Abstract
T cells compete against each other for access to molecules on antigen presenting cells (APC) in addition to peptide:MHC complexes. However, the identity of cell surface molecules that influence T cell competition, other than peptide:MHC, have yet to be defined. Here we identify CD70, a TNF ligand expressed on activated APCs, as an important mediator of T cell competition for APCs. Upon engagement of CD27 by CD70, CD27 is proteolytically cleaved from the surface of the interacting CD8+ T cell and captured by CD70 expressing dendritic cells. The capture of CD27 effectively masks CD70 on APCs, disallowing the interaction with CD27 on other competing T cells. Collectively, our data indicate that T cells compete against each other for access to the TNF-ligand CD70, an interaction that affects the duration and potency of T cell:DC interactions, thus influencing the repertoire of responding CD8+ T cells to self or foreign antigens.
Introduction
The ability to respond to self and foreign antigens requires a network of signals between antigen presenting cells (APCs) and responding T lymphocytes. The host typically contains a spectrum of T cells, ranging in frequency from a few hundred to a few thousand antigen specific T cells in the unprimed repertoire[1, 2]. Despite this diversity of antigen specific T cells, often it is only a limited repertoire of T cells that dominates the response to any antigen challenge[3]. The process by which T cell receptor diversity is limited following antigen exposure is known as clonal dominance[4]. Though we do not fully understand how clonal dominance is achieved, the timing of antigen expression, efficiency of antigen processing by the proteasome, epitope affinity for MHC, abundance of surface MHC:peptide, and TCR affinity for MHC:peptide are all known to contribute in various ways[5]. One specific mechanism of clonal dominance centers on competition between T cells for access to the MHC:peptide complexes on the APC surface[6, 7]. We [7] and others [8, 9] have demonstrated that T cells can alter the level of MHC:Peptide complexes on the surface of the APC which could change the efficiency by which one clone dominates the response. However, it has been additionally shown that T cells of different antigen specificities can also compete against each other for factors produced by APCs that are independent of MHC:Peptide complexes[10, 11]. Collectively these studies indicated that T cells compete against one another for some aspect of the APC surface that is independent of antigen specificity. Prior to the work presented herein, none of these competitive factors have been identified.
CD27 is a TNF receptor super-family member expressed uniformly by naïve T cells and in selective memory T cell subsets. Its ligand, CD70, is expressed by activated APCs and some cases on activated lymphocyte subsets[12]. Though originally described as a primary costimulatory molecule [13], its function has been refined to acting more as a “signal 3” mediator, enhancing T cell survival during the early phases of clonal expansion and differentiation into effectors[14, 15]. More recently, it was shown that T cells with lower affinity for nominal antigen require CD27 stimulation to participate in the response, such that in the absence of CD27, only high affinity T cells survive and differentiate into effectors and eventually memory[16]. These data suggest a mechanism by which limiting access of T cells to CD70:CD27 interactions would result in a more limited repertoire of responding T cells, ie. a greater degree of immunodominance.
Herein, we show that CD27 on naïve T cells is proteolytically cleaved from the T cell surface upon interaction with a CD70 bearing APC. Surprisingly, the cleaved CD27 remains bound to the CD70 expressed on the APC surface, effectively blocking its interaction with CD27 on any subsequently interacting T cell. Consistent with the conclusion that this contributes to immunodominance, CD27−/− T cells are unable to clonally dominate the response to antigen against T cells of similar specificity. These data highlight a novel, non-MHC associated mechanism by which a given T cell restricts the response of neighboring T cells, ultimately contributing to the formation of immunodominance and T cell clonal/affinity maturation.
Results
T cell surface expression of CD27 modulates CD70 and vice versa
We have published extensively on the use of a combined adjuvant, consisting of polyI:C and an agonistic CD40 antibody (PolyIC/αCD40), which elicits robust CD4 and CD8+ T cell responses after a single vaccination [17–19]. Our previous data demonstrated that a combined TLR/CD40 agonistic vaccination is able to induce an increase in CD70 expression on resident DC populations of 5–10 fold above resting levels[19]. Twenty-four hours after polyIC/αCD40 immunization and increased CD70 expression on the APCs, we observed a significant decrease in CD27 staining on bulk CD8+ T cells (Fig 1A and B). This was unexpected as this immunization ultimately leads to T cells with increased expression of CD27 [20]. Closer examination using CD70 deficient (CD70−/−) mice revealed that the decrease in CD27 staining on CD8+ T cells was CD70-dependent (Fig 1A). Indeed, CD8+ T cells in CD70−/− mice showed a slight, but significant, increase in CD27 expression (Fig 1B) consistent with in vitro stimulation assays[18]. These data suggested that, early after immunization, surface detection of CD27 was being regulated by its ligand, CD70.
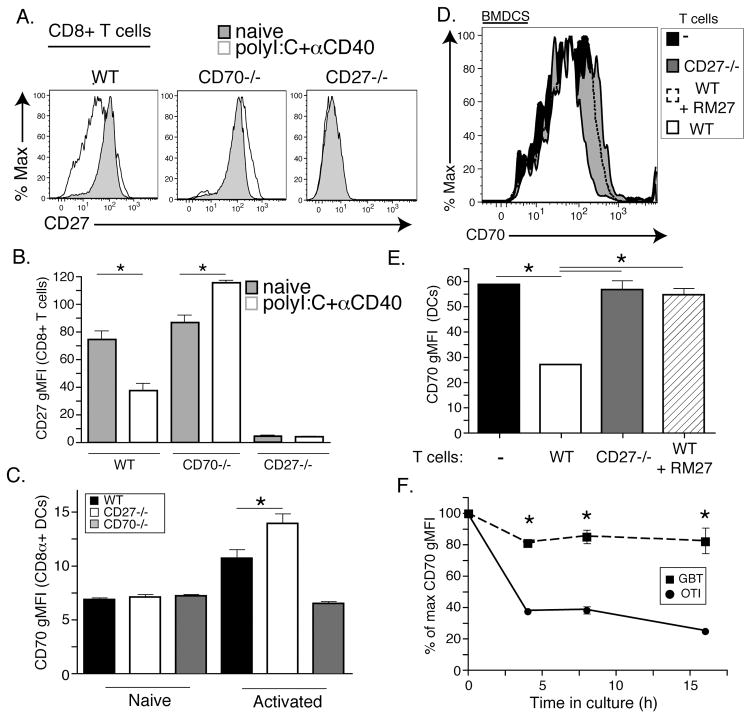
Figure 1. CD27 and CD70 regulate surface expression of their respective binding partner.
A. WT, CD70−/−, or CD27−/− T cells were immunized with 50μg polyI:C and 50 μg αCD40 intraperitoneally (polyI:C+αCD40) or not at all (naïve). 24 hours later mice were sacrificed and T cells were isolated from spleens. Shown is the expression of CD27 of B220−, CD8+ cells from indicated mice. (see supplemental Fig 3 for CD8+ T cell gating strategy) B. Geometric mean fluorescence intensity (gMFI) of CD27 expression on B220−, CD8+ T cells from mouse groups described in A where gray bars are naïve and white bars are polyI:C+αCD40 activated BMDCs. C. Dendritic cell activation 24 hours post immunization as in A and B. CD70 gMFI from WT (black bars), CD27−/− (white bars), and CD70−/− (gray bars) was calculated from CD11c+, B220−, CD11b−, CD8+ cells using flow cytometery. (see supplemental Fig 3 for DC gating strategy) D. CD70 expression profile of LPS activated bone marrow derived dendritic cells (BMDCs) co-cultured with WT (white histogram), CD27−/− (gray histogram), WT+ RM27 (CD27 blocking antibody) (dashed line) or without T cells (black histogram). E. Quantitation of CD70 gMFI from D. F. BMDCs activated with LPS and pulsed with either SIINFEKL (OT1 peptide) were co-cultured with CD8 T cells specific for SIINFEKL (OT1) or specific for SSIEFARL (GBT) or without T cells. Shown is the percent of maximum CD70 gMFI calculated from BMDCs at the respective time point without T cells. Each experiment was performed with at least three replicates per group and more than four independent times with similar results. Data shown is from representative experiments. Data shown is the mean +/−SEM. Asterisks represent values that are significantly different using a students t-test with a p-value less than 0.05. Only values that were significant across experiments are shown with an asterisk.
The use of both CD70−/− and CD27−/− mice as controls in the experiments described above revealed a second surprising finding; expression of CD27 by T cells also affected the detection of CD70 on the surface of the DC. When CD70 levels on DCs were compared between WT and CD27−/− mice, cells from CD27−/− mice had increased CD70 staining 24 hours after PolyIC/αCD40 immunization. Importantly, the increase in CD70 staining on CD8+ DCs from CD27−/− mice occurred at the same time point as the decrease in CD27 staining on T cells in WT mice (Fig 1C). To more closely examine the loss of CD70 staining on DCs, we utilized an in vitro system, co-culturing SIINFEKL-pulsed, activated BMDCs (Fig 1D and E) or splenic DCs (not shown) with WT or CD27−/− OTI T cells. OTI T cells reduced the detection of CD70 on the surface of the DCs (Fig 1E) in a manner directly related to CD27:CD70 interactions as reduced CD70 staining was not observed in the presence of either an antibody that blocks CD27 binding to CD70 (hatched bar/dotted histogram) or in the presence CD27-deficient OTI T cells (shaded bar/histogram). Consistent with the in vivo data (Fig 1A), the loss of CD70 staining on the DC was largely, though not exclusively, antigen dependent, since co-culture of SIINFEKL pulsed DCs with transgenic T cells specific for HSV glycoprotein B (gBT-1 T cells) resulted in only a limited loss of CD70 staining as compared to co-culture with OT1 T cells (Fig 1F). Thus, while the loss of CD70 staining can be seen between T cells and APCs interacting in an antigen non-specific fashion, this is dramatically increased under conditions of antigen specificity. Collectively, the data indicate that the detection of CD27 on CD8+ T cells is reduced in the presence CD70-bearing APCs and vice versa.
CD27 is proteolytically cleaved from T cells and binds to CD70 on DCs
There are two mechanisms by which CD27-CD70 interactions might lead to the loss of both molecules from their respective cell surfaces; down modulation or proteolytic cleavage. While CD27-mediated down modulation of CD70 from the surface of APCs has been reported previously [21], the identification of soluble CD27 (sCD27) in clinical settings[22–26] and in vitro cultures[27, 28] indicate that cleavage of CD27 from the cell surface also can occur. With these results in mind, we postulated that our data could be explained through an exchange of cleaved, sCD27 between the interacting T cells and APCs. If sCD27 were to remain bound to the CD70 on the cell surface of the APC, it would effectively block detection by the CD70 antibody used in our experiments. If true, then we speculated that we should not only observe sCD27 bound to CD70 expressing APCs, we should also regain detection of CD70 on the APC surface by using protease inhibitors to stop the cleavage of CD27.
To address whether CD27 is transferred from the surface of T cells to APCs we utilized a co-culture system in which CD27−/−, SIINFEKL-pulsed BMDCs were induced to express CD70 and then incubated with WT or CD27−/− OT1 T cells with or without an antibody that blocks CD27/CD70 interactions (RM27). 18–24 hours later, the BMDC/OT1 co-culture was stained with a different (non-competing) antibody against CD27 and analyzed by flow cytometry. Using this assay we found that the loss of CD27 by T cells occurs concurrently with the appearance of CD27 fluorescence on the APCs (Fig 2A and B). This was not because the BMDCs expressed CD27 as they were derived from CD27−/− bone marrow. Furthermore, detection of CD27 on the DCs was abrogated by the addition of RM27, indicating the ligand-dependency of DC CD27 staining (Fig 2B).
Figure 2. CD27 is proteolytically cleaved from the surface of T cells in a CD70 dependent manner.
A. CD27−/− BMDCs were matured in vitro with either LPS or αCD40/PolyIC and pulsed with the SIINFEKL peptide and then co-cultured with WT OT1 T cells (white histogram), CD27−/− OT1 T cells (gray histogram), WT OT1 T cells plus the blocking antibody RM27 (dashed line), or without T cells (black histogram). BMDCs were stained for the acquisition of CD27 and shown are the flow cytometry histograms. B. Quantitation of groups shown in A. C. WT and CD70−/− BMDCs were matured with LPS and pulsed with SIINFEKL as in A and B. BMDCs were then labeled with CFSE (green) and OT1 T cells were labeled with CMTMR (blue). 2–6 hours after co-culturing labeled OT1s with BMDCs cells were stained with an APC conjugated CD27 antibody (red) and paraformaldehyde fixed before visualizing on a spinning disk microscope. White scale bar is 10μm in length. The image was captured with a 40x objective. D. Microscope slides were divided into sections and imaged. Fold increase in CD27 staining over BMDCs cultured without T cells was calculated using based on all images for both WT and CD70−/− BMDCs. E. As above, matured BMDCs were co-cultured with T cells in the presence or absence of an MMP8 inhibitor or a CD70 blocking antibody. 18–24 hours later the T cells were stained with αCD27 antibody and the MFI compared to the αCD27 MFI on T cells before incubation with BMDCs (pre). Each experiment was repeated three or more times with at least three replicates per group with similar results. Data shown are representative of these experiments. Data shown is the mean +/−SEM.One asterisk represents differences with a p-value less than 0.05, three asterisks represent a p-value less than 0.001 by student’s t test. Only values that were significant across experiments are shown with an asterisk.
These data are only consistent with the interpretation that CD27 was cleaved from the T cell and transferred to the DC in a CD70-dependent fashion. Using spinning disc-confocal microscopy, we were able to directly visualize CD27 on the surface of APCs following the in vitro co-culture. In these experiments we incubated WT OT1 T cells with either WT or CD70−/− antigen pulsed BMDCs. We again found that CD27 was transferred from the surface of T cells to APCs in a CD70 dependent manner (Fig 2C and D). Finally, using protease inhibitors against matrix metalloproteinase 8 (previously shown to cleave CD27 into a soluble form [28, 29]), we blocked the loss of CD27 staining from the surface of T cells and the subsequent acquisition of CD27 staining on the APC (Fig 2E). Taken together, these findings identify a novel mechanism by which the expression of the TNFR-family member CD27 is regulated on the surface of T cells in a ligand-dependent manner; namely that by interacting with its ligand, CD27 becomes susceptible to MMP-mediated cleavage from the surface of the T cell into a soluble form which then remains bound to CD70 expressed on the APC cell surface.
CD27 expression confers a competitive advantage to CD8+ T cells
Transferred TCR transgenic T cells into a recipient host in increasing numbers have been well documented to compete against (ie. suppress) the response of the endogenous pool of antigen specific T cells, ultimately dominating the response to antigen [6, 30]. Our data above suggest that access to CD70 could be a primary determinant by which these transferred cells compete. Manipulating access to CD70 through the shedding of CD27 could effectively block CD27 stimulation on subsequently interacting (endogenous) T cells. If this is a means by which the transferred T cells compete against the endogenous repertoire, then the transfer of CD27−/− T cells should poorly compete against endogenous T cells with the same or similar specificity. To explore this hypothesis, congenically marked (CD45.1+) WT and CD27−/− OT1 T cells were adoptively transferred into WT recipient hosts in increasing numbers (Fig 3A). The recipient mice were immunized with LPS-activated BMDCs that were pulsed with antigens against which the transferred T cells had either high (SIINFEKL-N4) moderate (SAINFEKL-A2) or low (SIYNFEKL) affinity. Six days after DC immunization, the transferred T cell (CD45.1+) and endogenous T cell (CD45.2+) responses were monitored by MHC tetramer (Fig 3B), with the tetramer staining corresponding to the immunizing peptide. Thus, the endogenous N4-specific T cell response was monitored in the mice injected with N4-pulsed BMDCs, the endogenous A2-specific T cell response was monitored in mice injected with A2-pulsed BMDCs, and the endogenous Y3-specific response was monitored in mice injected with Y3 pulsed BMDCs.
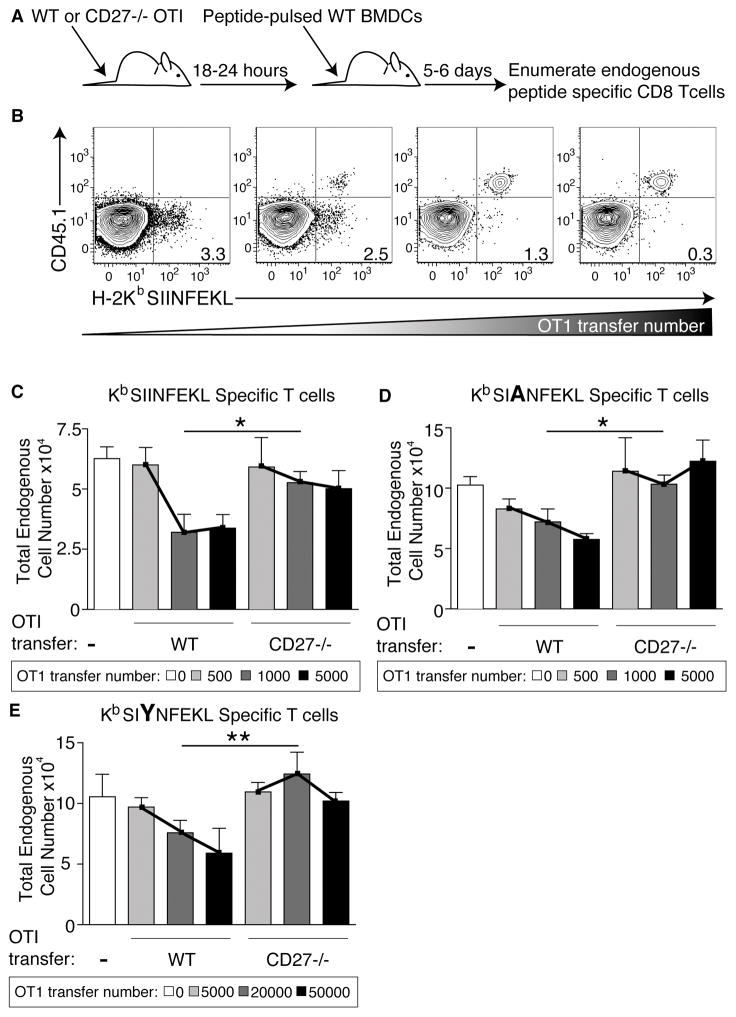
Figure 3. The CD27:CD70 interaction influences immunodominance.
A. Schematic of experimental design where WT or CD27−/− OT1 T cells were transferred into mice 18–24 hours before immunization with LPS matured peptide-pulsed WT BMDCs. 5–6 days post immunization mice were sacrifice and stained with peptide specific tetramer to evaluate the ability of endogenous peptide specific T cells to compete with the transferred cells. B. Example flow plots of the frequency of endogenous (CD45.2) tetramer staining cells over the course of 500–5000 transferred WT OT1 T cells (CD45.1). Numbers in lower right quadrant represent the percent tetramer staining T cells out of total endogenous T cells. The staining and gating strategy shown was used in the calculation of endogenous T cell numbers in C–E. C. Total number of endogenous SIINFEKL specific T cells after SIINFEKL loaded BMDC immunization followed by no OT1 T cell transfer (white bar), 500 WT or CD27−/− OT1 T cells transferred (light gray bars), 1000 WT or CD27−/− OT1 T cells transferred (medium gray bars), or 5000 WT or CD27−/− OT1 T cells transferred. Cells were visualized using flow cytometry and gated on B220-, CD8+, CD44+, tetramer+ cells and the congenic marker as described in part B. This experiment was done more than ten times with 3–7 mice per group. D. Total number of endogenous SAINFEKL specific T cells post SAINFEKL loaded BMDC immunization. Done exactly as described in C and repeated at least four times with 3–7 mice per group. E. Total number of endogenous SIYNFEKL specific T cells after SIYNFEKL loaded BMDC immunization followed by OT1 T cell transfer of 0 (white), 5000 (light gray), 20000 (dark gray), or 50000 (black) cells. Experiments in part E were repeated at least twice with 3 mice per group with similar results. All experiments in the figure are representative and one asterisk represents statistically significant differences in the lines as evaluated by two-way ANOVA with a p value <0.05, two asterisks represent a p-value <0.01. Data shown is the mean +/−SEM.
Consistent with published data [31], WT OT1 T cells competed effectively against either endogenous N4-specific, A2-specific, and Y3-specific T cells, observed as a decrease in the number of antigen specific endogenous T cells as the transferred cell number increased (Fig 3C–E). In contrast, CD27−/− OT1 cells were poorly competitive against endogenous T cell responses regardless of the affinity of peptide presented to them (Fig 3C–E). Given its low affinity for the Y3 ligand, competition against the endogenous Y3 response required the transfer of increased number of WT OT1 cells (Fig 3E, note OT1 transfer numbers). However, even at this higher number of transferred T cells, the CD27−/− OT1s failed to compete against the endogenous Y3 specific T cell response relative to their WT counterparts (Fig 3E).
It is important to reiterate that the results in Figure 3 are of the endogenous response, not of the transferred OT1 T cells. Thus the unexpected finding is that CD27−/− T cells fail to compete effectively against (clonally dominate) the endogenous responders. This inability to clonally dominate the response was not related to the expansion and/or survival of the competing CD27−/− OT1 T cells for a number of reasons. First, the number of CD27−/− OTI T cells is the same as the WT T cells over the course of the early events in T cell activation (through day 4), even under conditions of maximal DC CD70 expression [19] (supplemental Fig 1). This is also consistent with the data in the literature from Van Lier and colleagues showing that high affinity T cells do not require CD27 signaling for their survival [16]. Second, previous data showed that competition between T cells occurs within the first 24 hours after immunization/antigen encounter [11]. This is in agreement with the time frame in which CD27/CD70 interactions impact the magnitude of the T cell response [19]. Because CD27−/− OT1 T cell numbers are the same as WT OT1 T cells during the time points of maximal CD27–CD70 interactions and competition with the endogenous repertoire, we conclude that differences in competing T cell numbers cannot explain the differences in endogenous responders observed in Figure 3. Finally, statistical differences in competition between WT and CD27−/− OT1 T cells were eliminated when immunizing with CD70−/− BMDCs (Supplemental Fig 2), further focusing the capacity of a T cell for clonal dominance on CD27/CD70 interactions. Thus, over a broad range of TCR affinities for antigen, CD27–CD70 interactions influence the capacity of T cells to compete against each other in the presence of antigen bearing APCs.
Discussion
Our data, in combination with published reports, support a model by which a T cells’ response can be suppressed as a result of the proteolytic cleavage of CD27 from a neighboring T cell. The resulting soluble form of CD27 then binds to and blocks CD70 from productively providing a co-stimulus to membrane associated CD27 during subsequent T cell interactions. These data finally provide a mechanistic underpinning for how T cells compete against each other outside of peptide:MHC interactions. While T cells specific for a given antigen can compete against each other for access to peptide:MHC complexes, previous data indicated that T cells also compete for APC factors other than peptide:MHC [6, 7]. Since the discovery of this form of T cell competition, a number of lines of evidence suggested that CD70 might be one of these factors. First, the experimental models examining T cell competition have used either vaccination (anti-CD40-based adjuvants, BMDCs) or infectious challenge (VV, LM) in which the T cell responses to all of these forms of immunization are dependent upon the expression of CD70[32]. Second, T cell competition for access to DCs was shown to occur within 24 hours of initial immunization[11], a time frame similar to that observed for the costimulatory function of CD70 in instigating T cell immunity[19]. Lastly, CD27 influences the recruitment of lower affinity T cells into an active immune response[16], indicating that T cells without access to CD70 may be at a competitive disadvantage relative to T cells with access to CD70. Our data presented here now provide the mechanism by which access to CD70 can be manipulated by specific T cell clones, ultimately resulting in immunodominance following infection or vaccination.
It is worth noting that inverse correlations of CD27 and CD70 have been previously reported with the conclusion that CD70 is down-modulated by interaction with CD27 [21]. However, those data were derived mainly by examining DC CD70 staining in the presence or absence of a CD27 antibody, an antibody we now know can block the acquisition of cleaved CD27 by the CD70-expressing APCs. Indeed, actual internalization of CD70 by DCs was only shown using a CD70 antibody and was not shown in response to added soluble CD27. Thus, it is possible if not likely that at least some of the previously published data can be explained as a result of the CD27 cleavage mechanism we document here rather than down-modulation of CD70 on the APC.
The data we show here document a role for CD27 in the suppression of competing T cells responses. However, a positive role for sCD27 in augmenting tumor specific T cell responses has been suggested [33]. In this report, the addition of sCD27 to in vitro T cell cultures increased their proliferative response and survival, and their expression of activation markers. In addition, a positive correlation between patient prognosis and high levels of sCD27 in the serum was identified. However, the majority of reports documenting sCD27 do so during a host of diseased states [22, 25, 26, 28, 34–37], and collectively are consistent with the conclusion that sCD27 inhibits effective T cell function. Though it is unclear how a positive role for sCD27 may fit in with the larger conclusions from the literature, our observation that the production of sCD27 requires interaction with CD70 may again offer a solution to explain both positive and negative prognostic value of patient serum sCD27. Our data (Fig 2) suggest that the CD27:CD70 interaction must render the CD27 more accessible or susceptible to MMP proteolytic activity and thus production of more sCD27. While the cleavage of CD27 is dramatically enhanced in an antigen specific interaction between the T cell and APC, our data also show that non-antigen specific interactions in vitro (Fig 2F) and in vivo (Fig 1A) can also result in CD27 cleavage and CD70 masking. While increased CD70 expression can rescue T cell tolerance against chronic infections and cancer [38, 39], chronic CD70 expression results in clonal deletion and tolerance [40, 41]. Thus, serum sCD27 would simply be a proxy for increased patient CD70 expression, the prognostic benefits of which would have to be empirically determined, likely dictated by the immune status of the patient and their degree of immune exhaustion.
Given this important role for CD70/CD27 interactions in reversing tolerance [38, 39], our data provide further incentive to explore the use of agonistic CD27 antibodies in the clinic for treatment against chronic infections and/or cancer. The high levels of sCD27 observed in many of these diseased states, in conjunction with our data shown above, suggest that masking of DC CD70 might be a means by which the maintenance of endogenous responses are further compromised. Though membrane associated CD27 would be expected to interact with CD70 with an overall higher avidity than sCD27, the fact that the binding of sCD27 on CD70 expressing DCs is stable enough to be seen in vitro and direct ex vivo (Figure 2) and influences a T cells capacity for competition (Figure 3) suggests that sCD27 is capable of disruption of mCD27 ligation and signaling. Indeed, the levels of sCD27 observed in patients might well bind the CD27-specific antibody that is currently under clinical development [42–44], effectively distracting it from its intended role in augmenting T cell survival and function. Our data suggest that an investigation into the capacity of CD27 antibodies for binding sCD27 rather than membrane associated CD27 is warranted.
Finally, it remains to be determined whether this novel function for soluble CD27 in blocking productive DC:T cell interactions will be useful or detrimental in the setting of vaccination. On the one hand, the preferential response of higher affinity T cells, which CD27 shedding seems likely to facilitate, would be an advantage in the protection against a specific infection. However, lower affinity responses to a specific antigen also have increased cross reactivity to related antigens [16], important in a setting such as vaccination against influenza where cross protection against disparate strains is much preferred. Thus, CD27 shedding might be detrimental in this context and immunization strategies should be explored to take this into account. Given the importance of TNFR in dictating the course of T cell responses in general, and the propensity of these molecules for proteolytic cleavage, our data suggest a closer examination of other TNFR superfamily members is in order with respect to their influence on T cell responses after productive interaction with their respective ligands and outside of their proximal signaling events.
Materials and Methods
Mice
Wild-type C57bl/6 mice were purchased from the National Cancer Institute or Jackson labs. CD70−/− mice were made from knockout ES cells obtained through the KOMP (Knock Out Mouse Project) (University of California, Davis CA). Microinjections into albino B6 were performed by the National Jewish Mouse Genetics Core facility. Chimeric founders were bred and germline transmission of the knockout allele confirmed by PCR. CD27−/− mice were a kind gift from Jannie Borst (The Netherlands Cancer Institute, Amsterdam). OT-1 TCR transgenic mice specific to the SIINFEKL peptide of ovalbumin (OVA257-264) in the context of H-2Kb were purchased from Jackson labs and bred in house. gBT-1 (GBT) TCR transgenic mice specific for the SSIEFARL peptide of herpes simplex virus glycoprotein B (HSV-1gB 498–505) in the context of H-2Kb, were a kind gift from Bill Heath (University of Melbourne).
Dendritic cell isolation
Dendritic cells were harvested from the spleen of mice 18–24 hours post immunization with 50μg polyI:C and 50μg αCD40 given intraperitoneally in phosphate buffered saline (PBS) as described in [19]. Briefly, spleens were macerated using forceps and digested at 37 degrees Celsius (C) with collagenase and DNAse (Worthington, Lakewood, NJ) in EHAA media without L-glutamine (GIBCO, Grand Island, NY) for 45 minutes. The digestion was stopped with 0.1M EDTA in Hank’s buffered saline solution and incubated for 5min at 37 degrees C. Single cell suspensions were made by pushing residual tissue pieces through a screen and washed with 5mM EDTA in EHAA. Following ACK (Ammonium-Chloride-Potassium) lysis cells were washed and stained with CD11c clone N418, CD8 clone 53–6.7, CD11b clone M1/70 (eBioscience, San Diego, CA), CD27 clone Lg.3A10 and CD70 clone FR70. All antibodies were purchase from Biolegend (San Diego, CA) unless otherwise stated. Cells were run on the DakoCytomation CyAn ADP flow cytometer (Fort Collins, CO) and acquired using Summit acquisition software. Gating strategy for the identification of CD8+ and CD11b+ splenic DC subsets, as well as for the identification of CD8+ T cells, is shown in supplemental Fig 3. FlowJo software (Tree Star, Ashland, OR) was used to analyze flow cytometry data and cells were counted using a ViCell (Beckman, Coulter, Brea, CA).
In vitro co-culture assays
Bone marrow derived dendritic cells (BMDCs) were grown as described previously [6]. Briefly, bone marrow was isolated from the femur of WT or CD70−/− mice and cultured for 6–7 days in MEM and 1000U/ml of GM-CSF (from B78Hi/GMCSF.1 cell line provided by Dr. Hyam Levistsky, Johns Hopkins, Baltimore, MD). Every two days non-adherant cells were removed from the cultures and the resulting conditioned medium was replaced with half fresh media containing 2 times the GM-CSF and half conditioned medium. On day 6 BMDCs were matured with 1μg/ml of LPS or 50μg/ml polyI:C and 50μg/ml αCD40 for 18–24 hours. When peptide was used, either SIINFEKL or SSIEFARL, the peptide was added in the last 1.5 hours before washing. BMDCs were washed at least 3 times. Concurrently, OT1 or gBT, mice were sacrificed and spleens removed for CD8 T cell isolation using negative selection with the Miltenyi (Miltenyi, Auburn, CA) or Stem Cell Technologies (Vancouver, BC) kits as described previously [45]. DCs and T cells were mixed at a ratio of 1:10, respectively. 4, 8, or 16 hours post co-culturing of DCs and T cells into wells both DCs and T cells were stained for expression of canonical markers as well as CD27 and CD70 as described above. Antibodies used for T cell analysis were B220 (clone RA3-6B2) and CD8 (clone 53–6.7) from Biolegend. Antibodies for DC analysis were CD11c clone N418 (Biolegend), CD8 clone 53–6.7 (Biolegend), and CD11b clone M1/70 (eBioscience, San Diego, CA).
Spinning disk microscopy
In vitro co-cultures were prepared as above with the following exceptions. After BMDCs and OT1 T cells were isolated each cell type was labeled with a vital dye. BMDCs were labeled with 1μM carboxyfluorescein succinimidyl ester (CFSE) (Life Technologies, Grand Island, NY) in PBS for 10 minutes at 37 degrees C. OT1 T cells were labeled with 5μM (5-(and-6)-(((4-chloromethyl)benzoyl)amino)tetramethylrhodamine (CMTMR) (Life Technologies) for 20 minutes at 37 degrees C. The labeling was stopped by adding complete media (MEM with 10%FBS). 2–4 hours after co-culture all cells were stained for 30 minutes with an antibody against CD27 conjugated to APC. After washing, cells were fixed with 1% paraformaldehyde with 4% sucrose for 10 minutes. Cells were then placed on a slide and liquid was removed by spinning the cells onto the slide for 5 minutes at 1000RPM. Cells were visualized using a 3i Olympus spinning disk confocal microscope (Olympus, Waltham, MA) with a 40x oil objective and analyzed with slidebook software. This experiment was repeated 3 times with similar results.
Blocking Antibodies and Inhibitors
RM27, specific for CD27, and FR70, a CD70 blocking antibody, were obtained from Hideo Yagita. Both antibodies were used at 10μg/ml in vitro. MMP8 inhibitor was purchased from Calbiochem (Millipore, MA) and used at 50μg/ml in DMSO.
T cell Competition Assay
Congenically labeled (CD45.1/2) WT or CD27−/− OT1 T cells were isolated as described above using a CD8 negative selection kit. 500–50000 OT1 T cells were transferred intravenously into mice. BMDCs were isolated and grown in vitro as described above. On day 7 after culture BMDCs were removed from the dish by vigorous pipetting and matured with LPS (1μg/ml) for 2.5 hours in a 50ml conical tube. During the last 1.5 hours of LPS treatment peptide was added at a concentration of 10μg/ml. The day after OT1 transfer 0.5–1×106 matured, peptide loaded, BMDCs were used to immunize mice intravenously. 5–6 days after BMDC immunization mice were sacrificed and spleens were harvested. Spleens were processed using glass slides to grind up tissue and ACK to lyse red blood cells. After washing, cells were counted on a ViCell (Beckman Coulter, Brea, CA) and ~3×106 cells were stained with peptide specific MHC class I tetramer in media containing 2.5% FBS at 37 degrees C for 1.5 hours. In the last 0.5 hours of tetramer staining antibodies against CD8, B220, CD44, CD45.1, and CD27 were added. Cells were run on the DakoCytomation CyAn ADP flow cytometer (Fort Collins, CO) and acquired using Summit acquisition software. FlowJo software (Tree Star, Ashland, OR) was used to analyze flow cytometry data.
Supplementary Material
Acknowledgments
We would like to thank Jordan Jacobelli and Rachel Friedman for their guidance with the spinning disk confocal microscopy and Catherine Haluszczak for technical assistance. MAB was supported by a Cancer Research Institute Irvington Post Doctoral Fellowship, BAT was supported by an American Cancer Society fellowship, and RMK was supported by NIH grants AI099863 and AI06612.
Footnotes
Conflict of interest disclaimer. The authors declare no competing conflicts of interests for the published work.
References
- 1.Haluszczak C, Akue AD, Hamilton SE, Johnson LD, Pujanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T Cell Frequency Varies for Different Epitopes and Predicts Repertoire Diversity and Response Magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McHeyzer-Williams MG, Davis MM. Antigen-specific development of primary and memory T cells in vivo. Science. 1995;268:106–111. doi: 10.1126/science.7535476. [DOI] [PubMed] [Google Scholar]
- 4.Marrack P, Mitchell T, Hildeman D, Kedl R, Teague TK, Bender J, Rees W, Schaefer BC, Kappler J. Genomic-scale analysis of gene expression in resting and activated T cells. Curr Opin Immunol. 2000;12:206–209. doi: 10.1016/s0952-7915(99)00075-8. [DOI] [PubMed] [Google Scholar]
- 5.Kedl RM, Kappler JW, Marrack P. Epitope dominance, competition and T cell affinity maturation. Curr Opin Immunol. 2003;15:120–127. doi: 10.1016/s0952-7915(02)00009-2. [DOI] [PubMed] [Google Scholar]
- 6.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T cells compete for access to antigen-bearing antigen-presenting cells. J Exp Med. 2000;192:1105–1113. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kedl RM, Schaefer BC, Kappler JW, Marrack P. T cells down-modulate peptide-MHC complexes on APCs in vivo. Nat Immunol. 2002;3:27–32. doi: 10.1038/ni742. [DOI] [PubMed] [Google Scholar]
- 8.Huang JF, Yang Y, Sepulveda H, Shi W, Hwang I, Peterson PA, Jackson MR, Sprent J, Cai Z. TCR-Mediated internalization of peptide-MHC complexes acquired by T cells. Science. 1999;286:952–954. doi: 10.1126/science.286.5441.952. [DOI] [PubMed] [Google Scholar]
- 9.Hwang I, Huang JF, Kishimoto H, Brunmark A, Peterson PA, Jackson MR, Surh CD, Cai Z, Sprent J. T cells can use either T cell receptor or CD28 receptors to absorb and internalize cell surface molecules derived from antigen-presenting cells. J Exp Med. 2000;191:1137–1148. doi: 10.1084/jem.191.7.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kedl RM, Rees WA, Hildeman DA, Schaefer B, Mitchell T, Kappler J, Marrack P. T Cells Compete for Access to Antigen-bearing Antigen-presenting Cells. J Exp Med. 2000;192:1105–1114. doi: 10.1084/jem.192.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis RA, Kappler JW, Marrack PC. CD8 T cell competition for dendritic cells in vivo is an early event in activation. Proc Natl Acad Sci U S A. 2006;103:12063–12068. doi: 10.1073/pnas.0605130103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tesselaar K, Gravestein LA, van Schijndel GM, Borst J, van Lier RA. Characterization of murine CD70, the ligand of the TNF receptor family member CD27. J Immunol. 1997;159:4959–4965. [PubMed] [Google Scholar]
- 13.Hintzen RQ, Lens SM, Lammers K, Kuiper H, Beckmann MP, van Lier RA. Engagement of CD27 with its ligand CD70 provides a second signal for T cell activation. J Immunol. 1995;154:2612–2623. [PubMed] [Google Scholar]
- 14.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003;198:1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanchez PJ, Kedl RM. An alternative signal 3: CD8 T cell memory independent of IL-12 and type I IFN is dependent on CD27/OX40 signaling. Vaccine. 2012;30:1154–1161. doi: 10.1016/j.vaccine.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Gisbergen KP, Klarenbeek PL, Kragten NA, Unger PP, Nieuwenhuis MB, Wensveen FM, ten Brinke A, Tak PP, Eldering E, Nolte MA, van Lier RA. The costimulatory molecule CD27 maintains clonally diverse CD8(+) T cell responses of low antigen affinity to protect against viral variants. Immunity. 2011;35:97–108. doi: 10.1016/j.immuni.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Ahonen CL, Doxsee CL, McGurran SM, Riter TR, Wade WF, Barth RJ, Vasilakos JP, Noelle RJ, Kedl RM. Combined TLR and CD40 triggering induces potent CD8+ T cell expansion with variable dependence on type I IFN. J Exp Med. 2004;199:775–784. doi: 10.1084/jem.20031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurche JS, Burchill MA, Sanchez PJ, Haluszczak C, Kedl RM. Comparison of OX40 ligand and CD70 in the promotion of CD4+ T cell responses. J Immunol. 2010;185:2106–2115. doi: 10.4049/jimmunol.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanchez PJ, McWilliams JA, Haluszczak C, Yagita H, Kedl RM. Combined TLR/CD40 stimulation mediates potent cellular immunity by regulating dendritic cell expression of CD70 in vivo. J Immunol. 2007;178:1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 20.Edwards LE, Haluszczak C, Kedl RM. Phenotype and function of protective, CD4-independent CD8 T cell memory. Immunol Res. 2013;55:135–145. doi: 10.1007/s12026-012-8356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kuka M, Munitic I, Giardino Torchia ML, Ashwell JD. CD70 is downregulated by interaction with CD27. J Immunol. 2013;191:2282–2289. doi: 10.4049/jimmunol.1300868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Widney D, Gundapp G, Said JW, van der Meijden M, Bonavida B, Demidem A, Trevisan C, Taylor J, Detels R, Martinez-Maza O. Aberrant expression of CD27 and soluble CD27 (sCD27) in HIV infection and in AIDS-associated lymphoma. Clin Immunol. 1999;93:114–123. doi: 10.1006/clim.1999.4782. [DOI] [PubMed] [Google Scholar]
- 23.Yalcin B, Canpinar H, Kutluk MT, Varan A, Akyuz C, Buyukpamukcu M. Cerebrospinal fluid soluble CD27 levels in children with non-Hodgkin lymphomas. Pediatr Hematol Oncol. 2004;21:717–723. doi: 10.1080/08880010490514903. [DOI] [PubMed] [Google Scholar]
- 24.Kara IO, Sahin B, Gunesacar R. Expression of soluble CD27 and interleukins-8 and -10 in B-cell chronic lymphocytic leukemia: correlation with disease stage and prognosis. Adv Ther. 2007;24:29–40. doi: 10.1007/BF02849990. [DOI] [PubMed] [Google Scholar]
- 25.Kara IO, Sahin B, Gunesacar R. Levels of serum and cerebrospinal fluid soluble CD27 in the diagnosis of leptomeningeal involvement of hematolymphoid malignancies. Adv Ther. 2007;24:741–747. doi: 10.1007/BF02849967. [DOI] [PubMed] [Google Scholar]
- 26.Gattorno M, Prigione I, Vignola S, Falcini F, Chiesa S, Morandi F, Picco P, Buoncompagni A, Martini A, Pistoia V. Levels of soluble CD27 in sera and synovial fluid and its expression on memory T cells in patients with juvenile idiopathic arthritides. Clin Exp Rheumatol. 2002;20:863–866. [PubMed] [Google Scholar]
- 27.Loenen WA, De Vries E, Gravestein LA, Hintzen RQ, Van Lier RA, Borst J. The CD27 membrane receptor, a lymphocyte-specific member of the nerve growth factor receptor family, gives rise to a soluble form by protein processing that does not involve receptor endocytosis. Eur J Immunol. 1992;22:447–455. doi: 10.1002/eji.1830220224. [DOI] [PubMed] [Google Scholar]
- 28.Kato K, Chu P, Takahashi S, Hamada H, Kipps TJ. Metalloprotease inhibitors block release of soluble CD27 and enhance the immune stimulatory activity of chronic lymphocytic leukemia cells. Exp Hematol. 2007;35:434–442. doi: 10.1016/j.exphem.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Y, Liu X, Xu L, Tseng H, Cao Y, Jiang J, Ciccarelli BT, Yang G, Patterson CJ, Hunter ZR, Treon SP. Matrix metalloproteinase-8 is overexpressed in Waldenstrom’s macroglobulinemia cells, and specific inhibition of this metalloproteinase blocks release of soluble CD27. Clin Lymphoma Myeloma Leuk. 2011;11:172–175. doi: 10.3816/CLML.2011.n.041. [DOI] [PubMed] [Google Scholar]
- 30.Marzo AL, Klonowski KD, Le Bon A, Borrow P, Tough DF, Lefrancois L. Initial T cell frequency dictates memory CD8+ T cell lineage commitment. Nat Immunol. 2005;6:793–799. doi: 10.1038/ni1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burchill MA, Tamburini BA, Pennock ND, White JT, Kurche JS, Kedl RM. T cell vaccinology: exploring the known unknowns. Vaccine. 2013;31:297–305. doi: 10.1016/j.vaccine.2012.10.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang J, Jochems C, Anderson AM, Talaie T, Jales A, Madan RA, Hodge JW, Tsang KY, Liewehr DJ, Steinberg SM, Gulley JL, Schlom J. Soluble CD27-pool in humans may contribute to T cell activation and tumor immunity. J Immunol. 2013;190:6250–6258. doi: 10.4049/jimmunol.1300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Font J, Pallares L, Martorell J, Martinez E, Gaya A, Vives J, Ingelmo M. Elevated soluble CD27 levels in serum of patients with systemic lupus erythematosus. Clin Immunol Immunopathol. 1996;81:239–243. doi: 10.1006/clin.1996.0184. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Jochems C, Talaie T, Anderson A, Jales A, Tsang KY, Madan RA, Gulley JL, Schlom J. Elevated serum soluble CD40 ligand in cancer patients may play an immunosuppressive role. Blood. 2012;120:3030–3038. doi: 10.1182/blood-2012-05-427799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalayjian RC, Machekano RN, Rizk N, Robbins GK, Gandhi RT, Rodriguez BA, Pollard RB, Lederman MM, Landay A. Pretreatment levels of soluble cellular receptors and interleukin-6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201:1796–1805. doi: 10.1086/652750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swaak AJ, Hintzen RQ, Huysen V, van den Brink HG, Smeenk JT. Serum levels of soluble forms of T cell activation antigens CD27 and CD25 in systemic lupus erythematosus in relation with lymphocytes count and disease course. Clin Rheumatol. 1995;14:293–300. doi: 10.1007/BF02208342. [DOI] [PubMed] [Google Scholar]
- 38.Keller AM, Schildknecht A, Xiao Y, van den Broek M, Borst J. Expression of costimulatory ligand CD70 on steady-state dendritic cells breaks CD8+ T cell tolerance and permits effective immunity. Immunity. 2008;29:934–946. doi: 10.1016/j.immuni.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 39.Keller AM, Xiao Y, Peperzak V, Naik SH, Borst J. Costimulatory ligand CD70 allows induction of CD8+ T-cell immunity by immature dendritic cells in a vaccination setting. Blood. 2009;113:5167–5175. doi: 10.1182/blood-2008-03-148007. [DOI] [PubMed] [Google Scholar]
- 40.Arens R, Tesselaar K, Baars PA, van Schijndel GM, Hendriks J, Pals ST, Krimpenfort P, Borst J, van Oers MH, van Lier RA. Constitutive CD27/CD70 interaction induces expansion of effector-type T cells and results in IFNgamma-mediated B cell depletion. Immunity. 2001;15:801–812. doi: 10.1016/s1074-7613(01)00236-9. [DOI] [PubMed] [Google Scholar]
- 41.Beishuizen CR, Kragten NA, Boon L, Nolte MA, van Lier RA, van Gisbergen KP. Chronic CD70-driven costimulation impairs IgG responses by instructing T cells to inhibit germinal center B cell formation through FasL-Fas interactions. J Immunol. 2009;183:6442–6451. doi: 10.4049/jimmunol.0901565. [DOI] [PubMed] [Google Scholar]
- 42.He LZ, Prostak N, Thomas LJ, Vitale L, Weidlick J, Crocker A, Pilsmaker CD, Round SM, Tutt A, Glennie MJ, Marsh H, Keler T. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol. 2013;191:4174–4183. doi: 10.4049/jimmunol.1300409. [DOI] [PubMed] [Google Scholar]
- 43.Thomas LJ, He LZ, Marsh H, Keler T. Targeting human CD27 with an agonist antibody stimulates T-cell activation and antitumor immunity. Oncoimmunology. 2014;3:e27255. doi: 10.4161/onci.27255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitale LA, He LZ, Thomas LJ, Widger J, Weidlick J, Crocker A, O’Neill T, Storey J, Glennie MJ, Grote DM, Ansell SM, Marsh H, Keler T. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin Cancer Res. 2012;18:3812–3821. doi: 10.1158/1078-0432.CCR-11-3308. [DOI] [PubMed] [Google Scholar]
- 45.Tamburini BA, Burchill MA, Kedl RM. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nat Commun. 2014;5:3989. doi: 10.1038/ncomms4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.