack of information on their penetration into red cells suggests that further work is required to establish the clinical relevance of these findings. The more favorable drug interaction profile of the LPV/r arm and potential antimalarial activity of this protease inhibitor may have contributed to this effect. The limitations of this study design are that the study was conducted in healthy subjects (quinine exposure is known to be increased during acute-phase malaria infection). In addition, quinine was given as a single dose (standard treatment is multiple dose of 600 mg, every 7 hours for 7 days), and the unbound concentrations were measured only at a single point (2 hours). Further investigation in patients with malaria and HIV coinfection is required to clarify the magnitude and clinical significance of these potential interactions before any decision on quinine dose optimization can be recommended.
ACKNOWLEDGMENTS
We would like to thank all study participants in this study for their voluntary participation, and members of the clinical trial study team of Mae Sot hospital.
Footnotes
Financial support: The study was supported by The National Research Council of Thailand (grant no. 034/2556), Commission on Higher education (NRU Project), and Thammasat University (Center of Excellence in Pharmacology and Molecular Biology of Malaria and Cholangiocarcinoma). Siwalee Rattanapunya receives financial support from the Office of the Higher Education Commission (OHEC) (grant no. 036/554) for her PhD Program Thai Doctoral degree, Chiang Mai Rajabhat University.
Authors' addresses: Siwalee Rattanapunya, Faculty of Science and Technology, Chiang Mai Rajabhat University, Chiang Mai, Thailand, E-mail: sirk2012@gmail.com. Tim R. Cressey and Yardpiroon Tawon, Faculty of Associated Medical Sciences, Chiang Mai University, Chiang Mai, Thailand, and Program for HIV Prevention and Treatment (PHPT/IRD URI 174), Department of Medical Technology, Chiang Mai University, Chiang Mai, Thailand, E-mails: tim.cressey@phpt.org and yardpiroon.tawon@phpt.org. Ronnatrai Ruengweerayut, Department of Medicine, Mae Sot Hospital, Tak Province, Thailand, E-mail: ronnatrai@hotmail.com. Panida Kongjam, Chulabhorn International College of Medicine, Thammasat University, Pathumthani, Thailand, and Graduate Program in Bioclinical Sciences, Chulabhorn International College of Medicine, Pathumthani, Thailand, E-mail: panida210@hotmail.com. Kesara Na-Bangchang, Faculty of Allied Health Sciences, Thammasat University (Rangsit Campus), Pathumthani, Thailand, E-mail: kesaratmu@yahoo.com.
References
- 1.World Health Organization The Top 10 Causes of Death. 2015. http://www.who.int/mediacentre/factsheets/fs310/en/ Available at. Accessed January 6, 2015.
- 2.World Health Organization Malaria and HIV Interactions and Their Implications for Public Health Policy. 2004. http://www.who.int/hiv/pub/prev_care/malariahiv.pdf Consultation on Malaria and HIV Interactions and Public Health Policy 2004. Available at. Accessed February 12, 2015.
- 3.Laufer MK, van Oosterhout JJG, Thesing PC, Thumba F, Zijlstra EE, Graham SM, Taylor TE, Plowe CV. Impact of HIV-associated immunosuppression on malaria infection and disease in Malawi. J Infect Dis. 2006;193:872–878. doi: 10.1086/500245. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman I, Jere C, Taylor T, Munthali P, Dyer JR, Wirima JJ, Rogerson SJ, Kumwenda N, Eron JJ, Fiscus SA, Chakraborty H, Taha TE, Cohen MS, Molyneux ME. The effect of Plasmodium falciparum malaria on HIV-1 RNA blood plasma concentration. AIDS. 1999;13:487–494. doi: 10.1097/00002030-199903110-00007. [DOI] [PubMed] [Google Scholar]
- 5.Orlov M, Vaida F, Williamson K. Antigen-presenting phagocytic cells ingest malaria parasites and increase HIV replication in a tumor necrosis factor alpha-dependent manner. J Infect Dis. 2014;210:1562–1572. doi: 10.1093/infdis/jiu317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alemu A, Shiferaw Y, Addis Z, Mathewos B, Birhan W. Effect of malaria on HIV/AIDS transmission and progression. Parasit Vectors. 2013;6:18–22. doi: 10.1186/1756-3305-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalwe V, Mukwamataba D, Menten J, Kamalamba J, Mulenga M, D'Alessandro U. Increased risk for severe malaria in HIV-1-infected adults, Zambia. Emerg Infect Dis. 2009;15:749–755. doi: 10.3201/eid1505.081009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soyinka JO, Onyeji CO, Omoruyi SI, Owolabi AR, Sarma PV, Cook JM. Pharmacokinetic interactions between ritonavir and quinine in healthy volunteers following concurrent administration. Br J Clin Pharmacol. 2010;69:262–270. doi: 10.1111/j.1365-2125.2009.03566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byakika-Kibwika P, Lamorde M, Okaba-Kayom V, Mayanja-Kizza H, Katabira E, Hanpithakpong W, Pakker N, Dorlo TP, Tarning J, Lindegardh N, de Vries PJ, Back D, Khoo S, Merry C. Lopinavir/ritonavir significantly influences pharmacokinetic exposure of artemether/lumefantrine in HIV-infected Ugandan adults. J Antimicrob Chemother. 2012;67:1217–1223. doi: 10.1093/jac/dkr596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris CA, Lopez-Lazaro L, Jung D, Methaneethorn J, Duparc S, Borghini-Fuhrer I, Pokorny R, Shin CS, Fleckenstein L. Drug-drug interaction analysis of pyronaridine/artesunate and ritonavir in healthy volunteers. Am J Trop Med Hyg. 2012;86:489–495. doi: 10.4269/ajtmh.2012.11-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyunt MM, Lu Y, El-Gasim M, Parsons TL, Petty BG, Hendrix CW. Effects of ritonavir-boosted lopinavir on the pharmacokinetics of quinine. Clin Pharmacol Ther. 2012;91:889–895. doi: 10.1038/clpt.2011.326. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization Guidelines for the Treatment of Malaria. 3rd edition. 2015. http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf Available at. Accessed February 12, 2015. [PubMed]
- 13.Achan J, Talisuna AO, Erhart A, Tibenderana JK, Baliraine FN, Rosenthal PJ, D'Alessandro U. Quinine, an old anti-malarial drug in a modern world: role in the treatment of malaria. Malar J. 2011;10:1475. doi: 10.1186/1475-2875-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bamford A, Turkova A, Lyall H, Foster C, Klein N, Bastiaans D, Burger D, Bernadi S, Butler K, Chiappini E, Clayden P, Della Negra M, Giacomet V, Giaquinto C, Gibb D, Galli L, Hainaut M, Koros M, Marques L, Nastouli E, Niehues T, Noguera-Julian A, Rojo P, Rudin C. Paediatric European Network for Treatment of AIDS (PENTA) guidelines for treatment of paediatric HIV-1 infection 2015: optimizing health in preparation for adult life. HIV Med. 2015;3:12217. doi: 10.1111/hiv.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed BS, Phelps BR, Reuben EB, Ferris RE. Does a significant reduction in malaria risk make lopinavir/ritonavir-based ART cost-effective for children with HIV in co-endemic, low-resource settings? Trans R Soc Trop Med Hyg. 2014;108:49–54. doi: 10.1093/trstmh/trt108. [DOI] [PubMed] [Google Scholar]
- 16.Eagling VA, Back DJ, Barry MG. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997;14:190–194. doi: 10.1046/j.1365-2125.1997.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sevrioukova IF, Poulos TL. Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc Natl Acad Sci USA. 2010;107:18422–18427. doi: 10.1073/pnas.1010693107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao XJ, Yokoyama H, Chiba K, Chiba K, Wanwimolruk S, Ishizaki T. Identification of human cytochrome P450 isoforms involved in the 3-hydroxylation of quinine by human live microsomes and nine recombinant human cytochromes P450. J Pharmacol Exp Ther. 1996;279:1327–1334. [PubMed] [Google Scholar]
- 19.Lakhman SS, Ma Q, Morse GD. Pharmacogenomics of CYP3A: considerations for HIV treatment. Pharmacog. 2009;10:1323–1339. doi: 10.2217/pgs.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang H, Coville PF, Walker RJ, Miners JO, Birkett DJ, Wanwimolruk S. Evidence for involvement of human CYP3A in the 3-hydroxylation of quinine. Br J Clin Pharmacol. 1997;43:245–252. doi: 10.1046/j.1365-2125.1997.00556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wyen C, Fuhr U, Frank D, Aarnoutse RE, Klaassen T, Lazar A, Seeringer A, Doroshyenko O, Kirchheiner JC, Abdulrazik F, Schmeisser N, Lehmann C, Hein W, Schömig E, Burger DM, Fätkenheuer G, Jetter A. Effect of an antiretroviral regimen containing ritonavir boosted lopinavir on intestinal and hepatic CYP3A, CYP2D6 and P-glycoprotein in HIV-infected patients. Clin Pharmacol Ther. 2008;84:75–82. doi: 10.1038/sj.clpt.6100452. [DOI] [PubMed] [Google Scholar]
- 22.Kumar GN, Jayanti VK, Johnson MK, Uchic J, Thomas S, Lee RD, Grabowski BA, Sham HL, Kempf DJ, Denissen JF, Marsh KC, Sun E, Roberts SA. Metabolism and disposition of the HIV-1 protease inhibitor lopinavir (ABT-378) given in combination with ritonavir in rats, dogs, and humans. Pharm Res. 2004;21:1622–1630. doi: 10.1023/b:pham.0000041457.64638.8d. [DOI] [PubMed] [Google Scholar]
- 23.Schon A, del Mar Ingaramo M, Freire E. The binding of HIV-1 protease inhibitors to human serum proteins. Biophys Chem. 2003;105:221–230. doi: 10.1016/s0301-4622(03)00071-1. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima K, Kobuchi S, Mizuhara K, Aoyama H, Takada K, Sugioka N. Time-dependent interaction of ritonavir in chronic use: the power balance between inhibition and induction of P-glycoprotein and cytochrome P450 3A. J Pharm Sci. 2013;102:2044–2055. doi: 10.1002/jps.23545. [DOI] [PubMed] [Google Scholar]
- 25.Greman P, Parikh S, Lawrence J, Dorsey G, Rosenthat PJ, Havlir D, Charlebois E, Hanpitakpong W, Lindergardh N, Aweeka FT. Lopinavir/ritonavir affects pharmacokinetic exposure of artemether/lumefantrine in HIV-uninfected healthy volunteers. J Acquir Immune Defic Syndr. 2009;51:424–429. doi: 10.1097/QAI.0b013e3181acb4ff. [DOI] [PubMed] [Google Scholar]
- 26.Achan J, Kakuru A, Ikilezi G, Ruel T, Clark TD, Nsanabana C, Charlebois E, Aweeka F, Dorsey G, Rosenthal PJ, Havlir D, Kamya MR. Antiretroviral agents and prevention of malaria in HIV-infected Ugandan children. N Engl J Med. 2012;367:2110–2118. doi: 10.1056/NEJMoa1200501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karbwang J, Na Bangchang K, Molunto P, Bunnag D. Determination of quinine and quinidine in biological fluids by high performance liquid chromatography. Southeast Asian J Trop Med Public Health. 1989;20:65–69. [PubMed] [Google Scholar]
- 28.National Cancer Institute . The NCI Common Terminology Criteria for Adverse Events v3.0 (CTCAE) U.S. Department of Health and Human Service; 2003. pp. 1–71.http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf Available at. Accessed April 2, 2012. [Google Scholar]
- 29.Droste JA, Verweij-Van Wissen CP, Burger DM. Simultaneous determination of the HIV drugs indinavir, amprenavir, saquinavir, ritonavir, lopinavir, nelfinavir, the nelfinavir hydroxymetabolite M8, and nevirapine in human plasma by reversed-phase high-performance liquid chromatography. Ther Drug Monit. 2008;25:393–399. doi: 10.1097/00007691-200306000-00023. [DOI] [PubMed] [Google Scholar]
- 30.DiFrancesco R, Tooley K, Rosenkranz SL, Siminski S, Taylor CR, Pande P, Morse GD. Clinical pharmacology quality assurance for HIV and related infectious diseases research. Clin Pharmacol Ther. 2013;93:479–482. doi: 10.1038/clpt.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gibaldi M, Perrier D. Non-compartmental analysis based on statistical moment theory. Pharmacokinetics. 1982;2:409–417. [Google Scholar]
- 32.US FDA . Guidance for Industry: Drug Interaction Studies—Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations. Rockville, MD: Food and Drug Administration; 2012. pp. 39–54. [Google Scholar]
- 33.Bongiovanni M, Cicconi P, Landonio S. Predictive factors of lopinavir/ritonavir discontinuation for drug-related toxicity: results from a cohort of 416 multi-experienced HIV-infected individuals. Int J Antimicrob Agents. 2005;26:88–91. doi: 10.1016/j.ijantimicag.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Banhegyi D, Katlama C, da Cunha CA, Schneider S, Rachlis A, Workman C, De Meyer S, Vandevoorde A, Van De Casteele T, Tomaka F. Week 96 efficacy, virology and safety of darunavir/r versus lopinavir/r in treatment-experienced patients in TITAN. Curr HIV Res. 2012;10:171–181. doi: 10.2174/157016212799937218. [DOI] [PubMed] [Google Scholar]
- 35.Hermes A, Squires K, Fredrick L, Martinez M, Pasley M, Trinh R, Norton M. Meta-analysis of the safety, tolerability, and efficacy of lopinavir/ritonavir-containing antiretroviral therapy in HIV-1-infected women. HIV Clin Trials. 2012;13:308–323. doi: 10.1310/hct1306-308. [DOI] [PubMed] [Google Scholar]
- 36.AlKadi HO. Antimalarial drug toxicity: a review. Chemother. 2007;53:385–391. doi: 10.1159/000109767. [DOI] [PubMed] [Google Scholar]
- 37.Flexner C, Tierney C, Gross R. Comparison of once-daily versus twice-daily combination antiretroviral therapy in treatment-naive patients: results of AIDS clinical trials group (ACTG) A5073, a 48-week randomized controlled trial. Clin Infect Dis. 2010;50:1041–1052. doi: 10.1086/651118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mirghani RA, Hellgren U, Bertilsson L, Gustafsson LL, Ericsson O. Metabolism and elimination of quinine in healthy volunteers. Eur J Clin Pharmacol. 2003;59:423–427. doi: 10.1007/s00228-003-0637-8. [DOI] [PubMed] [Google Scholar]
- 39.Hull MW, Montaner JSG. Ritonavir-boosted protease inhibitors in HIV therapy. Ann Med. 2011;43:375–388. doi: 10.3109/07853890.2011.572905. [DOI] [PubMed] [Google Scholar]
- 40.Hesse LM, von Moltke LL, Shader RI, Greenblatt DJ. Ritonavir, efavirenz, and nelfinavir inhibit CYP2B6 activity in vitro: potential drug interactions with bupropion. Drug Metab Dispos. 2001;29:100–102. [PubMed] [Google Scholar]
- 41.Kharasch ED, Mitchell D, Coles R, Blanco R. Rapid clinical induction of hepatic cytochrome P4502B6 activity by ritonavir. Antimicrob Agents Chemother. 2008;52:1663–1669. doi: 10.1128/AAC.01600-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeh RF, Gaver VE, Patterson KB, Rezk NL, Baxter-Meheux F, Blake MJ, Eron JJ, Jr, Klein CE, Rublein JC, Kashuba AD. Lopinavir/ritonavir induces the hepatic activity of cytochrome P450 enzymes CYP2C9, CYP2C19, and CYP1A2 but inhibits the hepatic and intestinal activity of CYP3A as measured by a phenotyping drug cocktail in healthy volunteers. J Acquir Immune Defic Syndr. 2006;42:52–60. doi: 10.1097/01.qai.0000219774.20174.64. [DOI] [PubMed] [Google Scholar]
- 43.Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42:1048–1059. doi: 10.1345/aph.1K615. [DOI] [PubMed] [Google Scholar]
- 44.Annaert P, Ye ZW, Stieger B, Augustijn S. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica. 2010;40:163–176. doi: 10.3109/00498250903509375. [DOI] [PubMed] [Google Scholar]
- 45.Lubomirov R, di Iulio J, Fayet A, Colombo S, Martinez R, Marzolini C, Furrer H, Vernazza P, Calmy A, Cavassini M, Ledergerber B, Rentsch K, Descombes P, Buclin T, Decosterd LA, Csajka C, Telenti A, Swiss HIV Cohort Study ADME pharmacogenetics: investigation of the pharmacokinetics of the antiretroviral agent lopinavir coformulated with ritonavir. Pharmacogenet Genomics. 2010;20:217–230. doi: 10.1097/FPC.0b013e328336eee4. [DOI] [PubMed] [Google Scholar]
- 46.Hartkoorn RC, Kwan WS, Shallcross V, Chaikan A, Liptrott N, Egan D, Sora ES, James CE, Gibbons S, Bray PG, Back DJ, Khoo SH, Owen A. HIV protease inhibitors are substrates for OATP1A2, OATP1B1 and OATP1B3 and lopinavir plasma concentrations are influenced by SLCO1B1 polymorphisms. Pharmacogenet Genomics. 2010;20:112–120. doi: 10.1097/FPC.0b013e328335b02d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bierman WF, Scheffer GL, Schoonderwoerd A, Jansen G, van Agtmael MA, Danner SA, Scheper RJ. Protease inhibitors atazanavir, lopinavir and ritonavir are potent blockers, but poor substrates, of ABC transporters in a broad panel of ABC transporter-overexpressing cell lines. J Antimicrob Chemother. 2010;65:1672–1680. doi: 10.1093/jac/dkq209. [DOI] [PubMed] [Google Scholar]
- 48.Silamut K, Molunto P, Ho M, Davis TM, White NJ. Alpha 1-acid glycoprotein (orosomucoid) and plasma protein binding of quinine in falciparum malaria. Br J Clin Pharmacol. 1991;32:311–315. doi: 10.1111/j.1365-2125.1991.tb03904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pussard E, Merzouk M, Barennes H. Increased uptake of quinine into the brain by inhibition of P-glycoprotein. Eur J Pharm Sci. 2007;32:123–127. doi: 10.1016/j.ejps.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 50.Silamut K, White N, Looareesuwan S, Warrell DA. Binding of quinine to plasma proteins in falciparum malaria. Am J Trop Med Hyg. 1985;34:681–686. doi: 10.4269/ajtmh.1985.34.681. [DOI] [PubMed] [Google Scholar]
- 51.Nsanzabana C, Rosenthal P. In vitro activity of antiretroviral drugs against Plasmodium falciparum. Antimicrob Agents Chemother. 2011;55:5073–5077. doi: 10.1128/AAC.05130-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin R, Butterworth A, Gardiner D, Kirk K, McCarthy JS, Skinner-Adams TS. Saquinavir inhibits the malaria parasite's chloroquine resistance transporter. Antimicrob Agents Chemother. 2012;56:2283–2289. doi: 10.1128/AAC.00166-12. [DOI] [PMC free article] [PubMed] [Google Scholar]