Abstract
Background/Aims
Recent studies have indicated a link between impaired capacity of de novo protein synthesis and neurodegenerative diseases including Alzheimer’s disease (AD). Moreover, it has been established that eukaryotic elongation factor 1A (eEF1A) plays a critical role in maintaining long-term synaptic plasticity, a cellular model for learning and memory. The aim of the present study is to determine whether brain eEF1A protein levels are dysregulated in brain tissue from AD patients compared with controls.
Methods
Post mortem human brain samples collected from patients clinically diagnosed as AD, and from age-matched healthy controls, were utilized for this study. Both Western blot and immunohistochemistry approaches were utilized to investigate potential alteration of eEF1A protein levels by using specific antibody.
Results
Our data demonstrate that eEF1A expression is reduced in AD patients in hippocampus, but not in cerebellum or midfrontal gyrus. Furthermore, immunohistochemical experiments reveal that neuronal eEF1A reduction in AD hippocampus is localized to CA1 and dentate gyrus, but not in CA3.
Conclusion
Dysregulation of eEF1A and its associated signaling pathways might represent novel molecular mechanisms underlying AD pathogenesis. Further investigation is necessary to determine whether eEF1A is a viable therapeutic target for AD and other cognitive syndromes.
Keywords: Protein synthesis, elongation factors, eEF1A, mTOR, Alzheimer’s disease
Introduction
A better understanding of the molecular mechanisms underlying the pathophysiology of Alzheimer’s disease (AD) could help identify novel diagnostic and prognostic biomarkers and potential therapeutic targets. Recent studies have suggested a link between disruption of protein synthesis homeostasis and neurodegenerative diseases including prion disease and AD (1, 2). Mounting evidence has identified impairments of synaptic efficacy as an early and enduring key event in the process of AD-associated cognitive decline (3, 4), but understanding the mechanisms underlying this pathophysiology has been elusive. One possible mechanism may involve eukaryotic mRNA translational factor eEF1A and its associated signaling pathways, which play important roles in maintaining long-lasting synaptic plasticity such as long-term potentiation (LTP), which is well known as a cellular model for learning and memory (5–7).
As one of the most abundant translational factors, eEF1A is a GTP-binding protein and responsible for the delivery of all aminoacylated-tRNAs (aa-tRNA) to the ribosome, and thus the “elongation” of polypeptide chain (8, 9). Of interest, along with several other components of mRNA translational machinery, synthesis of eEF1A is known to be controlled by mammalian target of rapamycin complex 1 (mTORC1) signaling pathway (10). Dysregulation of mTORC1 signaling has been implicated in AD, albeit varying results have been reported (11, 12). Taken together, we hypothesize that eEF1A dysregulation may represent a novel molecular mechanism underlying AD pathophysiology. As the very first step to test our hypothesis, here we investigated eEF1A protein levels in different brain regions in post mortem human AD samples and compared them with age-matched controls. Our findings help lay the foundations for the future study of eEF1A-related therapeutics or diagnostics for AD, and potentially other age-related neurodegenerative processes that causes dementia.
Methods
Subjects and Tissues
De-identified post mortem human brain samples were obtained from the University of Washington (UW) Neuropathology Core in accordance with the UW and Wake Forest University School of Medicine Institutional Review Boards. Studies were performed using tissue from deceased patients in the UW Alzheimer’s Disease Research Center with clinically diagnosed (and neuropathologically confirmed) AD (n=5) as well as age-matched controls with low levels of AD neuropathology (n=5). Samples used in this study were from decedents who underwent a rapid autopsy shortly after death in which one half of the brain was dissected fresh and samples from diverse brain regions were flash frozen in liquid nitrogen. Non-frozen portions of the dissected hemisphere, and the entire contralateral hemisphere, were fixed for approximately two weeks in 10 % neutral buffered formalin and then underwent research quality diagnostic neuropathologic examination, where at least 22 routine samples are collected and processed into formalin-fixed, paraffin-embedded (FFPE) tissue blocks. Clinical and neuropathological diagnoses were based on neurocognitive testing and post mortem Braak neurofibrillary tangle staging and Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) scores, respectively. Patient characteristics are shown in Table 1. Mean age of death is 89.9 years. The post mortem interval (PMI) ranged between 3 and 10 h with a mean of 5.63 h.
Table 1.
Patient Demographics
| Case | Diagnosis | Age (yr) | Gender | PMI (h) | Braak stage | CERAD score |
|---|---|---|---|---|---|---|
| 1 | No dementia | 78 | M | 6 | II | Sparse |
| 2 | No dementia | 86 | M | 3.15 | III | Absent |
| 3 | No dementia | 91 | M | 5 | III | Absent |
| 4 | No dementia | 92 | F | 6 | III | Sparse |
| 5 | No dementia | 97 | F | 10 | III | Sparse |
| 6 | AD | 82 | F | 8 | VI | Frequent |
| 7 | AD | 88 | M | 4.4 | VI | Moderate |
| 8 | AD | 91 | M | 7 | V | Moderate |
| 9 | AD | 96 | F | 4.45 | VI | Frequent |
| 10 | AD | 98 | F | 2.3 | IV | Frequent |
Western blot
Frozen tissue samples received from the UW Neuropathology Core were kept on dry ice and sonicated in lysis buffer using a motorized Thermo Scientific (Waltham, MA) homogenizer. Lysis buffer was composed of T-Per® Tissue Extraction Reagent (Thermo Scientific) with Halt™ Phosphatase Inhibitor Cocktail (Thermo Scientific) and Halt™ Protease Inhibitor Cocktail (Thermo Scientific). Homogenates were then centrifuged in 4°C for 10 min at 15,000 rpm. The supernatant was collected for protein quantification using the Pierce™ BCA Protein Assay Kit (Thermo Scientific). Samples containing 20 µg protein were loaded into precast Mini-Protean® TGX gels (4–10%; Biorad, Hercules, CA) and resolved by standard gel electrophoresis. Protein was then transferred electrophoretically onto nitrocellulose membrane (0.2 µm; Bio-Rad) at room temperature. Membranes were blocked for 1 h in blocking buffer composed of 5% nonfat dry milk in tris buffered saline containing 0.1% Tween 20 (TBS-T). All primary and secondary antibodies were diluted in blocking buffer. Blots were probed with primary antibodies for eEF1A (1:5000; EMD Millipore, Billerica, MA) and GAPDH (1:10,000, Cell Signaling, Danvers, MA). Protein bands were visualized using chemiluminescence (Clarity™ ECL; Bio-Rad) and the Bio-Rad ChemiDoc™ MP Imaging System. Densitometric analysis was performed using ImageLab™ software (Bio-Rad). All data for eEF1A were normalized to GAPDH. Additionally, membranes were also probed for β-actin (1:10,000, Sigma Aldrich, St. Louis, MO) as another loading control (data not shown).
Immunohistochemistry
Tissue sections used for immunohistochemistry were prepared by the UW Neuropathology Core from FFPE tissue blocks described above. Sections were cut at 5 µm thickness, mounted on positively charged slides and baked for 30 min at 60°C. Sections were deparaffinized in xylene and rehydrated through a graded alcohol series. Antigen retrieval utilized citrate buffer (pH 6.0) in a standard 15 min microwave procedure. Endogenous peroxidase activity was blocked using 3% hydrogen peroxide for 25 min. Slides were incubated in a humidified chamber in primary antibody for eEF1A (mouse, 1:1000; EMD Millipore) overnight at 4°C or amyloid β (6E10, mouse, 1:5000; BioLegend, San Diego, CA) for 1 h at room temperature. Sections were then incubated in biotinylated α mouse secondary antibody (1:200; Vector Labs, Burlingame, CA) for 30 min at room temperature followed by Vectastain® Elite ABC reagent (Vector Labs) for another 30 minutes. Primary and secondary antibodies as well as ABC reagent were diluted in 1% BSA/PBS. Diaminobenzidine (DAB) was diluted in Tris buffer (pH 7.7) and 3% hydrogen peroxidase in a working DAB solution. Sections were developed in DAB for 10 min in a 42°C water bath. Slides were counterstained using Mayer’s hematoxylin and blued with 0.2% lithium carbonate. In between each step of immunohistochemistry, sections were rinsed using distilled water or PBS (pH 7.4). Negative controls were incubated in 1% BSA with no primary antibody. Sections were dehydrated in an alcohol series and cleared with xylene, coverslipped, and dried overnight. Imaging was performed using brightfield microscopy on a Zeiss Axioplan 2 Epiflourescent Microscope (Oberkochen, Germany) using Zen 2012 Imaging Software (Zeiss).
Data analysis
Data analysis was conducted using Prism 6 (Graphpad Software, San Diego, CA). All data sets were subjected to the D'Agostino-Pearson normality test and determined to have a normal Gaussian distribution. Data are presented as group means with SEM, with p<0.05 (*) considered significantly different from controls. Group differences were assessed using unpaired independent t-test.
Results
Levels of eEF1A proteins are decreased in hippocampi of human AD patients
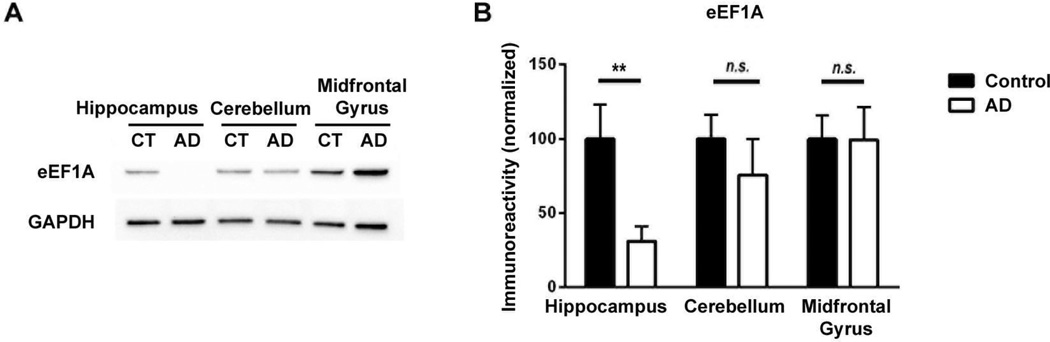
We set out to study whether eEF1A protein levels are altered in different brain regions by performing Western blot assays on protein extracts from post mortem human brain samples. Three brain regions were investigated including: hippocampus, cerebellum, and midfrontal gyrus. As shown in Figure 1, eEF1A levels in hippocampus were significantly decreased in AD patients, compared to age-match controls (p=0.0087). In contrast, eEF1A expression in either cerebellum or midfrontal gyrus of AD samples were not distinguishable from those of control samples (p=0.4458 and 0.9792, respectively).
Figure 1. Hippocampal levels of eEF1A are diminished in AD cases compared to age-matched controls.
(A) Representative Western blot shows reduced hippocampal eEF1A levels in AD patients (AD), compared to age-matched controls (CT). Cerebellum and midfrontal gyrus levels are similar in AD and control samples. (B) Cumulative data based on densitometric analysis for eEF1A Western blot experiments (n=10) are shown in bar graph. Levels of eEF1A are significantly decreased in hippocampi of AD patients compared to those of age-matched controls. Independent unpaired t-test, **p<0.01.
Hippocampal neuronal eEF1A levels are reduced in human AD patients
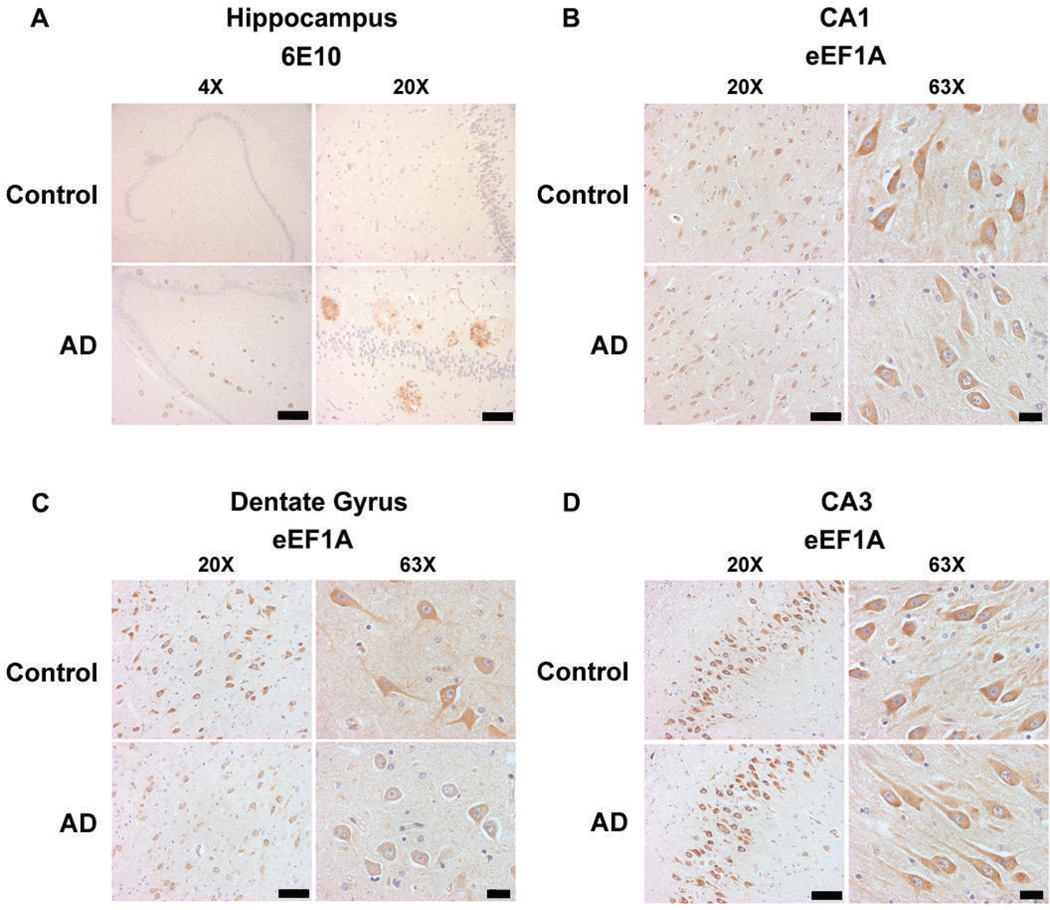
To gain insight into the cellular distribution of AD-associated eEF1A dysregulation, we performed immunohistochemistry on FFPE sections. First, we confirmed the presence of AD pathology (amyloid plaques) in AD samples, and absence in control tissues, using an antibody (6E10) for beta-amyloid (Aβ) (Fig. 2A). We then evaluated the pattern and intensity of eEF1A immunostains in adjacent sections, which revealed reduced eEF1A signals in CA1 pyramidal neurons and hilar neurons of the dentate gyrus (DG) in AD, compared to controls (Fig. 2B & C). We did not observed a clear difference in eEF1A immunostaining in CA3 pyramidal neurons (Fig. 2D), suggesting region- or neuron-specific regulation of eEF1A expression in AD. The results were consistent with the aforementioned findings from biochemical experiments and indicated impaired eEF1A expression and thus general protein synthesis in AD.
Figure 2. Hippocampal neuronal eEF1A levels are reduced in human AD patients.
(A) Immunohistochemistry with Aβ antibody (6E10) demonstrates abundant hippocampal “plaques” involving hilus, CA fields, and dentate gyrus of AD patients (AD), whereas controls (CT) have very little plaque deposition. Representative images are shown for 4× (scale bar 1 mm) and 20× (scale bar 200 µm) magnification. (B) Compared with controls, patients with AD have decreased eEF1A immunostaining in CA1 pyramidal neurons. (C) In hilar neurons (CA4) of the dentate gyrus, AD patients also displaya pronounced reduction in eEF1A levels. (D) Levels of eEF1A immunostaining in CA3 pyramidal neurons in AD patients are not different from controls. For (B), (C), and (D), representative images are shown for 20× (scale bar 200 µm) and 63× (scale bar 50 µm) magnification. Images shown represent results from four independent experiments.
Discussion
By all measures AD is a significant public health issue and will grow in severity with population aging and is thus an impending health care disaster (13). Unfortunately, there is still no effective intervention available to slow progression or cure the disease, which makes it urgent to search for new therapeutic strategies based on fundamental mechanistic studies. Here we have demonstrated, in AD brains, significantly reduced levels of hippocampal eEF1A protein. It is interesting that we find differences specific to hippocampus, which is critical for memory formation and considered to be involved relatively early in the progression of AD pathology. Further, the control tissues examined in this study were Braak II-III, which indicates involvement of CA1 and subiculum by neurofibrillary tangle pathology, while other CA regions (including CA3 and hilus of the dentate gyrus – CA4) are relatively uninvolved. This suggests that amyloid beta pathology, which is sparse or absent in control cases, but moderate to frequent in AD cases, may drive eEF1A differences more than tau pathology. Future experiments are needed to determine the association of eEF1A with specific AD pathologic processes and the specificity of eEF1A changes to AD and other neurodegenerative processes.
As one of the most abundant mRNA translational factors, eEF1At plays a critical role in the elongation phase of de novo protein synthesis. Numerous studies have firmly established that de novo protein synthesis is essential for maintenance of long-term synaptic plasticity and memory formation (14, 15). In agreement, recent findings indicate a link between compromised de novo protein synthesis and AD-associated impairments of neuronal plasticity and cognitive function (2). It is also worth mentioning that, the regulation at the elongation phase is especially valuable when mRNA translation has already been started but remains dormant until needed, thus accelerating new protein production by bypassing the relatively slow initiation step (16).
Furthermore, mounting evidence indicates a relationship between AD pathophysiology and mTORC1 signaling pathway (11, 12). The mTORC1 controls protein synthesis in part through regulating synthesis of new translational apparatus via p70 S6 kinase (p70S6K). Particularly, the process leads to the translation of a specific class of mRNAs characterized by terminal oligopyrimidine (TOP) in their 5’ untranslated regions (UTRs). Proteins encoded by such transcripts include ribosomal proteins and elongation factors such as eEF1A (10). The findings from the current study that eEF1A levels are down-regulated in AD suggest an impaired mTORC1 signaling, which is consistent with our previous report (11).
Our long-term goal is to determine whether neuronal eEF1A dysregulation may represent a heretofore unknown molecular mechanism underlying AD pathogenesis. Experiments to correct impaired eEF1A dysregulation in an AD mouse model are ongoing to determine whether de novo protein synthesis can be restored and cognitive impairments can be rescued using this approach. These studies will provide clarity on whether eEF1A is a feasible therapeutic target for AD. Further studies of eEF1A in other age-related models of neurodegeneration are planned to determine how specific eEF1A alterations are to AD.
Acknowledgement
This work was supported by USA National Institutes of Health Grant K99 AG044469 and a grant from the BrightFocus Foundation to T.M. Brain samples were obtained from the ADRC Neuropathology Core of the University of Washington (NIA P50 AG05136). We thank Allison Beller, Samantha Rice, and Kim Howard for administrative and technical assistance.
Footnotes
Disclosure Statement
The authors have no conflict of interests to disclose.
References
- 1.Moreno JA, et al. Sustained translational repression by eIF2α-P mediates prion neurodegeneration. Nature. 2012;485(7399):507–511. doi: 10.1038/nature11058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma T, et al. Suppression of eIF2α kinases alleviates Alzheimer's disease-related plasticity and memory deficits. Nat Neurosci. 2013;16(9):1299–1305. doi: 10.1038/nn.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 4.Ma T, Klann E. Amyloid b: Linking Synaptic Plasticity Failure to Memory Disruption in Alzheimer’s Disease. J Neurochem. 2012;120(Suppl 1):140–148. doi: 10.1111/j.1471-4159.2011.07506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsokas P, et al. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25(24):5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giustetto M, et al. Axonal transport of eukaryotic translation elongation factor 1alpha mRNA couples transcription in the nucleus to long-term facilitation at the synapse. Proc Natl Acad Sci U S A. 2003;100(23):13680–13685. doi: 10.1073/pnas.1835674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malenka RC. The long-term potential of LTP. Nat Rev Neurosci. 2003;4(11):923–926. doi: 10.1038/nrn1258. [DOI] [PubMed] [Google Scholar]
- 8.Jakob Nilsson PN. Elongation factors on the ribosome. Curr Opin Struct Biol. 2005;15(3):349–354. doi: 10.1016/j.sbi.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Wintermeyer W, et al. Mechanisms of elongation on the ribosome: dynamics of a macromolecular machine. Biochem Soc Trans. 2004;32(5):733–737. doi: 10.1042/BST0320733. [DOI] [PubMed] [Google Scholar]
- 10.Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem. 2000;267(21):6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- 11.Ma T, et al. Dysregulation of the mTOR pathway mediates impairment of synaptic plasticity in a mouse model of Alzheimer's disease. PLoS One. 2010;5(9):e12845. doi: 10.1371/journal.pone.0012845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285(17):13107–13120. doi: 10.1074/jbc.M110.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holtzman DM, Morris JC, Goate AM. Alzheimer's disease: the challenge of the second century. Sci Transl Med. 2011;3(77):77sr71. doi: 10.1126/scitranslmed.3002369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61(1):10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoeffer CA, Klann E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33(2):67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269(22):5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]