Abstract
Individuals with Rett syndrome have greatly impaired speech and language abilities. Auditory brainstem responses to sounds are normal, but cortical responses are highly abnormal. In this study, we used the novel rat Mecp2 knockout model of Rett syndrome to document the neural and behavioral processing of speech sounds. We hypothesized that both speech discrimination ability and the neural response to speech sounds would be impaired in Mecp2 rats. We expected that extensive speech training would improve speech discrimination ability and the cortical response to speech sounds. Our results reveal that speech responses across all four auditory cortex fields of Mecp2 rats were hyperexcitable, responded slower, and were less able to follow rapidly presented sounds. While Mecp2 rats could accurately perform consonant and vowel discrimination tasks in quiet, they were significantly impaired at speech sound discrimination in background noise. Extensive speech training improved discrimination ability. Training shifted cortical responses in both Mecp2 and control rats to favor the onset of speech sounds. While training increased the response to low frequency sounds in control rats, the opposite occurred in Mecp2 rats. Although neural coding and plasticity are abnormal in the rat model of Rett syndrome, extensive therapy appears to be effective. These findings may help to explain some aspects of communication deficits in Rett syndrome and suggest that extensive rehabilitation therapy might prove beneficial.
Keywords: Mecp2, Auditory processing, Speech therapy, Plasticity, Rett syndrome, Cortex
Introduction
Individuals with Rett syndrome acquire early developmental skills until 6-18 months of age, followed by rapid regression in speech, motor deficits, respiratory problems, and seizures (Chahrour and Zoghbi, 2007). Loss-of-function mutation of one copy of the MECP2 gene is responsible for Rett syndrome and imparts a 50% chance of an autism spectrum diagnosis (Amir et al., 1999; Wulffaert et al., 2009). Social impairments, problems with speech and language, and sensory processing difficulties are common in individuals with Rett syndrome (Kaufmann et al., 2012; Lenn et al., 1986; Urbanowicz et al., 2015). Recent studies have documented that speech and language abilities and the acquisition of early developmental skills are abnormal even in the pre-regression period; while some milestones are reached, acquisition of these skills is often delayed (Marschik et al., 2013; Neul et al., 2014).
The abnormal perception of auditory stimuli appears to play a critical role in the communication difficulties observed in individuals with Rett syndrome. Auditory brainstem responses are normal, which indicates that the early stages of the auditory system are intact (Bader et al., 1989; Kálmánchey, 1990; Stach et al., 1994; Verma et al., 1987). However, individuals with Rett syndrome exhibit severely degraded auditory cortex responses that have delayed onset latencies and weaker amplitudes compared to controls, indicating a decline in auditory processing at higher levels of the auditory system (Bader et al., 1989; Glaze, 2005; Stach et al., 1994; Stauder et al., 2006). While almost all studies of auditory processing in Rett syndrome use simple clicks or tones as the auditory stimuli, a recent study documented cortical responses to the word “hi” spoken by the mother and by female strangers. While a familiar voice normally evokes a stronger response than an unfamiliar voice (Beauchemin et al., 2011, 2006; Purhonen et al., 2004), this study found that individuals with Rett syndrome exhibit a stronger response to a stranger’s voice compared to their mother’s voice (Peters et al., 2014). More information about the brain’s response to speech in Rett syndrome may clarify potential rehabilitation therapies.
No study has ever reported whether rehabilitation training can improve behavioral performance and neural responses in individuals with Rett syndrome. Numerous studies have focused on autism, and have documented that behavior and neural responses in individuals with autism can improve with behavioral training. Many studies have documented that intensive training can ameliorate some autism symptoms (Dawson et al., 2010; Landa et al., 2011; McEachin et al., 1993). For example, children with autism who received 20 hours of intervention per week over a period of 2 years exhibit substantial improvements in IQ (+11 points), language scores, and adaptive behavior (Dawson et al., 2010). The children who received intensive intervention also exhibit neural responses that are both faster and stronger compared to children with autism who had received community intervention (Dawson et al., 2012, 2010; Russo et al., 2010).
The mouse Mecp2 knockout model of Rett syndrome mimics many of the symptoms seen in individuals with Rett syndrome, including seizures, poor motor function, anxiety, and object recognition deficits (Stearns et al., 2007). A recent study demonstrated that Mecp2 knockout mice exhibit normal auditory brainstem responses but delayed auditory cortex responses, consistent with the responses observed in individuals with Rett syndrome (Liao et al., 2012). In this study, we use the novel rat Mecp2 knockout model of Rett syndrome to document speech discrimination ability and the cortical processing of speech sounds. We hypothesized that both speech discrimination ability and the neural response to speech sounds would be impaired, as seen in individuals with autism. We then documented speech discrimination ability following extensive speech training as well as the neural responses to speech sounds following training. We expected that extensive speech training would improve both discrimination ability and the neural responses to speech sounds.
Methods and Materials
Speech sounds
The speech sounds ‘bad’, ‘chad’, ‘dad’, ‘dead’, ‘deed’, ‘dood’, ‘dud’, ‘gad’, ‘sad’, ‘shad’, and ‘tad’ were used in this study. These sounds were identical to the sounds used in our previous studies (C T Engineer et al., 2014a, 2014b, 2014c; Engineer et al., 2008; Perez et al., 2013). Each sound was spoken by a female native English speaker and recorded in a double-walled soundproof booth. Speech sounds were shifted up by 1 octave into the rat hearing range using the STRAIGHT vocoder (Kawahara, 1997). All sounds were presented so that the loudest 100 ms of the vowel was 60 dB SPL.
Mecp2 mutant rat model
Thirteen female heterozygous Mecp2 mutant rats were obtained from SAGE Labs (SD-Mecp2tm1sage, Boyertown, PA) (Wöhr and Scattoni, 2013). These rats have a 71 base pair deletion in Exon 4, and are maintained by breeding heterozygous females with wild type males, each with a Sprague Dawley background. All Mecp2 rats were adult rats past the onset of regression, and were between 5 – 11 months old. Previous studies have observed behavioral or neurophysiological deficits in female Mecp2 mice between 4 – 5 months old (Goffin et al., 2011; Liao et al., 2012), although some studies have detected differences in female Mecp2 mice as young as 5 – 7 weeks old (Johnston et al., 2014; Stearns et al., 2007). Many of the Mecp2 rats in this study had handling-related seizures (83% of the speech trained Mecp2 rats). Female age-matched Sprague Dawley control rats were obtained from Charles River Laboratories. The University of Texas at Dallas Institutional Animal Care and Use Committee approved all protocols and recording procedures.
Speech discrimination training
Six Mecp2 mutant rats and five experimentally naïve control rats were trained to discriminate speech sounds. All speech discrimination training tasks and procedures were identical to the speech discrimination training in our previous studies (C T Engineer et al., 2014b, 2014c; Engineer et al., 2015, 2008; Perez et al., 2013; Shetake et al., 2011). Rats were first trained to press a lever in response to the target speech sound ‘dad’. Rats were trained on this target detection task until they reached a performance criteria of a session d’ ≥ 1.5 for 10 sessions. Each rat trained for two 1 hour sessions per day, 5 days per week. Following the target detection task, rats were trained to discriminate speech sounds differing in initial consonant. For this consonant discrimination task, rats were trained to press the lever in response to the target sound ‘dad’, and refrain from pressing the lever in response to the non-target sounds (‘bad’, ‘gad’, ‘sad’, and ‘tad’). Rats received a 45 mg sugar pellet reward for a correct lever press in response to the target sound, and received a 6 second timeout where the training program paused and the booth lights were extinguished for an incorrect lever press in response to a non-target sound. Each rat trained on the consonant discrimination task for 20 days. Following the consonant discrimination task, rats were trained to discriminate speech sounds differing in vowel. For this vowel discrimination task, rats were trained to press the lever in response to the target sound ‘dad’, and refrain from pressing the lever in response to the non-target sounds (‘deed’, ‘dood’, and ‘dud’). Each rat trained on the vowel discrimination task for 15 days. Following the vowel discrimination task, rats were trained to discriminate speech sounds in the presence of varying levels of background noise. For this speech in noise task, rats were trained to press the lever in response to the target sound ‘dad’, and refrain from pressing the lever in response to any of the previously trained non-target sounds (‘bad’, ‘gad’, ‘sad’, ‘tad’, ‘deed’, ‘dood’, and ‘dud’). The speech-shaped background noise was presented in blocks at 0, 48, 54, 60, and 72 dB, as in our previous studies (Centanni et al., 2014; Engineer et al., 2015; Shetake et al., 2011). Each rat trained on the speech in noise task for 15 days. Throughout training, the 5 out of the 6 trained Mecp2 rats that experienced a seizure before a training session were not trained for that session, and were given full access to food and water.
Auditory cortex recordings
Auditory cortex responses were recorded from 1,660 driven auditory cortex recording sites in 23 rats: 7 untrained Mecp2 mutant rats (469 recording sites), 5 untrained experimentally naïve control rats (345 recording sites), 6 speech-trained Mecp2 mutant rats (413 recording sites), and 5 speech-trained control rats (433 recording sites). All recording procedures were identical to the recording procedures in our previous studies (C T Engineer et al., 2014a, 2014c; Engineer et al., 2015). In each rat, local field potential and multi-unit responses were recorded from 4 auditory fields: anterior auditory field (AAF), primary auditory cortex (A1), ventral auditory field (VAF), and posterior auditory field (PAF). Rats were anesthetized with sodium pentobarbital (50 mg/kg), and received supplemental doses of dilute pentobarbital (8 mg/mL) throughout the experiment. Responses were recorded from layers 4/5 of right auditory cortex using 4 simultaneously lowered Parylene-coated tungsten microelectrodes (1.5 – 2.5 MΩ, FHC). Sound presentation and data recording was performed using Tucker-Davis Technologies software (SigGen and BrainWare) and hardware (RP2 and RX5). At each recording site, 1440 tones were randomly interleaved and presented at varying tone frequencies (1 – 48 kHz in 0.125 octave steps) and intensities (0 – 75 dB in 5 dB steps). Noise burst trains were presented at 7, 10, 12.5, and 15 Hz (6 noise bursts per train, 20 repeats per speed). Twenty repeats of 11 speech sounds were also presented at each recording site, including 3 novel speech sounds (‘bad’, ‘chad’, ‘dad’, ‘dead’, ‘deed’, ‘dood’, ‘dud’, ‘gad’, ‘sad’, ‘shad’, and ‘tad’).
Data analysis
All data analysis was performed using custom MATLAB software that blinds the researcher to the experimental groups. The local field potential peak amplitudes and latencies were quantified by finding the minimum (N1 and N2) and maximum (P2 and P3) amplitude peaks and the response latency at each peak. The multi-unit response strength evoked by speech sounds was quantified for the 40 ms onset response to the initial consonant as well as the 300 ms response to the vowel. The response strength was the number of driven spikes evoked during each time window after first removing the spontaneous firing level. The onset latency was the latency of the first spike following the presentation of the speech sound. The peak latency was the latency of the maximum firing rate. For the noise burst trains, the firing rate was defined as the peak firing rate evoked for the first and second noise bursts within 30 ms after presentation of the noise burst. For each recording site, the frequency that evoked a response at the lowest intensity was defined as the characteristic frequency (CF). The intensity at the CF was defined as the threshold for that site. The range of frequencies that evoked a response 40 dB above the threshold was defined as the bandwidth for that site. The spontaneous firing rate at each site was defined as the firing rate recorded before presentation of the sound (during silence).
Behavioral performance was quantified in units of percent correct, which is the average of correct lever presses to the target sound and correct rejections to the non-target sounds. Two-way repeated measures ANOVAs with Tukey-Kramer post hoc tests were used to determine statistical significance. The metric d’ was used to quantify behavioral performance on the detection stage of training in order to advance to the discrimination stages of training. A d’ of 0 indicates an inability to distinguish between target and non-target sounds, while a d’ of 1.5 indicates that rats can reliably press the lever in response to the target sound and refrain from pressing in response to a non-target sound (Stanislaw and Todorov, 1999).
Results
Mecp2 mutation alters the auditory cortex response to speech sounds
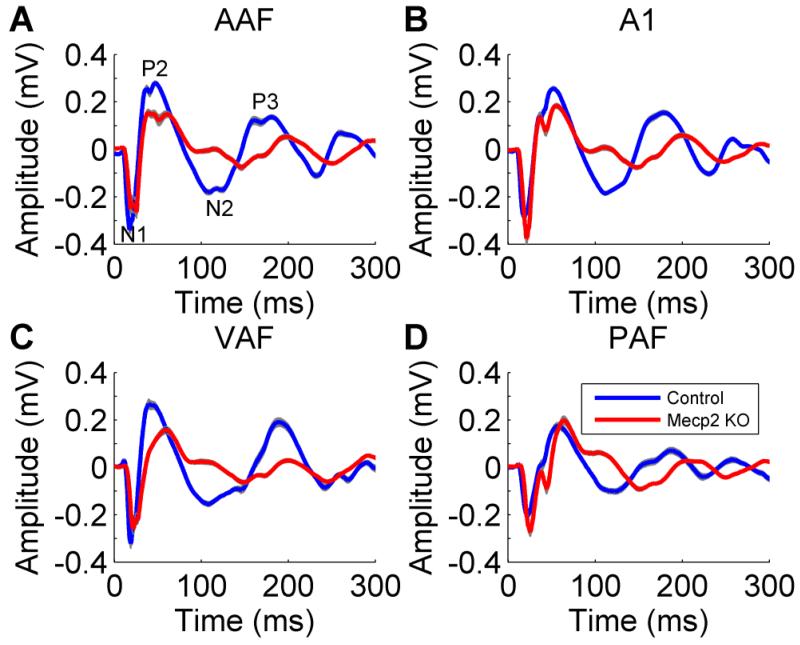
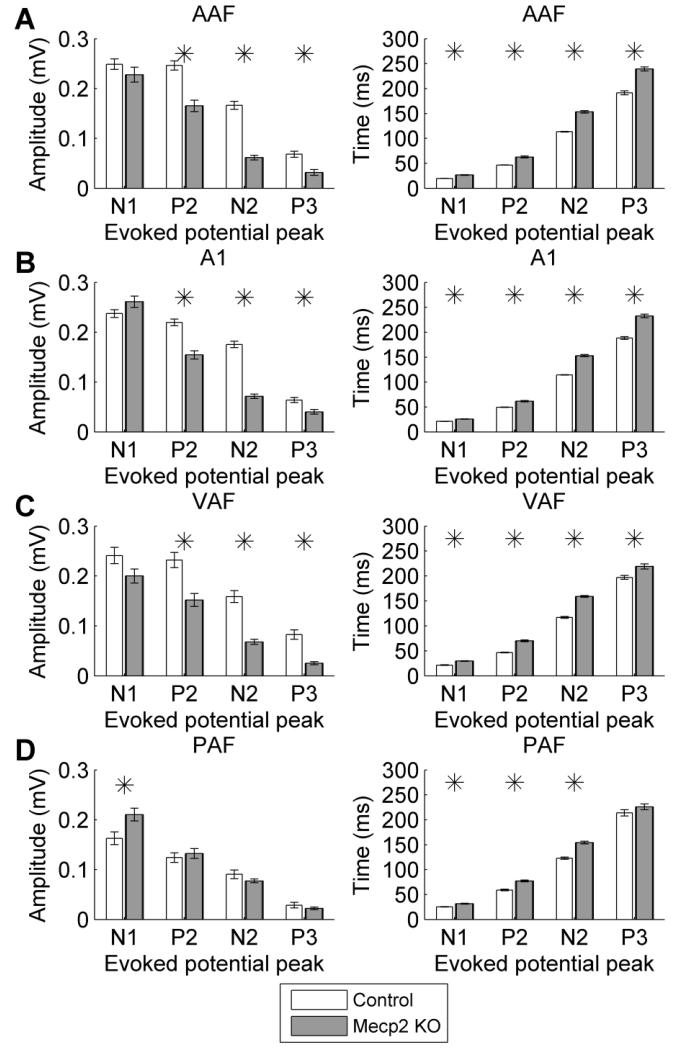
Previous literature has documented that cortical auditory evoked potentials are abnormal in individuals with Rett syndrome (Bader et al., 1989; Stach et al., 1994; Stauder et al., 2006). Each study documented prolonged response latencies. However, the response amplitude is less consistently documented. In this study, auditory cortex local field potential and multiunit responses were recorded from four auditory cortex fields in Mecp2 and control rats: anterior auditory field (AAF), primary auditory cortex (A1), ventral auditory field (VAF), and posterior auditory field (PAF). The N1 potential amplitude was unaltered in each of the auditory fields except PAF, where Mecp2 rats evoked a stronger response amplitude compared to control rats (Figures 1 & 2). In contrast to PAF, each of the other auditory cortex fields evoked a weaker P2, N2, and P3 response in Mecp2 rats compared to control rats (Figure 2a,b,c). Although the response amplitudes were not consistent across auditory fields, the response latency was consistently slower across all four fields (Figure 2). This finding that the response latency is consistently slower while the response amplitude is stronger or weaker may explain the response amplitude inconsistencies reported between studies in individuals with Rett syndrome.
Figure 1.
The local field potential response to speech sounds was altered in Mecp2 rats. Both the amplitude and the latency of the response to the speech sound ‘dad’ were impaired in Mecp2 rats compared to control rats in (a) AAF, (b) A1, (c) VAF, and (d) PAF. The gray shading behind each line indicates SEM across recording sites.
Figure 2.
The local field potential response amplitude and latency to speech sounds were altered in Mecp2 rats. In (a) AAF, (b) A1, and (c) VAF, the response amplitude was decreased and the response latency was increased in Mecp2 rats compared to control rats. (d) In PAF, both the response amplitude and latency were increased. Stars indicate statistically significant differences between Mecp2 and control rats (p < 0.05). Error bars indicate SEM across recording sites.
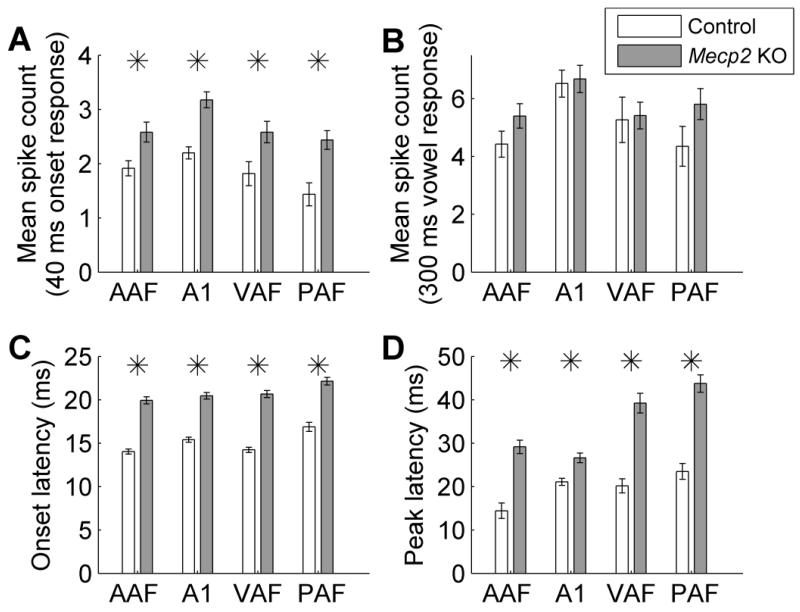
Across all four auditory fields, multi-unit responses to speech sounds were significantly hyperexcitable in Mecp2 rats compared to control rats. The response strength to the onset of the speech sounds was 35% stronger in AAF (p = 0.005), 45% stronger in A1 (p < 0.0001), 42% stronger in VAF (p = 0.02), and 70% stronger in PAF in Mecp2 rats compared to control rats (p = 0.0008, Figure 3a). In spite of this hyperexcitable response to the onset of speech sounds, the response strength to the vowel portion of the sound was not significantly different in Mecp2 rats compared to control rats (p > 0.05, Figure 3b). This finding is consistent with the hyperexcitable neural responses observed in individuals with Rett syndrome and the mouse model of Rett syndrome (Glaze, 2005; Liao et al., 2012).
Figure 3.
Speech evoked multiunit responses are altered in Mecp2 rats. (a) Speech sounds evoked a hyperexcitable response across all four auditory fields in Mecp2 rats compared to control rats. The driven response for the speech sound onset was calculated as number of spikes evoked in the 40 ms onset response to each speech sound. Error bars indicate SEM across recording sites. Stars indicate a statistically significant difference between Mecp2 rats and control rats (p < 0.05). (b) The response strength during the vowel portion of the speech sound was unaltered in Mecp2 rats compared to control rats. The (c) onset and (d) peak response latency to speech sounds were significantly delayed in Mecp2 rats compared to control rats.
In addition to having significantly more excitable responses, Mecp2 responses to speech sounds were also significantly delayed. The onset latency to speech sounds was 5.9 ms later in AAF (p < 0.0001), 5.1 ms later in A1 (p < 0.0001), 6.4 ms later in VAF (p < 0.0001), and 5.3 ms later in PAF in Mecp2 rats compared to control rats (p < 0.0001, Figure 3c). The latency to maximum (peak) firing in response to speech sounds was also significantly delayed in Mecp2 rats. The peak latency was 14.7 ms later in AAF (p < 0.0001), 5.5 ms later in A1 (p < 0.0001), 19.1 ms later in VAF (p < 0.0001), and 20.2 ms later in PAF (p < 0.0001) in Mecp2 rats compared to control rats (Figure 3d). In each of the fields, the response latency delay was greater for the peak latency compared to the onset latency, suggesting that Mecp2 neurons may have trouble following rapidly presented sounds.
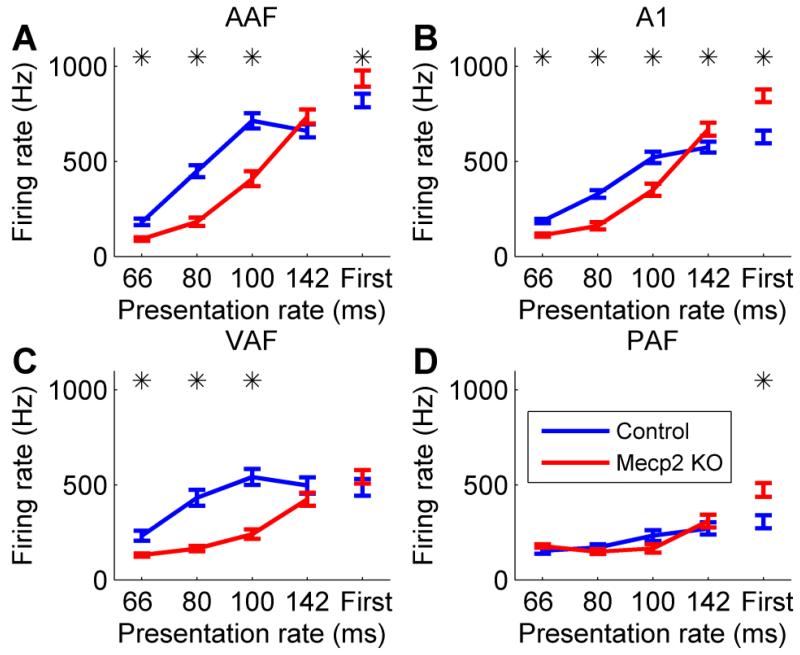
To test the hypothesis that Mecp2 neurons were unable to keep up with rapidly presented sounds, trains of noise bursts were presented at varying presentation rates (7, 10, 12.5, and 15 Hz). Mecp2 neurons were unable to follow rapidly presented noise burst trains compared to control neurons (Figure 4). In AAF, A1, and VAF, the response to the second noise burst in the train was significantly weaker in Mecp2 rats compared to control rats, particularly for the most rapidly presented sounds (p < 0.05, Figure 4). For example in Mecp2 rats, the response to the second of two noise bursts separated by 80 ms was less than half that of control rats. These results confirm that responses to temporally modulated sounds are impaired in Mecp2 rats.
Figure 4.
The response to rapidly presented sounds is impaired in Mecp2 rats. While the response to the first noise burst in a train of noise bursts was stronger in Mecp2 rats, the response to the second noise burst was significantly weaker in Mecp2 rats compared to control rats. There was greater suppression in (a) AAF, (b) A1, and (c) VAF, but not (d) PAF. Error bars indicate SEM across recording sites. Stars indicate a statistically significant difference between Mecp2 rats and control rats (p < 0.05).
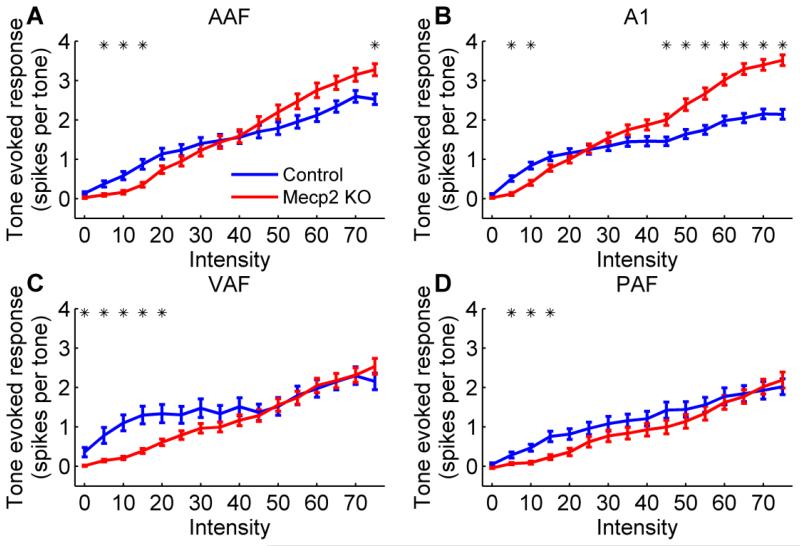
Receptive field properties were also significantly impaired in Mecp2 rats across all four auditory cortex fields. The response strength to tones was differentially altered depending on the tone intensity. In AAF and A1, Mecp2 rats exhibited a weaker response to quiet tones but a stronger response to louder tones compared to control rats (p < 0.0031 Bonferroni corrected, Figure 5a,b and Supplementary Figure 1). These fields are known to be tonotopically organized, and the organization of both fields was largely unaffected in Mecp2 rats. Each individual control rat had a statistically significant correlation between characteristic frequency and anterior-posterior (AP) location for AAF (0.59 mean R2 for individual control rats, p < 0.05) and A1 (0.72 mean R2 for individual control rats, p < 0.05). In Mecp2 rats, 86% of rats had a statistically significant correlation between CF and AP location for AAF (0.48 mean R2 for individual Mecp2 rats, p < 0.05), and 100% of rats had a statistically significant correlation between CF and AP location for A1 (0.76 mean R2 for individual Mecp2 rats, p < 0.05). Cortical neurons in Mecp2 KO rats responded to a wider range of tone frequencies, responded slower to tones, and had less spontaneous firing compared to control rats (Table 1). Mecp2 rats had tone frequency bandwidths that were 1.0 – 1.6 octaves wider than control rats across all four fields (p < 0.0001, Table 1). The peak response latency was significantly slower in Mecp2 rats, and was 2.6 ms slower in AAF (p < 0.0001), 2.3 ms slower in A1 (p < 0.0001), 16.2 ms slower in VAF (p < 0.0001), and 13.2 ms slower in PAF (p = 0.0007, Table 1). In addition, Mecp2 rats had significantly less spontaneous firing when no stimulus was present compared to control rats in each of the auditory fields (p < 0.0001, Table 1).
Figure 5.
The response to tones presented at varying tone intensities was altered in Mecp2 rats compared to control rats in (a) AAF, (b) A1, (c) VAF, and (d) PAF. Across all four fields, Mecp2 rats evoked a weaker response to low intensity tones compared to control rats. However, in AAF and A1, Mecp2 rats also evoked a stronger response to high intensity tones compared to control rats. Error bars indicate SEM across recording sites. Stars indicate a statistically significant difference between Mecp2 rats and control rats (p < 0.0031 Bonferroni corrected). The response at each tone frequency and tone intensity combination is shown in Supplementary Figure 1.
Table 1.
Receptive field properties were significantly altered in Mecp2 rats. Mecp2 rats had wider bandwidths, increased response latency, and decreased spontaneous firing in all four fields compared to control rats. Bold numbers marked with a star were significantly different in Mecp2 rats compared to control rats (p < 0.05).
| Threshold (dB) |
Bandwidth 40 (octaves) |
Peak latency (ms) |
Spontaneous rate (spikes) |
Driven rate (spikes/tone) |
||
|---|---|---|---|---|---|---|
| AAF | Control | 15.4 | 3.1 | 17.2 | 16.1 | 3.0 |
| Mecp2 KO | 19.4* | 4.1* | 19.8* | 7.7* | 3.5 | |
| A1 | Control | 11.0 | 2.7 | 18.5 | 16.8 | 3.4 |
| Mecp2 KO | 14.9* | 4.1* | 20.8* | 9.3* | 3.9* | |
| VAF | Control | 16.9 | 2.8 | 22.7 | 22.5 | 3.4 |
| Mecp2 KO | 20.8 | 4.4* | 38.9* | 8.7* | 4.0 | |
| PAF | Control | 19.8 | 3.5 | 28.5 | 15.7 | 2.8 |
| Mecp2 KO | 11.8* | 4.8* | 41.7* | 7.6* | 3.9* |
Mecp2 mutation impairs speech discrimination ability
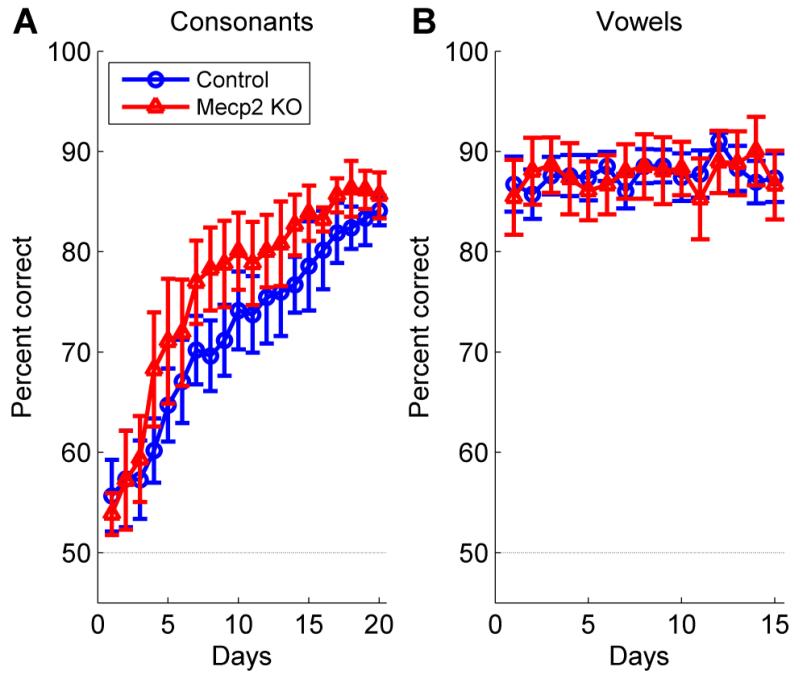
Eleven rats were trained to discriminate between speech sounds that differed in their initial consonant or vowel (n = 6 Mecp2 rats, n = 5 control rats). All rats were first trained on a consonant discrimination task, where they learned to press the lever in response to the target sound ‘dad’, and refrain from pressing the lever in response to the non-target sounds ‘bad’, ‘gad’, ‘sad’, and ‘tad’. Mecp2 KO rats did not perform significantly differently on the consonant discrimination task compared to control rats (F(1,171) = 1.27, p = 0.29, two-way repeated measures ANOVA, Figure 6a). Both Mecp2 rats and control rats improved at consonant discrimination over time, and were significantly more accurate after a month of training (F(19,171) = 40.34, p < 0.0001, two-way repeated measures ANOVA, Figure 6a). Following the consonant discrimination task, all rats switched to a vowel discrimination task. Rats were trained to press the lever in response to the target sound ‘dad’, and refrain from pressing the lever in response to the non-target sounds ‘deed’, ‘dood’, and ‘dud’. For the vowel discrimination task, Mecp2 rats did not perform significantly different compared to control rats (F(1,126) = 0, p = 0.99, two-way repeated measures ANOVA, Figure 6b). After 3 weeks of training, there was no improvement in vowel discrimination performance because both groups performed the task accurately on the first day (F(14,126) = 1.22, p = 0.27, two-way repeated measures ANOVA, Figure 6b). These findings demonstrate that Mecp2 rats are able to detect, respond to, and discriminate speech sounds as well as control rats.
Figure 6.
Consonant and vowel discrimination ability was unimpaired in Mecp2 mutant rats. (a) Mecp2 and control rats were trained to perform a consonant discrimination task, where they pressed a lever in response to the speech sound ‘dad’, and refrained from lever pressing to the speech sounds ‘bad’, ‘gad’, ‘sad’, and ‘tad’. Error bars indicate SEM across rats. The dashed line indicates chance performance (50%). (b) Mecp2 and control rats were trained to perform a vowel discrimination task, where they pressed a lever in response to the speech sound ‘dad’, and refrained from lever pressing to the speech sounds ‘deed’, ‘dood’, and ‘dud’.
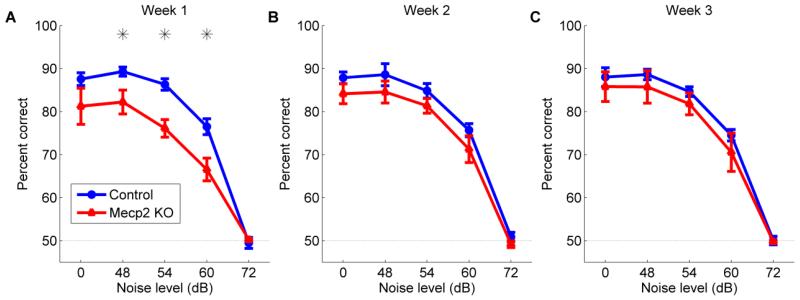
Despite normal speech discrimination abilities in quiet, discrimination of speech sounds was impaired in Mecp2 mutant rats in varying levels of background noise. For the speech in noise task, rats were trained to discriminate speech sounds by both the initial consonant and the vowel, and had to press the lever in response to the target sound ‘dad’, and refrain from pressing the lever in response to any of the non-target sounds (‘bad’, ‘deed’, ‘dood’, ‘dud’, ‘gad’, ‘sad’, and ‘tad’). During the first week of speech in noise training, Mecp2 rats were significantly impaired at discriminating the speech sounds compared to control rats (F(1,40) = 28.87, p < 0.0001, 2-way ANOVA, Figure 7a). Mecp2 rats were unimpaired during quiet blocks, but were significantly impaired when the signal to noise ratio was +12, +6, and 0 dB (48, 54, and 60 dB noise levels). Both Mecp2 rats and control rats were unable to perform the task above chance levels (p > 0.05, Figure 7a) when the signal to noise ratio was −12 dB (72 dB noise level). After 3 weeks of training on this speech in noise task, Mecp2 rats significantly improved their performance on this task, and were unimpaired at all noise levels compared to control rats (F(1,98) = 4.31, p = 0.08, 2-way repeated measures ANOVA, Figure 7c). Extensive speech training was able to ameliorate the speech discrimination deficit observed in Mecp2 rats in the presence of background noise and restore discrimination abilities to control levels.
Figure 7.
Discrimination of speech sounds in varying levels of background noise is impaired in Mecp2 rats. (a) Rats were tested on their ability to discriminate speech sounds in varying levels of background speech-shaped noise. While there was no significant difference in behavioral accuracy at discriminating speech sounds in quiet (0 dB noise level), Mecp2 rats were significantly impaired at speech sound discrimination in increasing levels of background noise compared to control rats during the first week of training. All speech sounds were presented so that the loudest 100 ms of the vowel was 60 dB. Error bars indicate SEM across rats (n = 5 control rats; n = 5 Mecp2 rats). Stars indicate a statistically significant difference between Mecp2 and control rats (p < 0.05). The dashed line indicates chance performance at 50% correct. Mecp2 rats improved at the speech in noise task, and there was no significant difference in performance between Mecp2 rats and control rats for (b) week 2 or (c) week 3.
Speech training alters the cortical response to speech sounds in Mecp2 rats
Auditory cortex responses were recorded in speech-trained rats following completion of 10 weeks of discrimination training in order to determine whether speech training alters cortical responses to speech sounds in Mecp2 rats. Although the tonotopic organization of the fields was unaltered in trained control rats, tonotopy was disrupted in trained Mecp2 rats. Following extensive training, 100% of trained control rats had a statistically significant correlation between CF and AP location for both AAF (0.65 mean R2 for individual trained control rats) and A1 (0.52 mean R2). However, only 40% of trained Mecp2 rats had a statistically significantly correlation between CF and AP location for AAF (0.28 mean R2 for individual trained Mecp2 rats) and only 60% of trained Mecp2 rats had a significant correlation for A1 (0.45 mean R2 for individual trained Mecp2 rats). Since tonotopy could not be used to reliably determine auditory cortex field boundaries for the speech-trained Mecp2 rats, responses from all four fields were pooled together for subsequent analysis of the four groups of rats.
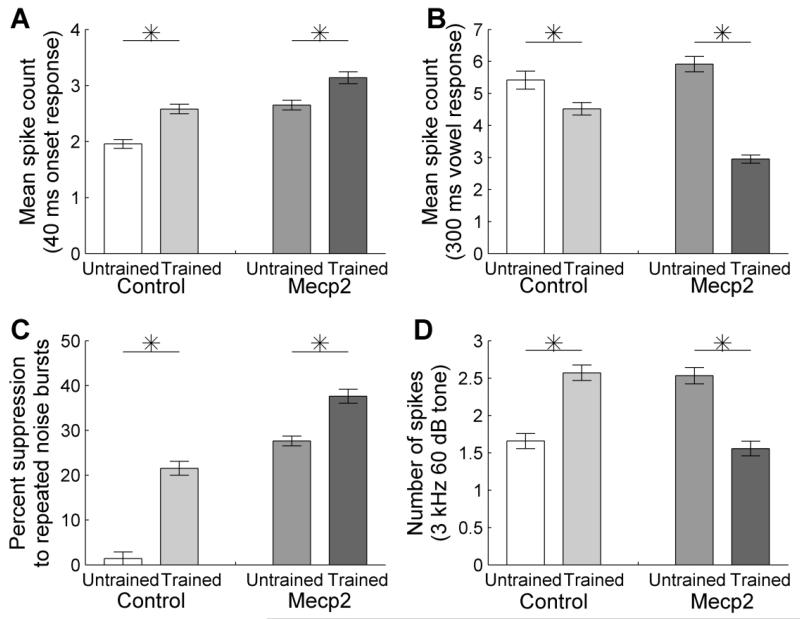
The auditory cortex response strength to the speech sound onset was 32% stronger in speech-trained control rats compared to untrained control rats (p < 0.0001, Figure 8a). As seen in control rats, the onset response strength was also significantly stronger in speech-trained Mecp2 rats compared to untrained Mecp2 rats (18% stronger, p = 0.0004, Figure 8a). In contrast, the auditory cortex response strength to the vowel portion of the speech sound was 17% weaker in speech-trained control rats compared to untrained control rats (p = 0.008, Figure 8b). Similarly, the vowel response strength was significantly weaker in speech-trained Mecp2 rats compared to untrained Mecp2 rats (50% weaker, p < 0.0001, Figure 8b). In addition to an altered response strength following training, speech-trained rats also exhibited delayed responses to speech sounds. The onset response latency was 2.2 ms longer in speech-trained control rats compared to untrained control rats (p < 0.0001). The onset latency was 2.8 ms longer in speech-trained Mecp2 rats compared to untrained Mecp2 rats (p < 0.0001). These findings suggest that speech training alters auditory cortex similarly in Mecp2 rats and control rats.
Figure 8.
The response strength to speech sounds was altered following speech training. (a) Following training, the onset response to speech sounds was stronger in trained compared to untrained control rats and was stronger in trained compared to untrained Mecp2 rats. Error bars indicate SEM across recording sites. Stars indicate a statistically significant difference between untrained and trained rats (p < 0.05). (b) Following training, the vowel response to speech sounds was weaker in trained compared to untrained control rats and was weaker in trained compared to untrained Mecp2 rats. (c) The percent suppression in response to a rapid noise burst train presented at 10 Hz increased in both trained groups following speech training. (d) The response strength to low frequency tones increased in trained compared to untrained control rats and decreased in trained compared to untrained Mecp2 rats.
The finding that the response to the onset of the speech sound was strengthened while the response to the vowel portion of the sound was weakened suggests that speech training may reduce the ability of auditory cortex to follow rapidly presented sounds. Consistent with this hypothesis, both groups of speech-trained rats were less able to follow rapidly presented noise burst trains compared to untrained rats. Speech-trained control rats had 15 times more suppression compared to untrained control rats to the second burst in the train compared to the first noise burst (43.1 ± 3.1% vs. 2.8 ± 3.0%, p < 0.0001, Figure 8c). Speech-trained Mecp2 rats also had significantly greater suppression compared to untrained Mecp2 rats (75.3 ± 3.1% vs. 55.3 ± 2.2%, p < 0.0001, Figure 8c).
Finally, the speech-trained control rats confirmed our earlier observation that speech training increases the cortical response to lower frequency tones, which are the most intense frequencies of the speech spectrum (2.6 ± 0.1 spikes in response to a 3 kHz 60 dB tone in trained control rats vs. 1.7 ± 0.1 spikes in untrained control rats, p < 0.0001, Figure 8d and Supplementary Figure 2a). Speech training failed to increase the response to low frequency sounds in trained Mecp2 rats compared to untrained Mecp2 rats. Instead, speech training significantly decreased the cortical response to low frequency sounds in Mecp2 rats (1.6 ± 0.1 spikes in trained Mecp2 rats vs. 2.5 ± 0.1 spikes in untrained Mecp2 rats, p < 0.0001, Figure 8d and Supplementary Figure 2b). This result suggests that although auditory cortex is plastic in Mecp2 rats, it does not respond to speech training entirely in the same way as in control rats.
Discussion
Previous studies have documented impaired auditory cortex responses to sound in individuals with Rett syndrome (Bader et al., 1989; Glaze, 2005; Stach et al., 1994; Stauder et al., 2006). In this study, we clarify clinical findings by documenting that local field potential amplitudes in Mecp2 rats were either stronger or weaker, depending on the auditory field and the peak. Our experiments were conducted in female rats with a heterozygous Mecp2 loss of function mutation in order to most closely match the heterozygous mutations found in girls with Rett syndrome. Multiunit responses to the onset of speech sounds were hyperexcitable in all four auditory fields tested. These neurons had significantly delayed response latencies, and were unable to follow rapidly presented sounds. Mecp2 rats were able to accurately discriminate speech sounds by both consonant and vowel when presented in quiet, but were significantly impaired at this task when the speech sounds were presented in background noise. Three weeks of training on this task significantly improved speech in noise discrimination ability. Following training, auditory cortex responses were significantly altered in trained rats compared to untrained rats. In both trained Mecp2 rats and trained control rats, the response strength to the sound onset increased, and the response strength to the sustained vowel portion of the sound decreased. Compared to untrained rats, both trained groups had delayed response latencies and greater suppression to rapidly presented stimuli. Mecp2 rats showed no evidence of the increase in the cortical response to low frequency sounds that normally results from extensive speech sound training, but instead had a significant decrease in the response to low frequency sounds.
Relationship to previous neurophysiology studies
Individuals with Rett syndrome exhibit delayed auditory cortex responses and normal auditory brainstem responses (Bader et al., 1989; Kálmánchey, 1990; Stach et al., 1994; Stauder et al., 2006). Mouse Mecp2 KO models exhibit normal brainstem responses, but longer latency cortical responses to auditory stimuli (Goffin et al., 2011; Liao et al., 2012). Our finding of delayed latencies to both tones and speech sounds across the four auditory cortex fields is consistent with previous findings in both individuals with Rett syndrome and rodent models of Rett syndrome. The observation that the non-primary auditory fields appear to have larger response latency deficits compared to primary auditory cortex is consistent with the previous finding that there is a systematic decline in auditory processing at increasing levels in the auditory pathway (Bader et al., 1989; Stach et al., 1994). Many previous studies have confirmed that increased response latencies are associated with language delays in autism spectrum disorders (Oram Cardy et al., 2008, 2005; Roberts et al., 2011; Russo et al., 2009). Further studies are needed to examine the relationship between auditory cortex response latency and speech and language deficits in individuals with Rett syndrome.
Previous studies documenting the cortical response strength in individuals with Rett syndrome have been much less clear. While some studies document an increased response strength (Yamanouchi et al., 1993), others have seen a decreased response strength (Stach et al., 1994; Stauder et al., 2006). Rodent models of Rett syndrome have also documented either an increased (Liao et al., 2012) or decreased (Goffin et al., 2011) auditory cortex response strength. The current study is the first to document both an increased and a decreased response strength. In this study, we documented that responses in Mecp2 rats are hyperexcitable when there is a long interstimulus interval and hypoexcitable when there is a short interstimulus interval. Additionally, responses in Mecp2 rats are hyperexcitable when sounds are presented at high intensities, and hypoexcitable when sounds are presented at low intensities. This interesting finding may explain why there are differences in the cortical response strength in individuals with Rett syndrome in previous studies. However, the current study was conducted in anesthetized animals; future experiments are needed to determine if these same findings are observed in awake animals. Many previous studies have documented GABAergic interneuron and LTP deficits in Rett syndrome, which may be the mechanism responsible for our finding of both hyper and hypoexcitable responses depending on the speed and intensity of the sounds (Chao et al., 2010; Della Sala and Pizzorusso, 2014; Goffin et al., 2014; Medrihan et al., 2008).
Speech training induced plasticity
Previous studies have documented that auditory cortex training-induced plasticity is dependent upon the demands of the training. For example, primary auditory cortex response latencies and bandwidths following training can either increase or decrease, depending on the difficulty level of the trained task (Engineer et al., 2012). Similarly, rats trained on a series of 6 speech discrimination tasks exhibit A1 map plasticity, while rats trained on a subset of 1 or 3 speech discrimination tasks did not exhibit A1 map plasticity (C T Engineer et al., 2014d). These findings suggest that both the extent and the difficulty level of training can greatly alter the brain’s responses.
It is unknown whether training-induced plasticity is observed in individuals with genetic conditions, such as Rett syndrome. Individuals with syndromes such as Rett and fragile X syndrome are often excluded from studies of autism therapy, despite the high prevalence of autism in these populations. Extensive intervention training in children with idiopathic autism improves behavior and normalizes brain activity (Dawson et al., 2012; Russo et al., 2010). Extensive speech training also normalizes auditory cortex activity to speech sounds in an environmentally induced model of autism, prenatal exposure to valproic acid (C T Engineer et al., 2014b). Many other models of communication disorders caused by environmental problems, such as exposure to lead or SSRIs, also exhibit cortical plasticity and behavioral recovery following rehabilitation training (Zhou et al., 2015; Zhu et al., 2014).
It is possible that genetic conditions associated with communication disorders are more difficult to treat with behavioral therapy alone. Extensive speech training was unable to restore the auditory cortex deficits observed in the Fmr1 KO rat model of fragile X syndrome (C T Engineer et al., 2014c). MECP2 is a gene involved in transcriptional regulation, and is known to modulate synaptic plasticity (Chahrour and Zoghbi, 2007; Na et al., 2013). The observation that Mecp2 rats exhibit some aspects of speech training induced cortical plasticity but not others suggests that the intensity of behavioral therapy required may depend on the genetic insult. Speech training can normalize auditory cortex responses in a rat model of dyslexia exposed to in utero RNAi of Kiaa0319 (Centanni et al., 2014). This dyslexia model may be less severely impaired because gene expression was abnormal in only a small percent of neurons. Collectively, these results are consistent with the hypothesis that the autism spectrum is explained at least in part by the severity of neural coding and plasticity deficits present in each individual.
Our observation that behavioral training can alter cortical responses in Mecp2 rats suggests the need to evaluate whether individuals with Rett syndrome can benefit from intensive behavioral therapy (Sigafoos et al., 2009). Our observation that Mecp2 rats exhibit similar neural and behavioral auditory processing problems that are observed in individuals with Rett syndrome suggests that this model could prove valuable for examining other potential therapies for Rett syndrome.
Supplementary Material
Highlights.
Mecp2 auditory cortex responses to speech sounds were hyperexcitable and delayed
Auditory cortex was less able to follow rapidly presented sounds in Mecp2 rats
Mecp2 rats were impaired at speech sound discrimination in background noise
Extensive speech training improved behavioral and neural responses
Extensive therapy appears to be effective although plasticity is abnormal
Acknowledgements
We would like to thank Elizabeth Hanacik for neurophysiology recording assistance and Bogdan Bordieanu for behavioral training assistance. This research was supported by a grant from the National Institutes of Health to MPK (Grant # R01DC010433).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat. Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Bader GG, Witt-Engerström I, Hagberg B. Neurophysiological findings in the Rett syndrome, II: Visual and auditory brainstem, middle and late evoked responses. Brain Dev. 1989;11:110–114. doi: 10.1016/s0387-7604(89)80078-6. [DOI] [PubMed] [Google Scholar]
- Beauchemin M, De Beaumont L, Vannasing P, Turcotte A, Arcand C, Belin P, Lassonde M. Electrophysiological markers of voice familiarity. Eur. J. Neurosci. 2006;23:3081–3086. doi: 10.1111/j.1460-9568.2006.04856.x. [DOI] [PubMed] [Google Scholar]
- Beauchemin M, González-Frankenberger B, Tremblay J, Vannasing P, Martínez-Montes E, Belin P, Béland R, Francoeur D, Carceller AM, Wallois F, Lassonde M. Mother and stranger: An electrophysiological study of voice processing in newborns. Cereb. Cortex. 2011;21:1705–1711. doi: 10.1093/cercor/bhq242. [DOI] [PubMed] [Google Scholar]
- Centanni TM, Chen F, Booker AM, Engineer CT, Sloan AM, Rennaker RL, LoTurco JJ, Kilgard MP. Speech sound processing deficits and training-induced neural plasticity in rats with dyslexia gene knockdown. PLoS One. 2014;9:e98439. doi: 10.1371/journal.pone.0098439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chao H-T, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu H-C, Heintz N, Ekker M, Rubenstein JLR, Noebels JL, Rosenmund C, Zoghbi HY. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Jones EJH, Merkle K, Venema K, Lowy R, Faja S, Kamara D, Murias M, Greenson J, Winter J, Smith M, Rogers SJ, Webb SJ. Early behavioral intervention is associated with normalized brain activity in young children with autism. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:1150–9. doi: 10.1016/j.jaac.2012.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Rogers S, Munson J, Smith M, Winter J, Greenson J, Donaldson A, Varley J. Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics. 2010;125:e17–23. doi: 10.1542/peds.2009-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Sala G, Pizzorusso T. Synaptic plasticity and signaling in rett syndrome. Dev. Neurobiol. 2014;74:178–196. doi: 10.1002/dneu.22114. [DOI] [PubMed] [Google Scholar]
- Engineer CT, Centanni TM, Im KW, Borland MS, Moreno NA, Carraway RS, Wilson LG, Kilgard MP. Degraded auditory processing in a rat model of autism limits the speech representation in non-primary auditory cortex. Dev. Neurobiol. 2014a;74:972–86. doi: 10.1002/dneu.22175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Centanni TM, Im KW, Kilgard MP. Speech sound discrimination training improves auditory cortex responses in a rat model of autism. Front. Syst. Neurosci. 2014b;8:137. doi: 10.3389/fnsys.2014.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Centanni TM, Im KW, Rahebi KC, Buell EP, Kilgard MP. Degraded speech sound processing in a rat model of fragile X syndrome. Brain Res. 2014c;1564:72–84. doi: 10.1016/j.brainres.2014.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Perez CA, Carraway RS, Chang KQ, Roland JL, Kilgard MP. Speech training alters tone frequency tuning in rat primary auditory cortex. Behav. Brain Res. 2014d;258:166–78. doi: 10.1016/j.bbr.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Perez CA, Chen YH, Carraway RS, Reed AC, Shetake JA, Jakkamsetti V, Chang KQ, Kilgard MP. Cortical activity patterns predict speech discrimination ability. Nat. Neurosci. 2008;11:603–8. doi: 10.1038/nn.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer CT, Rahebi KC, Buell EP, Fink MK, Kilgard MP. Speech training alters consonant and vowel responses in multiple auditory cortex fields. Behav. Brain Res. 2015;287:256–264. doi: 10.1016/j.bbr.2015.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engineer ND, Engineer CT, Reed AC, Pandya PK, Jakkamsetti V, Moucha R, Kilgard MP. Inverted-U function relating cortical plasticity and task difficulty. Neuroscience. 2012;205:81–90. doi: 10.1016/j.neuroscience.2011.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaze DG. Neurophysiology of Rett syndrome. J. Child Neurol. 2005;20:740–6. doi: 10.1177/08830738050200090801. [DOI] [PubMed] [Google Scholar]
- Goffin D, Allen M, Zhang L, Amorim M, Wang I-TJ, Reyes A-RS, Mercado-Berton A, Ong C, Cohen S, Hu L, Blendy J. a, Carlson GC, Siegel SJ, Greenberg ME, Zhou Z. Rett syndrome mutation MeCP2 T158A disrupts DNA binding, protein stability and ERP responses. Nat. Neurosci. 2011 doi: 10.1038/nn.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin D, Brodkin ES, Blendy J. a, Siegel SJ, Zhou Z. Cellular origins of auditory event-related potential deficits in Rett syndrome. Nat. Neurosci. 2014;17:804–806. doi: 10.1038/nn.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston MV, Ammanuel S, O’Driscoll C, Wozniak A, Naidu S, Kadam SD. Twenty-four hour quantitative-EEG and in-vivo glutamate biosensor detects activity and circadian rhythm dependent biomarkers of pathogenesis in Mecp2 null mice. Front. Syst. Neurosci. 2014;8:1–13. doi: 10.3389/fnsys.2014.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kálmánchey R. Evoked potentials in the Rett syndrome. Brain Dev. 1990;12:73–76. doi: 10.1016/s0387-7604(12)80181-1. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, Tierney E, Rohde C. a, Suarez-Pedraza MC, Clarke M. a, Salorio CF, Bibat G, Bukelis I, Naram D, Lanham DC, Naidu S. Social impairments in Rett syndrome: characteristics and relationship with clinical severity. J. Intellect. Disabil. Res. 2012;56:233–47. doi: 10.1111/j.1365-2788.2011.01404.x. [DOI] [PubMed] [Google Scholar]
- Kawahara H. Speech representation and transformation using adaptive interpolation of weighted spectrum: Vocoder revisited. Proc. ICASSP. 1997;2:1303–1306. [Google Scholar]
- Landa RJ, Holman KC, O’Neill AH, Stuart E. a. Intervention targeting development of socially synchronous engagement in toddlers with autism spectrum disorder: a randomized controlled trial. J. Child Psychol. Psychiatry. 2011;52:13–21. doi: 10.1111/j.1469-7610.2010.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenn NJ, Olsho LW, Turk WR. Auditory processing deficit in a patient with Rett syndrome. Am. J. Med. Genet. Suppl. 1986;1:153–6. doi: 10.1002/ajmg.1320250517. [DOI] [PubMed] [Google Scholar]
- Liao W, Gandal MJ, Ehrlichman RS, Siegel SJ, Carlson GC. MeCP2+/− mouse model of RTT reproduces auditory phenotypes associated with Rett syndrome and replicate select EEG endophenotypes of autism spectrum disorder. Neurobiol. Dis. 2012;46:88–92. doi: 10.1016/j.nbd.2011.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschik PB, Kaufmann WE, Sigafoos J, Wolin T, Zhang D, Bartl-Pokorny KD, Pini G, Zappella M, Tager-Flusberg H, Einspieler C, Johnston MV. Changing the perspective on early development of Rett syndrome. Res. Dev. Disabil. 2013;34:1236–1239. doi: 10.1016/j.ridd.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachin J, Smith T, Lovaas OI. Long-term outcome for children with autism who received early intensive behavioral treatment. Am. J. Ment. …. 1993;97:359–372. [PubMed] [Google Scholar]
- Medrihan L, Tantalaki E, Aramuni G, Sargsyan V, Dudanova I, Missler M, Zhang W. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J. Neurophysiol. 2008;99:112–121. doi: 10.1152/jn.00826.2007. [DOI] [PubMed] [Google Scholar]
- Na ES, Nelson ED, Kavalali ET, Monteggia LM. The impact of MeCP2 loss- or gain-of-function on synaptic plasticity. Neuropsychopharmacology. 2013;38:212–9. doi: 10.1038/npp.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neul JL, Lane JB, Lee H-S, Geerts S, Barrish JO, Annese F, Baggett LM, Barnes K, Skinner S. a, Motil KJ, Glaze DG, Kaufmann WE, Percy AK. Developmental delay in Rett syndrome: data from the natural history study. J. Neurodev. Disord. 2014;6:20. doi: 10.1186/1866-1955-6-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Roberts TP. Delayed mismatch field for speech and non-speech sounds in children with autism. Neuroreport. 2005;16:521–525. doi: 10.1097/00001756-200504040-00021. [DOI] [PubMed] [Google Scholar]
- Oram Cardy JE, Flagg EJ, Roberts W, Roberts TP. Auditory evoked fields predict language ability and impairment in children. Int J Psychophysiol. 2008;68:170–175. doi: 10.1016/j.ijpsycho.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Perez CA, Engineer CT, Jakkamsetti V, Carraway RS, Perry MS, Kilgard MP. Different timescales for the neural coding of consonant and vowel sounds. Cereb. Cortex. 2013;23:670–83. doi: 10.1093/cercor/bhs045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters SU, Gordon RL, Key AP. Induced Gamma Oscillations Differentiate Familiar and Novel Voices in Children With MECP2 Duplication and Rett Syndromes. J. Child Neurol. 2014 doi: 10.1177/0883073814530503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purhonen M, Kilpeläinen-Lees R, Valkonen-Korhonen M, Karhu J, Lehtonen J. Cerebral processing of mother’s voice compared to unfamiliar voice in 4-month-old infants. Int. J. Psychophysiol. 2004;52:257–266. doi: 10.1016/j.ijpsycho.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Roberts TPL, Cannon KM, Tavabi K, Blaskey L, Khan SY, Monroe JF, Qasmieh S, Levy SE, Edgar JC. Auditory magnetic mismatch field latency: a biomarker for language impairment in autism. Biol. Psychiatry. 2011;70:263–269. doi: 10.1016/j.biopsych.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo N, Zecker S, Trommer B, Chen J, Kraus N. Effects of background noise on cortical encoding of speech in autism spectrum disorders. J Autism Dev Disord. 2009;39:1185–1196. doi: 10.1007/s10803-009-0737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Hornickel J, Nicol T, Zecker S, Kraus N. Biological changes in auditory function following training in children with autism spectrum disorders. Behav. Brain Funct. 2010;6:60. doi: 10.1186/1744-9081-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetake JA, Wolf JT, Cheung RJ, Engineer CT, Ram SK, Kilgard MP. Cortical activity patterns predict robust speech discrimination ability in noise. Eur. J. Neurosci. 2011;34:1823–38. doi: 10.1111/j.1460-9568.2011.07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigafoos J, Green V. a., Schlosser R, O’eilly MF, Lancioni GE, Rispoli M, Lang R. Communication intervention in Rett syndrome: A systematic review. Res. Autism Spectr. Disord. 2009;3:304–318. [Google Scholar]
- Stach BA, Stoner WR, Smith SL, Jerger JF. Auditory evoked potentials in Rett syndrome. J. Am. Acad. Audiol. 1994;5:226–30. [PubMed] [Google Scholar]
- Stanislaw H, Todorov N. Calculation of signal detection theory measures. Behav. Res. Methods. Instrum. Comput. 1999;31:137–149. doi: 10.3758/bf03207704. [DOI] [PubMed] [Google Scholar]
- Stauder J.E. a, Smeets EEJ, van Mil SGM, Curfs LGM. The development of visual- and auditory processing in Rett syndrome: an ERP study. Brain Dev. 2006;28:487–94. doi: 10.1016/j.braindev.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Stearns N. a, Schaevitz LR, Bowling H, Nag N, Berger UV, Berger-Sweeney J. Behavioral and anatomical abnormalities in Mecp2 mutant mice: a model for Rett syndrome. Neuroscience. 2007;146:907–21. doi: 10.1016/j.neuroscience.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Urbanowicz A, Downs J, Girdler S, Ciccone N, Leonard H. Aspects of speech-language abilities are influenced by MECP2 mutation type in girls with Rett syndrome. Am. J. Med. Genet. Part A. 2015;167:354–362. doi: 10.1002/ajmg.a.36871. [DOI] [PubMed] [Google Scholar]
- Verma NP, Nigro MA, Hart ZH. Rett syndrome--a gray matter disease? Electrophysiologic evidence. Electroencephalogr. Clin. Neurophysiol. 1987;67:327–9. doi: 10.1016/0013-4694(87)90118-0. [DOI] [PubMed] [Google Scholar]
- Wöhr M, Scattoni ML. Behavioural methods used in rodent models of autism spectrum disorders: current standards and new developments. Behav. Brain Res. 2013;251:5–17. doi: 10.1016/j.bbr.2013.05.047. [DOI] [PubMed] [Google Scholar]
- Wulffaert J, Van Berckelaer-Onnes IA, Scholte EM. Autistic disorder symptoms in Rett syndrome. Autism. 2009;13:567–81. doi: 10.1177/1362361309338184. [DOI] [PubMed] [Google Scholar]
- Yamanouchi H, Kaga M, Arima M. Abnormal cortical excitability in Rett syndrome. Pediatr. Neurol. 1993;9:202–206. doi: 10.1016/0887-8994(93)90085-q. [DOI] [PubMed] [Google Scholar]
- Zhou X, Lu JY-F, Darling RD, Simpson KL, Zhu X, Wang F, Yu L. Behavioral training reverses global cortical network dysfunction induced by perinatal antidepressant exposure. Proc Natl Acad Sci U S A. 2015;112:201416582. doi: 10.1073/pnas.1416582111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Liu X, Wei F, Wang F, Merzenich MM, Schreiner CE, Sun X, Zhou X. Perceptual Training Restores Impaired Cortical Temporal Processing Due to Lead Exposure. Cereb. Cortex. 2014:1–12. doi: 10.1093/cercor/bhu258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.