Abstract
Background
The role of insulin resistance (IR) on fibrosis progression in HCV patients has not been systematically evaluated. Therefore, this systemic review aimed to summarize the available epidemiologic evidence to evaluate the strength of association between IR and advanced liver fibrosis in these patients.
Methods
We performed a systemic literature search in PubMed, OvidSP and MEDLINE from January 1990 to April 2015 without language restriction using the following search terms: insulin resistance, liver fibrosis, cirrhosis, diabetes mellitus and chronic hepatitis C. Publication bias was assessed using the Begg and Egger’s tests and with a visual inspection of funnel plot. All analyses were performed using Comprehensive Meta-Analysis version 2 software.
Results
A total of 3,659 participants with HCV infection from 14 studies were included in the analysis. After adjusting for publication bias, the RR for significant hepatic fibrosis among HCV subjects with IR was 1.63 (95% CI 1.34-2.01). Subgroup analysis by genotypes showed RR of 2.16 (95% CI 1.52-3.06) for genotype 1; however, the association was no longer significant when we analyzed the data for HCV genotype 3; RR 1.40 (95% CI 0.8-2.45).
Conclusion
Our study showed significant association between IR and significant hepatic fibrosis in patients with HCV genotype 1 infection.
Keywords: insulin resistance, liver fibrosis, chronic hepatitis C
INTRODUCTION
Hepatitis C virus (HCV) infection is a major global public health problem and it is estimated that ~2%-3% of the world population are infected with the virus1. It can now be successfully eradicated with the new therapeutic regimens; which are safe and highly efficacious2. However, the cost of these new drugs prohibits their use in many countries with limited resources around the world1. Undoubtedly, the morbidity and mortality from HCV infection continue to increase1.
Most patients who acquire HCV develop chronic HCV infection. However, the rate of disease progression from the time of infection till the development of advanced fibrosis or cirrhosis is variable3. In fact, the natural history of chronic HCV infection is difficult to determine because of the difficulty in estimating the time and duration of infection and other factors that can affect disease course. Though the mechanism is poorly understood, it is likely that both host and viral factors play an important role in the disease progression. Several factors have been reported to influence the fibrosis progression, including age4, ethnic background5, gender6, and alcohol use7.
Recent epidemiological studies suggested that HCV infection is an independent predictor for the development of type 2 diabetes mellitus (DM), and that type 2 DM is more prevalent among patients with chronic HCV infection than in those with other causes of liver diseases8–10. It is likely that the HCV itself or the inflammatory response to HCV infection contributes to the development of insulin resistance (IR) and thus increasing the risk for type 2 DM11. The presence of IR and type 2 DM are independent predictors of severe fibrosis in patients with non-alcoholic fatty liver disease12, 13. The role of IR and advanced fibrosis in HCV patients has not been systematically evaluated. Therefore, this systemic review aimed to summarize the available epidemiologic evidence to evaluate the strength of association between IR and liver fibrosis in these patients.
METHODS
Study selection/search Strategy
We performed a systemic literature search in PubMed, OvidSP and MEDLINE from January 1990 to April 2015 without language restriction using the following search terms: insulin resistance, liver fibrosis, cirrhosis, diabetes mellitus and chronic hepatitis C. The reference list of each included study was comprehensively searched to further identify relevant studies. The process of systematic review was conducted in adherence to standards of quality for reporting meta-analyses14.
Inclusion and exclusion criteria
Relevant studies were included if they met the following criteria: (1) the studies were either case-control or cohort study designs; (2) the study participants were ≥ 18 years old; and (3) the relative risk (RR) estimate or odds ratio was reported for significant hepatic fibrosis in those with HCV infection.
Definition of hepatic fibrosis/cirrhosis
The following pathological classification for hepatic fibrosis was used; METAVIR, Scheuer, Ishak and histological activity index (HAI)4, 15–17. METAVIR scoring system was specifically designed for patients with HCV infection4. The fibrosis score is assigned a number from F0-F4 where F3-F4 representing advanced fibrosis. Scheuer classification defines stages of fibrosis from 0 to 4 where stages 2-4 indicate significant17. Ishak scale assesses liver fibrosis in 7 categories, ranging from normal (stage 0) to cirrhosis (stage 6)15. For this scoring system, stages 4-6 indicate significant fibrosis. Lastly, HAI classifies fibrosis from stage A to D and gives a score to each stage where score 3 or more indicates advanced fibrosis16.
Definition of insulin resistance
Insulin resistance was measured using the HOMA (Homeostasis Model Assessment). The cut-off for the HOMA to define the presence of IR in each study is shown in Table 1.
Table 1.
Study characteristics and RR with CI for individual studies
| No | Study Name |
Year of publication |
Country | Type | Male: Female |
Single center Vs Multi Center |
N-O scale* |
Cases of CHC |
Genotype | Fibrosis definition |
IR definition |
Total RR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hui et al 24 | 2003 | AUS | Case- control | 175:85 | Single | 7 | 260 | 3 and Non 3 | Scheuer | HOMA | 1.3 (1.1– 1.4) |
| 2 | Sud et al 34 | 2004 | AUS | Cohort | 115:61 | Multi | 6 | 176 | ** | Scheuer | HOMA | 1.47 (1.14– 1.89) |
| 3 | Bugi anesi et al25 | 2006 | AUS | Cohort | 101:31 | Single | 7 | 132 | 3 | Scheuer | HOMA ≥2.7 | 2.98 (1.13– 7.89) |
| 4 | Baroni et al 33 | 2007 | Italy | Cohort | 62:28 | Multi | 7 | 90 | 3 and Non 3 | Ishak | HOMA >2.7 | 0.36 (0.06– 2.05) |
| 5 | Cua et al 21 | 2008 | AUS | Cohort | 235:111 | Single | 5 | 346 | 1 and 3 | Scheuer | HOMA>2 | 3.15 (1.56– 6.35) |
| 6 | Chu et al 26 | 2008 | China | Cohort | ** | Single | 5 | 192 | ** | Metavir | HOMA >2.5 | 2.46 (1.24– 4.9) |
| 7 | Petta et al 22 | 2008 | Italy | Cohort | 105:96 | Single | 6 | 201 | 1 | Scheuer | HOMA >2.7 | 2.69 (1.46–4.95) |
| 8 | Moucari et al 27 | 2008 | France | Cohort | 254:200 | Single | 6 | 454 | 1 and 4 | Metavir | HOMA >3 | 1.8 (1.16– 2.82) |
| 9 | Halfon et al 29 | 2009 | France | Cohort | 121:49 | Single | 5 | 170 | ** | Metavir | HOMA >2 | 2.44 (1.15–5) |
| 10 | Moucari et al 28 | 2009 | France | Cohort | 168:58 | Single | 6 | 226 | 4 | Metavir | HOMA>3 | 3.86 (1.86– 8.03) |
| 11 | Hsu et al 30 | 2010 | Taiwan | Cohort | 288:246 | Single | 5 | 528 | 1 and 2 | Metavir | HOMA >2.4 | 1.23 (0.78– 1.95) |
| 12 | Patel et al 31 | 2011 | Asia | Cohort | 141:122 | Multi | 5 | 263 | 2 and 3 | Metavir | HOMA>2 | 2.80 (1.4– 5.4) |
| 13 | Petta et al 23 | 2012 | Italy | Cohort | 239:242 | Single | 5 | 481 | 1 | Scheuer | HOMA>3 | 1.69 (1.04– 2.73) |
| 14 | Ziada et al 32 | 2013 | Egypt | Case-control | 75:65 | Single | 6 | 140 | ** | HAI | HOMA≥2 | 1.92 (0.97– 3.4) |
Newcastle-Ottawa Scale
Data extraction
The following information were extracted from each study: publication data (such as first author’s last name and first name initials, year of publication and country of origin), sample size, participants’ demographic data, types of study design (case-control/cohort), number of cases and controls (for case-control studies), number of exposed and unexposed (for cohort studies), criteria used to define significant hepatic fibrosis stratified by different pathological classification, the levels of HOMA scores to define IR, risk estimates with their corresponding confidence intervals (CIs), and the covariates (if any) which were used in the multivariate modeling. We carefully reviewed the potential confounders; that might be associated with the risk of liver fibrosis in the studied population. In this study, odds ratios (ORs) from case-control studies were considered as estimate of relative risk. This is based on the assumption that the prevalence of HCV infection is <10%, and in this case the odds ratio and relative risk will be approximately the same. Two independent reviewers (SP and RJ) reviewed the studies and any discrepancies regarding inclusion/exclusion or risk estimates were resolved through the discussion by authors. We used Cohen’s kappa coefficient to assess the agreement among reviewers for inclusion/exclusion of specific studies18.
Assessment of methodological quality
To assess methodological quality of all the publications that were included in the final analysis, the Newcastle-Ottawa Scale was used19. The scale allocates stars, maximum of nine, for the following categories: quality of selection, comparability, exposure and outcome of study participants. Any studies with the scale < 5 were excluded.
Statistical analysis
Summaries of relative risk (RR) estimates were evaluated using both fixed- and random-effects methods. Initial analysis was performed to look for association between IR and significant liver fibrosis. We used Cochran’s Q-test and I2-statistic to determine the heterogeneity of the publications. Publication bias was assessed by (i) construction and visual inspection of funnel plot and (ii) employing the Egger’s and Begg and Mazumdar tests. Duval and Tweedie’s trim and fill method was utilized to obtain RR after adjustments of the publication bias. The p value of < 0.05 indicated statistical significance. All analyses were performed using Comprehensive Meta-analysis Version 2 (Biostat, Englewood, New Jersey)20.
RESULTS
Literature Search and Study Characteristics
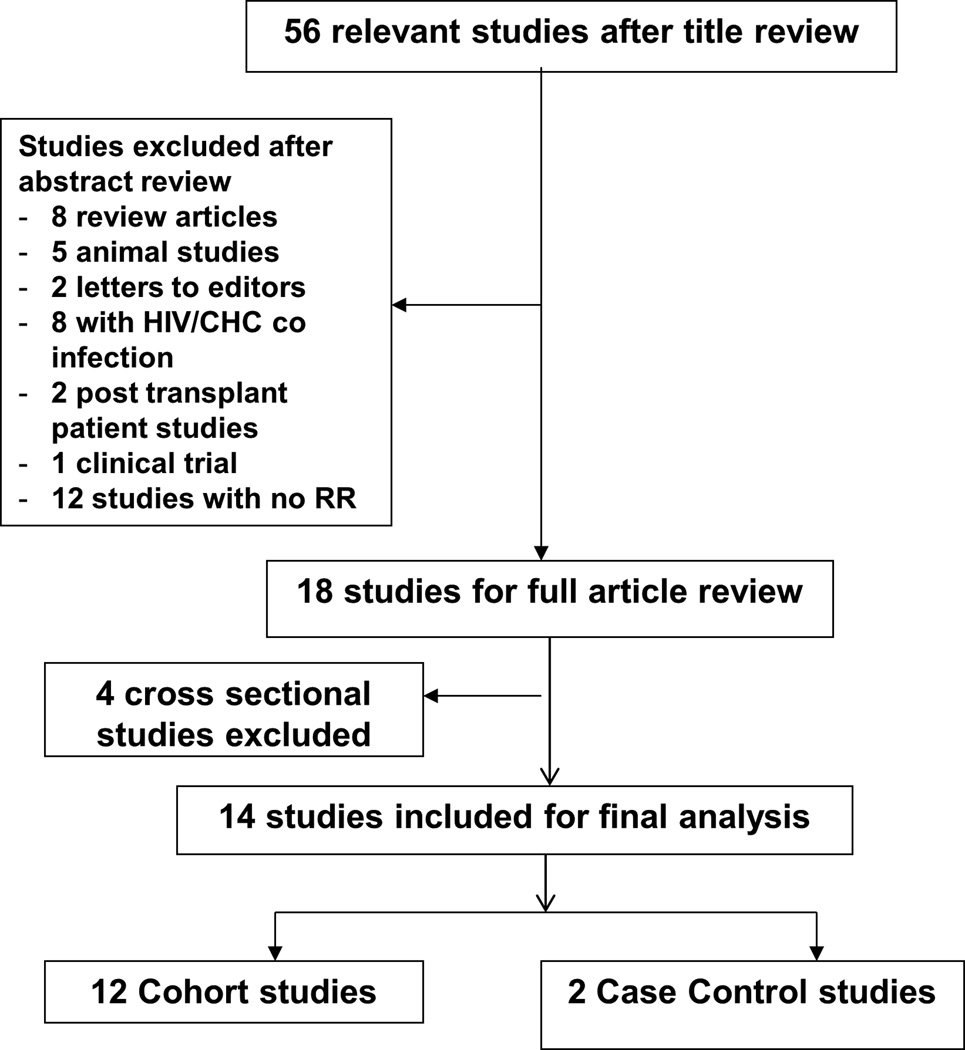
The schematic diagram of the detailed literature selection is shown in Figure 1. We identified 380 studies from different databases, either in full publications or in abstract forms. After title appraisal and extensive review, 56 publications were considered to be potentially relevant. Of these, we excluded 8 review articles, 5 animal studies, 2 letters to the editors, 8 studies which described HIV/HCV co-infections, 2 studies with post liver transplant HCV patients, 1 clinical trial, and 12 studies which did not provide RR estimate. Four additional studies were excluded as they were cross sectional studies. Fourteen studies (12 cohort and 2 case-control studies) were considered for full article assessment and included in the final analysis.
Figure 1.
Schematic diagram for study selection of the relevant articles
Publication quality and bias
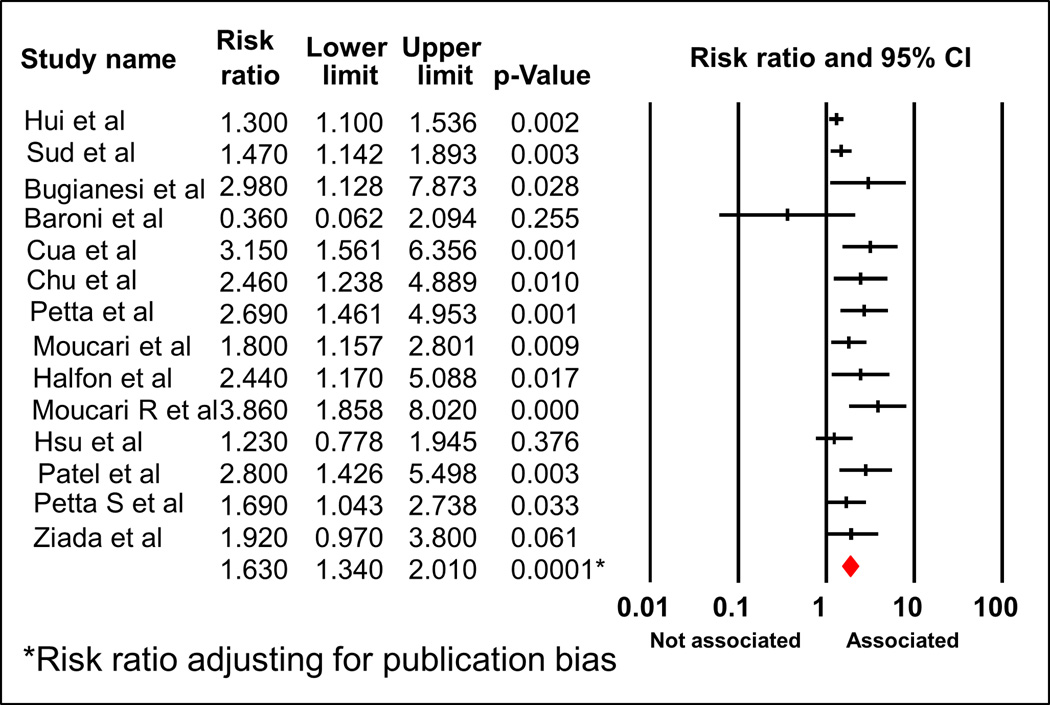
The Newcastle-Ottawa Scale (range, 1-9 stars) to assess the publication quality revealed average 5.6 stars for the twelve cohort studies and 6.5 for the two case-control studies. The detail for the scale for each study is shown in Table 1. Due to the presence of publication bias (funnel plot, Supplemental Fig 1, Begg and Mazumdar test: p = 0.04; Egger’s test: p = 0.006), adjusted RR was used to report the results (Figure 2).
Figure 2.
Forest plot of meta-analyses demonstrating the association between IR and significant fibrosis of all 14 studies
Association between insulin resistance and liver fibrosis
A total of 3,659 participants with HCV infection were included in the analysis. Of these, 12 were cohort studies consisting of 3,259 subjects. Due to evidence of heterogeneity (Q=29.83, p value for heterogeneity 0.005, I2= 56.42%), we used random-effect model to report the pooled RR. The pooled RR for significant hepatic fibrosis among HCV subjects with IR was 1.89 (95% CI 1.54 -2.33) (Figure 2 and Fig S1). After adjusting for publication bias, the association remained significant with the adjusted RR 1.63 (95% CI 1.34-2.01).
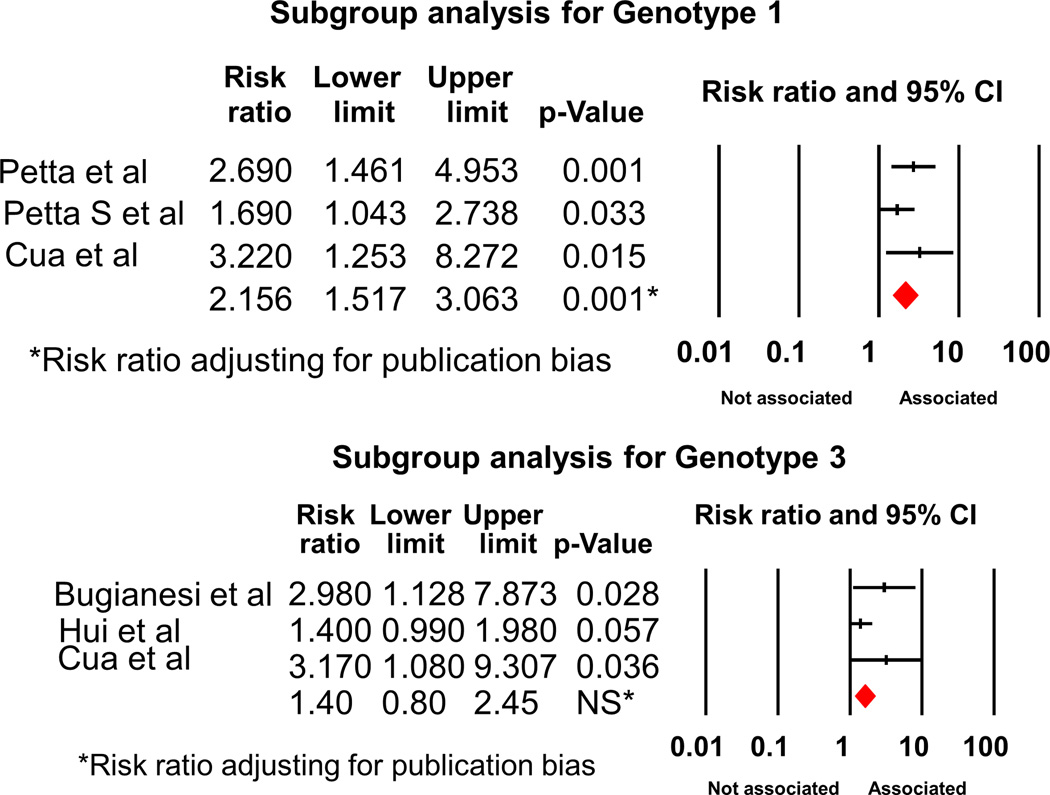
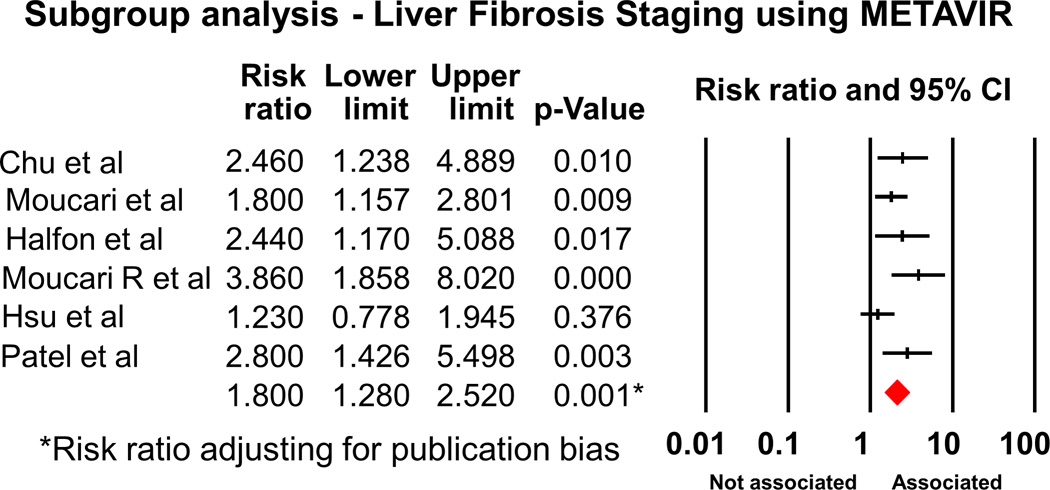
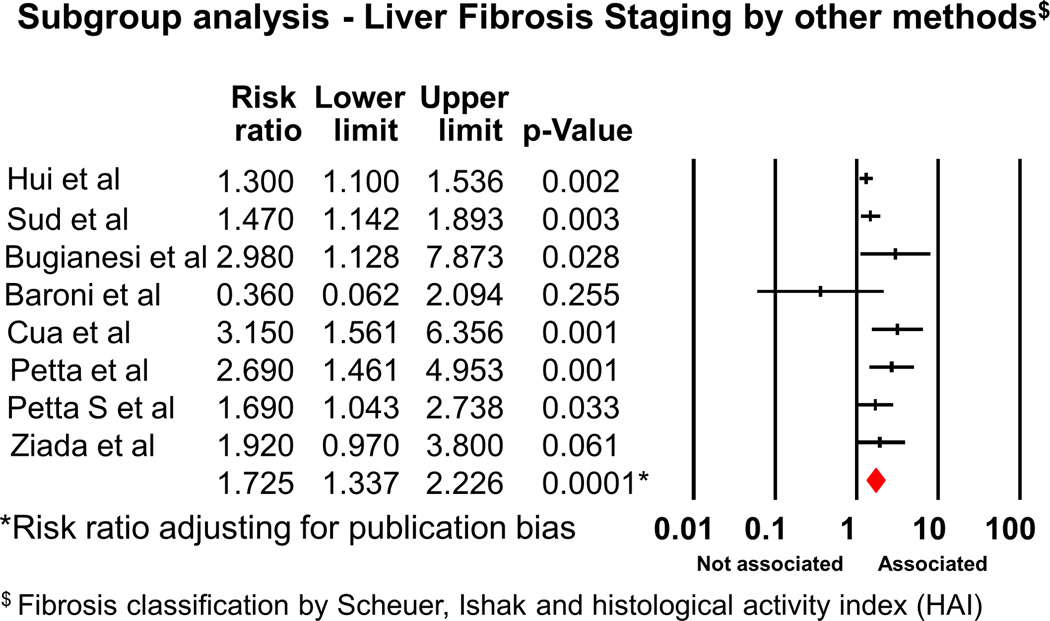
We also performed subgroup analysis. Analysis of the 12 cohort studies showed RR of 2.02 (95% CI 1.6-2.55, p<0.001). Subgroup analysis by genotypes showed RR of 2.16 (95% CI 1.52-3.06) for Genotype 1 (Figures 3, 3 studies21–23); however, the association was no longer significant when we analyzed the data for HCV genotype 3 (3 studies21, 24, 25); RR 1.40 (95% CI 0.8-2.45)(Figure 3 and Fig S2). We also analyzed the strength of association between IR and significant fibrosis, stratified by the different pathological classification. We found that the RR was 1.80 (95%CI 1.28-2.52), when considered the studies using METAVIR method (Figure 4 and Fig S3, 6 studies26–31). The association was still significant when other classifications (Figure 5, 8 studies21–25, 30, 32, 33) were used, RR 1.73 (95% CI 1.34-2.23).
Figure 3.
Forest plot demonstrating association between IR and significant fibrosis, subgroup analysis stratified by genotypes
Figure 4.
Forest plot demonstrating association between IR and significant fibrosis, subgroup analysis for the studies using METAVIR as the pathological classification for fibrosis
Figure 5.
Forest plot demonstrating association between IR and significant fibrosis, subgroup analysis for the studies using non-METAVIR methods as the pathological classification for fibrosis
Subgroup analysis was also performed based on the geographical location. There were no geographical differences in the association between IR and hepatic fibrosis; RR 1.69 (95% CI 1.32-2.17) for the studies from Europe and Australia (Figure S4, 10 studies21–25, 27–29, 33, 34) and RR 1.90 (95% CI 1.27-2.83) for those from Asia (Figure S5, 4 studies26, 30, 32).
DISCUSSION
The major findings of our study are the followings: 1) the presence of IR is a significant risk factor for advanced hepatic fibrosis in HCV patients and 2) whereas no association is observed for those infected with HCV genotype 3, the risk for significant fibrosis is increased for those infected with HCV genotype 1.
Association between hepatitis C infection and IR
The causal relationship of HCV infection and IR development has been demonstrated by the increased prevalence of IR in chronic HCV infection. The prevalence of IR in those infected with HCV is significantly higher than that in the general population35, 36. The mechanism of HCV-induced IR is complex. Following inflammatory response in the liver to HCV infection, a profound impairment of insulin signaling occurs at the level of insulin receptor substrate (IRS) tyrosine phosphorylation and phosphoinositide 3-kinase activation37. The increase in the levels of tumor necrotic factor-alpha by HCV core protein may also lead to proteasomal degradation of IRS1 and IRS2, resulting in the alteration of insulin function and the development of IR37.
In addition to the direct effect of HCV on insulin signaling, the development of IR can also mediated through hepatic steatosis. This can coexist with HCV, regardless of genotype, in patients with risk factors such as obesity and hyperlipidemia. Hepatic steatosis can also be related to the direct hepatopathic effect of genotype 3 viral infection38. In this scenario, the relationship between IR and HCV infection is bidirectional37; HCV induces steatosis and the latter could also cause IR39. HCV-associated hepatic steatosis is mainly virus-induced in genotype 3 infected patients due to the impairment in very low-density lipoprotein (VLDL) secretion38. However, in non-genotype 3, the development of IR is likely play a major role in steatosis37, 38.
Insulin resistance and hepatic fibrosis
Once developed, IR plays an important role in promoting hepatic fibrosis. Hyperinsulinemic state associated with IR directly activates stellate cells47–50. Furthermore, IR-induced hepatic lipid accumulation and generation of ROS can also indirectly activate stellate cells and initiate the cellular signaling cascades triggering hepatic fibrosis. Our findings that the progression of fibrosis in patients with IR is genotype-specific deserve further comments. Although the interference with the insulin sensitivity shows some HCV genotype-specificity, IR has been reported to occur in all HCV genotypes, but to a different extent37, 40. Patients infected with the genotype 3 virus have a lower prevalence of IR when compared with those infected with the other viral genotypes, even after adjustment for the effects of body mass index (BMI) and other confounders41, 42. However, genotype 1 infection was found to be a significant determinant of severe IR, even in patients without underlying diabetes mellitus37. The effect of different genotypes of HCV on the severity of IR is likely explained our findings.
Limitation
Our systemic review has some limitations. First, there are factors which were not taken into consideration for the adjusted RR analysis such as age, gender, body mass index, duration of HCV infection and family history, primarily due to unavailability of these data in the original studies. Second, information on the history of alcohol intake was not uniformly provided. In the studies that mentioned alcohol intake, there were discrepancies in the amount as well as the cut-off levels for hazardous alcohol use. One study excluded any amount of alcohol users23 while others26, 28, 33, 34 used different levels of alcohol intake as the cut-off and one study31 did not consider alcohol consumption in exclusion criteria.
Summary and Clinical implications
This study elucidates the important relation for a genotype-specific association between IR and significant fibrosis in patients with HCV infection. Improvement in IR either by weight loss, life style change or insulin sensitizer can significantly improve SVR rates and treatment outcomes43. At present, it is unclear how insulin resistance will impact the response to treatment with the newly effective anti-viral agents for HCV. However, given the limited access to these new medications in other parts of the world, strategies to improve insulin sensitivity should be explored as they might mitigate against the progression of fibrosis.
Supplementary Material
Acknowledgments
Financial support/Sources of funding: This study is supported by K08 AA016570 from the NIH/NIAAA, 1I01CX000361-01 from the Veterans Affairs Research and Administration, and W81XWH-12-1-0497 from United States Department of Defense (All to S.L)
Abbreviations
- HCV
Hepatitis C virus
- HOMA
Homeostasis model assessment
- IR
Insulin Resistance
- OR
Odds Ratio
- RR
Relative Risk
Footnotes
Conflict of interest: All authors reported no conflicts of interest to the content of this manuscript
Authors contribution:
SP and SL – study design
SP and RP – literature search, review and collection of data
SP and RJ – analysis and interpretation of data
SP, RJ and RP – drafted manuscript
SL – made critical revisions to manuscript
Reference List
- 1.Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55(Suppl 1):S10–S15. doi: 10.1093/cid/cis361. [DOI] [PubMed] [Google Scholar]
- 2.Dhingra A, Kapoor S, Alqahtani SA. Recent advances in the treatment of hepatitis C. Discov Med. 2014;18(99):203–208. [PubMed] [Google Scholar]
- 3.McCaughan GW, George J. Fibrosis progression in chronic hepatitis C virus infection. Gut. 2004;53(3):318–321. doi: 10.1136/gut.2003.026393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet. 1997;349(9055):825–832. doi: 10.1016/s0140-6736(96)07642-8. [DOI] [PubMed] [Google Scholar]
- 5.Wiley TE, Brown J, Chan J. Hepatitis C infection in African Americans: its natural history and histological progression. Am J Gastroenterol. 2002;97(3):700–706. doi: 10.1111/j.1572-0241.2002.05555.x. [DOI] [PubMed] [Google Scholar]
- 6.Marabita F, Aghemo A, De NS, et al. Genetic variation in the interleukin-28B gene is not associated with fibrosis progression in patients with chronic hepatitis C and known date of infection. Hepatology. 2011;54(4):1127–1134. doi: 10.1002/hep.24503. [DOI] [PubMed] [Google Scholar]
- 7.Ostapowicz G, Watson KJ, Locarnini SA, Desmond PV. Role of alcohol in the progression of liver disease caused by hepatitis C virus infection. Hepatology. 1998;27(6):1730–1735. doi: 10.1002/hep.510270637. [DOI] [PubMed] [Google Scholar]
- 8.Mehta SH, Brancati FL, Sulkowski MS, Strathdee SA, Szklo M, Thomas DL. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133(8):592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 9.Allison ME, Wreghitt T, Palmer CR, Alexander GJ. Evidence for a link between hepatitis C virus infection and diabetes mellitus in a cirrhotic population. J Hepatol. 1994;21(6):1135–1139. doi: 10.1016/s0168-8278(05)80631-2. [DOI] [PubMed] [Google Scholar]
- 10.Yoneda M, Saito S, Ikeda T, et al. Hepatitis C virus directly associates with insulin resistance independent of the visceral fat area in nonobese and nondiabetic patients. J Viral Hepat. 2007;14(9):600–607. doi: 10.1111/j.1365-2893.2006.00836.x. [DOI] [PubMed] [Google Scholar]
- 11.Hui JM, Sud A, Farrell GC, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125(6):1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Dixon JB, Bhathal PS, O'Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121(1):91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 13.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30(6):1356–1362. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 15.Ishak K, Baptista A, Bianchi L, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 16.Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1(5):431–435. doi: 10.1002/hep.1840010511. [DOI] [PubMed] [Google Scholar]
- 17.Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13(3):372–374. doi: 10.1016/0168-8278(91)90084-o. [DOI] [PubMed] [Google Scholar]
- 18.Cohen's Kappa calculator http://vassarstats.net/kappa.html
- 19.Newcastle-Ottawa scale http://www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf
- 20.Comprehensive Meta-analysis http://www.meta-analysis.com/index.php#
- 21.Cua IH, Hui JM, Kench JG, George J. Genotype-specific interactions of insulin resistance, steatosis, and fibrosis in chronic hepatitis C. Hepatology. 2008;48(3):723–731. doi: 10.1002/hep.22392. [DOI] [PubMed] [Google Scholar]
- 22.Petta S, Camma C, Di MV, et al. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. 2008;103(5):1136–1144. doi: 10.1111/j.1572-0241.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 23.Petta S, Macaluso FS, Barcellona MR, et al. Serum gamma-glutamyl transferase levels, insulin resistance and liver fibrosis in patients with chronic liver diseases. PLoS One. 2012;7(12):e51165. doi: 10.1371/journal.pone.0051165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hui JM, Sud A, Farrell GC, et al. Insulin resistance is associated with chronic hepatitis C virus infection and fibrosis progression [corrected] Gastroenterology. 2003;125(6):1695–1704. doi: 10.1053/j.gastro.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 25.Bugianesi E, Marchesini G, Gentilcore E, et al. Fibrosis in genotype 3 chronic hepatitis C and nonalcoholic fatty liver disease: Role of insulin resistance and hepatic steatosis. Hepatology. 2006;44(6):1648–1655. doi: 10.1002/hep.21429. [DOI] [PubMed] [Google Scholar]
- 26.Chu CJ, Hung TH, Hwang SJ, et al. Association of insulin resistance with hepatic steatosis and progression of fibrosis in Chinese patients with chronic hepatitis C. Hepatogastroenterology. 2008;55(88):2157–2161. [PubMed] [Google Scholar]
- 27.Moucari R, Asselah T, Cazals-Hatem D, et al. Insulin resistance in chronic hepatitis C: association with genotypes 1 and 4, serum HCV RNA level, and liver fibrosis. Gastroenterology. 2008;134(2):416–423. doi: 10.1053/j.gastro.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 28.Moucari R, Ripault MP, Martinot-Peignoux M, et al. Insulin resistance and geographical origin: major predictors of liver fibrosis and response to peginterferon and ribavirin in HCV-4. Gut. 2009;58(12):1662–1669. doi: 10.1136/gut.2009.185074. [DOI] [PubMed] [Google Scholar]
- 29.Halfon P, Penaranda G, Carrat F, et al. Influence of insulin resistance on hepatic fibrosis and steatosis in hepatitis C virus (HCV) mono-infected compared with HIV-HCV co-infected patients. Aliment Pharmacol Ther. 2009;30(1):61–70. doi: 10.1111/j.1365-2036.2009.03995.x. [DOI] [PubMed] [Google Scholar]
- 30.Hsu CS, Liu CH, Liu CJ, et al. Association of metabolic profiles with hepatic fibrosis in chronic hepatitis C patients with genotype 1 or 2 infection. J Gastroenterol Hepatol. 2010;25(5):970–977. doi: 10.1111/j.1440-1746.2009.06186.x. [DOI] [PubMed] [Google Scholar]
- 31.Patel K, Thompson AJ, Chuang WL, et al. Insulin resistance is independently associated with significant hepatic fibrosis in Asian chronic hepatitis C genotype 2 or 3 patients. J Gastroenterol Hepatol. 2011;26(7):1182–1188. doi: 10.1111/j.1440-1746.2011.06722.x. [DOI] [PubMed] [Google Scholar]
- 32.Ziada DH, El SS, Enaba M, Ghazy M, Hasan A. The interaction between insulin resistance, liver fibrosis and early virological response in Egyptian patients with chronic hepatitis C. Can J Gastroenterol. 2012;26(6):325–329. doi: 10.1155/2012/291457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svegliati-Baroni G, Bugianesi E, Bouserhal T, et al. Post-load insulin resistance is an independent predictor of hepatic fibrosis in virus C chronic hepatitis and in non-alcoholic fatty liver disease. Gut. 2007;56(9):1296–1301. doi: 10.1136/gut.2006.107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sud A, Hui JM, Farrell GC, et al. Improved prediction of fibrosis in chronic hepatitis C using measures of insulin resistance in a probability index. Hepatology. 2004;39(5):1239–1247. doi: 10.1002/hep.20207. [DOI] [PubMed] [Google Scholar]
- 35.Harrison SA. Insulin resistance among patients with chronic hepatitis C: etiology and impact on treatment. Clin Gastroenterol Hepatol. 2008;6(8):864–876. doi: 10.1016/j.cgh.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 36.Ferrannini E, Natali A, Capaldo B, Lehtovirta M, Jacob S, Yki-Jarvinen H. Insulin resistance, hyperinsulinemia, and blood pressure: role of age and obesity. European Group for the Study of Insulin Resistance (EGIR) Hypertension. 1997;30(5):1144–1149. doi: 10.1161/01.hyp.30.5.1144. [DOI] [PubMed] [Google Scholar]
- 37.El-Zayadi AR, Anis M. Hepatitis C virus induced insulin resistance impairs response to anti viral therapy. World J Gastroenterol. 2012;18(3):212–224. doi: 10.3748/wjg.v18.i3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon EJ, Hu KQ. Hepatitis C virus (HCV) infection and hepatic steatosis. Int J Med Sci. 2006;3(2):53–56. doi: 10.7150/ijms.3.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Farese RV, Jr, Zechner R, Newgard CB, Walther TC. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab. 2012;15(5):570–573. doi: 10.1016/j.cmet.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muzzi A, Leandro G, Rubbia-Brandt L, et al. Insulin resistance is associated with liver fibrosis in non-diabetic chronic hepatitis C patients. J Hepatol. 2005;42(1):41–46. doi: 10.1016/j.jhep.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 41.Knobler H, Schihmanter R, Zifroni A, Fenakel G, Schattner A. Increased risk of type 2 diabetes in noncirrhotic patients with chronic hepatitis C virus infection. Mayo Clin Proc. 2000;75(4):355–359. doi: 10.4065/75.4.355. [DOI] [PubMed] [Google Scholar]
- 42.Mason AL, Lau JY, Hoang N, et al. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29(2):328–333. doi: 10.1002/hep.510290235. [DOI] [PubMed] [Google Scholar]
- 43.Khattab M, Emad M, Abdelaleem A, et al. Pioglitazone improves virological response to peginterferon alpha-2b/ribavirin combination therapy in hepatitis C genotype 4 patients with insulin resistance. Liver Int. 2010;30(3):447–454. doi: 10.1111/j.1478-3231.2009.02171.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.