Abstract
Many receptors signal via adaptors to the IKK–NF-κB axis, transducing extracellular cues to transcriptional regulation. In this issue, Meng et al. (2015. J. Cell Biol. http://dx.doi.org/10.1083/jcb.201505091) reveal that the IKK regulator NLRC5 shapes NF-κB activity through a feedforward loop of NLRC5 ubiquitination and deubiquitination, highlighting a new pathway modulating IKK–NF-κB activity.
NF-κB is required for lymphoid cell and organ development, innate and adaptive immunity, and cell survival. The NF-κB family contains several members, which form heterodimers or homodimers that execute their functions as transcriptional factors in the nucleus. Regulation of NF-κB activation is crucial to many biological functions, and dysregulation of NF-κB activity has been involved in the pathogenesis of immune deficiency, infectious diseases, inflammation, and cancer (Liu et al., 2006; Pannicke et al., 2013). The IKK complex is central to the regulation of NF-κB activity (Ghosh and Karin, 2002). It is composed of the kinases IKKα and IKKβ, as well as of the regulatory subunit IKKγ (NEMO). After the activation of many receptors, IKK phosphorylates IκB, which physically retains NF-κB in the cytoplasmic compartment. This phosphorylation induces IκB degradation through the ubiquitin-proteasome machinery, allowing NF-κB translocation to the nucleus and its activation to regulate gene expression.
Multiple receptors, including TNFRs, IL-1R, Toll-like receptors (TLRs), Nod-like receptors (NLRs or NLRCs), RIG-like receptors (RLRs), TCRs, and BCRs, depend on the IKK–NF-κB pathway to transduce external cellular signals to the transcriptional machinery. It is known that such diverse and important receptors require IKK–NF-κB; however, the regulatory mechanisms by which distinct receptors signal to IKK in different cell types are not fully understood. NLRs, TLRs, and RLRs specifically recognize pathogen-associated molecules, which provide the innate immune response as the first line of defense against invading microbes (Akira et al., 2001). NLRs are intracellular pattern-recognition receptors that feature a central nucleotide-binding and oligomerization domain. Although they were originally thought to initiate inflammasome formation, recent works showed that several NLR family members, including NLRC5, negatively regulate TLR and RLR signaling (Benko et al., 2010; Cui et al., 2010). Indeed, Cui et al., 2010 previously revealed that NLRC5 negatively regulates the NF-κB pathway by blocking the phosphorylation of IKKα and IKKβ in LPS-stimulated, TLR4–activated signaling in macrophages. In this process, NLRC5 competes with IKKγ to bind to IKKα and IKKβ, thereby inhibiting IKK and NF-κB activity. The exact mechanism by which NLRC5 regulates IKK and NF-κB activation and its regulation needs to be further investigated.
In this issue, Meng et al. build a mathematical model based on the competition for IKKβ binding between NLRC5 and IKKγ–NEMO to predict the role of NLRC5 in NF-κB signaling upon LPS stimulation. Interestingly, they observed that the experimental temporal dynamics of IKK–NLRC5 complex formation did not exactly overlap with the prediction from the mathematical model, suggesting that modulation of the NLRC5–IKK interaction, other than that based on competitive binding, might exist. The authors used coimmunoprecipitation analyses in HEK 293T cells expressing TLR4 and other cell types to delineate whether NLRC5 undergoes posttranslational modification after TLR4 activation. Meng et al. (2015) discovered that NLRC5 is ubiquitinated with K63 linkage. Remarkably, the levels of ubiquitinated NLRC5 inversely correlated to the levels of IKKβ–NLRC5 complex formation, suggesting that modification of NLRC5 affects its binding to IKKβ, which the authors confirmed in silico based on their initial model. Indeed, the researchers found that treatment with LPS recruits E3 ligases and NF-κB signaling adaptors TRAF2/6 into the complex with NLRC5 and IKKβ. SiRNA-mediated knockdown of TRAF2 or TRAF6 abolished NLRC5 polyubiquitination and increased the interaction of NLRC5 with IKKβ after LPS treatment. The authors therefore propose that NLRC5 is targeted for degradation through K63-linked ubiquitination at a specific site mapped by analyzing truncated constructs, i.e., lysine 1178. These results also suggest that TRAF2/6-mediated NLRC5 degradation removes the IKK–NF-κB negative regulator NLRC5, which allows IKKγ to replace NLRC5 in the complex with IKKα and IKKβ to activate NF-κB.
Interestingly, the in silico analyses by Meng et al. (2015) also suggested that deubiquitination of NLRC5 might be involved in NF-κB signaling regulation by restoring the pool of unmodified NLRC5. The authors tested various ubiquitin-specific proteases (USPs), which belong to a subclass of deubiquitinases (DUBs), for their ability to bind NLRC5 and enhance its interaction with IKKβ in vitro. They show that three USPs—USP14, USP18, and USP22—fulfilled these criteria, but focused on USP14, as USP18 and USP22 could also inhibit IKKβ activation directly in the absence of NLRC5. The authors confirmed that USP14, but not a catalytically inactive mutant, directly interacted with NLRC5 to modulate its function and that cells deficient for USP14 generated via CRISPR/Cas9 technology displayed lower NF-κB activity when expressing WT NLRC5, but not when expressing a version of NLRC5 mutated on its ubiquitination site. The researchers propose that, after the ubiquitination of NLRC5 at lysine 1178 is catalyzed by TRAF2/6, USP14 specifically removes the polyubiquitin chains from NLRC5 to enhance NLRC5-mediated inhibition of IKK–NF-κB signaling, thus forming a coherent feedforward loop to regulate IKK–NF-κB activation (Fig. 1). This new regulatory mechanism is different from other ubiquitination-based modes of regulation of IKK–NF-κB, such as those involving A20, CYLD, or IKKγ (Chen, 2012). Further investigation is needed to dissect these mechanisms and their specific biological significance. The DUB superfamily is composed of many enzymes, and the IKK and NF-κB families also comprise multiple members, so it may be challenging to delineate the physiological function of all possible combinations in the context of a particular pathway or stimulus.
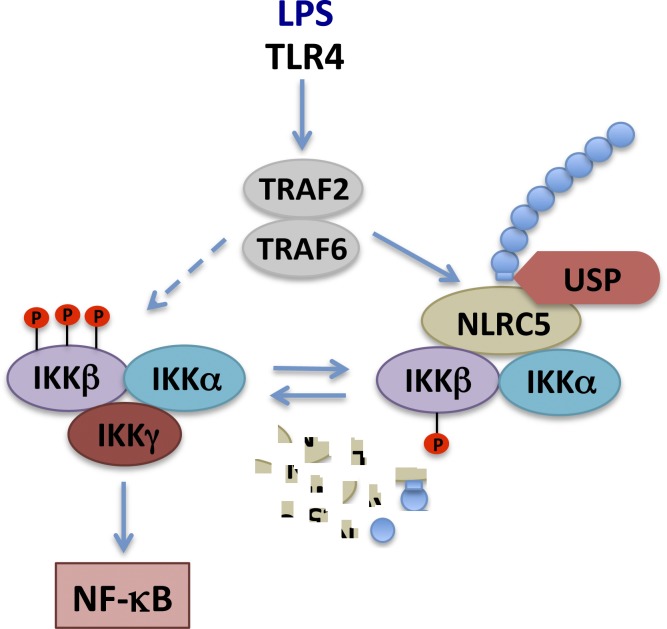
Figure 1.
A working model of how the NLRC5 ubiquitination and deubiquitination feedforward loop shapes IKK/NF-κB activity. NLRC5 interacts with IKKα and IKKβ to block IKK activation. After LPS stimulation, TRAF2/6-mediated NLRC5 ubiquitination frees IKKα and IKKβ, resulting in the formation of the activated IKK complex containing IKKα, IKKβ, and IKKγ, which activates NF-κB. USPs such as USP14 deubiquitinate NLRC5, enhancing NLRC5-mediated inhibition of IKK–NF-κB signaling. Arrows direct the signaling pathway suggested by Meng et al. (2015), and the dashed arrow indicates the canonical pathway resulting in NF-κB activation. P with circles, phosphorylation; blue circles, ubiquitin; irregular fragments, degraded NLRC5.
Moreover, Meng et al. (2015) found that USPs are differentially expressed in various immune cell types. These differences may determine the cell type–specific activities of IKK–NF-κB. For example, USP14, USP18, and USP22 were highly expressed in macrophages. The researchers tested whether NLRC5 levels and NLRC5 ubiquitination after LPS treatment were also cell type specific. Consistent with varying levels of USPs, the kinetics and dynamics of NLRC5 ubiquitination, as well as NLRC5 expression levels, fluctuated in several immune cell types, suggesting that the impact of NLRC5 on NF-κB is cell type specific. The origin of some previously reported contradictory findings obtained from Nlrc5-deficient mice (Kumar et al., 2011; Tong et al., 2012; Yao et al., 2012) remains to be further investigated.
Despite its thorough characterization of NRLC5 modification, this work does not further dissect the downstream partners and effectors of NRLC5 necessary for NF-κB activation, and in particular, it raises questions as to which IKK subunit is required to mediate its effects. Indeed, treatment with certain stimuli such as TNF, which activates TNFR1, induces the phosphorylation of both IKKα and IKKβ (Xia et al., 2013), whereas LPS stimulation, which activates TLR4, mainly induces phosphorylation of IKKβ, but not of IKKα (Cui et al., 2010; Meng et al., 2015). These results suggest that the mechanism connecting TLR4 to the IKK complex differs from that linking TNFR1 to the same complex. Tools are available to investigate this possibility, as, for instance, Western blotting allows the separation of IKKα, IKKβ, and their phosphorylated forms thanks to their different molecular weights, although the antibody used in this work to identify phosphorylated IKK recognizes both IKKα and IKKβ. In addition, other experimental set-ups may limit the interpretation and robustness of the conclusions, including artificial effects of IKKα and IKKβ ectopic overexpression, which might mask cell type–specific phenotypes. Lastly, several lines of evidence point to different biological activities for IKKα and IKKβ, including their different knockout phenotypes and the differences in which molecules or pathways can rescue them (Pasparakis et al., 2002; Liu et al., 2008). For example, in T cells, deletion of IKKβ, but not of IKKα, causes apoptosis, reducing T cell numbers (Senftleben et al., 2001; Schmidt-Supprian et al., 2003; Balkhi et al., 2012; Chen et al., 2015). Similarly, Tnfr1 knockout rescues the lethality of Ikkβ−/− mice and the skin phenotype of mice lacking IKKβ in their keratinocytes (Pasparakis et al., 2002), but it neither rescues the lethality of Ikkα−/− mice nor the skin phenotype of mice lacking IKKα in their keratinocytes (Li et al., 1999a; Liu et al., 2008). IKKα-inducible deletion in keratinocytes is known to cause spontaneous skin tumors, and it can be rescued in the Egfr heterozygous (Egfr+/−) genetic background (Liu et al., 2008). The phenotype of IKKβ deletion on skin tumorigenesis remains to be examined, but keratinocyte-specific deletion of p65, a major NF-κB target for IKKβ, inhibits chemical carcinogen-induced skin carcinogenesis (Kim and Pasparakis, 2014), which stands in contrast to the IKKα deletion phenotype. As dysregulation of IKKα and IKKβ has been implicated in several human diseases, it will be important to identify the differences in their biological functions and downstream signaling to design targeted therapeutics.
Regulation and timely termination of the immune responses triggered by NLRs, TLRs, and RLRs are crucial to prevent inflammation-associated diseases. Understanding the dynamic control of these pathways is therefore necessary to produce adequate therapeutics for inflammation-induced pathologies. Through computational and experimental approaches, this work takes an important step in delineating the temporal and dynamic modulation of NF-κB signaling, as well as its cell type specificity. The reversible ubiquitination of NLRC5 shapes NF-κB activation by allowing efficient activation and termination of innate immune signaling; by creating a feedforward loop that sets a threshold for robust innate immune responses; and, lastly, by altering the cellular sensitivity to NLRC5 ablation. This work opens the door to the future investigation of other dynamic modes of regulation of these pathways and of the impact of the intracellular (de)ubiquitination environment on inflammatory responses.
Acknowledgments
The author declares no competing financial interests.
References
- Akira S., Takeda K., and Kaisho T.. 2001. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat. Immunol. 2:675–680. 10.1038/90609 [DOI] [PubMed] [Google Scholar]
- Balkhi M.Y., Willette-Brown J., Zhu F., Chen Z., Liu S., Guttridge D.C., Karin M., and Hu Y.. 2012. IKKα-mediated signaling circuitry regulates early B lymphopoiesis during hematopoiesis. Blood. 119:5467–5477. 10.1182/blood-2012-01-401547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benko S., Magalhaes J.G., Philpott D.J., and Girardin S.E.. 2010. NLRC5 limits the activation of inflammatory pathways. J. Immunol. 185:1681–1691. 10.4049/jimmunol.0903900 [DOI] [PubMed] [Google Scholar]
- Chen Z.J. 2012. Ubiquitination in signaling to and activation of IKK. Immunol. Rev. 246:95–106. 10.1111/j.1600-065X.2012.01108.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Willette-Brown J., Wu X., Hu Y., Howard O.M., Hu Y., and Oppenheim J.J.. 2015. IKKα is required for the homeostasis of regulatory T cells and for the expansion of both regulatory and effector CD4 T cells. FASEB J. 29:443–454. 10.1096/fj.14-259564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J., Zhu L., Xia X., Wang H.Y., Legras X., Hong J., Ji J., Shen P., Zheng S., Chen Z.J., and Wang R.F.. 2010. NLRC5 negatively regulates the NF-κB and type I interferon signaling pathways. Cell. 141:483–496. 10.1016/j.cell.2010.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S., and Karin M.. 2002. Missing pieces in the NF-κB puzzle. Cell. 109(2, Suppl):S81–S96. 10.1016/S0092-8674(02)00703-1 [DOI] [PubMed] [Google Scholar]
- Kim C., and Pasparakis M.. 2014. Epidermal p65/NF-κB signalling is essential for skin carcinogenesis. EMBO Mol. Med. 6:970–983. 10.15252/emmm.201303541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar H., Pandey S., Zou J., Kumagai Y., Takahashi K., Akira S., and Kawai T.. 2011. NLRC5 deficiency does not influence cytokine induction by virus and bacteria infections. J. Immunol. 186:994–1000. 10.4049/jimmunol.1002094 [DOI] [PubMed] [Google Scholar]
- Li Q., Lu Q., Hwang J.Y., Büscher D., Lee K.F., Izpisua-Belmonte J.C., and Verma I.M.. 1999a IKK1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 13:1322–1328. 10.1101/gad.13.10.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Park E., Zhu F., Bustos T., Liu J., Shen J., Fischer S.M., and Hu Y.. 2006. A critical role for IκB kinase α in the development of human and mouse squamous cell carcinomas. Proc. Natl. Acad. Sci. USA. 103:17202–17207. 10.1073/pnas.0604481103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B., Xia X., Zhu F., Park E., Carbajal S., Kiguchi K., DiGiovanni J., Fischer S.M., and Hu Y.. 2008. IKKα is required to maintain skin homeostasis and prevent skin cancer. Cancer Cell. 14:212–225. 10.1016/j.ccr.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q., Cai C., Sun T., Wang Q., Xie W., Wang R.-F., and Cui J.. 2015. Reversible ubiquitination shapes NLRC5 function and modulates NF-κB activation switch. J. Cell Biol. 10.1083/jcb.201505091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannicke U., Baumann B., Fuchs S., Henneke P., Rensing-Ehl A., Rizzi M., Janda A., Hese K., Schlesier M., Holzmann K., et al. . 2013. Deficiency of innate and acquired immunity caused by an IKBKB mutation. N. Engl. J. Med. 369:2504–2514. 10.1056/NEJMoa1309199 [DOI] [PubMed] [Google Scholar]
- Pasparakis M., Courtois G., Hafner M., Schmidt-Supprian M., Nenci A., Toksoy A., Krampert M., Goebeler M., Gillitzer R., Israel A., et al. . 2002. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 417:861–866. 10.1038/nature00820 [DOI] [PubMed] [Google Scholar]
- Schmidt-Supprian M., Courtois G., Tian J., Coyle A.J., Israël A., Rajewsky K., and Pasparakis M.. 2003. Mature T cells depend on signaling through the IKK complex. Immunity. 19:377–389. 10.1016/S1074-7613(03)00237-1 [DOI] [PubMed] [Google Scholar]
- Senftleben U., Li Z.W., Baud V., and Karin M.. 2001. IKKbeta is essential for protecting T cells from TNFα-induced apoptosis. Immunity. 14:217–230. 10.1016/S1074-7613(01)00104-2 [DOI] [PubMed] [Google Scholar]
- Tong Y., Cui J., Li Q., Zou J., Wang H.Y., and Wang R.F.. 2012. Enhanced TLR-induced NF-κB signaling and type I interferon responses in NLRC5 deficient mice. Cell Res. 22:822–835. 10.1038/cr.2012.53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X., Liu S., Xiao Z., Zhu F., Song N.Y., Zhou M., Liu B., Shen J., Nagashima K., Veenstra T.D., et al. . 2013. An IKKα-nucleophosmin axis utilizes inflammatory signaling to promote genome integrity. Cell Reports. 5:1243–1255. 10.1016/j.celrep.2013.10.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y., Wang Y., Chen F., Huang Y., Zhu S., Leng Q., Wang H., Shi Y., and Qian Y.. 2012. NLRC5 regulates MHC class I antigen presentation in host defense against intracellular pathogens. Cell Res. 22:836–847. 10.1038/cr.2012.56 [DOI] [PMC free article] [PubMed] [Google Scholar]