Abstract
Radiation therapy is a highly effective tool for treating all stages of prostate cancer (PCa), from curative approaches in localized disease to palliative care and enhanced survival for patients with distant bone metastases. The therapeutic index of these approaches may be enhanced with targeted radiation-sensitizing agents. Aptamers are promising nucleic-acid delivery agents for short interfering RNAs (siRNA) and short hairpin RNAs (shRNA). We have previously developed a radiation-sensitizing RNA aptamer-shRNA chimera which selectively delivers DNA-PK targeting shRNAs to Prostate Specific Membrane Antigen (PSMA) positive cells in the absence of transfection reagents. Although these chimera are effective, their synthesis requires in vitro transcription and their evaluation was limited to intratumoral administration. Here we have developed a second generation aptamer-siRNA chimera that can be assembled through the annealing of three separate chemically synthesized components. The resulting chimera knocked-down DNA-PK in PSMA-positive prostate cancer cells, without the need of additional transfection reagents, and enhanced the efficacy of radiation mediated cell death. Following intravenous injection, the chimera effectively knocked down DNA-PK in established subcutaneous PSMA-positive tumors. Systemic treatment with these radiation-sensitizing agents selectively enhanced the potency of external beam radiation therapy for established PSMA-positive tumors.
Keywords: Prostate Cancer, PSMA, RNA Aptamers, DNA repair, Radiation Sensitization
INTRODUCTION
Ionizing radiation (IR) is a widely utilized tool in the management of prostate cancer (PCa) (1,2). External beam radiation therapy (EBRT) is applied to treat low-risk primary PCa as well as intermediate to high-risk primary PCa, in combination with androgen deprivation therapy or as an adjuvant after surgery. EBRT or systemic radionuclides, such as strontium-89 or samarium-153, can further be utilized for the management of pain associated with PCa bone metastases. More recently, the bone seeking alpha-emitting particle, radium 223 dichloride, was shown to enhance the overall survival of men with castration resistant metastatic PCa to bone (3). While IR therapy is commonly utilized and effective, the long-term benefits can be limited for some patients. For example, the estimated ten year disease-free-survival rate for locally advanced PCa following IR therapy is less than 50% (4,5). The survival benefits of radium are also limited, providing only three months of additional survival in men who previously received standard chemotherapy (3). Thus, there are several opportunities to improve IR therapies.
Improvements in IR therapy can be achieved through new mechanisms in delivery or dosing, or through IR-sensitizing strategies. Radiation sensitization is an attractive approach because it has the potential to either improve the potency of existing therapies, or to reduce the amount of IR required. Importantly, to improve the therapeutic index tumor-targeted or tumor-selective IR-sensitization is required, to spare healthy tissues from similar IR-sensitization (6).
Radiation therapy primarily causes DNA double-strand breaks (DSB), which are considered to be the most lethal lesion (7-9). A critical pathway of DSB repair is non-homologous end joining, which involves signaling through the DNA Protein Kinase (DNA-PK) complex (10). The catalytic subunit of DNA-PK was recently identified as one of the most potent targets for PCa IR-sensitization in a high throughput siRNA library screen (11). In light of this we developed a DNA-PK-targeted radiation-sensitizing agent. Prostate-selective targeting was achieved through a previously developed PSMA-targeting RNA aptamer, A10-3 (12). A short hairpin RNA (shRNA), targeting DNA-PK, was then affixed to the end of this aptamer, generating an internalizing aptamer-shRNA chimera (11,13). When these chimera were intratumorally administered into established PSMA-positive human xenograft tumors, the therapeutic effect of IR was significantly enhanced (11). While these results are promising, the clinical translation of this strategy may be challenging.
The chemical synthesis of long, 2’-modified RNA aptamers can be costly and are generally limited to a maximum product length of 50-60 nucleotides (14). Consequently, aptamer targeted therapeutics above this size have been generated by in vitro transcription (11,13). Recently, a truncated form of the A10-3 aptamer was identified and shown to be sufficiently small for chemical synthesis and targeted siRNA knock-down of Polo-like kinase 1 (15). Intraperitoneal injection of these aptamer-siRNA chimera caused a pronounced regression of established PSMA-expressing tumors, revealing the potential for systemically administered PSMA aptamer-targeted siRNAs. In light of these results, and other successful systemic applications of aptamer-siRNAs (16,17), we sought to develop a chemically-synthesized aptamer-siRNA chimera for systemic PCa radiation sensitization. Here we describe the development and characterization of this chimera in a human PCa xenograft model of EBRT.
MATERIALS AND METHODS
Materials
Aptamers were purchased from IDT Technologies (Leuven, Belgium) and RNA oligonucleotides from Sigma Aldrich (St. Louis, MO). Anti-DNA-PK (AB2) and Anti-human ACTB (AC-15) were purchased from Millipore (Billerica, MA) and Sigma-Aldrich (St. Louis, MO). Powervision Poly-HRP anti-mouse IgG was purchased from Leica Biosystems (Newcastle Ltd. UK).
Cell culture
LNCaP and PC-3 cells were originally obtained from ATCC in 2006 (Manassas, VA). Parental cells were authenticated and verified Mycoplasma free by DDC medical (Fairfield, OH) in November of 2014. LNCaP-MLuc cell derivation is previously described (18). All cells are verified mycoplasma free semiannually by MycoAltert Mycoplasma Detection Kit (Lonza, Anaheim, CA), last performed September of 2014. Cells were grown in RPMI 1640 supplemented with 10% FBS and maintained at 37°C and 5% CO2.
Aptamer-siRNA chimeras
Aptamers A10-3 (PSMA-targeting) or Neg (control) were chemically synthesized with 2’Fluoro-pyrimidine, 5’amine, and deoxy-T 3’-3’ cap modifications (12). DNAPK antisense (DNA-PK targeting) and Con antisense (control) siRNA were synthesized as non-modified RNA. Bridge oligos DNAPK (sense)-link A10-3, Con siRNA (sense)-link A10-3, DNAPK (sense)-link Neg, and Con siRNA (sense)-link Neg were synthesized as non-modified RNA for annealing to A10-3 and Neg aptamers, respectively. Aptamer-siRNA chimera were annealed with equimolar concentrations by brief denaturation at 95 °C followed by gentle cooling to room temperature. Sequences are provided in Supplementary Table S1.
Dicer assay
1 μg of annealed chimera was incubated with recombinant Dicer following the manufacturer’s recommendations (Recombinant Human Turbo Dicer Kit; GTS, San Diego, CA) (11). Products were resolved by ethidium bromide stained agarose gel electrophoresis.
In vitro gene silencing and radiosensitization
2 × 105 cells (LNCaP, LNCaP-MLuc) were Hiperfect (Qiagen, Germantown, MD) transfected with 100 nM siRNA in 6-well plates or treated with 400 nM of aptamer-shRNA. After 48 hours, cells were either collected for RNA or protein extraction or seeded in 96-well plates (500-1,000 cells/well). For cell viability studies, 96 well plates were irradiated 24 h later with 0 or 6 Gy using a Gammacell 40 (Nordion) 137Cs radiator (0.6 Gy/min). Viability was assessed after 7 days by MLuc assay (18) or Cell Titer-Blue assay (Promega, Madison, WI) per manufacturer’s instructions. Gene silencing was assessed by quantitative RT-PCR as previously described (11). Briefly, 1 µg RNA was reverse-transcribed using QuantiTect Reverse Transcription Kit (Qiagen) and SYBR Green (Biorad, Hercules, CA) with a Biorad iCycler. Standard curves were generated by serial dilution of each sample and the relative amount of target gene mRNA was normalized to GAPDH mRNA. Details of primers are provided in Supplementary Table S2.
Immunoblotting
Cells were lysed by Nupage LDS Sample Buffer (Life Technologies), heated at 70°C for 10 minutes and separated by 4-15% Criterion Tris-HCL Gel and transferred to Immu-Blot PVDF (BioRad). Membranes were probed with primary and secondary antibodies at optimized concentrations and protein visualized by ChemiDoc SRS+ and Image Lab software (Bio-Rad).
A10-3-siRNA stability assay
A10-3-DNAPK chimera (1 µM final concentration) was incubated in 200ul RPMI 1640 medium containing 50% or 5% mouse serum (Jackson ImmunoResearch Laboratories) or human serum (Sigma-Aldrich) at 37°C. Between 2 hr and 72 hrs, 20 ul aliquots of the reaction were withdrawn and then stored at −80°C until all incubations were completed. Each mixture was then analyzed by nondenaturing polyacrylamide gel electrophoresis with ethidium bromide staining.
Animal model studies
Studies were performed according to the protocols approved by the Animal Care and Use Committee at Johns Hopkins University. 8-week-old athymic nude mice (nu/nu; Harlan Laboratories, Indianapolis, IN) were obtained and housed at the Animal Center Isolation Facility at Johns Hopkins University. Mice were inoculated with 5 × 106 (50% Matrigel) cells subcutaneously, and tumors grown to at least 0.8 cm in diameter. At least four animals were included in each study group. Animals were injected via tail vein with 1nmol aptamer-chimeras on days −3 and −2. On day 0, the tumor was harvested and partitioned for RNA extraction or formalin fixation. Chimera treatment doses were based on preliminary studies (200 pmol-1 nmole) to identify functional doses and existing literature (15). For radiosensitization, animals with established tumors were randomized and intravenously treated with aptamer-siRNA chimeras as described above on days −3 and −2. On day 0, animals were mock irradiated or treated 6 Gy local IR (5.8 Gy/min) to the tumor-bearing leg from a J.L. Shepherd Mark 137Cs irradiator, with the remainder of the body shielded. Tumors were measured every 2 days to calculate tumor volume: (w × l × h) × 0.52. Tumor response was determined as reaching 4 times its volume at the start of treatment.
Immunohistochemistry
Slides were deparaffinized, rehydrated and stained for DNA-PK as previously described (11). Stains were developed with diaminobenzidine (DAB kit, Vector laboratories. US) and counterstained with Haemotoxylin Mayers. Images were captured by Nikon 50i microscopy with Nikon NIS-Elements software and CCD digital camera. For quantification of DNA-PK, whole DAB staining slides were scanned via ScanScope CS system at the Tissue Micro Array Core of Johns Hopkins University School of Medicine, and total DNA-PK expression per cell nucleus was measured from multiple randomized areas of each tissue specimen using Framework for Image Dataset Analysis (FrIDA) software as previously described (19).
5′RACE assay
5’ RACE was performed as previously described (11). Briefly, mRNA (5 μg) from treated cells or tumor was ligated to GeneRacer adaptor (Invitrogen, Carlsbad, CA). Ligated RNA was reverse transcribed with GSP[DNAPK] reverse-1 and PCR amplified with GR 5’primer and GSP[DNAPK] reverse-2 (Supplementary Table S2). Products were resolved by ethidium bromide stained agarose gel electrophoresis.
Statistical analysis
Tumor size was evaluated by two-way ANOVA. P value of 0.05 or less was considered significant. Extensions to tumor quadrupling: Events (tumor volume < 4 fold from injection) were plotted by Kaplan-Meier curve and analyzed by log-rank (Mantel-Cox) test. Paired samples were evaluated by student’s t-test.
RESULTS
Chemically-synthesized aptamer-siRNA chimera
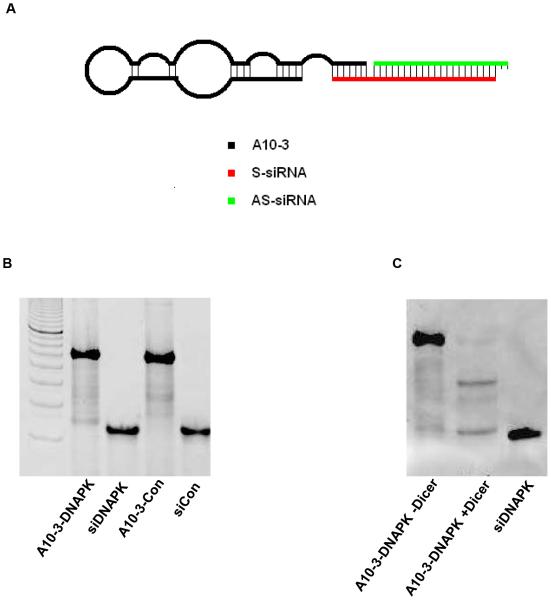
The A10-3-DNAPK-siRNA chimera was generated through a three-part chemical synthesis and annealing process (Fig 1A). Previous truncations of the A10-3 aptamer had identified the 5’-terminal 39 nucleotides (nt) as the minimally functional region (15). We therefore hypothesized that a portion of the 3’-terminus of A10-3 was unnecessary and could be used as a ‘bridge’ to anneal radiation-sensitizing siRNAs. A similar approach has been successful with other aptamer-siRNA chimera (15,20). A complementary ‘bridge’ RNA oligonucleotide was designed by fusing the sense strand of the DNA-PK-targeting siRNA to a 13 nt extension with complementarity to the 3’-terminus of the aptamer (Fig 1A, AS-siRNA). A dinucleotide uridine spacer was placed between the bridge and sense siRNA portions to mimic a 3’-overhang. A complementary anti-sense DNA-PK siRNA was then applied and also designed to contain a 3’ dinucleotide uridine overhang (Fig 1A, S-siRNA). Control chimera containing non-targeting aptamers (Neg-DNAPK) or non-specific siRNA (A10-3-Con) were also generated using the same ‘bridge’ strategy as above (Supplementary Table S1). The efficiency of generating the annealed aptamer-siRNA chimera is high, with over 90% efficiency (Fig 1B). The chimera are functional substrates of Dicer, resulting in the desired siRNA product (Fig 1C).
Figure 1. Chemically synthesized A10-3-DNAPK siRNA chimera.
A, Schematic of three parts annealed chimera. A10-3: PSMA targeting 2’-Fluorpyrimdine aptamer; S-siRNA: Non-modified sense siRNA with a 3’ extended bridge for aptamer annealing; AS-siRNA: Non-modified antisense siRNA. Both sense and antisense siRNA contain unpaired 3’-UU-dinucleotides. B, Annealed products. A10-3-DNAPK: A10-3, DNA-PK sense siRNA with bridge and DNA-PK antisense RNA; siDNAPK: DNA-PK sense siRNA with bridge and DNA-PK antisense RNA; A10-3-Con: A10-3, control sense siRNA with bridge and control antisense RNA; siCon: control sense siRNA with bridge and control antisense RNA. C, A10-3-DNAPK siRNA +/− Dicer processing in vitro. ds-siDNAPK applied for size reference. Images (B,C) represent ethidium bromide stained polyacrylamide gels.
Aptamer-siRNAs knock-down DNA-PK and sensitize human PCa cells to IR
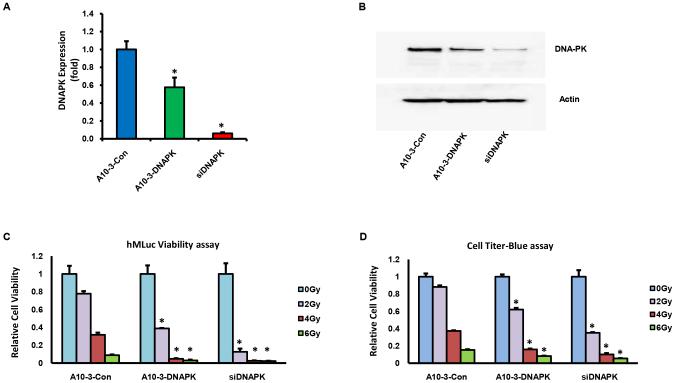
Chemically synthesized and annealed aptamer-siRNA chimera were evaluated for the ability to knock-down DNA-PK and to sensitize PSMA expressing cells to IR. A previously developed PSMA-positive reporter cell line, LNCaP-MLuc, was applied for these studies (18). Cells were treated with 400 nM of A10-3-DNAPK or a negative control chimera with non-specific siRNA (A10-3-Con), in the absence of transfection reagents, and relative DNA-PK expression levels were quantified by real time RT-PCR. Within 48 hours of treatment, A10-3-DNAPK treated cells had ~40% less DNA-PK mRNA levels when compared to cells treated with A10-3-Con (Fig 2A). siRNA transfection was utilized as a positive control for these studies (Fig 2A, siDNAPK). Knock-down of DNA-PK protein was verified by immunoblotting (Fig 2B). 5’-rapid amplification of cDNA ends (5’-RACE) was then applied to verify that DNA-PK knock-down was mediated by RNAi-induced mRNA cleavage, as detected by the predicted cleavage product (Supplementary Fig S1).
Figure 2. Aptamer mediated DNA-PK silencing and IR sensitization.
A, LNCaP-MLuc cells treated with 400 nM A10-3-Con or A10-3-DNAPK, in the absence of transfection reagents, or transfected with 100 nM siDNAPK. Relative DNA-PK expression quantified by qRT-PCR. Mean ± SEM (n = 3). *P < 0.05, relative to A10-3-Con. B, DNA-PK protein quantification of the same treatments by Western blot. C, In vitro radiosensitization of LNCaP-MLuc cells treated with 400 nM A10-3-DNAPK or A10-3-Con, in the absence of transfection reagents, or transfected with 100 nM siDNAPK. Cells were irradiated 72 hours after treatment (0-6 Gy) and relative cell viability detected by secreted MLuc Activity. Percent cell viability relative to 0 Gy for each treatment. Mean ±SEM (n = 3) *P < 0.05 relative to A10-3-Con at each dose. D, Relative cell viability of treated cells quantified by CellTiter-blue assay. Percent cell viability relative to 0 Gy for each treatment. Mean ±SEM (n = 3). *P < 0.05 relative to A10-3-Con at each dose.
LNCaP-MLuc cells express a secreted Metridia Luciferase (MLuc) which is driven by a β-actin promoter and enhancer to provide a signal that is representative of viable cell numbers (18). These reporter cells were treated with 400 nM of aptamer-siRNA chimera in the absence of transfection reagents, or transfected with the positive control DNA-PK siRNA. Two days after treatment, cells were irradiated with 2-6 Gy of IR. Cell viability and proliferation was then measured by secreted MLuc activity (Fig 2C) and verified by the commercial Cell Titer-Blue assay (Fig 2D). Both assays detected significantly enhanced IR-mediated cytotoxicity in A10-3-DNAPK treated and siDNAPK transfected cells, when compared to A10-3-Con control treated cells. Thus, in an in vitro cell model, the chemically synthesized aptamer-siRNA chimeras were confirmed to function as radiation sensitizers.
Systemic knock down of DNA-PK in PSMA positive tumors
Sufficient nuclease stability is required for the systemic application of aptamer-siRNA chimera. Prior to in vivo application, the stability of A10-3-DNAPK chimera were therefore analyzed in media supplemented with mouse or human serum for several days. Results indicate that over half of the chimera remain stable for up to 12 hours at 37 °C (Supplementary Fig S2). Therefore, sufficient chimera should be available for tumor delivery.
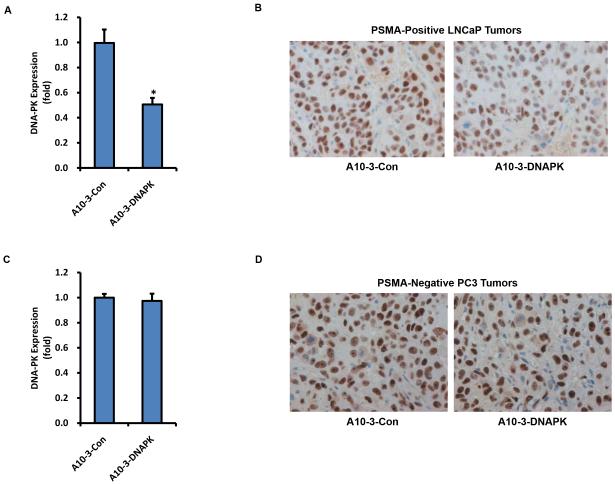
The chemically synthesized and annealed chimera were then evaluated as systemic siRNA delivery agents in PSMA-positive and PSMA-negative mouse tumor xenograft models. On two consecutive days, 1 nmol of each chimera was administered intravenously into athymic nude mice hosting established subcutaneous PSMA-positive LNCaP or PSMA-negative PC-3 tumors. Tumors were then harvested for analysis two days post-injection. PSMA-positive LNCaP tumors from mice treated with A10-3-DNAPK had significantly reduced DNA-PK mRNA and protein levels, as quantified by qRT-PCR (Fig 3A) and immunohistochemistry (Fig 3B), when compared to A10-3-Con treated animals. Targeted DNA-PK mRNA and protein knock-down was further confirmed in LNCaP-MLuc xenograft tumor models (Supplementary Fig S3). Conversely, A10-3-DNAPK treatment did not reduce DNA-PK mRNA or protein levels in PSMA-negative PC-3 tumors (Fig 3C-D). These results support PSMA-selective targeting by the A10-3-DNA-PK chimera.
Figure 3. Systemic DNA-PK silencing in PSMA positive tumor models.
Mice with establish subcutaneous PSMA-positive LNCaP or PSMA-negative PC-3 tumors were intravenously injected with aptamer-siRNA chimeras (1nmol/injection) on days −3 and −2. Tumors were harvested on day 0 and relative DNA-PK expression was quantified by qRT-PCR and immunohistochemistry. A, DNA-PK qRT-PCR from LNCaP tumors. Mean ± SEM (n = 3). *P < 0.05 relative to A10-3-Con. B, Immunohistochemistry of DNA-PK in treated animals with LNCaP tumors. C, DNA-PK qRT-PCR from PC-3 tumors. Mean ± SEM (n = 3). D, Immunohistochemistry of DNA-PK in treated animals with PC-3 tumors.
Systemic radiosensitization of PSMA positive tumors
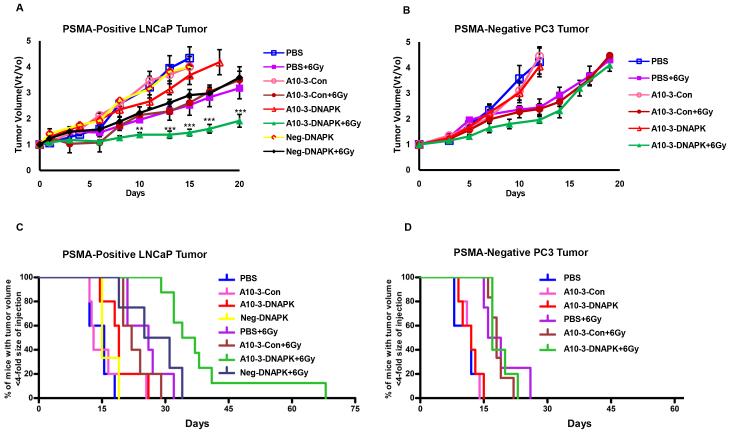
Subcutaneous human PSMA-positive LNCaP or PSMA-negative PC3 prostate tumor xenografts were again established in nude mice. Mice were then treated with 1 nmol of A10-3-DNAPK, A10-3-Con, NEG-DNAPK (a control chimera containing a non-targeted aptamer with DNA-PK targeting siRNA), or PBS by tail vein injection on two consecutive days. Two days after the last injection, half of each treatment group received direct tumor irradiation with a single non-ablative dose of 6 Gy. Tumor growth was then measured for several weeks after treatment. The therapeutic effect of IR was significantly greater in A10-3-DNAPK treated PSMA-positive LNCaP tumors when compared to irradiated animals treated with control chimera (Fig 4A). On the other hand, A10-3-DNAPK treatment did not enhance IR therapy in PSMA-negative PC-3 tumors (Fig 4B). The tumor volumes of non-irradiated cohorts were similar, indicating that A10-3-DNAPK treatment alone has no significant therapeutic effect in the absence of irradiation for either LNCaP or PC-3 tumors. The combination of A10-3-DNAPK and IR significantly extended the time for PSMA-positive tumor volume quadrupling by approximately 6 weeks, when compared to 3.5 weeks for animals with irradiated tumors that were treated with A10-3-Con control or Neg-DNAPK chimeras (Fig 4C). This effect was not observed in PSMA-negative PC-3 tumors, indicating PSMA selective radiation sensitization (Fig 4D).
Figure 4. Systemic and targeted radiosensitization in tumor models.
Mice bearing established subcutaneous tumors were intravenously injected with aptamer-siRNA chimeras (1nmol/injection) or PBS on days −3 and −2. On day 0, tumors were either untreated or irradiated (0 or 6 Gy IR). A, Systemic radiosensitization of established PSMA-positive LNCaP tumors. Relative tumor volume over time (Vt/Vo). Radiation similarly affected growth in all treatment groups except irradiated A10-3-DNAPK. **P < 0.05, ***P < 0.001, A10-3-DNAPK + 6 Gy vs. A10-3-Con + 6 Gy and Neg-DNAPK + 6 Gy; 2-way ANOVA. Mean ± SEM. B, Systemic radiosensitization of established PSMA-negative PC3 tumors. Relative tumor volume over time (Vt/Vo) of established PSMA-negative PC-3 tumors. Mean ± SEM. C, Extension of tumor quadrupling for PSMA-positve LNCaP tumors. Events (animals whose tumor volume was not yet 4-fold the size at the time of injection) were plotted by Kaplan-Meier curve. P < 0.0001, A10-3-DNAPK + 6 Gy vs. A10-3-Con + 6 Gy and A10-3-DNAPK + 6 Gy vs. Neg-DNAPK + 6 Gy; log-rank (Mantel-Cox) test. D, Extension of tumor quadrupling for PSMA-negative PC3 tumors. Events (animals whose tumor volume was not yet 4-fold the size at the time of injection) were plotted by Kaplan-Meier curve.
DISCUSSION
Efficient delivery and cellular internalization has been a challenge for RNA interference (RNAi) therapeutics. Over the last decade aptamers have evolved as promising RNAi delivery vehicles, capable of binding a variety of specific cell-surface ligands and shuttling associated RNAi agents into cells (21,22). The use of modified nucleic acids has provided sufficient stability for RNA aptamer-siRNA chimera to perform well in multiple complex and challenging in vivo disease models. PSMA-targeting RNA aptamers have successfully delivered polo-like kinase 1 targeting siRNAs to established prostate tumors after intraperitoneal injection, resulting in marked tumor regression (15). In a different systemic delivery model, aptamers targeting the HIV-1 envelope protein gp120 delivered tat/rev-targeted siRNAs by intravenous injection, causing significant decreases in viral loads and resulting in the recovery of CD4+ T cells (17). In a model of HIV transmission, the intravagnial application of gel-formulated CD4-targeting aptamers delivered antiviral siRNAs and blocked the sexual transmission of virus (23). Here we have demonstrated in another challenging in vivo model that RNA aptamers are capable of targeting established PSMA-positive tumors, following intravenous injection, to cause sufficient siRNA-mediated DNA-PK knock-down and tumor radiation sensitization.
There are several strategies for generating aptamer-siRNA chimera including the continuous in vitro transcription of aptamer-shRNAs, the solid phase synthesis of aptamers and siRNAs followed by complementary annealing, or a combination thereof. The goal of this project was to develop radiation-sensitizing aptamer-siRNA chimera using fully synthesized and annealed components (Fig 1). This has previously been accomplished through a similar annealing strategy, where the truncated PSMA aptamer A10-3.2 was extended to include the guide or passenger strand of the siRNA, and the complementary siRNA strand was then separately annealed (15). Similarly, the addition of complementary sticky bridge sequences to aptamers and siRNAs has resulted in successful assembly and fully functional chimera (20). Here we utilized a region of the 3’-terminus of the A10-3 aptamer as a bridging region for chimera assembly. Complementary sequences were incorporated into the sense siRNA to bridge and join the aptamer, sense and antisense siRNA (Fig 1). In addition, we intentionally engineered dinucleotide overhangs onto the 3’-ends of both sense and anti-sense siRNAs as previous studies have reported enhanced activity in chimera containing dinucleotide overhangs (15). Notably, we utilized non-modified RNA for the sense and anti-sense components. Nonetheless, the annealed chimera demonstrated significant stability in human and mouse serum supplemented media (Supplementary Fig S2), suggesting the aptamer may provide some protection to the conjugated siRNA duplex.
In summary, these data support that the pre-treatment of animals bearing PSMA-positive tumors with chemically synthesized and systemically administered aptamer-siRNA chimeras (two days prior to IR therapy) can significantly enhance tumor IR responses. The experimental design intentionally applied a single non-ablative IR treatment, so that additional therapeutic efficacy from IR-sensitization could be observed. Nonetheless we have observed one animal, treated with A10-3-DNAPK and 6 Gy IR, to have complete tumor ablation for more than three months after the termination of therapy. We anticipate that higher or longer IR doses would have resulted in more long-term tumor responses. Fractionated IR doses may also benefit if longer term gene knock-down is achieved, such as those reported in aptamer-siRNA chimera protective models of HIV transmission (23).
RNA aptamers are a unique class of targeting agents that have potential for clinical translation. There is currently one FDA-approved RNA aptamer therapeutic which targets VEGF for the treatment of macular degeneration. In addition, there are several aptamer-mediated therapeutic strategies in development for the treatment of cancer, and other diseases, where aptamers function as selective enzyme inhibitors or delivery vehicles for nanoparticles, drugs, and RNA interference agents (24). Here we sought to develop a first-in-class chemically synthesized IR-sensitizing aptamer-siRNA chimera. This design was pursued to overcome potential hurdles in GMP synthesis associated with plasmid purification, large scale in vitro transcription and full-length product purification of longer aptamer-shRNA chimera (11). We felt that chemical synthesis may be more amendable to larger scale production in a GMP environment. If these chimeras can be safely administered in the clinic, they may enhance a variety of IR therapies for PCa. For example, the chimera could be systemically administered prior to EBRT or brachytherapy for locally advanced PCa. Similarly, pre-treatment with the chimera may enhance the palliative or cytotoxic potential of systemic radionuclides in the treatment of bone metastatic PCa. However, such studies would need to be mindful of PSMA expression in non-target tissues, such as in the kidney, to avoid potential IR sensitization of healthy tissues. Although these tissues would theoretically receive lower doses due to reduced PSMA expression, when compared to tumors, and due to the targeted nature of external beam radiation therapy and systemically targeted radiotherapeutics, such as the bone seeking radium 223 dichloride. In addition to targeting specificity, it is notable that larger double stranded RNAs may non-specifically induce inflammatory responses through toll-like receptors. Previous studies of aptamer-siRNA chimera in human cells and in mouse models have not yet observed any significant inflammatory responses to date (11,13,15,17,20,25,26). Further, interferons are well-known enhancers of radiation therapy (27). However, there were no observed increases in the potency of radiation our studies of PSMA-negative A10-3-DNAPK treated tumors or in any cells or tumors treated with control aptamer-siRNA chimeras. Nonetheless, these pre-clinical results do not preclude the possibility of aptamer-siRNA chimera mediated inflammatory reactions in humans. Therefore, additional studies will be needed to verify the safety and efficacy of these of these and other IR-sensitizing agents in phase I trials with safety-focused endpoints.
Supplementary Material
ACKNOWLEDGMENTS
We thank Alan Meeker and the IHC Core for assistance with analysis. This work was supported by NIH grant 5P50CA058236-15 (to T.L.D and S.E.L), the David H. Koch Charitable Foundation (to S.E.L), the National Natural Science Foundation of China 81372768 (to N.X), and Shanghai Pujiang Program 13pj1407100 (to N.X).
Footnotes
Disclosure Statement: S.E.L, X.N., Y.Z., and T.L.D. are co-inventors on US patent 9,029,340.
REFERENCES
- 1.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. Journal of the National Comprehensive Cancer Network : JNCCN. 2010;8(2):145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 2.Mohler JL, Armstrong AJ, Bahnson RR, Boston B, Busby JE, D'Amico AV, et al. Prostate cancer, Version 3.2012: featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10(9):1081–7. doi: 10.6004/jnccn.2012.0114. [DOI] [PubMed] [Google Scholar]
- 3.Parker C, Nilsson S, Heinrich D, Helle SI, O'Sullivan JM, Fossa SD, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. The New England journal of medicine. 2013;369(3):213–23. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 4.Michalski JM, Winter K, Purdy JA, Parliament M, Wong H, Perez CA, et al. Toxicity after three-dimensional radiotherapy for prostate cancer on RTOG 9406 dose Level V. International journal of radiation oncology, biology, physics. 2005;62(3):706–13. doi: 10.1016/j.ijrobp.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Zietman AL, DeSilvio ML, Slater JD, Rossi CJ, Jr., Miller DW, Adams JA, et al. Comparison of conventional-dose vs high-dose conformal radiation therapy in clinically localized adenocarcinoma of the prostate: a randomized controlled trial. JAMA : the journal of the American Medical Association. 2005;294(10):1233–9. doi: 10.1001/jama.294.10.1233. [DOI] [PubMed] [Google Scholar]
- 6.Alcorn S, Walker AJ, Gandhi N, Narang A, Wild AT, Hales RK, et al. Molecularly targeted agents as radiosensitizers in cancer therapy--focus on prostate cancer. International journal of molecular sciences. 2013;14(7):14800–32. doi: 10.3390/ijms140714800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411(6835):366–74. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 8.van Gent DC, Hoeijmakers JH, Kanaar R. Chromosomal stability and the DNA double-stranded break connection. Nat Rev Genet. 2001;2(3):196–206. doi: 10.1038/35056049. [DOI] [PubMed] [Google Scholar]
- 9.Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(22):12871–6. doi: 10.1073/pnas.2135498100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lieber MR, Ma Y, Pannicke U, Schwarz K. Mechanism and regulation of human non-homologous DNA end-joining. Nat Rev Mol Cell Biol. 2003;4(9):712–20. doi: 10.1038/nrm1202. [DOI] [PubMed] [Google Scholar]
- 11.Ni X, Zhang Y, Ribas J, Chowdhury WH, Castanares M, Zhang Z, et al. Prostate-targeted radiosensitization via aptamer-shRNA chimeras in human tumor xenografts. The Journal of clinical investigation. 2011;121(6):2383–90. doi: 10.1172/JCI45109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lupold SE, Hicke BJ, Lin Y, Coffey DS. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer research. 2002;62(14):4029–33. [PubMed] [Google Scholar]
- 13.McNamara JO, 2nd, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, et al. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nature biotechnology. 2006;24(8):1005–15. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- 14.Ni X, Castanares M, Mukherjee A, Lupold SE. Nucleic acid aptamers: clinical applications and promising new horizons. Current medicinal chemistry. 2011;18(27):4206–14. doi: 10.2174/092986711797189600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dassie JP, Liu XY, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, et al. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nature biotechnology. 2009;27(9):839–49. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pastor F, Kolonias D, Giangrande PH, Gilboa E. Induction of tumour immunity by targeted inhibition of nonsense-mediated mRNA decay. Nature. 2010;465(7295):227–30. doi: 10.1038/nature08999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neff CP, Zhou J, Remling L, Kuruvilla J, Zhang J, Li H, et al. An aptamer-siRNA chimera suppresses HIV-1 viral loads and protects from helper CD4(+) T cell decline in humanized mice. Science translational medicine. 2011;3(66):66ra6. doi: 10.1126/scitranslmed.3001581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lupold SE, Johnson T, Chowdhury WH, Rodriguez R. A real time Metridia luciferase based non-invasive reporter assay of mammalian cell viability and cytotoxicity via the beta-actin promoter and enhancer. PloS one. 2012;7(5):e36535. doi: 10.1371/journal.pone.0036535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurel B, Iwata T, Koh CM, Jenkins RB, Lan F, Van Dang C, et al. Nuclear MYC protein overexpression is an early alteration in human prostate carcinogenesis. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2008;21(9):1156–67. doi: 10.1038/modpathol.2008.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J, Neff CP, Swiderski P, Li H, Smith DD, Aboellail T, et al. Functional in vivo delivery of multiplexed anti-HIV-1 siRNAs via a chemically synthesized aptamer with a sticky bridge. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21(1):192–200. doi: 10.1038/mt.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Esposito CL, Catuogno S, de Franciscis V. Aptamer-mediated selective delivery of short RNA therapeutics in cancer cells. Journal of RNAi and gene silencing : an international journal of RNA and gene targeting research. 2014;10:500–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Takahashi M, Burnett JC, Rossi JJ. Aptamer-siRNA chimeras for HIV. Advances in experimental medicine and biology. 2015;848:211–34. doi: 10.1007/978-1-4939-2432-5_11. [DOI] [PubMed] [Google Scholar]
- 23.Wheeler LA, Vrbanac V, Trifonova R, Brehm MA, Gilboa-Geffen A, Tanno S, et al. Durable knockdown and protection from HIV transmission in humanized mice treated with gel-formulated CD4 aptamer-siRNA chimeras. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21(7):1378–89. doi: 10.1038/mt.2013.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dassie JP, Giangrande PH. Current progress on aptamer-targeted oligonucleotide therapeutics. Therapeutic delivery. 2013;4(12):1527–46. doi: 10.4155/tde.13.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wullner U, Neef I, Eller A, Kleines M, Tur MK, Barth S. Cell-specific induction of apoptosis by rationally designed bivalent aptamer-siRNA transcripts silencing eukaryotic elongation factor 2. Curr Cancer Drug Targets. 2008;8(7):554–65. doi: 10.2174/156800908786241078. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler LA, Trifonova R, Vrbanac V, Basar E, McKernan S, Xu Z, et al. Inhibition of HIV transmission in human cervicovaginal explants and humanized mice using CD4 aptamer-siRNA chimeras. The Journal of clinical investigation. 2011;121(6):2401–12. doi: 10.1172/JCI45876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang AY, Keng PC. Potentiation of radiation cytotoxicity by recombinant interferons, a phenomenon associated with increased blockage at the G2-M phase of the cell cycle. Cancer research. 1987;47(16):4338–41. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.