Abstract
Objective
While the safety and efficacy of peripheral nerve blocks for postoperative pain management has been established in several well controlled prospective trials, the local anesthetic (LA) concentration and volume used in these studies was associated with a significant increase muscle weakness due to motor nerve block. The purpose of the present retrospective study of patients undergoing total knee arthroplasty (TKA) was to assess the relative analgesic efficacy and functional outcomes of the low concentration, low volume of LA used in peripheral nerve blocks for postoperative pain management.
Methods
Twenty-four months of de-identified patient data were extracted from an electronic medical record system. All patients received opioids with or without continuous femoral and sciatic nerve block infusions for postoperative analgesia. Pain (resting and with activity), cumulative opioid and local anesthetic (LA) use were primary endpoints, participation in physical therapy, muscle strength deficits and length of hospital stay were secondary endpoints.
Results
Postoperative pain and opioid use were significantly lower in patients with peripheral nerve blocks (n = 1,329) than those with opioids alone (n = 439). There was no detectable decrease in strength associated with nerve blocks, while a significantly greater proportion of patients with nerve blocks were able to participate in physical therapy (PT) on postoperative day 1 (96.4% vs. 57.1%). These differences were not due to the impact of the surgeon per se, but whether or not the surgeon used nerve blocks for pain management. There was a small but statistically significant decrease in the average length of hospital stay in patients with blocks.
Conclusion
This analysis supports the use of low concentration, low volume of LA based peripheral nerve blocks for post-operative pain management.
Introduction
Available evidence suggests that peripheral nerve block with local anesthetics (LA) is an effective component of postoperative pain management particularly for patients undergoing complex procedures on peripheral limbs (1). Reported advantages of the use of such regional anesthesia techniques include the decrease in the need for systemic analgesics, in particular opioids, thereby minimizing opioid related side effects such as somnolence, nausea and respiratory depression (2). Opioid sparing enables earlier initiation of ambulation and physical therapy, resulting in earlier discharge times and overall high levels of patient satisfaction (3). While the safety and efficacy of peripheral nerve blocks has been established in several well controlled prospective trials (4–6), the LA concentrations and volumes used in these studies were relatively high, ranging from 0.1 to 0.5% ropivocaine and 0.1 to 0.2% bupivacaine at flow rates of up to 10ml/hr, and consequently associated with a significant increase muscle weakness due to motor nerve block. This is potentially problematic because these undesirable side effects may increase the risks of falls and preclude access to physical therapy (7, 8). Concerns about the relative efficacy of peripheral nerve blocks and early participation in physical therapy are particularly important in light of evidence that these issues may impact the functional outcomes of TKA procedures (9–11). A strategy with which to minimize the potential deleterious consequences associated with motor block while preserving analgesic efficacy and opioid sparing is to use continuous nerve block techniques with low concentration (0.03 to 0.0625% bupivacaine), low volume (3–5 ml/hr) of LAs. The purpose of the present retrospective study of patients undergoing total knee arthroplasty (TKA) was therefore to assess the relative analgesic efficacy and functional outcomes of the low concentration, low volume of LA used in peripheral nerve blocks for postoperative pain management.
Materials and Methods
The UPMC electronic medical records system was originally screened by the UPMC Center for Assistance in Research using eRecord (CARe) for all patients who underwent TKA procedures, as defined by the ICD-9 procedural code 81.54 and 81.55/00.80. The screen spanned the dates from September 2010 to August 2012 and was restricted to a single Hospital: UPMC Shadyside. Data for all patients identified by these initial screening criteria included the surgical procedure performed, demographic data (age, sex, race/ethnicity), surgeon, pain scores, drug use, and physical therapy data. Two Good Faith Brokers, independent researchers who did not directly participate in data collection and analysis, checked the integrity of the data set generated from this screen prior to de-identification and subsequent analysis.
Patients in the data set were considered for further analysis subject to the following inclusion and exclusion criteria. Inclusion criteria included: 1) Age 18 and older, to avoid complications associated the management of pain in adolescent patients; and 2) males and females of all races. Exclusion criteria included: 1) TKA due to trauma; 2) patients undergoing an uni-compartment, a bilateral procedure or a revision of a previous procedure; 3) patients in which there were missing values for assessments of pain, local anesthetic, and/or opioid use (as indicated by gaps in the medical record of > 12 hrs).
Data for each patient was analyzed by day, where all data on the first day of the record was considered to be associated with the post-anesthesia care unit (PACU), and each subsequent day was considered postoperative day (POD) 1, 2, 3 etc. Postoperative pain was based of patient reports recorded by nurses or physical therapists using the 11-point visual analog scale (VAS) where 0 is no pain and 10 is the worst pain imaginable. Pain assessed by nurses was considered to be a measure of pain at rest and that assessed by the physical therapists was considered to be a measure of pain with activity. Pain at rest was determined from the average of the VAS values reported in the PACU and on POD 1, 2 and 3. Ongoing pain was assessed on a regular basis in conjunction with the assessment of other vital signs throughout the hospital stay at a frequency of at least once every 12 hours, although the frequency was considerably higher in the PACU and on POD1. An average VAS score was used in an effort to counter the impact of fluctuations in VAS scores in association with events such as the bolus administration of analgesic or a physical therapy. As a control for the potential impact of sampling bias associated with averaging VAS values over a 24 hour period, or at least to account for the impact of changes in pain over time, we also calculated a time weighted pain score for each patient. This was determined as
where the sum of product of the average VAS and the time period during which VAS were recorded, is divided by the sampling interval (i.e. total time in PACU, 24hr on POD1, etc). The average VAS values recorded by the physical therapist in association with physical therapy were considered the pain with activity.
All patients were visited twice by a physical therapist on POD1 and at least once per day on all subsequent days throughout the hospital stay. If possible, physical therapy (PT) was initiated on POD1. PT consisted of progressive knee ROM (Range of Motion), active and passive stretching of quadriceps muscles, neuromuscular re-education, and functional muscular training. Muscle strength was evaluated and compared with that on the contralateral side. Muscle strength was recorded as a modified Bromage scale (0–5; 0: unable to move feet or knees; 1: able to move feet only; 2: just able to move knees; 3: detectable weakness of quadriceps muscles while supine with full extension of knees; 4: no detectable weakness of quadriceps muscles while supine; 5: able to perform knee bend). The difference between sides ipsi- and contralateral to the surgery was used to determine if there was a strength deficit in the treated leg, ranging from 0 (no deficit) to 5 (complete deficit). A single averaged VAS score was used to estimate the pain associated with all manipulations. Patients were considered to have been unable to participate in PT if therapist notes included “Not able to participate” and no pain scores were provided.
Opioid use was calculated by taking the sum of all opioids administered via PCA and/or p.o. over each time interval (PACU, POD1, 2 and 3), for each patient. This value was converted to equi-analgesic dosage of intravenous morphine (12). Combination opioid and non-opioid analgesic medications were sub-divided into each component (opioid/non-opioid), and only the opioid component was included in the total opioid consumption. For example, Percocet (Endo Pharmaceuticals, Chadds Ford, PA, USA, 5 mg oxycodone/325 mg acetaminophen) was separated into the opioid (oxycodone) and non-opioid (acetaminophen) categories. The 5 mg oxycodone was converted into a 0.75 mg intravenous morphine equivalent.
Local anesthetic (LA) use was calculated by taking the sum of the bupivacaine used in milligrams through continuous nerve block catheters and boluses at each time interval (PACU, POD1, 2 and 3).
While the focus of this retrospective study was on the impact of peripheral nerve blocks on the analgesia and functional outcomes following TKA, we also analyzed the impact of additional analgesic administered to each patient, which included acetaminophen, ketorolac, celecoxib, and gabapentin/pregabalin. While ketamine has been folded into the standard post-op analgesia protocol, this is a relative recent change. Consequently, a very small number of patients (n = 10) received ketamine. Because of the synergistic interaction between ketamine and opioids, data from these patients were excluded from further analysis.
Surgical notes were not extracted as part of the electronic medical record. This is because the intraoperative anesthesia procedures used for orthopedic procedures at UPMC Shadyside have been standardized. These include regional anesthesia with spinal block with 0.75% bupivacaine and 50–75 mcg /kg/ min propofol.. Intraoperative analgesia includes ketamine (20 mg i.v.) and acetaminophen (1000 mg i.v.).
There are two standard protocols used for postoperative pain management of orthopedic patients at UPMC Shadyside. Patients for whom the surgeon elected not to use a regional nerve block were started in the PACU with a Patient Controlled Analgesia (PCA unit) set to deliver hydromorphone 0.2 mg, q 8 min, 1.2 mg as one hour limit, with 0.3 mg / 30 min as RN boluses, together with non-opioid analgesics, such as acetaminophen 325 mg, q 6 hr, Celecoxib 200 mg, b.i.d. and pregabalin 150 mg b.i.d. PCA hydromorphone is usually stopped on POD1 and patients are switched to p.o. opioids, such as oxycodone (5 or 10 mg, q 4 hr.).
Patients for whom the surgeon elected to use systemic opioids in combination with regional nerve block had perineural catheters placed preoperatively with nerve stimulation and/or under ultrasound guidance. Sciatic nerve block catheters were placed between the great trochanter and ischial tuberocity below the gluteal maximus (gluteal approach), and therefore above the surgical tourniquet. The femoral catheter was flushed one-time with 20 ml of 0.2% ropivacaine, while the sciatic catheter was flushed with 10 ml of normal saline. Continuous LA infusions were started postoperatively in the PACU in patients responding normally to sensory and motor function tests with 0.0625% bupivacaine, 5 ml/h for femoral nerve block and 0.03% bupivacaine, 3 ml/h for sciatic nerve block. Patients received up to an additional 3 ml/h of LA in boluses through nerve block catheters as needed. Sciatic nerve infusion was usually terminated and the catheter removed on POD2, and femoral nerve block infusion was usually terminated and the catheter removed on POD3 to maximize the patients' mobility. Opioid based systemic analgesia was provided via a protocol identical to those patients who did not receive a peripheral nerve block. Patients were followed postoperatively by the Acute Interventional Perioperative Pain Service (AIPPS) team so as to receive necessary titration of the nerve block infusion and / or boluses. As part of the opioid sparing strategy behind the use of peripheral nerve blocks, the standard protocol was to bolus the nerve block catheters as the first step toward addressing a patients pain control needs. The PCA was only used if adequate pain relief was not achieved with peripheral nerve block. As post-op pain decreases, patients were transitioned from peripheral nerve blocks to PO analgesics.
Statistics
Statistical analysis was performed with SPSS 19.0 statistical software package (SPSS, Chicago, IL, USA). Data were categorized with the Shapiro-Wilk test to determine the distribution of the dataset. Because the length of hospital stay and muscle strength data were not normally distributed, these data were log transformed prior to statistical analysis. Group by day comparisons were made with a mixed design two-way ANOVA. The impact of surgeon on postoperative pain and opioid consumption was analyzed with a one-way ANOVA, as well as a mixed ANOVA with surgeon and use of nerve block included as independent variables. The Holm-Sidak test was used for post-hoc comparisons. Single day group comparisons were made with a t-test. Categorical variables (PT participation) were compared using the Chi- square test with Pearson correction or Fisher's exact test as appropriate. A test with a two sided P- value < 0.05 was considered statistically significant
Results
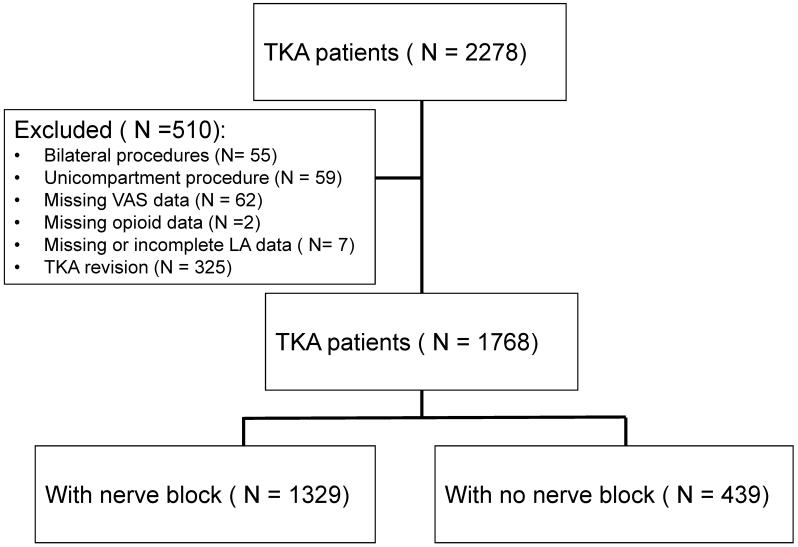
The initial data set contained 2278 patients. Of these, 55 were excluded because they underwent bilateral TKA, 59 were excluded because they underwent uni-compartment surgery, and 325 were excluded because they underwent a TKA revision. An additional 62 patients were excluded because of missing VAS data, and two were excluded because of missing opioid data. Three patients received LA, but were excluded because of missing LA data and 4 patients were excluded because LA infusions were stopped on POD1 for reasons such as low blood pressure. This left 1,768 patients for subsequent analysis. Of these, 1,329 patients received peripheral nerve blocks, and 439 did not. The selection of the patients participating in the data analysis is summarized in Figure 1. The demographic data for the patients are summarized in Table 1. Fifty-two percent of the patients in the data set were women (n = 920). The average age was comparable between the two groups. The racial and ethnic mix of the patient population was consistent with the population at large in the western Pennsylvania area (Table 1).
Figure 1.
Flow of screening process used inclusion of patient data extracted from the electronic medical record system in the retrospective analysis.
Table 1.
Demographics
| Variable | Block (n= 1329) | No block (n = 439) | P value | |
|---|---|---|---|---|
| Age | Years (mean ± SEM) | 65.2 ± 0.5 | 63.9 ± 1.4 | 0.273 |
| Sex | male: female | 648: 681 | 200: 239 | 0.268 |
| Race/ethnicity (%) | African American | 210 (16) | 74(17) | <0.01 |
| Asian | 5 (0.3) | 2(0.5) | ||
| Caucasian | 944 (71) | 343(78) | ||
| Hispanic | 3 (0.2) | - | ||
| Other | 167 (13) | 20(4.6) |
Block is with nerve block and No block is patients managed on opioids alone. While there was no significant influence of race/ethnicity on pain, local anesthetic or opioid use within a group, the distribution of patients was significantly different between groups. It is unlikely, however, that this difference is clinically meaningful.
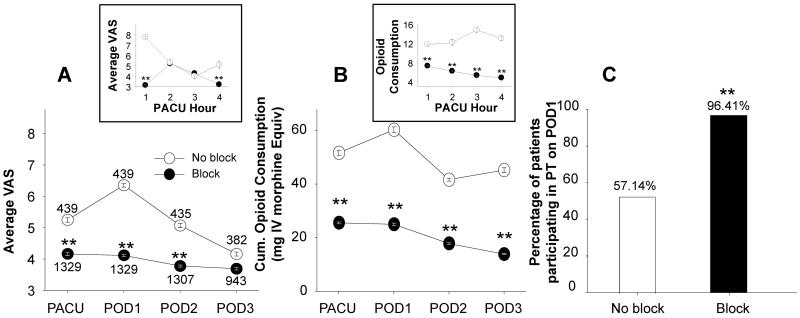
Postoperative pain, measured as VAS at rest and VAS with activity, and postoperative opioid use were analyzed in TKA patients with or without peripheral nerve blocks. TKA patients with peripheral nerve blocks had significantly lower resting pain in PACU and on POD1 and 2 (p < 0.01, Two way ANOVA with Holm-Sidak post-hoc test). Although pain with activity, was comparable between the two groups: it was 6.93 ± 0.04 and 7.06 ± 0.08 in the block and no-block groups, respectively (p >0.05). The opioid consumption was significantly lower in patients with nerve blocks than those without (p <0 .01) (Figure 2B).
Figure 2.
Pain, opioid use and PT participation in TKA patients with and without peripheral nerve blocks. Patient report of pain intensity on the visual analog scale (VAS) was used to assess average pain at rest (A), in patient with and without peripheral nerve blocks in the post-anesthesia care unit (PACU), and on post-operative day (POD), 1, 2 and 3. Data were analyzed with a mixed design two-way ANOVA which revealed patients with nerve blocks were associated with significantly less pain (A) and less opioid use (B) (p<0.01). Percentage of patients participating in PT on POD 1 was compared between those with blocks and those without using Chi-square test (C). The analysis indicated that significantly more patients with blocks were able to participate in PT on POD1 (C) (p<0.01). ** is p < 0.01
In order to assess the impact of pre-operative LA administration on pain scores and opioid consumptions in PACU, data were analyzed on an hourly basis over the ~4 hours when most of the patients stayed in the PACU (Figure 2 insets). Patients with missing data were excluded. Analysis of this data set revealed a statistically significant (p < 0.05, two-way mixed design ANOVA) difference between block and no block groups with respect to pain scores and opioid consumption over this four hour window. There does not appear to be a dramatic effect of the pre-operative LA bolus on pain, as pain increased in the block group in the second hour in the PACU. This observation argues against a significant contribution of the pre-operative block on post-operative pain or opioid consumption.
To assess the impact of peripheral nerve blocks on patients' ability to participate in physical therapy, we compared block vs no-block groups with respect to the number of patients who were “not able to participate in PT” on POD 1. The number of patients who were able to participate in PT on POD 1 was significantly higher in the block patients (96.41%) compared to those with no blocks (57.14%, p < 0.01) (Figure 2C).
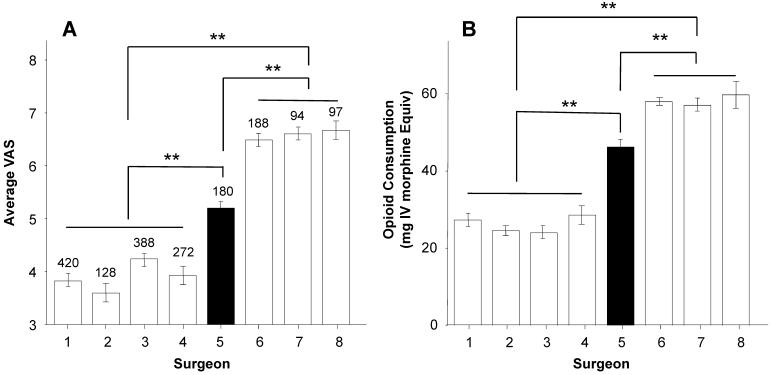
To assess the relative impact of surgeon on pain score and opioid use, pain score and opioid use on POD1 for block and no-block patients were analyzed by surgeon (Figure 3). Results of this analysis revealed significant (p < 0.01, one-way ANOVA) differences between surgeons with respect to both pain (Figure 3A) and opioid consumption (Figure 3B). Higher resting pain and opioid consumption were associated with surgeons who did not use nerve blocks for their TKA patients (Surgeons 6,7,8), compared with those that did (Surgeons 1,2,3,4). Resting pain and opioid use in the patients from surgeon 5 were significantly different from that of any other surgeon. This difference appeared to be due to the fact that this surgeon had roughly equal numbers of patients with and without nerve blocks. Given that there were largely different surgeons who did and did not use nerve blocks, these data were reanalyzed with a mixed ANOVA in which surgeon and nerve block were included as independent variables. Results of this analysis confirmed that there was a significant influence of surgeon, but this depended on the use of nerve block for both pain and opioid consumption (Figure 3, inset). Post-hoc analysis indicated that with the exception of surgeon 5, who was different from all other surgeons, the only differences between surgeons were between those who did and those who did not use nerve blocks.
Figure 3.
Average resting pain (A) and opioid use (B) on POD1 in TKA patients analyzed as a function of the surgeon who performed the procedure. Surgeon 1,2, 3 and 4 used nerve blocks for their TKA patients, while surgeon 6,7 and 8 did not. Surgeon 5 had roughly equal numbers of patients who received nerve blocks vs. no blocks. The number of patients for each surgeon is indicated above the bars. + indicates patients with peripheral nerve blocks, − indicates patients with no nerve blocks; ** is p < 0.01
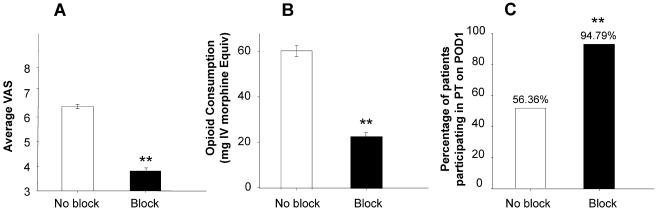
To further rule out the possibility that the differences between block and no-block patients were due to the post-operative pain management strategy rather than surgeon, we further analyzed the patients from surgeon 5. Results of this analysis were consistent with the results of the analysis of the total population of block and no-block patients with resting pain and opioid use significantly (p < 0.01, t-test) lower in the patients with blocks than those without (Figure 4). Data from POD1 have been plotted for clarity. Pain with activity data from the patients from surgeon 5 also were comparable to the total population of block and no-block patients: pain with activity was 7.04 ± 0.14 and 7.03 ± 0.18 in the block and no-block groups, respectively (p >0.05). Finally, PT participation rates on POD1 for block and no-block patients from surgeon 5 were also comparable to those observed for all surgeons combined (Figure 4C).
Figure 4.
Pain (A), opioid use (B) and PT participation (C) on POD1 for patients from surgeon #5 in Figure 3 analyzed as a function of whether or not the received peripheral nerve blocks. The number of patients in each group is indicated in parentheses above the columns. ** is p < 0.01
To begin to address the possibility that peripheral nerve blocks may be associated with an increased risk of falls because of an increase in muscle weakness, we compared muscle strength data recorded during the PT assessment. On POD1, there was no significant difference between the block and no block groups with respect to the magnitude (p > 0.05, t-test) of muscle weakness (Table 2). The average difference score (treated – untreated leg) was less than 1 for both groups, indicating that muscle strength and motor function are largely preserved in TKA patients regardless of whether or not they received peripheral nerve blocks.
Table 2.
Muscle strength and PT participation: POD 1
| Block | No block | P value | ||
|---|---|---|---|---|
| All surgeons | N = 1329 | N = 439 | ||
| Strength deficit * | 0.96 (0.89–1.03) | 0.91 (0.80–1.03) | 0.102 | |
| Surgeon 5 | N = 96 | N = 110 | ||
| Strength deficit * | 0.95 (0.88–1.02) | 0.92 (0.82–1.07) | 0.95 |
Block is with nerve block and No block is patients managed on opioids alone.
Muscle strength deficit (Strength deficit) was determined by the difference in Modified Bromage scale between the surgery side and contralateral side: 0 = no deficit; 5 = complete deficit
Mean (95% CI)
It has been suggested that early mobilization with improved pain control after TKA procedures can accelerate hospital discharge (13, 14). We, therefore assessed whether there were differences between the block and no-block groups with respect to length of hospital stay (LOS). The results of this analysis indicated that while the difference was small, LOS was significantly shorter for block than for no-block patients (p < 0.03, Table 3). The LOS difference between these two groups of patients from surgeon 5 was also significant (p < 0.02, Table 3).
Table 3.
Length of hospital stay (LOS)
| Block | No block | P value | ||
|---|---|---|---|---|
| All surgeons | N = 1329 | N = 439 | ||
| Length of stay * | 3.61 (3.56–3.67) | 3.76 (3.67–3.86) | <0.02 | |
| Surgeon 5 | N = 96 | N = 110 | ||
| Length of stay * | 3.63 (3.59–3.70) | 3.72 (3.68–3.81) | <0.03 |
Block is with nerve block and No block is patients managed on opioids alone.
Mean (95% CI)
In addition to the potential impact of surgeon on pain and opioid use, we assessed the impact of age (15), sex (16) and BMI (17). Pearce correlation analyses revealed that there was no significant influence of any of these three factors on postoperative pain, opioid or local anesthetic use (p > 0.05, data not shown).
Finally, because patients did not necessarily receive the same analgesics in addition to LA and/or opioids we assessed the possibility that these additional drugs influenced resting pain and/or opioid/LA use. We analyzed data as a function of whether or not patients received additional analgesics as well as by the type of analgesic received. Results of this analysis are summarized in Table 4. Strikingly, despite a relatively high percentage of patients receiving a variety of additional pain related medications, none of these had a significant influence on pain, opioid or LA use (Table 4). Further analysis of the results from the patients who received the highest doses of the additional analgesics still revealed no significant impact of these medications on pain scores and opioid use.
Table 4.
Influence of other pain medications
| Block | P Value | No block | P Value | ||||
|---|---|---|---|---|---|---|---|
| + | − | + | − | ||||
| Average VAS | Acetaminophen | 4.15 ± 0.4 | 4.2 ± 0.1 | 0.893 | 6.6 ± 0.2 | 6.2 ± 0.3 | 0.445 |
| Celecoxib | 4.1± 0.1 | 4.3 ± 0.1 | 0.158 | 6.4 ± 0.1 | 6.4 ± 0.3 | 1.000 | |
| Ketorolac | 4.3 ± 0.2 | 4.2 ± 0.6 | 0.975 | 7.0 ± 0.2 | 6.4 ± 0.2 | 0.235 | |
| Gabapentin / pregabalin | 4.2 ± 0.1 | 4.3 ± 0.1 | 0.747 | 6.5 ± 0.2 | 6.3 ± 0.2 | 0.692 | |
| Opioid use (mg) | Acetaminophen | 27.5 ± 1.4 | 27.5 ± 1.1 | 1.000 | 58.9 ± 2.7 | 68.0 ± 4.8 | 0.409 |
| Celecoxib | 25.8 ± 1.2 | 28.5 ± 1.3 | 0.128 | 61.4 ± 2.8 | 65.5 ± 5.9 | 0.781 | |
| Ketorolac | 24.9 ± 2.8 | 26.5 ± 0.7 | 0.675 | 55.4 ± 3.7 | 59.3 ± 2.4 | 0.527 | |
| Gabapentin / pregabalin | 25.7 ± 0.95 | 27.9 ± 1.2 | 0.466 | 59.3 ± 2.5 | 63.0 ± 2.6 | 0.572 | |
Block is with nerve block and No block is patients managed on opioids alone. "+" is with the additional pain medication and "−" is without additional pain medication.
Mean ± SE
Of note, for patients who received nerve blocks (n=1329), 1022 patients received acetaminophen (500–625 mg, q6h), 904 patients received celecoxib (200 mg, q12h), 431 patients received ketorolac (15 mg, i.v q6h), and 935 patients received gabapentin or pregabalin (gabapentin: 300–600 mg, q8h; pregabalin: 50–75 mg, q12h). For patients who did not receive nerve blocks (n= 439), 407 patients received acetaminophen, 412 received celecoxib, 217 received ketorolac, and 426 received gabapentin or pregabalin.
Discussion
The purpose of the study was to assess the relative analgesic efficacy and functional outcomes of the low concentration, low volume of LA used in peripheral nerve blocks for postoperative pain management. Results of our retrospective analysis indicated that postoperative resting pain and opioid use were significantly reduced in patients receiving peripheral nerve blocks. A significantly greater proportion of patients with nerve blocks were able to participate in physical therapy in POD1 yet no significant influence of peripheral nerve block on muscle strength was detected. In addition, the length of hospital stay was significantly shorter for patients receiving nerve blocks than those without. Finally, there was no influence of sex, age, obesity, surgeon or the use of additional analgesics on post-operative pain scores and opioid use.
Clinical benefits of peripheral nerve block in managing postoperative pain in TKA patients have been reviewed in the literature, and were supported by a variety of organizations such as the American Society of Anesthesiologists (2012) (18). The clinical benefits of peripheral nerve blocks previously described were further substantiated with the results of the present study. These were most readily demonstrated by improved pain control where regional block was associated with maximal decrease in VAS greater than 2 points, a change which has been described as at least moderately clinically meaningful (19). As previously documented, this better pain control was achieved in concert with significant opioid sparing, where opioid consumption of POD1 in the block group was less than half of that in the no-block group. While not assessed in the present study, such a reduction in opioid consumption has previously been shown to be associated with a significant reduction in deleterious opioid-induced side effects (2).
Given evidence that peripheral nerve blocks may cause muscle weakness, precluding early participation in physical therapy and increasing the risks of falls, low concentration and low volume of LA (bupivacaine 0.0626% 5ml /h for femoral nerve block, and bupivacaine 0.03% 3 ml/h for sciatic nerve block) were used in this study. Available physical therapy data indicate that low concentration and low volume blocks have no impact on lower extremity muscle strength. While we did not assess the incidence of falls, the absence of a detectable decrease in muscle strength would suggest that any increased risk of falls because of the block is likely to be negligible. Consistent with this suggestion are the results of a recently published retrospective study in which peripheral nerve blocks were reported to have no influence on inpatient falls in TKA patients (20). Our results also demonstrate that 10 times more patients with nerve blocks were able to participate in physical therapy on POD 1 than those without, suggesting that peripheral nerve blocks increase rather than decrease participation in physical therapy. There are likely to be multiple reasons for higher early PT participation rate in the block patients, but improved pain and / or less opioid –induced side effects (e.g. sedation) were likely the contributing factors.
Length of hospital stay (LOS) is an important functional outcome associated with TKA procedures because it is related to both patient satisfaction and cost effectiveness. The issue of LOS, however, is complex because it is not only determined by clinical parameters such as pain control, but also the reimbursement schedules and pre-established clinical pathways. Nevertheless, previous studies have shown that nerve blocks are associated with shorter hospital stay for TKA procedures (6). Our results are consistent with previous findings, although a ~ 0.1 day reduction in LOS is unlikely to have any meaningful financial impact under most reimbursement schedules.
In this regard, there are two primary sources of dollar cost associated with the use of peripheral nerve blocks. The first is the impact of the time associated with catheter placement on operating room throughput. However, because dedicated team members can place catheters preoperatively, catheter placement should have no real impact on patient flow through the operating room. The second is the added cost in terms of personnel and material for catheter placement and follow-up. Based on current Medicare/Medicaid CPT codes and reimbursement schedules, the actual cost of catheter placement and postoperative pain management is approximately $521.39 ($79.02 for femoral block catheter, $88.05 for sciatic block catheter, $117.50 for consultation and $ 236.82 for three days of in-patient visits) (20, 21)
There were several other interesting results from our study beyond their implications for our functional outcomes analysis. First, while the concentration and volume of local anesthetics used in the patients studied was specifically titrated to minimize the possibility of motor block, an average VAS from 4.2 to 4.5 at rest would suggest patients were still experiencing a moderate level of pain. This observation underscores the need to identify additional approaches to maximize pain relief while minimizing potentially deleterious consequences. Second, pain with activity was moderate to severe in both groups of patients. This observation further underscores the need to improve post-operative pain management, but the observation that patients still participated in physical therapy with such high pain scores suggests that it is pain at rest that primarily determines the patient's willingness to participate in physical therapy. However, because pain scores recorded by physical therapists generally reflects the maximal pain tolerance for the patient participation in physical therapy, it would be interesting to determine whether there were differences between groups with respect to how much patients are able to do in physical therapy. Third, there was no detectable evidence that a variety of pain related medications, even at their highest doses used, had a beneficial influence on pain scores or opioid consumption. This was surprising, in light of evidence from several studies suggesting that several of these compounds contribute to effective pain management in the postoperative setting (22, 23). The simplest explanation for the negative results obtained in the present study is that the additional medications administered, despite the relative high doses used in some cases, were not titrated to efficacy. This would suggest that if these compounds are to be used in the postoperative setting, it may be only useful to do so if it is possible to perform such a titration. Alternatively, as we did not distinguish single administration of additional analgesics from those administered repeated throughout the day, the impact of these compounds may have been missed in pain scores averaged over the entire day. Similarly, if the mechanisms underlying the analgesic efficacy of the peripheral nerve block are downstream of the mechanisms underlying the actions of the other analgesics, the efficacy of the block may have masked that of the alternative medication. Consistent with this suggestion, there was a small but significant decrease in opioid use in no block patients receiving celecoxib, but no such difference in the block group.
This study is unavoidably limited by its retrospective nature. Many potential confounding factors, such as pre-operative medical status and medications that might influence the results were not controlled. More data, such as incidence of falls could have been extracted to assess the functional outcomes in a greater detail. Furthermore, it was not possible to assess the long term consequences to the two post-operative pain management strategies, where even more substantial differences may be manifest, if as suggested, the efficacy of post-operative pain management impacts the likelihood that patients will go on to develop chronic pain (23). Nevertheless, the standardized practice of postoperative pain management as well as the utilization of a clinical pathway featuring efficient rehabilitation and rapid hospital discharge make the results less ambiguous.
In conclusion, the clear clinical advantages in addition to the potentially significant dollar savings associated with the use of peripheral nerve blocks, in the face of relatively limited evidence of disadvantages argue for peripheral nerve blocks as part of standard care for TKA patients.
Acknowledgments
This study was supported by the Department of Anesthesiology, University of Pittsburgh, and National Institutes of Health grant 1R01DE018252-02A2 (MSG). We wish to thank Mr. Johnathan Korpon and Ms. Greta Volpedo for assistance with data analysis.
List of abreviations
- LA
Local Anesthetic
- LOS
Length of Stay
- PACU
Post Anesthesia Care Unit
- POD
Post-operative Day
- TKA
(Total Knee Arthroplasty)
Footnotes
None of the authors have a conflict of interest with the context of this manuscript.
Results from an initial analysis of the data in this manuscript were presented at the 38th Annual Regional Anesthesia Meeting on May 3, 2013.
References
- 1.Chelly JE, Greger J, Gebhard R, Coupe K, Clyburn TA, Buckle R, et al. Continuous femoral blocks improve recovery and outcome of patients undergoing total knee arthroplasty. J Arthroplasty. 2001;16:436–45. doi: 10.1054/arth.2001.23622. [DOI] [PubMed] [Google Scholar]
- 2.Richman JM, Liu SS, Courpas G, Wong R, Rowlingson AJ, McGready J, et al. Does continuous peripheral nerve block provide superior pain control to opioids? A meta-analysis. Anesth Analg. 2006;102:248–57. doi: 10.1213/01.ANE.0000181289.09675.7D. [DOI] [PubMed] [Google Scholar]
- 3.Klein SM, Evans H, Nielsen KC, Tucker MS, Warner DS, Steele SM. Peripheral nerve block techniques for ambulatory surgery. Anesth Analg. 2005;101:1663–76. doi: 10.1213/01.ANE.0000184187.02887.24. [DOI] [PubMed] [Google Scholar]
- 4.Chelly JE, Ghisi D, Fanelli A. Continuous peripheral nerve blocks in acute pain management. Br J Anaesth. 2010;105(Suppl 1):i86–96. doi: 10.1093/bja/aeq322. [DOI] [PubMed] [Google Scholar]
- 5.Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011;113:904–25. doi: 10.1213/ANE.0b013e3182285e01. [DOI] [PubMed] [Google Scholar]
- 6.Paul JE, Arya A, Hurlburt L, Cheng J, Thabane L, Tidy A, et al. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: a meta-analysis of randomized controlled trials. Anesthesiology. 2010;113:1144–62. doi: 10.1097/ALN.0b013e3181f4b18. [DOI] [PubMed] [Google Scholar]
- 7.Kandasami M, Kinninmonth AW, Sarungi M, Baines J, Scott NB. Femoral nerve block for total knee replacement - a word of caution. The Knee. 2009;16:98–100. doi: 10.1016/j.knee.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 8.Memtsoudis SG, Danninger T, Rasul R, Poeran J, Gerner P, Stundner O, et al. Inpatient falls after total knee arthroplasty: the role of anesthesia type and peripheral nerve blocks. Anesthesiology. 2014;120:551–63. doi: 10.1097/ALN.0000000000000120. [DOI] [PubMed] [Google Scholar]
- 9.Capdevila X, Barthelet Y, Biboulet P, Ryckwaert Y, Rubenovitch J, d'Athis F. Effects of perioperative analgesic technique on the surgical outcome and duration of rehabilitation after major knee surgery. Anesthesiology. 1999;91:8–15. doi: 10.1097/00000542-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Johnson RL, Kopp SL, Hebl JR, Erwin PJ, Mantilla CB. Falls and major orthopaedic surgery with peripheral nerve blockade: a systematic review and meta-analysis. Br J Anaesth. 2013;110:518–28. doi: 10.1093/bja/aet013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279:847–52. doi: 10.1001/jama.279.11.847. [DOI] [PubMed] [Google Scholar]
- 12.Gordon DB, Stevenson KK, Griffie J, Muchka S, Rapp C, Ford-Roberts K. Opioid equianalgesic calculations. Journal of palliative medicine. 1999;2:209–18. doi: 10.1089/jpm.1999.2.209. [DOI] [PubMed] [Google Scholar]
- 13.Renkawitz T, Rieder T, Handel M, Koller M, Drescher J, Bonnlaender G, et al. Comparison of two accelerated clinical pathways--after total knee replacement how fast can we really go? Clinical rehabilitation. 2010;24:230–9. doi: 10.1177/0269215509353267. [DOI] [PubMed] [Google Scholar]
- 14.Schneider M, Kawahara I, Ballantyne G, McAuley C, Macgregor K, Garvie R, et al. Predictive factors influencing fast track rehabilitation following primary total hip and knee arthroplasty. Arch Orthop Trauma Surg. 2009;129:1585–91. doi: 10.1007/s00402-009-0825-9. [DOI] [PubMed] [Google Scholar]
- 15.Ip HY, Abrishami A, Peng PW, Wong J, Chung F. Predictors of postoperative pain and analgesic consumption: a qualitative systematic review. Anesthesiology. 2009;111:657–77. doi: 10.1097/ALN.0b013e3181aae87a. [DOI] [PubMed] [Google Scholar]
- 16.Kalkman CJ, Visser K, Moen J, Bonsel GJ, Grobbee DE, Moons KG. Preoperative prediction of severe postoperative pain. Pain. 2003;105:415–23. doi: 10.1016/S0304-3959(03)00252-5. [DOI] [PubMed] [Google Scholar]
- 17.Stone AA, Broderick JE. Obesity and pain are associated in the United States. Obesity (Silver Spring, Md) 2012;20:1491–5. doi: 10.1038/oby.2011.397. [DOI] [PubMed] [Google Scholar]
- 18.Management ASoATFoAP Practice guidelines for acute pain management in the perioperative setting: an updated report by the American Society of Anesthesiologists Task Force on Acute Pain Management. Anesthesiology. 2012;116:248–73. doi: 10.1097/ALN.0b013e31823c1030. [DOI] [PubMed] [Google Scholar]
- 19.Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9:105–21. doi: 10.1016/j.jpain.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Health Care Cost and Utilization Project. [Google Scholar]
- 21.Physician Fee Schedule. 2013. [Google Scholar]
- 22.Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain--a systematic review of randomized controlled trials. Pain. 2006;126:91–101. doi: 10.1016/j.pain.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 23.Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. 2006;367:1618–25. doi: 10.1016/S0140-6736(06)68700-X. [DOI] [PubMed] [Google Scholar]