Abstract
Background
The fibronectin splicing variant containing extra domain A (Fn-EDA) is present in negligible amounts in the plasma of healthy humans, but markedly elevated in patients with comorbid conditions including diabetes and hypercholesterolemia, which are risk factors for stroke. It remains unknown, however, whether Fn-EDA worsens stroke outcomes in such conditions. We determined the role of Fn-EDA in stroke outcome in a model of hypercholesterolemia, the apolipoprotein E-deficient (Apoe−/−) mouse.
Methods and Results
In a transient cerebral ischemia/reperfusion injury model, Apoe−/− mice expressing Fn deficient in EDA (Fn-EDA−/−Apoe−/− mice) exhibited smaller infarcts and improved neurological outcomes at days 1 and 8 (P<0.05 vs. Apoe−/− mice). Concomitantly, intracerebral thrombosis (assessed by fibrin (ogen) deposition) and postischemic inflammation (phospho-NF-κB p65, phospho IKKα/β, IL1-β and TNFα) within lesions of Fn-EDA−/−Apoe−/− mice were markedly decreased (P<0.05 vs. Apoe−/− mice). In a FeCl3 injury-induced carotid artery thrombosis model, thrombus growth rate and the time to occlusion were prolonged in Fn-EDA−/−Apoe−/− mice (P<0.05 vs. Apoe−/− mice). Genetic ablation of TLR4 improved stroke outcome in Apoe−/− mice (P<0.05) but had no effect on stroke outcome in Fn-EDA−/−Apoe−/− mice. Bone marrow transplantation experiments revealed that non-hematopoietic cell-derived Fn-EDA exacerbates stroke through TLR4 expressed on hematopoietic cells. Infusion of a specific inhibitor of Fn-EDA into Apoe−/− mouse 15 minutes after reperfusion significantly improved stroke outcome.
Conclusions
Hypercholesterolemic mice deficient in Fn-EDA exhibit reduced cerebral thrombosis and less inflammatory response after ischemia/reperfusion injury. These findings suggest that targeting Fn-EDA could be an effective therapeutic strategy in stroke associated with hypercholesterolemia.
Keywords: Fibronectin, Thrombosis, Inflammation, Ischemic stroke
Introduction
Despite advances in prevention and therapy during the last 20 years, ischemic stroke continues to be the fourth leading cause of death worldwide. Other than mechanical recanalization, the only FDA-approved therapy for acute ischemic stroke is tissue plasminogen activator, which triggers fibrinolysis of clot in the occluded vessels, thus promoting reperfusion and salvage of the ischemic brain. Although prompt reperfusion can improve clinical outcomes, evidence from human subjects and animal models suggests that cerebral reperfusion also promotes oxidative stress and inflammation, which can cause neuronal death in the ischemic penumbra.1, 2 It is therefore critical to identify pathways that underlie postischemic inflammation as well as thromboembolism. Such studies may lead to novel preventive strategies and thereby improve stroke outcomes.
Accumulating evidence suggests that the fibronectin (Fn) may contribute to both inflammation and thrombosis, two processes that are pivotal to stroke pathogenesis.3–7 Fn is a dimeric, multifunctional, adhesive glycoprotein that plays an important role in several cellular processes by providing a substrate for cell adhesion and spreading. Fn has multiple isoforms generated by alternative processing of a single primary transcript at three sites: Extra Domain A (EDA), Extra Domain B (EDB), and the Type III Homologies Connecting Segment (IIICS) (Figure S1). Two major Fn isoforms exist in humans and mice: 1) plasma Fn, which is a soluble dimeric form that lacks EDA and EDB, and is mainly synthesized by hepatocytes and secreted into the blood stream, and 2) cellular Fn, which is a dimeric or cross-linked multimeric form containing either EDA or EDB, or both, in varying proportions. Cellular Fn is synthesized by fibroblasts and other cells including macrophages and endothelial cells, and is deposited as fibrils in the extracellular matrix. Of note, the alternative splicing of EDA and EDB is developmentally regulated; inclusion of EDA and EDB in cellular Fn increases during embryonic development, but decreases substantially after birth.8, 9 Inclusion of the EDA exon in the Fn gene is temporally reestablished during adulthood in several biological and pathological processes, including wound healing,10, 11 vascular hypertension,12 and fibrotic disorders of the lung, liver, and skin.13–15 Fn containing the EDA (Fn-EDA) is present in negligible amounts in the plasma of healthy humans, but it becomes significantly elevated in the plasma of patients with diabetes,16 and atherosclerosis.17 Interestingly, the EDA of Fn, but not other Fn domains, is known to activate human TLR4 when expressed in HEK293 cells.18 EDA activation of TLR4 requires the myeloid differentiation-2 (MD-2) receptor.18
We have shown previously that constitutive inclusion of the EDA in Fn gene product of WT mice (C57BL6/J) promotes arterial thrombosis6, 7 and worsens brain injury in a transient middle cerebral artery occlusion model (tMCAO).19 Although murine stroke models have been very useful in dissecting molecular and cellular mechanisms of brain ischemia/reperfusion injury, most of this previous work has been performed using inbred strains of WT mice, which do not model the effects of pathophysiological conditions such as hypercholesterolemia, which is a risk factor for human stroke. To address this limitation of preclinical studies of acute stroke, the updated Stroke Therapy Academic Industry Roundtable (STAIR) recommends that murine studies be performed in mice with comorbid conditions such as hypertension, diabetes, and hypercholesterolemia.20 In the current study, we investigated the functional role of Fn-EDA in experimental stroke outcomes in a murine model of hypercholesterolemia, the apolipoprotein E-deficient (Apoe−/−) mouse. Novel strains of Apoe−/− mice that are also deficient in Fn-EDA and/or TLR4 were generated to assess the mechanistic role of Fn-EDA in transient cerebral ischemia/reperfusion injury in the context of hypercholesterolemia. We provide evidence for the first time that Fn-EDA exacerbates adverse stroke outcome in Apoe−/− mice by promoting thrombosis and post-ischemic inflammation through TLR4 expressed on hematopoietic cells. Importantly, we show that targeting Fn-EDA by specific inhibitor significantly improves stroke outcome.
Methods
An expanded version of the Methods section is available in the Online Data Supplementary section.
Experimental Animals
Fn-EDA−/− mice have been described previously.11 Briefly, to generate Fn-EDA−/−Apoe−/− mice, Fn-EDA−/− mice11 (backcrossed >15 times to C57BL/6J) were crossed to Apoe−/− mice (The Jackson Laboratory, Bar Harbor, ME). To generate TLR4−/−Apoe−/− mice, Apoe−/− mice were crossed to TLR4−/− mice. To generate Fn-EDA−/−TLR4−/−Apoe−/− mice, Fn-EDA−/−Apoe−/− mice were crossed to TLR4−/−Apoe−/− mice. Whenever possible, littermate control mice were studied. Mice were genotyped by PCR according to protocols from the Jackson laboratory and as described previously.11 All the mice used in the present study were on C57BL/6J background. Both male and female mice (littermates of age approximately 8–10 weeks) weighing 22–26 grams were utilized. The University of Iowa Animal Care and Use Committee approved all procedures and studies were performed according to the current Animal Research: Reporting of In Vivo Experiment guidelines (http://www.nc3rs.org/ARRIVE).
Cerebral ischemia and reperfusion injury
Focal cerebral ischemia was induced by transiently occluding the right middle cerebral artery for 60 minutes with a 7.0 siliconized filament (Doccol Corp.) followed by 23 hours or 8 days of reperfusion as described.19 Briefly, male and female mice were anesthetized with 1–1.5% isoflurane mixed with medical air. Body temperature was maintained at 37°C ± 1.0 using a heating pad. Laser Doppler flowmetry (Perimed instruments, Sweden) was used for each mouse, which showed that regional cerebral blood flow was reduced by 80–90% and recovered to 80 to 95% of baseline (100%) after removal of the filament, suggesting adequate occlusion and reperfusion of the vascular beds. Blood gases were measured in vitro using TruPoint Blood analysis system. A detailed description of Doppler flowmetry, blood gases, and assessment of neurological outcome and infarct volume is provided in the online-data supplement.
Statistical analysis
Results are reported as mean ± SEM except for the neurological score, which is expressed as median with 25th percentile and 75th percentile in parentheses. The number of experimental animals in each group was based on power calculations for the primary parameter (infarct volume) with mean differences and standard deviations taken from pilot data at power 80% with alpha of 0.05. For statistical analysis, Prism Graph software, Version 6.0 was used. The statistical significance was assessed using either unpaired Student's t test (normally distributed) or non-parametric Mann Whitney test (not normally distributed), analysis of variance (ANOVA) followed by Holm-Sidak multiple comparison test (comparison of more than two groups) and repeated measures ANOVA. Analysis of variance on ranks was applied to test for significant differences in the neurological score. Log-rank (Mantel-Cox) test was used to assess the significance in mortality rate up to 8 days following stroke. P<0.05 was considered to be statistically significant.
Results
Fn-EDA−/−Apoe−/− mice exhibit improved stroke outcome
Similar to healthy humans, Fn-EDA is present at only negligible levels in the plasma of wild-type (WT) mice.4, 11 However, Fn-EDA reappears in the plasma and becomes deposited in the arteries of adult hypercholesterolemic Apoe−/− mice, a response that is similar to that seen in patients with atherosclerosis.4, 17 To confirm that the low level of Fn-EDA seen in the plasma of WT mice (Table S1) does not contribute measurably to experimental stroke, we examined the effect of Fn-EDA deletion in WT (C57BL6/J) mice. Fn-EDA−/− and WT C57BL6/J male mice were subjected to 60 minutes of tMCAO followed by 23 hours of reperfusion. Not surprisingly, infarct volume and neurological outcome were comparable between Fn-EDA−/− and WT male mice (Figure S2).
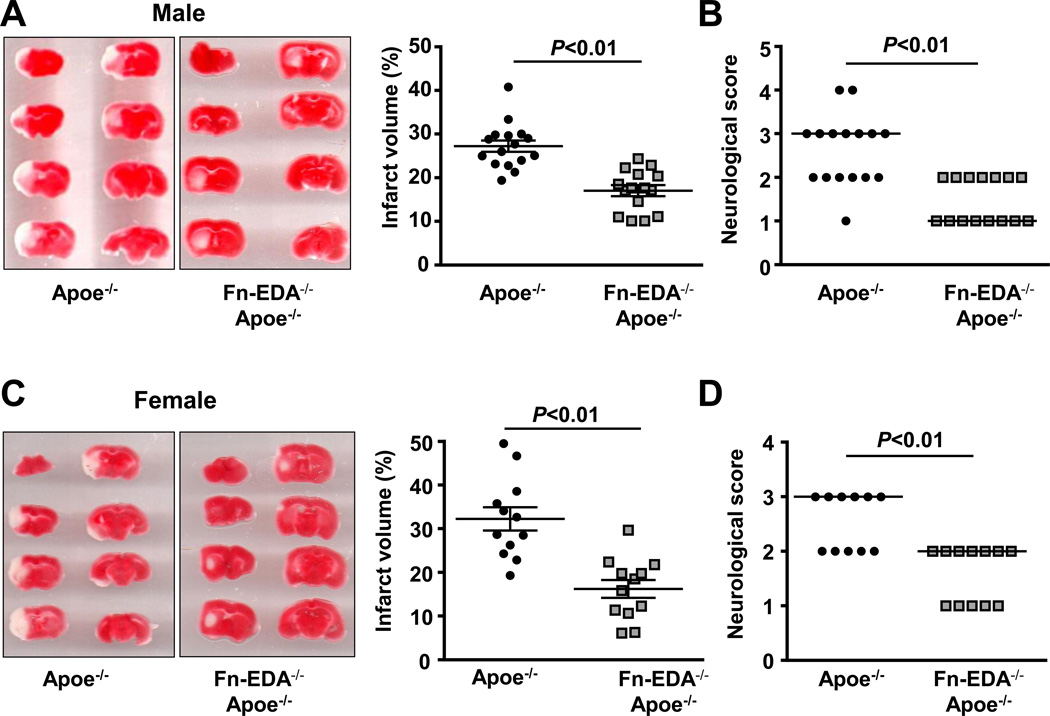
To determine whether the elevated levels of Fn-EDA present in hypercholesterolemic Apoe−/− mice contribute to experimental stroke, we next examined the effect of Fn-EDA deletion in Apoe−/− mice. To minimize the potential confounding effect of advanced atherosclerotic lesions in Apoe−/− mice, which can impair collateral flow and indirectly exacerbate the effect of tMCAO, we fed Fn-EDA−/−Apoe−/− and control Apoe−/− mice a normal chow diet until 8–10 weeks of age, an age at which no significant vascular lesions are found (not shown). In agreement with a previous report,4 we observed significantly elevated Fn-EDA levels in the plasma of Apoe−/− mice (Table S1). Fn-EDA was not detected in the plasma of Fn-EDA−/−Apoe−/− mice. Infarct volumes were markedly decreased (~ 40%) in Fn-EDA−/−Apoe−/− mice when compared with Apoe−/− mice (17.1 ± 1.4 % vs. 27.3 ± 1.6 %, P<0.01; Figure 1A). Reduced infarct volumes in Fn-EDA−/−Apoe−/− mice were associated with improved functional outcomes as assessed by neurological score (1.0 [1.0, 2.0] vs. 3.0 [2.0, 3.0], P<0.01, Figure 1B). Laser Doppler flow measurements (Table S2) and physiological parameters (Table S3) were similar among groups before, during and after ischemia. Cholesterol levels were also comparable among groups (Table S4). To establish that there were no gross anatomic differences in collateralization of the cerebral circulation, the circle of Willis was visualized by intravenous injection of India ink. An intact circle of Willis, including bilateral posterior communicating arteries, was documented in all mice, which suggests that there were no gross differences in cerebrovascular anatomy that could have accounted for smaller infarct volumes in Fn-EDA−/−Apoe−/− mice (Figure S3).
Figure 1.
Irrespective of gender, deletion of EDA of Fn in Apoe−/− mice improves acute stroke outcome and survival. A&C. Left panel shows representative 2, 3, 5-triphenyl-tetrazolium chloride stained serial coronal brain sections from one mouse of each genotype. Right panel shows corrected mean infarct volumes of each genotype (N=12–16/group). Data are mean ± SEM. B&D. Neurological score of male and female mice from each genotype as assessed prior to sacrifice on day 1 are depicted as scatter plots including median (N=12–16/group). Analysis of variance on ranks was applied to test for significant differences in the neurological score. E. Mortality rate between day 0 and day 8 after 60 minutes transient ischemia (N = 8 –9/ group). Survival curve: *P = 0.002, log-rank test compared with Apoe−/− mice. F. Neurological score of male mice from each genotype on day 3 and day 5 are depicted as scatter plots including median.
According to updated STAIR recommendations, we next determined whether Fn-EDA exacerbates stroke outcome independent of gender. Female Fn-EDA−/−Apoe−/− and Apoe−/− mice were subjected to 60 minutes of tMCAO followed by 23 hours of reperfusion. Fn-EDA−/−Apoe−/− mice exhibited significantly smaller infarct and better neurological outcome when compared with Apoe−/− mice (P<0.01, Figure 1C&D) suggesting that, irrespective of gender, Fn-EDA exacerbates stroke outcome.
Next, to determine whether Fn-EDA−/−Apoe−/− mice have improved stroke outcome not only at 24 hours but also at later time points, we followed male mice for survival up to eight days following 60 minutes of transient ischemia. We found that at day 8, Fn-EDA−/−Apoe−/− mice had a better survival rate (70%) when compared with Apoe−/− mice (10%, P=0.002; Figure 1E). Importantly, Fn-EDA−/−Apoe−/− mice also had improved neurological outcomes at day 3 and day 5 compared with Apoe−/− mice (Figure 1F).
Fn-EDA−/−Apoe−/− mice have improved local cerebral blood flow in the core region
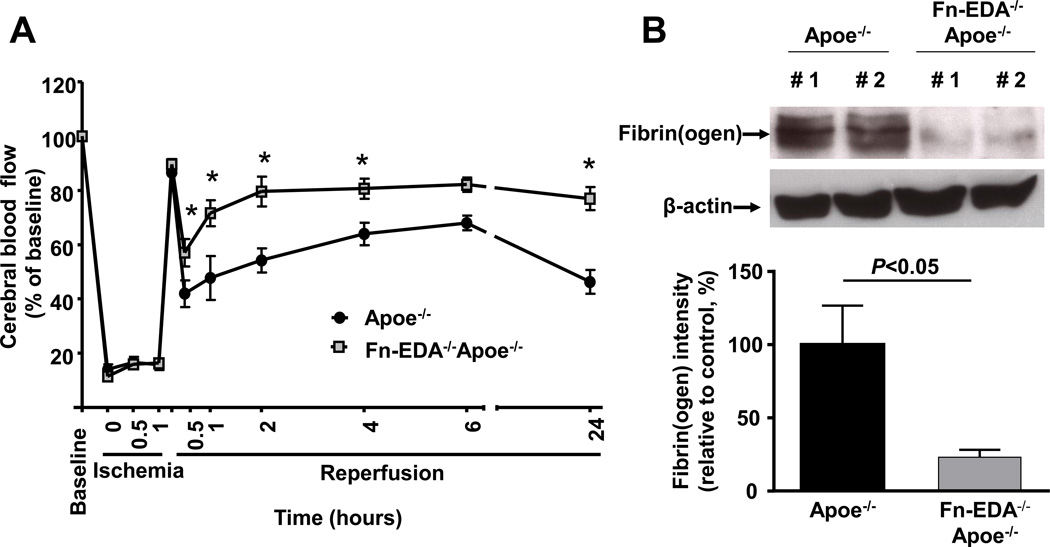
To determine whether improved stroke outcome in Fn-EDA−/−Apoe−/− mice correlated with improved local cerebral blood flow (CBF), laser Doppler flowmetry was performed at different time points (0.5–24 hours). We found that local CBF was significantly improved at 1, 2, 4, and 24 hours after reperfusion in Fn-EDA−/−Apoe−/− mice (P<0.05 vs. Apoe−/− mice, Figure 2A). In addition, we observed that intracerebral fibrin (ogen) deposition, as determined by Western, was markedly reduced in Fn-EDA−/−Apoe−/− mice (P<0.05 vs. Apoe−/− mice, Figure 2B) suggesting that Fn-EDA may promote intracerebral thrombosis, and thereby exacerbate stroke outcome.
Figure 2.
Fn-EDA−/−Apoe−/− mice has improved local cerebral blood flow and reduced intracerbral fibrin(ogen) deposition. A. Doppler flow measurements of local cerebral blood flow in the territory of the right middle cerebral artery at 0.5, 1, 2, 4, 6 and 24 hours of reperfusion (*P < 0.01 vs. Apoe−/− mice, repeated measures ANOVA; N=8–9 mice/group). B. Quantification of fibrin(ogen) in brain homogenates as determined by immunoblotting and densitometric quantification of the bands (N=3 mice/group). Actin was used as loading control.
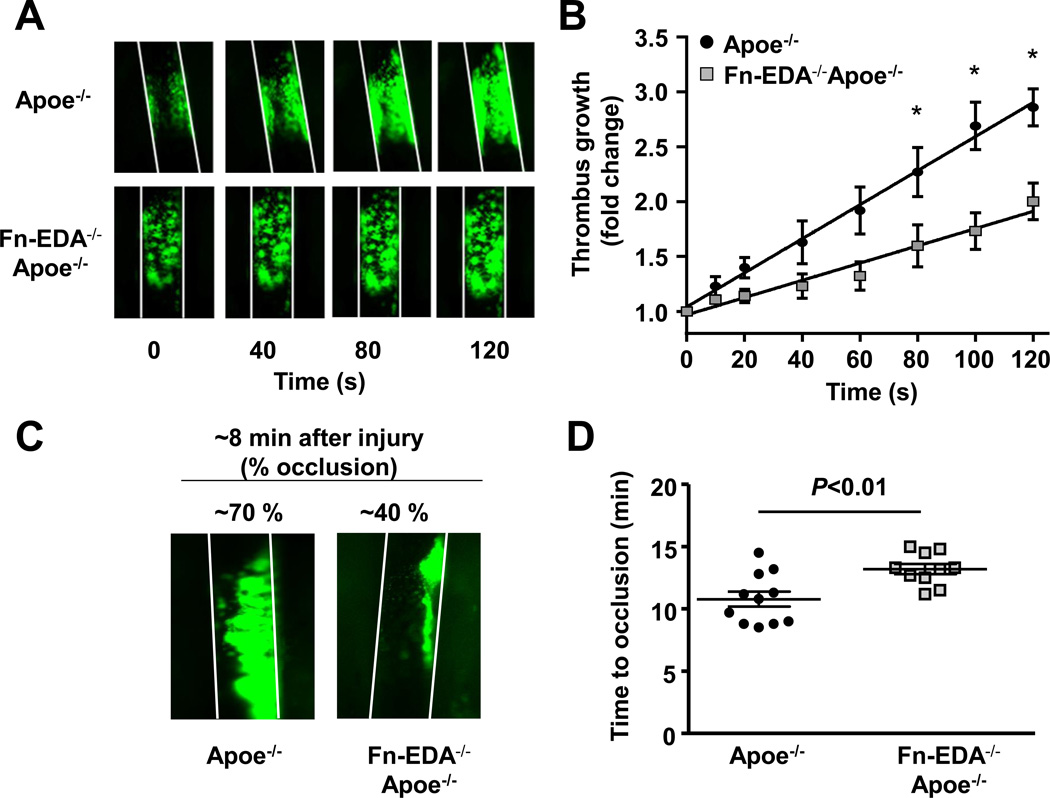
Fn-EDA−/−Apoe−/− mice exhibit delayed thrombus growth in injured carotid artery
To test the hypothesis that Fn-EDA promotes cerebral thrombosis and thereby exacerbates stroke outcome, Fn-EDA−/−Apoe−/− and control Apoe−/− mice were subjected to an experimental model of thrombosis (FeCl3-injury induced carotid artery thrombosis). All mice used were male and have similar age (8–10 weeks). Using intravital microscopy, we measured thrombus growth kinetics and time to occlusion of the injured carotid artery. The diameter of carotid arteries studied was similar among the groups (not shown). The rate of thrombus growth was markedly decreased in Fn-EDA−/−Apoe−/− mice (P<0.05 vs. Apoe−/− mice, Figure 3A&B). Consistent with these results, Fn-EDA−/−Apoe−/− mice developed smaller thrombi (~40% occlusion) when compared with Apoe−/− mice (~70% occlusion) eight minutes after FeCl3 injury (Figure 3C). The mean time to complete occlusion was significantly prolonged in Fn-EDA−/−Apoe−/− mice (P<0.01 vs. Apoe−/− mice, Figure 3D). All of the injured carotid vessels occluded at the site of injury.
Figure 3.
Fn-EDA−/−Apoe−/− mice are protected from experimental thrombosis of the carotid artery. A. Representative microphotographs of thrombus growth in FeCl3-injured carotid arteries as visualized by upright intravital microscopy. Platelets were labeled with calcein green. White lines delineate the arteries. B. The fold increase in diameter was calculated by dividing the diameter of the thrombus at time (n) by the diameter of the same thrombus at time (0) (defined as the time point at which the thrombus diameter first reached 100 µm). Slopes over time showed that the rate of thrombus growth in Fn-EDA−/−Apoe−/− mice (slope: 0.008 ± 0.001) was decreased when compared with Apoe−/− mice (slope: 0.016 ± 0.001). C. Representative microphotographs depicting percentage occlusion ~8 minutes after FeCl3-induced injury. Platelets were labeled with calcein green. White lines delineate the arteries D. Mean time to complete occlusion of FeCl3injured carotid artery. Data are presented as mean ± SEM. N=9–10 mice/group.
Fn-EDA−/−Apoe−/− mice exhibited reduced postischemic inflammation within the ischemic region
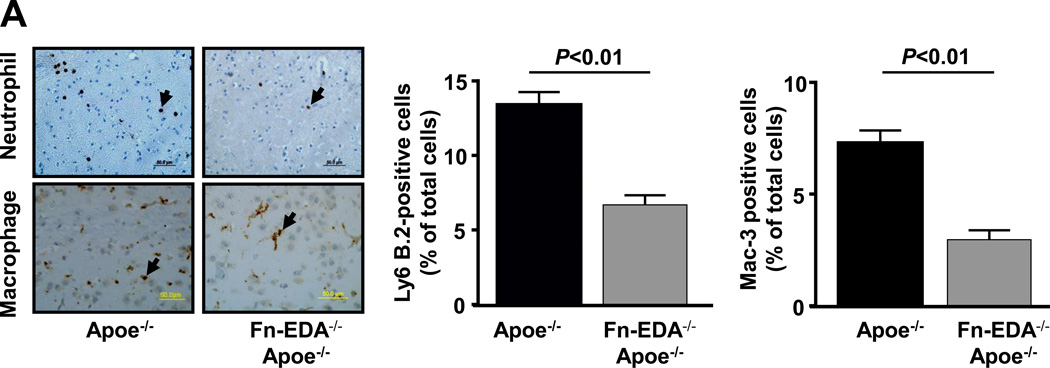
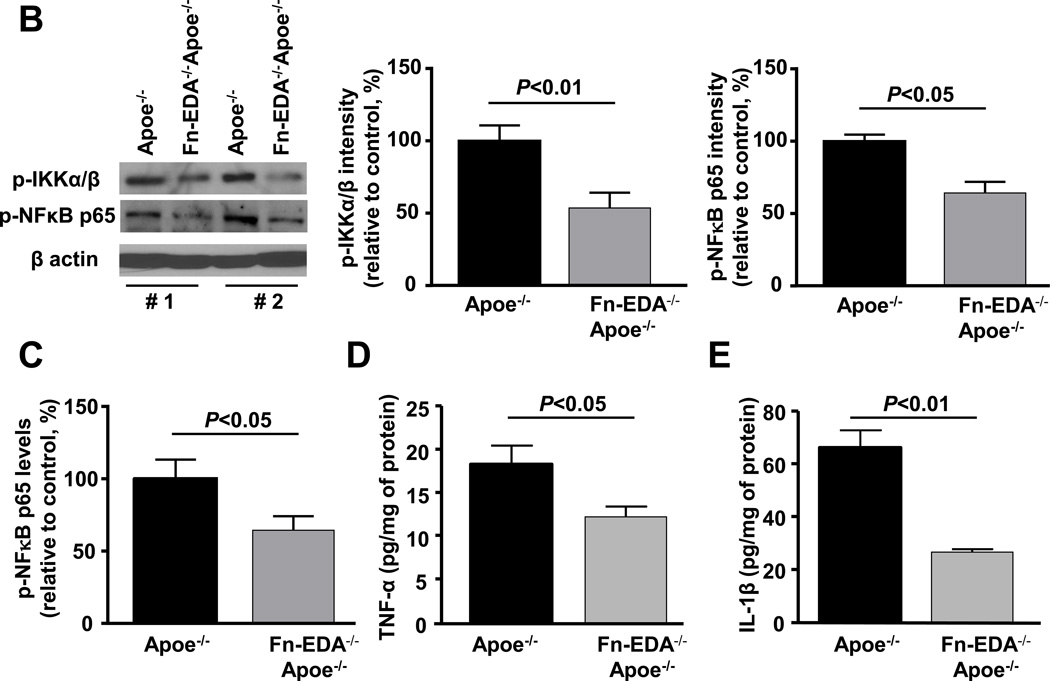
Evidence suggests that brain injury is modulated by the postischemic inflammatory response following focal ischemia of cerebral vessels.21 To determine whether improved stroke outcome in the Fn-EDA−/−Apoe−/− mice was associated with reduced postischemic inflammation, we measured neutrophil and macrophage influx within the infarct and peri-infarct region of the perfused brain after 60 minutes of tMCAO followed by 23 hours of reperfusion. Immunostained sections of the ischemic region revealed markedly reduced neutrophil and macrophage infiltration in Fn-EDA−/−Apoe−/− mice (P<0.01 vs. Apoe−/− mice; Figure 4A), and an absence of infiltration in the non-ischemic region of the contralateral hemisphere or in sham-operated animals (not shown). Additionally, we confirmed neutrophil and macrophage infiltration within the infarcted region by flow cytometer using specific antibodies (not shown). Total leukocyte counts were similar among genotypes (Table S5). Several previous studies, including some from our own group, have shown that Fn-EDA interacts with TLR4.5, 7, 15, 18 We hypothesized, therefore, that Fn-EDA may upregulate TLR4 signaling in Apoe−/− mice after the ischemic insult. We measured levels of phospho IKKα/β and phospho-NFκB p65, which are components of the canonical signaling pathway downstream of TLR4, in brain homogenates prepared from the infarcted and surrounding areas following tMCAO. Immunoblotting experiments showed a ~2-fold reduction in phospho IKKα/β and phospho-NFκB p65 intensity levels in Fn-EDA−/−Apoe−/− mice (P<0.05 vs. Apoe−/− mice; Figure 4B). Accordingly, ELISA experiments showed a ~2-fold reduction in phospho NFκB p65 (Figure 4C), TNFα and IL1β levels in Fn-EDA−/−Apoe−/− mice (P<0.05 vs. Apoe−/− mice, Figure 4D&E).
Figure 4.
Fn-EDA−/−Apoe−/− mice exhibit reduced postischemic inflammation. A. Left panel shows representative coronal brain sections from one mouse of each genotype stained for neutrophils (Ly6 B.2 positive cells stained as brown are indicated by arrow) and macrophages/microglia (Mac-3 positive cells stained as brown are indicated by arrow), and counterstained with hematoxylin (blue). The scale bar = 50 µm. Middle and right panels show quantification. The ratio of immunoreactive cells to total number of cells was used to calculate the mean fraction of immunoreactive cells within the ischemic region. Mean for individual mouse was calculated from 4 coronal sections/mouse (separated by 100 µm). Data are presented as mean ± SEM. N = 5 mice/group. B. Left panel shows representative immunoblots of phospho IKKα/β and phospho-NFκB p65 in brain homogenates prepared from the infarcted and surrounding areas. β-actin was used as a loading control. Middle and right panels show quantification of phospho IKKα/β and phospho-NFκB p65 intensity by densitometry. C, D &E. Quantification of phospho-NFκB p65, TNFα and IL-1β levels by ELISA in brain homogenates. Data are presented as mean ± SEM. N = 5 mice/group.
Fn-EDA mediated inflammatory brain injury is TLR4-dependent
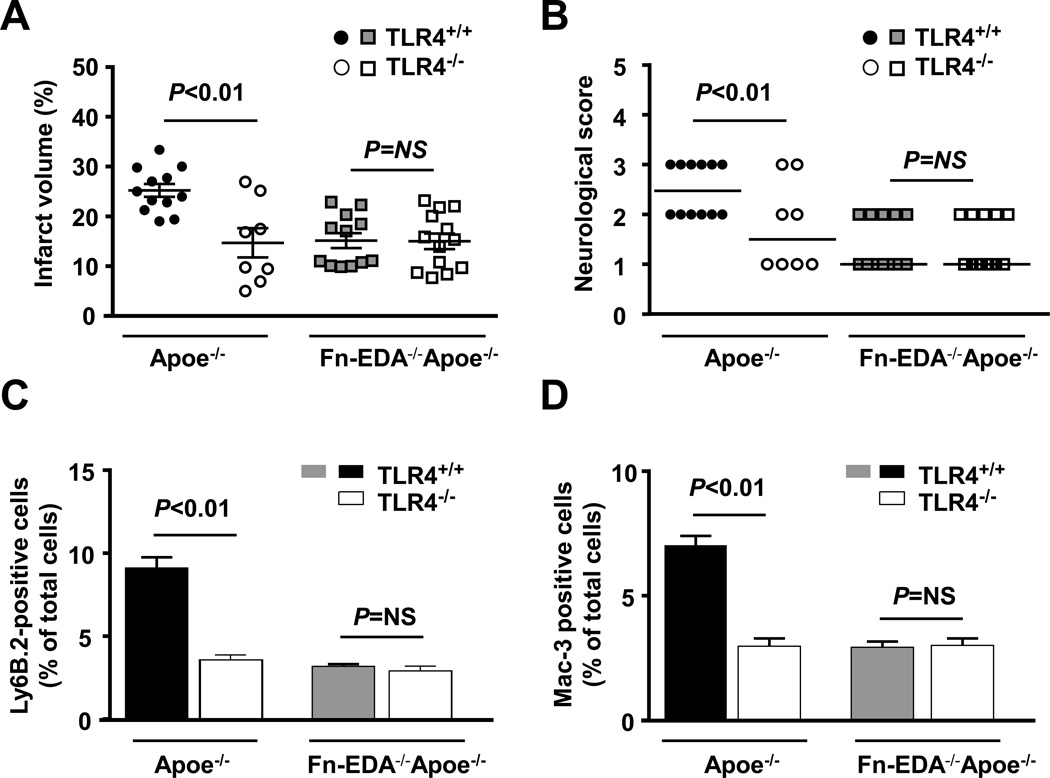
To directly test the hypothesis that Fn-EDA promotes inflammatory brain injury through TLR4, we generated Apoe−/− and Fn-EDA−/−Apoe−/− mice on a TLR4−/− background. Controls included Apoe−/− and Fn-EDA−/−Apoe−/− littermates. Male mice were subjected to tMCAO. Compared with Apoe−/− mice, infarct volumes were significantly decreased in TLR4−/−Apoe−/− mice (25.3 ± 4.5 versus 14.7 ± 8.3 %, P<0.01; Figure 5A). Reduced infarct volumes in TLR4−/−Apoe−/− mice were associated with improved neurological outcome (P<0.01; Figure 5B). Immunostained sections of the infarcted and peri-infarcted regions revealed a significant decrease in neutrophil and macrophage infiltration in TLR4−/−Apoe−/− mice (P<0.01 vs. Apoe−/− mice, Figure 5 C&D). To rule out the possibility that global deletion of TLR4 may have improved stroke outcome in Apoe−/− mice independently of Fn-EDA, we compared stroke outcome in Fn-EDA−/−Apoe−/− and Fn-EDA−/−TLR4−/−Apoe−/− mice. No significant differences in the infarct volume, neurological score and neutrophil and macrophage infiltration were observed between Fn-EDA−/−TLR4−/−Apoe−/− mice and Fn-EDA−/−Apoe−/− mice (Figure 5 A–D), suggesting that majority of the adverse effect on stroke outcome caused by Fn-EDA are occurring through TLR4.
Figure 5.
Genetic ablation of TLR4 improves acute ischemic stroke outcome in Apoe−/− mice, but not in Fn-EDA−/−Apoe−/− mice. A. Corrected mean infarct volumes of each genotype (N=8–12/group) following 60 minutes of transient ischemia and 23 hours of reperfusion. Data are mean ± SEM. B. Neurological score of all mice from each genotype as assessed prior to sacrifice on day 1 are depicted as scatter plots including median. (N=8–12/group). Analysis of variance on ranks was applied to test for significant differences in the neurological score. C&D. Quantification of neutrophils (Ly6 B.2 positive cells) and macrophages/microglial cells (Mac-3 positive cells). The ratio of immunoreactive cells to the total number of cells was used to calculate the mean fraction of immunoreactive cells within the ischemic region. Mean for individual mouse was calculated from 4 coronal sections/mouse (separated by 100 µm). Data are presented as mean ± SEM. N = 5 mice/group.
Fn-EDA exacerbates adverse stroke outcome through TLR4 expressed on cells of hematopoietic origin
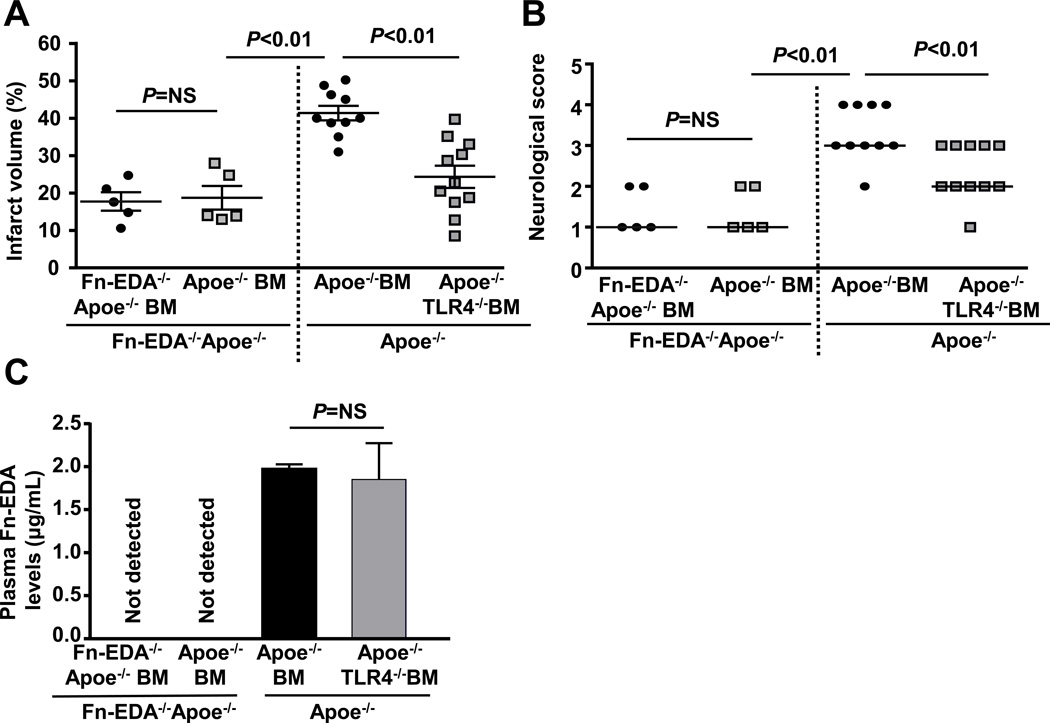
Cells of hematopoietic origin, including activated macrophages and platelets in addition to stressed/stimulated endothelial cells and other non-hematopoietic cells, express Fn-EDA.22 To determine the cellular source of Fn-EDA that contributes to Fn-EDA mediated stroke exacerbation, we transplanted irradiated Fn-EDA−/−Apoe−/− mice with bone marrow (BM) from either Fn-EDA−/−Apoe−/− or Apoe−/− mice. This BM transplantation (BMT) protocol resulted in chimeric mice (Apoe−/−-BM→ Fn-EDA−/−Apoe−/−) that express Fn-EDA in only in cells of hematopoietic origin. Complete blood counts were comparable suggesting that BMT did not affect the number of BM-derived blood cells (Table S6). Infarct volume and neurological score were comparable between Fn-EDA−/−Apoe−/−-BM→ Fn-EDA−/−Apoe−/− mice and Apoe−/−-BM→ Fn-EDA−/−Apoe−/− mice. In contrast, significantly larger infarcts and poorer neurological outcomes were found in Apoe−/− BM→ Apoe−/− mice when compared with Apoe−/−-BM→ Fn-EDA−/−Apoe−/− mice (P<0.01; Figure 6 A&B). This finding suggests that the Fn-EDA contributing to stroke exacerbation is derived from cells of non-hematopoietic cell, most likely from endothelial cells. In line with this finding, immunostaining demonstrated the presence of Fn-EDA in endothelial cells of Apoe−/− mice (Figure S4). To investigate the cellular source of TLR4 that contributes to Fn-EDA-mediated stroke exacerbation, we transplanted irradiated Apoe−/− mice with BM from TLR4−/− Apoe−/− mice. This BMT protocol resulted in chimeric mice that express TLR4 on non-hematopoietic cells, but lack TLR4 in cells of hematopoietic origin. Compared with Apoe−/− BM→ Apoe−/− mice, TLR4−/− Apoe−/−-BM→ Apoe−/− mice showed a significant decrease in infarct volume and improved neurological outcome (P<0.01; Figure 6A&B). Plasma Fn-EDA levels were comparable in Apoe−/− BM→Apoe−/− mice, TLR4−/− Apoe−/−-BM→ Apoe−/− mice (Figure 6C). To exclude the possibility that these in vivo effects are not simply mediated by TLR4 deletion in hematopoietic cells, we compared stroke outcome in Fn-EDA−/−Apoe−/−-BM→ Fn-EDA−/−Apoe−/− mice and Fn-EDA−/−TLR4−/−Apoe−/−-BM → Fn-EDA−/−Apoe−/− mice. Infarct volume and neurological score were comparable between these groups (Figure S5) suggesting that Fn-EDA promotes adverse stroke outcome via TLR4 expressed on cells of hematopoietic origin.
Figure 6.
Non-hematopoietic cell-derived Fn-EDA exacerbates stroke through TLR4 expressed on cells of hematopoietic origin. A. Corrected mean infarct volumes of each genotype. Data are mean ± SEM. B. Neurological score of all mice from each genotype as assessed on day 1 prior to sacrifice are depicted as scatter plots including median. C. Quantification of plasma Fn-EDA levels by ELISA (n = 6 mice/group).
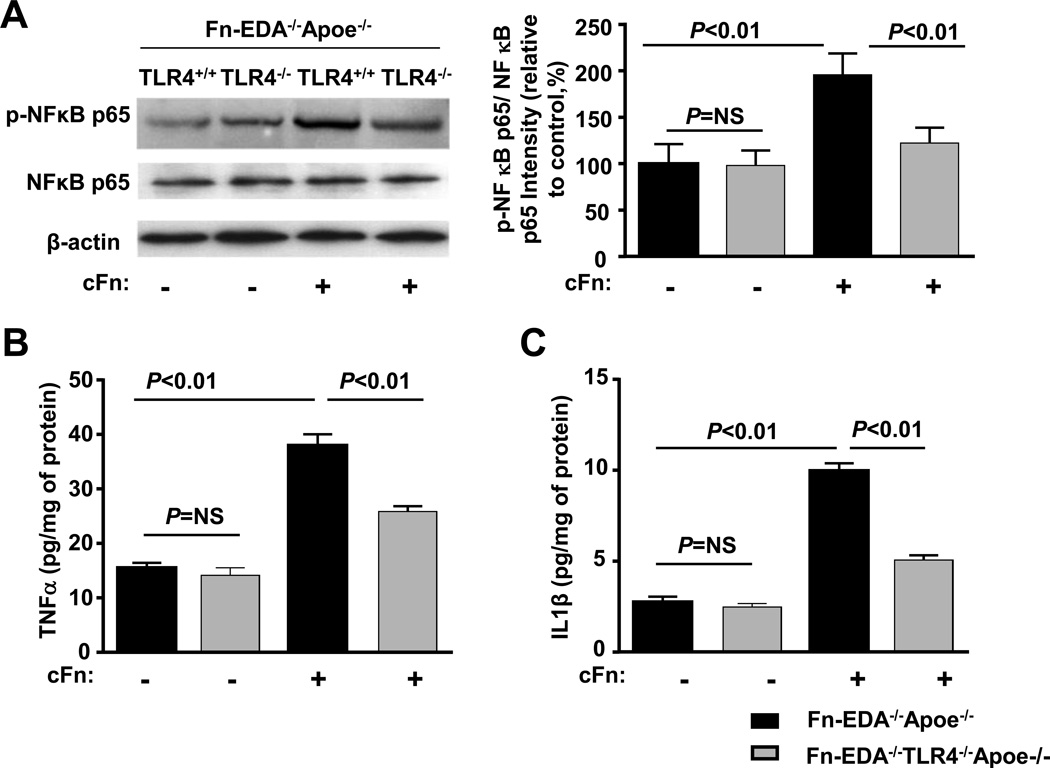
There is accumulating evidence that among of the various types of leukocytes, neutrophils are the first to infiltrate brain after ischemia/reperfusion in injury.21 We determined whether exogenous Fn-EDA interaction with activated neutrophils will further potentiate TLR4 signaling pathway. Neutrophils from Fn-EDA−/−Apoe−/− mice were activated with a sub-threshold dose (20 ng/mL) of phorbol myristate acetate (PMA) for 24 hrs in the presence or absence of exogenous human cellular Fn (cFn), which contains EDA. Immunoblotting experiments showed a significant increase in phospho-NFκB p65/total NFκB p65 levels in cFn treated neutrophils from Fn-EDA−/−Apoe−/− mice compared with untreated control Fn-EDA−/−Apoe−/− mice (P<0.05, Figure 7A). Concomitantly, TNFα and IL1β protein expression levels were also significantly increased (P<0.01, Figure 7B&C). These differences were not seen in cFn treated neutrophils from Fn-EDA−/− TLR4−/−Apoe−/− mice (P<0.05, Figure 7A–C). Phospho-NFκB p65/total NFκB p65, TNFα and IL1β protein expression levels were comparable in PMA-treated Fn-EDA−/−TLR4−/−Apoe−/− neutrophils vs. PMA-treated Fn-EDA−/−Apoe−/− neutrophils, which strongly suggests that the in vitro effects were not simply mediated by TLR4 deletion, but rather by a specific effect of Fn-EDA (Figure 7A–C). Similarly, we found that purified cFn potentiated inflammation (increased phospho-NFκB p65/ total NFκB p65, TNFα and IL1β) in PMA-treated bone marrow-derived macrophages from EDA−/−Apoe−/− mice but not from EDA−/−TLR4−/−Apoe−/− mice (Figure S6).
Figure 7.
Fn-EDA potentiates canonical NFκB signaling via TLR4. Bone marrow-derived neutrophils from Fn-EDA−/−Apoe−/− and Fn-EDA−/−TLR−/−Apoe−/− mice were stimulated with 20 ng phorbol myristate acetate in the presence or absence of cFn (10µg/well) for 24 hours. Left panel shows representative immunoblots of phospho- NFκB p65 and total NFκB p65. β actin was used as a loading control. The right panel represents quantification of intensity of phospho-NFκB p65 to total NFκB p65 in the presence or absence of cFn. Data are presented as mean ± SEM. N = 5/group. B &C. Quantification of TNFα and IL-1β by ELISA. Data are presented as mean ± SEM. N = 5/group.
Infusion of anti-Fn-EDA Ig improves stroke outcome in Apoe−/− mice
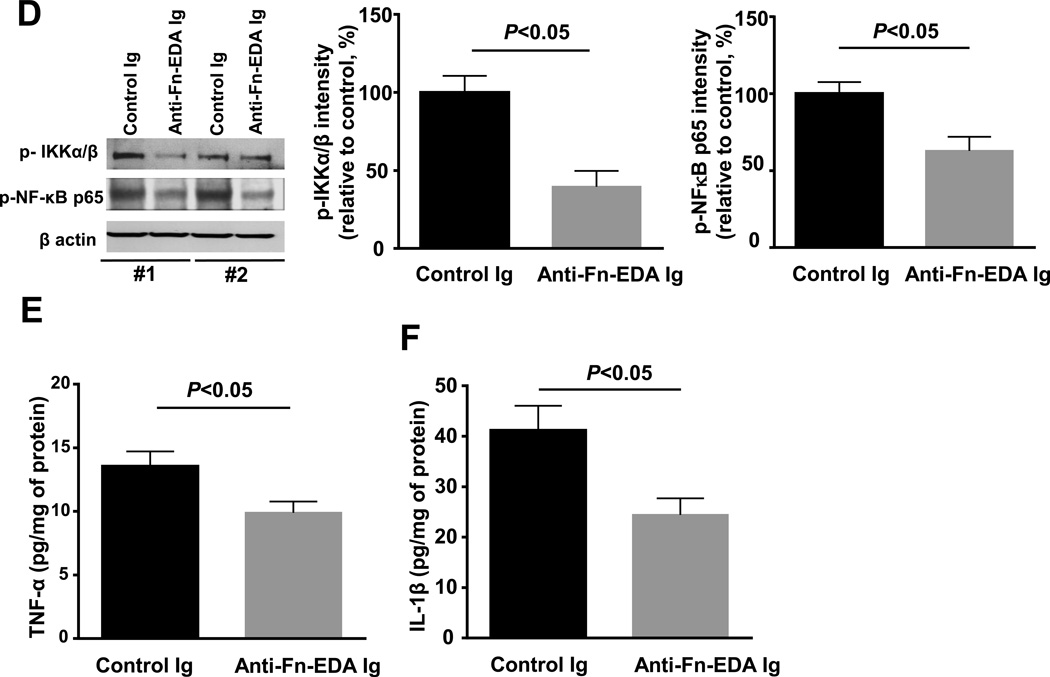
We next evaluated the therapeutic potential of targeting plasma Fn-EDA using specific antibodies to the EDA of Fn. Male mice were subjected to 60 minutes of tMCAO followed by 23 hours of reperfusion. To mimic clinical conditions of acute stroke therapy, we infused anti-Fn-EDA Ig intravenously into Apoe−/− mice 15 minutes after reperfusion. Apoe−/− mice treated with control Ig served as controls. Both groups of mice appeared normal during and after treatment. Infusion of anti-Fn-EDA Ig or control Ig did not significantly affect total fibronectin levels in the plasma (not shown). Body weight was comparable among treated and control mice (not shown). Infarct volumes were reduced by ~2-fold in anti-Fn-EDA Ig-treated Apoe−/− mice compared with control Ig-treated Apoe−/− mice (P<0.01; Figure 8 A). Reduced infarct volume in anti-Fn-EDA Ig-treated Apoe−/− mice was associated with improved neurological outcome (Figure 8B) that was concordant with improved local CBF (Figure 8C). Furthermore, we found a significant reduction in phospho IKKα/β and phospho-NFκB p65 intensity levels (by Western blot) and a decrease in levels of inflammatory cytokines TNFα and IL1β (by ELISA) in anti-Fn-EDA Ig-treated Apoe−/− mice (P<0.05 vs. control Ig-treated Apoe−/− mice; Figure 8D–F).
Figure 8.
Targeting plasma Fn-EDA with anti-Fn-EDA Ig after reperfusion significantly improves stroke outcome. A. Left panel shows representative 2, 3, 5-triphenyltetrazolium chloride stained serial coronal brain sections from control and anti-Fn-EDA Ig treated Apoe−/− mice. The right panel shows corrected mean infarct volumes of each genotype (N=9–14/group). Data are mean ± SEM. B. Neurological scores of all mice from each genotype as assessed on day 1 prior to sacrifice are depicted as scatter plots including median (N=9–14/group). Analysis of variance on ranks was applied to test for significant differences in the neurological score. C. Doppler flow measurements of cerebral blood flow in the territory of the right middle cerebral artery following reperfusion (*P < 0.01 vs. Apoe−/− mice, repeated measures ANOVA; N=8–9 mice/group). D. Left panel shows representative immunoblots of phospho IKKα/β and phospho-NFκB p65 in brain homogenates prepared from the infarcted and surrounding areas from 2 mice/group. β-actin was used as a loading control. Middle and right panels show quantification of phospho IKKα/β and phospho-NFκB p65 intensity by densitometry. E &F. Quantification of TNFα and IL-1β levels by ELISA in brain homogenates. Data are presented as mean ± SEM. N = 5 mice/group.
Discussion
Herein, we report that cellular Fn-EDA exacerbates adverse stroke outcome in the context of hypercholesterolemia. We believe that the findings of this study are novel and may have clinical significance for the following reasons: First, human stroke occurs in the context of comorbid conditions such as hypertension, diabetes and hypercholesterolemia. Fn-EDA is specifically elevated during these conditions. Irrespective of gender, we show for the first time that genetic ablation of Fn-EDA in hypercholesterolemic Apoe−/− mice markedly improves stroke outcome by reducing cerebral thrombo-inflammation. Second, we found that Apoe−/− mice lacking Fn-EDA were protected from increased susceptibility to mortality and had better neurological outcomes at later stages of recovery (day 8) following focal transient cerebral ischemia. Third, mechanistically, we provide evidence that endothelial-derived Fn-EDA aggravates stroke outcome by enhancing post-ischemic inflammation through TLR4 expressed on hematopoietic cells. Fourth, as a potential therapeutic strategy, we show that infusion of an antibody specific to Fn-EDA 15 minutes after reperfusion improves stroke outcome in Apoe−/− mice.
Following ischemic stroke, microvascular dysfunction and intracerebral thrombosis may cause secondary infarct growth after initial vessel recanalization accomplished mechanically or through fibrinolysis. We found that the deletion of Fn-EDA in Apoe−/− mice resulted not only in improved stroke outcomes but also in higher local CBF as estimated by non-invasive laser Doppler flowmetry. Although the temporal and spatial resolution of laser Doppler flowmetry is limited, this finding does suggest that deletion of Fn-EDA results in improved local CBF at a number of isolated points in the cortical area. In addition, we found markedly decreased intracerebral fibrin (ogen) deposition in Fn-EDA−/−Apoe−/− mice. Together, these findings suggest that Fn-EDA promotes intracerebral thrombosis and decreased regional CBF. Consistent with these observations, Fn-EDA−/−Apoe−/− mice were protected in a model of carotid artery thrombosis. Moreover, our data suggest that the molecular mechanisms by which Fn-EDA promotes thrombosis in the setting of hypercholesterolemia involve TLR4. Using an adoptive platelet transfer approach that allowed us to rapidly generate Fn-EDA mice specifically lacking platelet TLR4, we recently showed that Fn-EDA present in the plasma of WT mice (C57BL6/J) is able to promote arterial thrombosis in vivo through platelet TLR4. Additionally, we demonstrated direct interaction of Fn-EDA with platelet TLR4.7 Based on these findings, we speculate that elevated Fn-EDA in the plasma of Apoe−/− mice may potentiate cerebral thrombosis through the platelet TLR4 pathway. Platelets have all the molecular machinery necessary for signal transduction downstream of TLR4, including components of the canonical NF-κB pathway, which has been shown to play a role in platelet function.23 However, it remains possible that TLR4-independent mechanisms, such as an increased tendency of Fn-EDA to deposit as fibrils,24 or an alteration in the RGD motif present in the Fn type III-10 domain that results in enhanced binding to platelet integrin’s αIIbβ3, ανβ3 and α5β1,24, 25 may also contribute to Fn-EDA-mediated accelerated thrombosis. The new evidence presented here that Fn-EDA is prothrombotic in a murine model of hypercholesterolemia suggests that the presence of elevated levels of Fn-EDA in the circulation of patients with atherosclerosis may promote in situ cerebral thrombosis, and, thereby trigger or exacerbate ischemic stroke.
Cerebral ischemia/ reperfusion injury elicits a strong inflammatory response that promotes brain tissue damage in the ischemic penumbra.21, 26, 27 We found that Apoe−/− mice lacking Fn-EDA exhibited markedly reduced NF-κB activation and postischemic inflammation within the ischemic region. Our findings are in line with a previous study showing that recombinant EDA significantly increased components of NF-κB in vitro.18 Activation of NF-κB is known to induce proinflammatory genes encoding enzymes, cytokines, and other adhesion molecules, which are known to promote migration of inflammatory cells including neutrophils and macrophages causing inflammatory tissue injury.28 In agreement with these previous findings, we found that Apoe−/− mice lacking Fn-EDA had markedly reduced infiltration of neutrophils and macrophages within the ischemic regions. We also investigated the molecular mechanism by which Fn-EDA promotes postischemic inflammation in the context of hypercholesterolemia. We hypothesized that TLR4 signaling contributes to Fn-EDA mediated stroke exacerbation for the following reasons. First, using specific TLR4 inhibitors, we and others have demonstrated that Fn-EDA activates TLR4 signaling.5, 15, 18, 19 Second, TLR4-deficient mice have improved stroke outcomes after transient brain ischemia/reperfusion injury.29 Third, Fn-EDA−/−Apoe−/− mice exhibited reduced NF-κB activation and postischemic inflammation. Most importantly, we now provide evidence for first time that global deletion of TLR4 significantly improved stroke outcome in Apoe−/− mice, but had no effect on stroke outcome in Fn-EDA−/−Apoe−/− mice. We initially were surprised to see similar stroke outcomes in Fn-EDA−/−Apoe−/−TLR4−/− and Fn-EDA−/−Apoe−/− mice because multiple endogenous ligands, such as heat-shock proteins30 released from necrotic cells and fibrinogen and fibrin deposited during vascular injury,31 are known to activate TLR4 and generate an inflammatory response. Although our studies indicate that TLR4 signaling significantly contributes to Fn-EDA-mediated stroke exacerbation in the setting of the comorbid condition of hypercholesterolemia, it remains possible that some of the pro-inflammatory effects of Fn-EDA are TLR4-independent, perhaps mediated by binding sites for leukocyte integrin’s α4β1 and α9β1 in the EDA domain (Figure S1).32
Fn-EDA is expressed by cells of hematopoietic origin including macrophages and platelets in addition to activated endothelial cells.22, 33 Using a BMT approach, we demonstrated a pivotal role for Fn-EDA derived from non-hematopoietic cells, presumably endothelial cells (Figure S4), in stroke exacerbation. Multiple types of cells including endothelial cells, and inflammatory cells such as neutrophils and monocytes express TLR4. Additionally, TLR4 is expressed by neurons, and neuronal TLR4 has been proposed to play a detrimental role in ischemic stroke.34 However, the endogenous ligands that may activate TLR4 in neurons under ischemic conditions have not been identified, and our data from BMT experiments clearly show that TLR4 expressed on hematopoietic cells, rather than endothelium or neurons, contributes to Fn-EDA-mediated stroke exacerbation. In line with these findings, we also found that purified exogenous cFn potentiated NFκB activation and inflammation in neutrophils and macrophages from Fn-EDA−/−Apoe−/− mice, but not from Fn-EDA−/− TLR4−/−Apoe−/− mice. On the basis of these in vitro and in vivo studies, we propose a mechanistic model in which infiltrating activated neutrophils interact with Fn-EDA via TLR4, and, thereby potentiate the post-ischemic inflammatory response. These signals may further amplify the inflammatory microenvironment in the infarcted region, thereby promoting additional neutrophil influx and macrophage/microglia activation, resulting in stroke exacerbation. More complex studies will be required to precisely dissect the specific role of each cell type in Fn-EDA-mediated stroke exacerbation.
Secondary infarct growth is typically observed in stroke patients following initial vessel recanalization via mechanical intervention or fibrinolysis of clot by infusing tPA. The exact mechanisms that contribute to secondary infarct growth are not precisely determined, but they most likely include both post-thrombotic and post-inflammatory processes. Therefore, there is an unmet need for effective and safe therapeutic strategies to prevent secondary thrombosis and inflammation following acute ischemic stroke. We evaluated whether specific inhibition of Fn-EDA after tMCAO will improve stroke outcome. We provide evidence that targeting Fn-EDA in Apoe−/− mice by administration of a specific anti-Fn-EDA Ig 15 minutes after reperfusion significantly improved stroke outcome. One of the limitations of this study is that it does not model human stroke caused by thromboembolic disease. Future studies are warranted to determine whether infusion of low doses of tPA in combination with anti-Fn-EDA Ig will improve stroke outcome at early and later stages following transient ischemia.
In conclusion, our findings demonstrate that Fn-EDA promotes thrombo-inflammation and plays a causative role in stroke exacerbation in the setting of hypercholesterolemia. The mechanistic insights provided by the current study suggest that monitoring plasma Fn-EDA levels may have prognostic value for patients at high risk for stroke. Targeting Fn-EDA with specific inhibitors is a promising potential approach to reduce ischemia/reperfusion brain injury associated with hypercholesterolemia.
Supplementary Material
Acknowledgments
We thank Cynthia Lynch for technical assistance with analyzing blood gases.
Funding Sources: This work was supported by National Institutes of Health grants R01HL118246 and R01HL118742 to A.K.C and P01 HL062984 to S.R.L.
Footnotes
Disclosures: None.
References
- 1.Warach S, Latour LL. Evidence of reperfusion injury, exacerbated by thrombolytic therapy, in human focal brain ischemia using a novel imaging marker of early blood-brain barrier disruption. Stroke. 2004;35:2659–2661. doi: 10.1161/01.STR.0000144051.32131.09. [DOI] [PubMed] [Google Scholar]
- 2.Aronowski J, Strong R, Grotta JC. Reperfusion injury: Demonstration of brain damage produced by reperfusion after transient focal ischemia in rats. J Cereb Blood Flow Metab. 1997;17:1048–1056. doi: 10.1097/00004647-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Reheman A, Spring CM, Kalantari J, Marshall AH, Wolberg AS, Gross PL, Weitz JI, Rand ML, Mosher DF, Freedman J, Ni H. Plasma fibronectin supports hemostasis and regulates thrombosis. J Clin Invest. 2014;124:4281–4293. doi: 10.1172/JCI74630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan MH, Sun Z, Opitz SL, Schmidt TE, Peters JH, George EL. Deletion of the alternatively spliced fibronectin eiiia domain in mice reduces atherosclerosis. Blood. 2004;104:11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- 5.Lefebvre JS, Levesque T, Picard S, Pare G, Gravel A, Flamand L, Borgeat P. Extra domain a of fibronectin primes leukotriene biosynthesis and stimulates neutrophil migration through activation of toll-like receptor 4. Arthritis Rheum. 2011;63:1527–1533. doi: 10.1002/art.30308. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan AK, Kisucka J, Cozzi MR, Walsh MT, Moretti FA, Battiston M, Mazzucato M, De Marco L, Baralle FE, Wagner DD, Muro AF. Prothrombotic effects of fibronectin isoforms containing the eda domain. Arterioscler Thromb Vasc Biol. 2008;28:296–301. doi: 10.1161/ATVBAHA.107.149146. [DOI] [PubMed] [Google Scholar]
- 7.Prakash P, Kulkarni PP, Lentz SR, Chauhan AK. Cellular fibronectin containing extra domain a promotes arterial thrombosis in mice through platelet toll-like receptor 4. Blood. 2015;125:3164–3172. doi: 10.1182/blood-2014-10-608653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan AK, Iaconcig A, Baralle FE, Muro AF. Alternative splicing of fibronectin: A mouse model demonstrates the identity of in vitro and in vivo systems and the processing autonomy of regulated exons in adult mice. Gene. 2004;324:55–63. doi: 10.1016/j.gene.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Pagani F, Zagato L, Vergani C, Casari G, Sidoli A, Baralle FE. Tissue-specific splicing pattern of fibronectin messenger rna precursor during development and aging in rat. J Cell Biol. 1991;113:1223–1229. doi: 10.1083/jcb.113.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ffrench-Constant C, Van de Water L, Dvorak HF, Hynes RO. Reappearance of an embryonic pattern of fibronectin splicing during wound healing in the adult rat. J Cell Biol. 1989;109:903–914. doi: 10.1083/jcb.109.2.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muro AF, Chauhan AK, Gajovic S, Iaconcig A, Porro F, Stanta G, Baralle FE. Regulated splicing of the fibronectin eda exon is essential for proper skin wound healing and normal lifespan. J Cell Biol. 2003;162:149–160. doi: 10.1083/jcb.200212079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takasaki I, Chobanian AV, Mamuya WS, Brecher P. Hypertension induces alternatively spliced forms of fibronectin in rat aorta. Hypertension. 1992;20:20–25. doi: 10.1161/01.hyp.20.1.20. [DOI] [PubMed] [Google Scholar]
- 13.Muro AF, Moretti FA, Moore BB, Yan M, Atrasz RG, Wilke CA, Flaherty KR, Martinez FJ, Tsui JL, Sheppard D, Baralle FE, Toews GB, White ES. An essential role for fibronectin extra type iii domain a in pulmonary fibrosis. Am J Respir Crit Care Med. 2008;177:638–645. doi: 10.1164/rccm.200708-1291OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarnagin WR, Rockey DC, Koteliansky VE, Wang SS, Bissell DM. Expression of variant fibronectins in wound healing: Cellular source and biological activity of the eiiia segment in rat hepatic fibrogenesis. J Cell Biol. 1994;127:2037–2048. doi: 10.1083/jcb.127.6.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhattacharyya S, Tamaki Z, Wang W, Hinchcliff M, Hoover P, Getsios S, White ES, Varga J. Fibronectineda promotes chronic cutaneous fibrosis through toll-like receptor signaling. Sci Transl Med. 2014;6:232ra250. doi: 10.1126/scitranslmed.3008264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kanters SD, Banga JD, Algra A, Frijns RC, Beutler JJ, Fijnheer R. Plasma levels of cellular fibronectin in diabetes. Diabetes Care. 2001;24:323–327. doi: 10.2337/diacare.24.2.323. [DOI] [PubMed] [Google Scholar]
- 17.van Keulen JK, de Kleijn DP, Nijhuis MM, Busser E, Velema E, Fijnheer R, van der Graaf Y, Moll FL, de Vries JP, Pasterkamp G. Levels of extra domain a containing fibronectin in human atherosclerotic plaques are associated with a stable plaque phenotype. Atherosclerosis. 2007;195:e83–e91. doi: 10.1016/j.atherosclerosis.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 18.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., 3rd The extra domain a of fibronectin activates toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 19.Khan MM, Gandhi C, Chauhan N, Stevens JW, Motto DG, Lentz SR, Chauhan AK. Alternatively-spliced extra domain a of fibronectin promotes acute inflammation and brain injury after cerebral ischemia in mice. Stroke. 2012;43:1376–1382. doi: 10.1161/STROKEAHA.111.635516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.del Zoppo GJ. Acute anti-inflammatory approaches to ischemic stroke. Ann N Y Acad Sci. 2010;1207:143–148. doi: 10.1111/j.1749-6632.2010.05761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes JL, Hastings RR, De la Garza MA. Sequential expression of cellular fibronectin by platelets, macrophages, and mesangial cells in proliferative glomerulonephritis. Am J Pathol. 1994;145:585–597. [PMC free article] [PubMed] [Google Scholar]
- 23.Spinelli SL, Casey AE, Pollock SJ, Gertz JM, McMillan DH, Narasipura SD, Mody NA, King MR, Maggirwar SB, Francis CW, Taubman MB, Blumberg N, Phipps RP. Platelets and megakaryocytes contain functional nuclear factor-kappab. Arterioscler Thromb Vasc Biol. 2010;30:591–598. doi: 10.1161/ATVBAHA.109.197343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan JL, Trevithick JE, Hynes RO. Retroviral expression of alternatively spliced forms of rat fibronectin. J Cell Biol. 1990;110:833–847. doi: 10.1083/jcb.110.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White ES, Baralle FE, Muro AF. New insights into form and function of fibronectin splice variants. J Pathol. 2008;216:1–14. doi: 10.1002/path.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoll G, Kleinschnitz C, Nieswandt B. Combating innate inflammation: A new paradigm for acute treatment of stroke? Ann N Y Acad Sci. 2010;1207:149–154. doi: 10.1111/j.1749-6632.2010.05730.x. [DOI] [PubMed] [Google Scholar]
- 27.Nieswandt B, Kleinschnitz C, Stoll G. Ischaemic stroke: A thrombo-inflammatory disease? J Physiol. 2011;589:4115–4123. doi: 10.1113/jphysiol.2011.212886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: Putative role for cytokines, adhesion molecules and inos in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- 30.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: Heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 31.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167:2887–2894. doi: 10.4049/jimmunol.167.5.2887. [DOI] [PubMed] [Google Scholar]
- 32.Liao YF, Gotwals PJ, Koteliansky VE, Sheppard D, Van De Water L. The eiiia segment of fibronectin is a ligand for integrins alpha 9beta 1 and alpha 4beta 1 providing a novel mechanism for regulating cell adhesion by alternative splicing. J Biol Chem. 2002;277:14467–14474. doi: 10.1074/jbc.M201100200. [DOI] [PubMed] [Google Scholar]
- 33.Peters JH, Sporn LA, Ginsberg MH, Wagner DD. Human endothelial cells synthesize, process, and secrete fibronectin molecules bearing an alternatively spliced type iii homology (ed1) Blood. 1990;75:1801–1808. [PubMed] [Google Scholar]
- 34.Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.