Abstract
Background
Few effective community-based interventions exist for early childhood obesity. Parent mentors have been successful as an intervention for other conditions, but have not been used for childhood obesity. We designed an intervention for early childhood obesity using parent mentors and a positive outlier approach to assess potential efficacy, feasibility, and acceptability.
Methods
This trial enrolled obese (≥95th BMI percentile for age and gender) 2-5-year-old children in a Head Start program and their parents, with allocation to either parent mentors trained in positively deviant behaviors regarding childhood obesity, or community health workers delivering health education on obesity-related behaviors. The primary outcome is body mass index z-score change at the six-month follow-up assessment. Secondary outcomes include feeding behaviors and practices, health-related quality of life, dietary intake, and participation levels.
Results
We enrolled three parent mentors and 60 parent-child dyads. The population is 100% Hispanic; 44% of parents speak Spanish as their primary language and 45% were not high-school graduates. Children had a reported median vegetable and fruit intake of 0.3 and 1.1 cups per day, respectively, at baseline, and a median daily screen time of three hours. There was no intergroup difference in quality-of-life scores at baseline. Retention has been high, at 90% at three months.
Conclusions
In this randomized trial of the effects of parent mentors on early childhood obesity, parent-child dyads from an underserved, Hispanic population were successfully enrolled through a partnership with a Head Start organization, with a high retention rate.
Keywords: child, preschool, obesity, overweight, positive deviance
1. Introduction
Interventions for early childhood obesity with proven efficacy are limited. The few interventional clinical trials with demonstrated efficacy used labor-intensive designs with multidisciplinary teams [1,2]. These designs are challenging to replicate because of their resource-intensity, particularly for families who have limited access to healthcare resources. Unfortunately, those with limited healthcare access are often the same racial and ethnic minorities at highest risk for obesity [3]. In contrast, a positive deviance (outlier) approach addresses complex behavioral health problems using existing community resources. The core concept of positive deviance is that there are individuals who are able to thrive or be “deviant” in a positive way, despite being at high risk for poor health outcomes [4]. Positive deviance has been successfully used to reduce childhood malnutrition in the developing world [5], a problem analogous to obesity in the United States in terms of certain shared cultural, environmental, and behavioral challenges.
In south Texas, the prevalence of early childhood obesity is higher than the national average, with 40% of 2-5 year-old children overweight or obese [6], but there are very limited resources in this region for childhood obesity management outside of the primary-care setting. We recently completed a pilot study to examine the potential for identifying positively deviant behaviors and practices regarding childhood weight status in south Texas [7]. We subsequently designed the randomized clinical trial (RCT) reported herein to test whether these identified behaviors and practices could be implemented in an intervention using peer mentors. While Texas may experience higher rates of early childhood obesity, the fundamental dynamic of a lack of resources to address obesity among high-risk communities is not unique to Texas [8]. Therefore the implications of this model for clinical intervention in other settings across the United States are significant.
Peer mentoring is a successful model for educating and supporting families and changing health behaviors in a culturally acceptable way. Parents mentors, a special category of peer mentors, have been used in interventions for increasing insurance enrollment [9], improving asthma outcomes [10], recovering from malnutrition [11], and, in limited applications, improving weight-associated behaviors [12,13]. Two studies used peer mentors to address weight-related behaviors in children; one targeted school-age children in an ongoing program with primarily indirect contact with parents, and found statistically significant improvements in knowledge and some nutritional outcomes [13]. The other is an ongoing trial using health professionals in mentoring roles as the primary means of following-up in a multidisciplinary intervention employing motivational interviewing [12].
A major challenge for clinicians, researchers, and public-health advocates is how to implement large-scale initiatives and programs that prevent or reduce childhood obesity. Serving about one million low-income children nationwide, Head Start programs offer a unique opportunity to address this problem, although limited resources are still an issue [14]. Prior studies examining interventions for obesity in Head Start have faced challenges to integrating the program, given the already full schedule and demands of Head Start programs [15,16].
We describe the design and baseline characteristics of an RCT of the effects of parent mentors trained to use a positive-deviance approach on early childhood obesity in a high-risk population of low-income Latino children in south Texas enrolled in Head Start.
2. Methods
2.1. Overall design
This RCT compared parent mentors trained in positive-deviance behaviors (intervention) with community health workers providing education (control).
2.2. Recruitment and enrollment
Parent-child dyads enrolled in Head Start centers in Cameron or Willacy counties in south Texas were recruited through posted advertisements and letters. Children 2-5 years old at the time of enrollment who were obese (body mass index [BMI] ≥95th percentile for age and gender) were eligible for inclusion. Exclusion criteria were parental report of the child having been diagnosed with prematurity, mental retardation, severe developmental delay, seizure disorder, diabetes, genetic disorders, or cerebral palsy, given the potential for these conditions to influence weight status; and parents who were not proficient in English or Spanish.
2.3. Data management and randomization
Data were collected and managed using REDCap [17]. Computerized randomization was done in blocks of six, using REDCap. The randomization sequence was concealed from the primary investigator and the staff conducting enrollment.
2.4. Intervention
Parent-child dyads randomized to the intervention were paired with a trained parent mentor. The parent mentor conducted a baseline home assessment with the parent and child, using a standardized approach (Supplement 1), and conducted follow-up phone calls at least monthly to encourage healthy habits and behaviors identified as foci for that particular parent. Intervention parents also participated in monthly community meetings at Head Start centers; meetings were facilitated by parent mentors and focused on encouraging behaviors previously identified as positively deviant [7] and healthy habits identified as goals by the individuals participating in the group, in consultation with their assigned parent mentor. The community meetings for intervention and control participants were separately scheduled and located.
2.5. Parent mentor training
Parent mentors were recruited from Head Start centers via referrals from Head Start staff who identified potential parents whom the staff assessed as engaged and excellent potential candidates, based on the study description, and who had an actively enrolled child whose BMI was in the healthy weight range. Parent mentors could be of any weight status. Potential parent mentors were interviewed by two investigators to assess interest and their background and experience. Four parent mentors were chosen to complete an intensive, oneday training using the parent mentor manual (Supplement 2). All parent mentors were Latino and bilingual in spoken English and Spanish. The parent-mentor manual was developed using the American Academy of Pediatrics guidelines on obesity prevention [18] and the previous study on identified positive deviance practices [7]. Five primary areas of behavior change were emphasized: 1) accurate perceptions of weight (young children can be obese), 2) healthy snacking strategies (leaving healthy snacks available for children to access), 3) non-food based ways of dealing with behaviors problems and emotions, 4) organization and taking control (planning meals, shopping strategies, engaging grandparents), and 5) internalizing why healthy habits are important (see Supplement 2 for more detail). A pre-training knowledge assessment was administered that contained basic questions on obesity diagnosis, epidemiology, and health coaching. Following the training, a post-training questionnaire was administered using the same items and question order. In addition to the educational activities on childhood obesity provided in the manual, instruction was provided on health coaching, including the use of role playing. We did not explicitly train the mentors in cultural competence but rather focused on skills such as active listening, reflecting and supporting behaviors, and providing positive feedback. Finally, instruction was provided on basic health-information privacy and use of REDCap. One parent-mentor candidate was excluded after it was revealed that she previously had been employed as a health coach, given that the focus was on novice parents’ efficacy with mentoring other parents. The remaining three parent mentors were enrolled as research subjects in the study and provided participation honoraria as detailed below under incentives.
2.6. Control group
Parent-child dyads randomized to the control group were invited to attend monthly community meetings at Head Start centers. These meetings were facilitated by local promotoras, or community health workers, who used the EatPlayGrow™ curriculum to teach about healthy habits. EatPlayGrow™ uses an interactive format to engage parents and children, was developed jointly by the National Institutes of Health and the Children's Museum of Manhattan, and has been piloted in multiple early childhood settings among diverse populations[19]. Sample topics from the EatPlayGrow™ curriculum include the health benefits of fruits, vegetables, energy balance and portion control with activities designed to educate and engage including songs, stories and art activities. Control parent-child dyads did not receive home visits nor follow-up phone calls, but participated in other usual Head Start activities.
2.7. Ethics and incentives
This study was approved by the University of Texas Health Science Center at San Antonio Institutional Review Board. Both parent mentors and parents of obese children provided written informed consent to participate. All parents were provided with a monthly participation stipend of $20, with a final month stipend of $50. Parent mentors received a participation honorarium of $50 per month per parent-child dyad followed.
2.8. Sample size, power, and randomization
A total of three parent mentors were chosen to account for potential variations in interpersonal dynamics in assessing intervention acceptability (i.e., the use of only one mentor would limit evaluation of intervention acceptability). We used a 1:1 randomization of participants to the parent mentor intervention or controls, and a 1:10 ratio of parent mentors to parents, consistent with prior parent-mentor studies [10]. We designed this study to examine the potential effect size of this intervention in preparation for a larger study. Weight maintenance in this age group with continued normal growth in height leads to a decrease in BMI z-score among obese children of about 0.5 units over 6 months; this approximates a moderate effect size. With an expected mean BMI z-score of 2.5 at baseline and an α of 0.05, 30 participants in each group provides a 48% power to detect a difference of moderate effect size, 86% power to detect a large effect size (reduction in BMI z-score of 0.8) and 12% power to detect a small effect size (reduction in BMI z-score of at least 0.2) at six months follow-up.
2.9. Measures
All measures described below were administered in either English or Spanish dependent on the preference of the individual participant.
2.9.1. Food-frequency questionnaire
We administered the Block Kids Food Screener (BKFS) to parents at baseline and six months follow-up. This questionnaire, developed by NutritionQuest (Berkeley, CA, USA), has 41 items, and has been validated in the Mexican-American population specific to Texas [20]. It was designed to evaluate dietary intake of nutrients and food groups in children 2 -17 years old, based on lists of foods from the National Health and Nutrition Examination Survey. The frequency of consumption ranges from “none” to “every day.” Nutrient and food-group analysis was done by NutritionQuest. Given prior data suggesting yogurt as a food associated with a healthy weight in this community and its potential role in intestinal microbiome development [7,21], we assessed yogurt intake separately given that it is not separately assessed by the BKFS. We used the same frequency of consumption as the rest of the BKFS with one container designated as the reference unit for intake.
2.9.2 Quality of life
We administered the Pediatric Quality of Life Inventory (PedsQL 4.0, Mapi Research Trust, Lyon, France) at baseline and six months follow-up [22].
2.9.3. Anthropometrics
Height and weight were measured in the clinical research unit using a standardized stadiometer and weight scale. Children were weighed in light clothes and without shoes, and all height measurements were taken while standing. Blood pressure was measured on both arms using an automated sphygmomanometer with appropriate cuff size, with the average value of both arms’ measurements reported here.
2.9.4. Demographics
We used the format of the Behavioral Risk Factor Surveillance System Survey Questionnaire to assess demographic characteristics, including self-reported race/ethnicity, education, family income, employment, and parental height and weight [23].
2.9.5. Feeding behaviors
Given that parent mentors were trained to target specific behaviors and the role of feeding behaviors in nutritional intake, we administered the Comprehensive Feeding Practices Questionnaire (CFPQ) at baseline and six months follow-up [24]. This questionnaire consists of 49 questions and 12 sub-scales, and is a validated measure of feeding practices for a variety of populations [25–27]. The CFPQ sub-scales of child control (parents allow the child control of his/her eating behaviors and parent–child feeding interactions) and environment (parents make healthy foods available in the home) were used as proxy measures for the behaviors targeted by the parent mentors regarding control and organization. The CFPQ sub-scales of emotion regulation (parents use food to regulate the child's emotional states) and food as reward (parents use food as a reward for child behavior) were used as proxy measures for the intervention target of regulating behavior without the use of food. Likert scales were used to assess parental reports of the frequency or agreement with described practices and behaviors with a range of 1 (never or disagree) to 5 (always or agree).
2.9.6. Screen time, sleep, and outside play
Screen time was assessed with two questions addressing weekday and weekend duration separately using a standard question from the National Survey of Early Childhood Health [28]. Activity was assessed with two questions adapted from other early childhood studies [29]: “In a typical week, how many days does your child play outside or do other physical activity?” And: “On the days when your child plays outside or does physical activity, about how long do they play or do other activity?” Child sleep was assessed by parental report using standardized questions[30].
3. Results
3.1. Demographics and anthropometrics
There were no significant differences in demographic or anthropometric characteristics between the intervention and control groups. The mean child age was 52.3 months, and the mean parental age was 31.1 years (Table 1). The mean parental BMI was obese, at 32.2. Only one parent reported an annual household income of > $50,000. Just over half were employed, and there was a relatively wide range of reported parental educational attainment, from “not a high-school graduate” (45.0%) to “some college/college graduate” (38.3%).
Table 1.
Baseline demographic characteristics of study children and parents.
| Characteristic | All (N=59) | Controls (N=29) | Intervention Group (N=30) | p* |
|---|---|---|---|---|
| Child age, months, mean (SD) | 52.3 (7.6) | 50.5 (6.9) | 53.8 (8.0) | 0.09 |
| Child sex, % female (n)‡ | 42.4% (25) | 48.3% (14) | 36.7% (11) | 0.37 |
| Child BMI z-score, mean (SD) | 2.7 (0.8) | 2.7 (0.9) | 2.8 (0.7) | 0.77 |
| Blood pressure, systolic, mean (SD) | 102.2 (7.2) | 102.7 (5.9) | 101.7 (8.3) | 0.59 |
| Blood pressure, diastolic, mean (SD) | 65.7 (5.8) | 66.1 (5.0) | 65.4 (6.6) | 0.69 |
| Parental age, years, mean (SD) | 31.1 (7.2) | 31.5 (6.9) | 30.7 (7.6) | 0.66 |
| Parent sex, % female (n)‡ | 94.9% (56) | 96.6% (28) | 93.3% (28) | 0.57 |
| Preferred language, % English (n)‡ | 55.9% (33) | 55.2% (16) | 56.7% (17) | 0.91 |
| Parent BMI, mean (SD) | 32.2 (7.6) | 33.0 (6.7) | 31.4 (8.3) | 0.49 |
| Latino, % (n) | 100% (59) | 100% (29) | 100% (30) | |
| Household size, median, (IQR)+ | 5.0 (2.0) | 5.0 (3.0) | 5.0 (1.0) | 0.22 |
| Income, % (n)‡ | 0.76 | |||
| Less than $10,000 | 30.5% (18) | 31.0% (9) | 30.0% (9) | |
| $10,000-$25,000 | 35.6% (21) | 41.4% (12) | 30.0% (9) | |
| $25,000-$50,000 | 8.5% (5) | 6.9% (2) | 10% (3) | |
| Greater than $50,000 | 1.7% (1) | 0% (0) | 3.3% (1) | |
| Don't know/not sure | 23.7% (14) | 20.7% (6) | 26.7% (8) | |
| Employment, % (n)‡ | 0.43 | |||
| Employed/self-employed | 55.0% (33) | 63.3% (19) | 46.7% (14) | |
| Unemployed/unable to work/student | 25.0% (15) | 20.0% (6) | 30.0% (9) | |
| Homemaker | 20.0% (12) | 16.7% (5) | 23.3% (7) | |
| Education, % (n)‡ | 0.66 | |||
| Less than high school education | 45.0% (27) | 46.7% (14) | 43.3% (13) | |
| High school graduate/GED | 16.7% (10) | 20.0% (6) | 13.3% (4) | |
| Some college or technical school/college graduate | 38.3% (23) | 33.3% (10) | 43.3% (13) |
p-value for comparison between intervention and control groups
Mann-Whitney U
Chi Square Test
IQR = interquartile range; GED = general education development test; BMI = body mass index
3.2. Health-related quality of life
There were no intergroup differences in PedsQL scores (Table 2).
Table 2.
Health-related quality of life, child feeding, activity, and behavioral assessments. PedsQL scales shown with total possible score of 100 (range 0-100) indicating highest rated quality of life. The CFPQ scales (range 1-5) with higher scores indicating more agreement with the feeding behavior or higher frequency of the practice.
| Measure | All (N=59) | Controls (N=29) | Intervention (N=30) | p* |
|---|---|---|---|---|
| PedsQL scales, mean (SD): | ||||
| Overall | 84.4 (12.1) | 82.4 (12.5) | 86.2 (11.6) | 0.23 |
| Physical functioning | 84.6 (14.1) | 82.7 (15.9) | 86.6 (12.0) | 0.29 |
| Emotional functioning | 81.1 (17.3) | 78.4 (17.0) | 83.7 (17.4) | 0.25 |
| Social functioning | 87.5 (16.4) | 84.8 (19.1) | 90.0 (13.1) | 0.23 |
| School functioning | 83.0 (15.1) | 82.3 (12.8) | 83.7 (17.3) | 0.73 |
| Psychosocial health | 84.1 (12.8) | 82.2 (12.1) | 86.0 (13.4) | 0.26 |
| CFPQ scales, mean (SD): | ||||
| Child control (allow child control of eating behavior) | 2.6 (0.8) | 2.7 (0.9) | 2.5 (0.7) | 0.54 |
| Emotion regulation (parent uses food to regulate emotion) | 1.6 (0.7) | 1.7 (0.8) | 1.5 (0.6) | 0.23 |
| Encourage balance and variety in diet | 4.4 (0.6) | 4.3 (0.7) | 4.4 (0.6) | 0.53 |
| Environment (make healthy foods available in home) | 3.5 (1.0) | 3.4 (0.9) | 3.7 (1.0) | 0.17 |
| Food as reward for behavior | 2.5 (1.0) | 2.6 (1.0) | 2.4 (1.1) | 0.49 |
| Encourage child involvement in meal planning/preparation | 3.1 (1.0) | 3.1 (1.1) | 3.1 (1.0) | 0.81 |
| Modeling (parent demonstrates healthy eating) | 3.8 (1.0) | 3.8 (1.0) | 3.8 (1.0) | 0.85 |
| Monitoring (parent tracks less healthy foods) | 3.6 (0.9) | 3.5 (0.9) | 3.7 (0.9) | 0.47 |
| Pressure (parent pressures child to eat more food at meals) | 2.4 (1.0) | 2.2 (0.9) | 2.6 (1.1) | 0.16 |
| Restriction for health (parent controls intake to restrict less healthy foods) | 3.8 (0.9) | 3.8 (0.9) | 3.9 (0.9) | 0.86 |
| Restriction for weight control (parent controls intake to influence weight) | 3.0 (1.0) | 3.2 (1.0) | 2.8 (1.0) | 0.18 |
| Teaching about nutrition (explicit instruction to encourage healthy foods) | 3.7 (0.9) | 3.6 (0.9) | 3.9 (0.9) | 0.20 |
| Screen time, hours, per weekday+ | 2.0 (1.5) | 2.0 (2.0) | 3.0 (1.6) | 0.01 |
| Screen time, hours, per weekend day+ | 3.5 (3.0) | 2.0 (3.8) | 4.0 (2.6) | 0.01 |
| Screen time, hours, per all days+ | 3.0 (2.1) | 2.0 (2.6) | 3.5 (1.8) | 0.01 |
| Active play time, minutes per day, mean (SD) | 120.0 (90.0) | 120.0 (120.0) | 120.0 (90.0) | 0.83 |
| Sleep, hours per day, mean (SD) | 10.7 (1.4) | 10.6 (1.4) | 10.7 (1.4) | 0.90 |
p-value for comparison between intervention and control groups
Denotes use of Median (Interquartile Range) calculated by Independent Samples Mann-Whitney U Test
CFPQ = child feeding practices questionnaire
3.3. Dietary assessment
The intervention and control groups did not differ in intake of any macronutrient group at baseline, except for higher fiber intake by the intervention group (Table 3). Intake of macronutrients proportional to overall caloric intake was appropriate for age, based on standard recommendations[31]. Fiber, fruit, and vegetable intakes were lower than recommended [31] in both groups.
Table 3.
Basic nutritional information at baseline regarding daily intake of study children, derived from the Block Kids Food Screener; values shown are median (interquartile range) for daily intake estimates, unless otherwise indicated.
| Dietary Item | All subjects (N=59) | Control (N=29) | Intervention (N=30) | p* |
|---|---|---|---|---|
| Fruit (cups) | 1.1 (1.0) | 1.1 (0.6) | 1.1 (1) | 0.86 |
| Vegetables (cups) | 0.3 (0.3) | 0.3 (0.3) | 0.4 (0.3) | 0.36 |
| Potatoes, including French fries (cups) | 0.1 (0.2) | 0.1 (0.2) | 0.1 (0.2) | 0.69 |
| Whole grains (ounces) | 0.2 (0.5) | 0.1 (0.2) | 0.3 (0.5) | 0.01 |
| Saturated fat (grams) | 11.4 (8.0) | 9.9 (8.7) | 11.4 (8.9) | 0.90 |
| Meat, poultry, fish (ounces) | 1.3 (1.0) | 1.3 (1.2) | 1.3 (1.0) | 0.90 |
| Dairy (cups) | 1.4 (1.0) | 1.2 (0.8) | 1.5 (1.2) | 0.15 |
| Legumes (cups) | 0.1 (0.2) | 0.2 (0.2) | 0.1 (0.2) | 0.37 |
| Sugar added to foods/drink (tsp) | 4.2 (4.3) | 3.8 (4.3) | 4.3 (4.9) | 0.69 |
| Sugars occurring in foods, juice (grams) | 55 (30) | 55 (29) | 60 (45) | 0.69 |
| Protein (grams) | 38 (23) | 33 (23) | 39 (24) | 0.69 |
| Fat (grams) | 32 (24) | 30 (27) | 34 (23) | 0.69 |
| Carbohydrate (grams) | 106 (64) | 104 (62) | 111 (74) | 0.36 |
| Fiber (grams) | 8 (6) | 8 (6) | 9 (6) | 0.69 |
| Yogurt (containers per week) | 2.5 (3.2) | 2.1 (3.5) | 2.9 (3.0) | 0.40 |
| Sugary beverages (servings) | 0.1 (0.5) | 0.1 (0.4) | 0.1 (0.5) | 0.88 |
| Energy from sugary beverages (kcals) | 14 (51) | 14 (58) | 14 (51) | 0.88 |
| Total estimated energy intake (kcals) | 841 (560) | 795 (668) | 955 (480) | 0.36 |
p-value for comparison between intervention and control groups
tsp = teaspoon; kcals = kilocalories
3.4. Screen time, sleep, and outside play
At baseline, the median screen time was significantly higher for the intervention group, at 3.5 hours per day vs. 2.0 hours per day for controls (p <0.05) (Table 2). Both groups had a mean of approximately two hours of active play time per day.
3.5. Feeding behaviors
The two groups did not differ at baseline for any CFPQ subscale (Table 2). The mean emotion regulation sub-scale score was 1.6, signifying that parents, on average, report never or rarely using food to regulate the child's emotional states, and the mean for the food-as-reward sub-scale was 2.5, indicating a more moderate set of responses at baseline. The CFPQ scales of child control and environment had intermediate mean scores. The mean score for the encourage-balance-and-variety sub-scale was 4.4, indicating that parents, on average, strongly agree with promoting well-balanced food intake, including consumption of varied foods and healthy food choices.
3.6. Parent mentor knowledge
There was no significant difference between the pre-test and post-test knowledge scores of parent mentors, with a pre-test mean score (±SD) of 80.0%±5.4 and a post-test mean score of 88.3%±3.3 (p=0.08).
3.7. Recruitment and retention
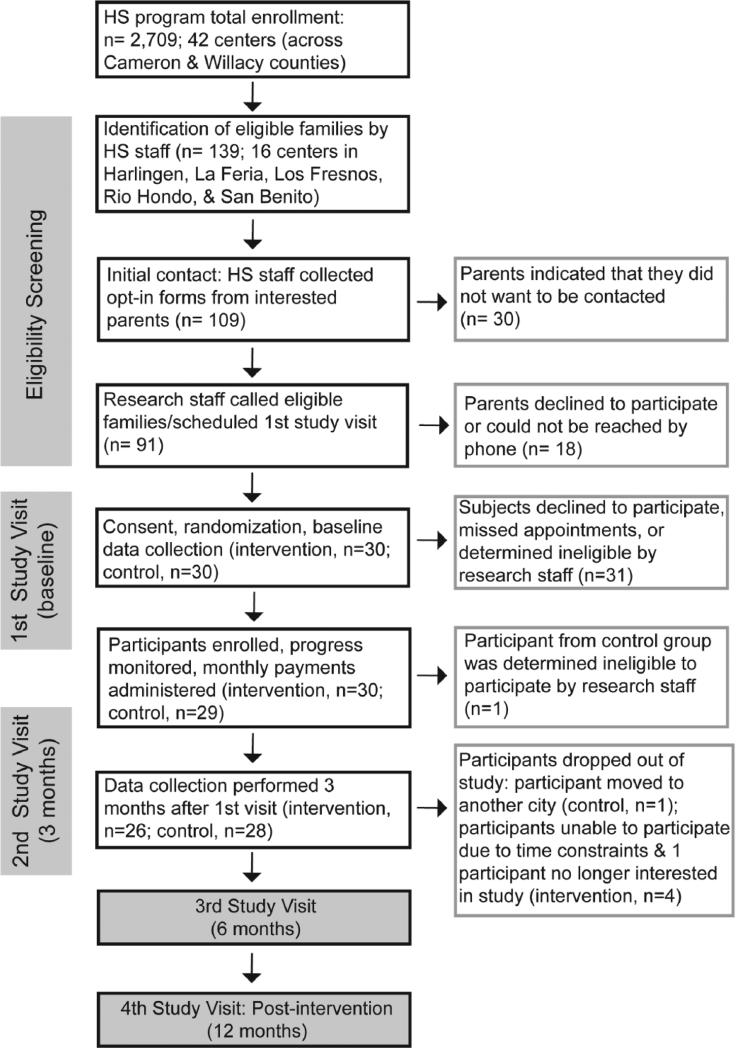
The target sample size of 60 parent-child dyads was recruited in two weeks (Figure 1). Attrition has been reasonably low, with only 10% of participants (n=6) lost to follow-up or withdrawn at three months into the study. Parent mentors have worked with Head Start staff to coordinate meetings and activities with their mentees, and research staff contacted mentors on a regular basis to check on progress and provide logistical support.
Figure 1.
Flow diagram of study recruitment, enrollment and follow-up through the study protocol with n indicating number of parent-child dyads (1 parent per dyad counted though more than one parent potentially involved in the study).
Discussion
This is the first randomized clinical trial (to our knowledge) to examine the efficacy and feasibility of parent mentors in early childhood obesity and to use a positive deviance approach as the theoretical basis for the intervention. Parent mentors have been shown to be effective in the treatment of childhood asthma, and, in an ongoing study, in improving insurance coverage for poor, minority children [9,10]. Although other recent trials have been conducted of educational and behavioral interventions for early childhood obesity [2,32,33], the advantage of the intervention described herein is the potential for sustainability, given that parent mentors provide the intervention with only minor support from Head Start and research staff. A benefit of conducting this trial in Cameron and Willacy Counties is the participation of underrepresented minorities in clinical research, a major objective of the National Institutes of Health [34]. Both counties are located in the Rio Grande Valley in south Texas, a region that has a predominantly Latino population with low rates of health-insurance coverage and a high prevalence of obesity [35]. Indeed, approximately one-third of all children in the study Head Start centers are overweight or obese, and the mean BMI of parents of children enrolled in this study was obese.
The CFPQ scales were largely in the neutral/sometimes range of scores, with the notable exceptions of emotional regulation and encourage balance and variety. This finding is in contrast to our initial study, in which we found that parents of obese children in the same geographical area and from a similar population often reported using food to regulate emotions [7]. This may be due to different methods of assessment or reflect actual differences in parental behaviors.
Participant PedsQL scores were lower than those reported for healthy populations of comparable age in other studies [22,36], and similar to a previously described obese population of the same age [37]. We did not conduct a direct comparison of quality of life for healthy children in the same Head Start centers as it was outside of the scope of this study. These findings are consistent with prior work demonstrating that obese children experience considerable limitations in quality of life. To our knowledge, health-related quality of life has not previously been assessed longitudinally as part of a weight-based intervention in 2-5 year old children; the association between change in health-related quality of life and weight over time will be assessed in this study.
The reported dietary intake of study children was notable for lower vegetable, whole-grain, and fiber intake, compared with the national Dietary Reference Intakes [31]. Although soda intake is not recommended at all in this age group, the median daily soda intake of 0.1 servings is comparable to that in prior studies of Latino children of this age [38]. The assessment of physical activity using parental report rather than more objective methods such as accelerometers is a limitation of this study.
The mission of Head Start programs includes empowering families in order to further children's cognitive, social, and emotional development [39]. An advantage of collaborating with Head Start programs is this mission of supporting parents, which likely contributed to high retention rates in our study. Notably, a recent study in Michigan showed that usual participation in Head Start was associated with a reduction in BMI z-score among overweight and obese children [40]. Whether a parent mentor program targeting obesity behaviors would be feasible in a pediatrician's office, other community-based organizations, or a school remains to be determined.
Parent mentors have now been used in multiple different contexts with increasing evidence that they are an effective community-based intervention [9,10]. The parent peer mentor model's application and scope is likely to be similar to that of the community health-worker model, with particularly appropriate application in under-served and minority communities given the intimate knowledge parents may have of local resources and the ability to engage participants. Parent mentors may be particularly well-suited for use in Head Start programs, given that Head Start has been designed to emphasize parental involvement. Importantly, both the positive deviance approach and the parent-mentor model are designed to empower communities and parents and to have the potential to reduce health disparities among disadvantaged populations.
Supplementary Material
Acknowledgements
We gratefully acknowledge the support and partnership with Neighbors in Need of Services (NINOS Inc.), in particular Ms. Manuela Rendón, Ms. Lusi Ortega, and Dr. Raul Garza. We thank the parent mentors for their dedication and involvement as well as the parents who enrolled and engaged in the project. This research was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant KL2 TR001118. The content is solely the responsibility of the authors, and does not necessarily represent the official views of the NIH.
Abbreviations
- BMI
body mass index
- SD
standard deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quattrin T, Roemmich JN, Paluch R, Yu J, Epstein LH, Ecker M a. Efficacy of family-based weight control program for preschool children in primary care. Pediatrics. 2012;130:660–6. doi: 10.1542/peds.2012-0701. doi:10.1542/peds.2012-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stark LJ, Spear S, Boles R, Kuhl E, Ratcliff M, Scharf C, et al. A pilot randomized controlled trial of a clinic and home-based behavioral intervention to decrease obesity in preschoolers. Obesity (Silver Spring) 2011;19:134–41. doi: 10.1038/oby.2010.87. doi:10.1038/oby.2010.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flores G, Vega LR. Barriers to health care access for Latino children: a review. Fam Med. 1998;30:196–205. [PubMed] [Google Scholar]
- 4.Marsh DR, Schroeder DG, Dearden KA, Sternin J, Sternin M. The power of positive deviance. BMJ Br Med J. 2004;329:1177–9. doi: 10.1136/bmj.329.7475.1177. doi:10.1136/bmj.329.7475.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mackintosh UAT, Marsh DR, Schroeder DG. Sustained positive deviant child care practices and their effects on child growth in Viet Nam. Food Nutr Bull. 2002;23:18–27. [PubMed] [Google Scholar]
- 6.Piziak V, Morgan-Cox M, Tubbs J, Rajab MH. Elevated body mass index in Texas Head Start children: a result of heredity and economics. South Med J. 2010;103:1219–22. doi: 10.1097/SMJ.0b013e3181faeb4d. doi:10.1097/SMJ.0b013e3181faeb4d. [DOI] [PubMed] [Google Scholar]
- 7.Foster BA, Farragher J, Parker P, Hale DE. A Positive Deviance Approach to Early Childhood Obesity: Cross-Sectional Characterization of Positive Outliers. Child Obes. 2015 doi: 10.1089/chi.2014.0098. doi:10.1089/chi.2014.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prevention SC on CO, Board F and N. Medicine I of. Creating Equal Opportunities for a Healthy Weight. 2013 [Google Scholar]
- 9.Flores G, Walker C, Lin H, Lee M, Fierro M, Henry M, et al. Design, methods, and baseline characteristics of the Kids’ Health Insurance by Educating Lots of Parents (Kids' HELP) trial: A randomized, controlled trial of the effectiveness of parent mentors in insuring uninsured minority children. Contemp Clin Trials. 2015;40:124–37. doi: 10.1016/j.cct.2014.11.015. doi:10.1016/j.cct.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flores G, Bridon C, Torres S, Perez R, Walter T, Brotanek J, et al. Improving asthma outcomes in minority children: a randomized, controlled trial of parent mentors. Pediatrics. 2009;124:1522–32. doi: 10.1542/peds.2009-0230. doi:10.1542/peds.2009-0230. [DOI] [PubMed] [Google Scholar]
- 11.Le Roux IM, le Roux K, Comulada WS, Greco EM, Desmond KA, Mbewu N, et al. Home visits by neighborhood Mentor Mothers provide timely recovery from childhood malnutrition in South Africa: results from a randomized controlled trial. Nutr J. 2010;9:56. doi: 10.1186/1475-2891-9-56. doi:10.1186/1475-2891-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor RW, Brown D, Dawson AM, Haszard J, Cox A, Rose EA, et al. Motivational interviewing for screening and feedback and encouraging lifestyle changes to reduce relative weight in 4-8 year old children: design of the MInT study. BMC Public Health. 2010;10:271. doi: 10.1186/1471-2458-10-271. doi:10.1186/1471-2458-10-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haire-Joshu D, Nanney MS, Elliott M, Davey C, Caito N, Loman D, et al. The use of mentoring programs to improve energy balance behaviors in high-risk children. Obesity (Silver Spring) 2010;18(Suppl 1):S75–83. doi: 10.1038/oby.2009.435. doi:10.1038/oby.2009.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes CC, Gooze RA, Finkelstein DM, Whitaker RC. Barriers to obesity prevention in Head Start. Health Aff. 2010 doi: 10.1377/hlthaff.2009.0499. doi:10.1377/hlthaff.2009.0499. [DOI] [PubMed] [Google Scholar]
- 15.Davis SM, Sanders SG, FitzGerald CA, Keane PC, Canaca GF, Volker-Rector R. CHILE: an evidence-based preschool intervention for obesity prevention in Head Start. J Sch Health. 2013;83:223–9. doi: 10.1111/josh.12018. doi:10.1111/josh.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams CL, Squillace MM, Bollella MC, Brotanek J, Campanaro LA, D'Agostino C, et al. Healthy Start: a comprehensive health education program for preschool children. Prev Med (Baltim) 1998;27:216–23. doi: 10.1006/pmed.1998.0278. doi:10.1006/pmed.1998.0278. [DOI] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. doi:10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl):S164–92. doi: 10.1542/peds.2007-2329C. doi:10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 19.EatPlayGrow. 2013 NIH Publ No 13-7818 http://www.nhlbi.nih.gov/health/educational/wecan/downloads//eatplaygrow.pdf.
- 20.Garcia-Dominic O, Treviño RP, Echon RM, Mobley C, Block T, Bizzari A, et al. Improving quality of Food Frequency Questionnaire response in low-income Mexican American children. Health Promot Pract. 2012;13:763–71. doi: 10.1177/1524839911405847. doi:10.1177/1524839911405847. [DOI] [PubMed] [Google Scholar]
- 21.Uyeno Y, Sekiguchi Y, Kamagata Y. Impact of consumption of probiotic lactobacilli-containing yogurt on microbial composition in human feces. Int J Food Microbiol. 2008;122:16–22. doi: 10.1016/j.ijfoodmicro.2007.11.042. doi:10.1016/j.ijfoodmicro.2007.11.042. [DOI] [PubMed] [Google Scholar]
- 22.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Prevention C for DC and. Behavioral Risk Factor Surveillance System Survey Questionnaire. Centers for Disease Control and Prevention; Atlanta: 2009. [Google Scholar]
- 24.Musher-Eizenman D, Holub S. Comprehensive Feeding Practices Questionnaire: validation of a new measure of parental feeding practices. J Pediatr Psychol. 2007;32:960–72. doi: 10.1093/jpepsy/jsm037. doi:10.1093/jpepsy/jsm037. [DOI] [PubMed] [Google Scholar]
- 25.Kiefner-Burmeister AE, Hoffmann DA, Meers MR, Koball AM, Musher-Eizenman DR. Food consumption by young children: a function of parental feeding goals and practices. Appetite. 2014;74:6–11. doi: 10.1016/j.appet.2013.11.011. doi:10.1016/j.appet.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Shohaimi S, Yoke Wei W, Mohd Shariff Z. Confirmatory Factor Analysis of the Malay Version Comprehensive Feeding Practices Questionnaire Tested among Mothers of Primary School Children in Malaysia. ScientificWorldJournal. 2014;2014:676174. doi: 10.1155/2014/676174. doi:10.1155/2014/676174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melbye EL, Øgaard T, Øverby NC. Validation of the Comprehensive Feeding Practices Questionnaire with parents of 10-to-12-year-olds. BMC Med Res Methodol. 2011;11:113. doi: 10.1186/1471-2288-11-113. doi:10.1186/1471-2288-11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blumberg SJ, Halfon N, Olson LM. The National Survey of Early Childhood Health . Pediatrics. 2004;113:1899–906. [PubMed] [Google Scholar]
- 29.Remmers T, Broeren SML, Renders CM, Hirasing RA, van Grieken A, Raat H. A longitudinal study of children's outside play using family environment and perceived physical environment as predictors. Int J Behav Nutr Phys Act. 2014;11:76. doi: 10.1186/1479-5868-11-76. doi:10.1186/1479-5868-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iglowstein I, Jenni OG, Molinari L, Largo RH. Sleep duration from infancy to adolescence: reference values and generational trends. Pediatrics. 2003;111:302–7. doi: 10.1542/peds.111.2.302. [DOI] [PubMed] [Google Scholar]
- 31.Otten JJ, Hellwig JP, Meyers LD. Editors. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. 2006 [Google Scholar]
- 32.Bocca G, Corpeleijn E, Stolk RP, Sauer PJJ. Results of a multidisciplinary treatment program in 3-year-old to 5-year-old overweight or obese children: a randomized controlled clinical trial. Arch Pediatr Adolesc Med. 2012;166:1109–15. doi: 10.1001/archpediatrics.2012.1638. doi:10.1001/archpediatrics.2012.1638. [DOI] [PubMed] [Google Scholar]
- 33.Quattrin T, Roemmich JN, Paluch R, Yu J, Epstein LH, Ecker MA. Treatment outcomes of overweight children and parents in the medical home. Pediatrics. 2014;134:290–7. doi: 10.1542/peds.2013-4084. doi:10.1542/peds.2013-4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Freedman LS, Simon R, Foulkes MA, Friedman L, Geller NL, Gordon DJ, et al. Inclusion of Women and Minorities in Clinical Trials and the NIH Revitalization Act of 1993 -The Perspective of NIH Clinicai Trialists. Control Clin Trials. 1995;16:277–85. doi: 10.1016/0197-2456(95)00048-8. [DOI] [PubMed] [Google Scholar]
- 35.Xu F, Town M, Balluz LS, Bartoli WP, Murphy W, Chowdhury PP, et al. Surveillance for certain health behaviors among states and selected local areas - United States, 2010. MMWR Surveill Summ. 2013;62:1–247. [PubMed] [Google Scholar]
- 36.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL 4.0 as a pediatric population health measure: feasibility, reliability, and validity. Ambul Pediatr. 3:329–41. doi: 10.1367/1539-4409(2003)003<0329:tpaapp>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 37.Kuhl ES, Rausch JR, Varni JW, Stark LJ. Impaired health-related quality of life in preschoolers with obesity. J Pediatr Psychol. 37:1148–56. doi: 10.1093/jpepsy/jss090. doi:10.1093/jpepsy/jss090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.De Hoog MLA, Kleinman KP, Gillman MW, Vrijkotte TGM, van Eijsden M, Taveras EM. Racial/ethnic and immigrant differences in early childhood diet quality. Public Health Nutr. 2014;17:1308–17. doi: 10.1017/S1368980013001183. doi:10.1017/S1368980013001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Head Start Program Office n.d. http://eclkc.ohs.acf.hhs.gov/hslc (accessed May 14, 2015)
- 40.Lumeng JC, Kaciroti N, Sturza J, Krusky AM, Miller AL, Peterson KE, et al. Changes in Body Mass Index Associated With Head Start Participation. Pediatrics. 2015;135:e449–56. doi: 10.1542/peds.2014-1725. doi:10.1542/peds.2014-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.