Abstract
Merocyanine 540-mediated photodynamic therapy (MC540-PDT) has been used in clinical trials for the purging of autologous hematopoietic stem cells grafts. When the same combinations of dye and light were applied to human peripheral blood lymphocytes, a broad range of T- and B-cell functions were impaired, prompting speculations about a potential role of MC540-PDT in the prophylaxis of graft-versus-host disease (GVHD). We here report on the effects of MC540-PDT on in vitro functions of murine lymphocytes as well as a preliminary evaluation of MC540-PDT for the prevention of GVHD in murine models of allogeneic bone marrow transplantation. Mixed lymphocyte reactions, proliferative responses to lectins, interleukin-2 and lipopolysaccharide, T-cell-mediated lysis, and NK activity were all inhibited by moderate doses of MC540-PDT. Whether MC540-PDT reduced the incidence and/or the severity of GVHD in murine models of allogeneic hematopoietic stem cell transplantation depended on the composition of the mismatched grafts and the intensity of the preparative regimen. MC540-PDT was only beneficial (i.e. reduced the incidence and/or severity of GVHD) when the spleen cell content of grafts was low and/or the radiation dose of the preparative regimen was not myeloablative, and, therefore, may have encouraged mixed chimerism.
Keywords: Photodynamic therapy, Merocyanine 540, Hematopoietic stem cell transplantation, Graft-versus-host disease, Mixed chimerism
1. Introduction
The introduction of T-cell depletion in allogeneic hematopoietic stem cell transplantation has reduced the incidence and severity of acute and chronic graft-versus-host disease (GVHD) and has encouraged a greater utilization of grafts from unrelated and mismatched donors (reviewed by 1–3). Despite these and other advances, GVHD remains a major challenge in hematopoietic stem cell transplantation, as many recipients of T-cell-depleted allogeneic grafts still experience acute and/or chronic GVHD. Furthermore, some of the gains achieved by T-cell depletion are offset by higher relapse rates (loss of graft-versus-tumor effect) as well as higher incidences of graft failures, post-transplant lymphoproliferative disorders, and prolonged immunodeficiencies [4–9]. These unintended consequences of T-cell depletion have been explained by inadequate depletions of donor cells that cause GVHD, inadequate depletions of donor cells that inhibit engraftment and immune reconstitution, excessive depletions of hematopoietic stem and progenitor cells, excessive depletions of cells that support hematopoietic engraftment, excessive depletions of cells that provide protective immunity after the transplant, and/or excessive depletions of cells that are responsible for graft-versus-tumor effects. This situation has motivated many transplant centers to explore alternative methods of T-cell depletion. Reports of successful applications of phototherapy (light therapy) and photochemotherapy (light therapy in the presence of photosensitizing agents) in the treatment of GVHD and autoimmune diseases, as well as the prevention of allosensitization and graft rejection [10–15] have prompted investigations into possible roles of phototherapy and photochemotherapy in the prevention of GVHD [16–18].
Immunological studies performed as part of a phase I/II clinical trial of Merocyanine 540-mediated photodynamic therapy (MC540-mediated PDT) for the extracorporeal purging of autologous bone marrow grafts from leukemia and lymphoma patients [19] showed that combinations of dye and light that did not prevent hematopoietic reconstitution after marrow-ablative therapy inhibited a broad range of human B- and T-cell functions [20], prompting speculations about a potential role of MC540-mediated PDT in the prophylaxis of GVHD in allogeneic hematopoietic stem cell transplantation. We have now extended these investigations to murine lymphocytes and to several murine models of allogeneic bone marrow transplantation.
2. Materials and methods
2.1. Materials
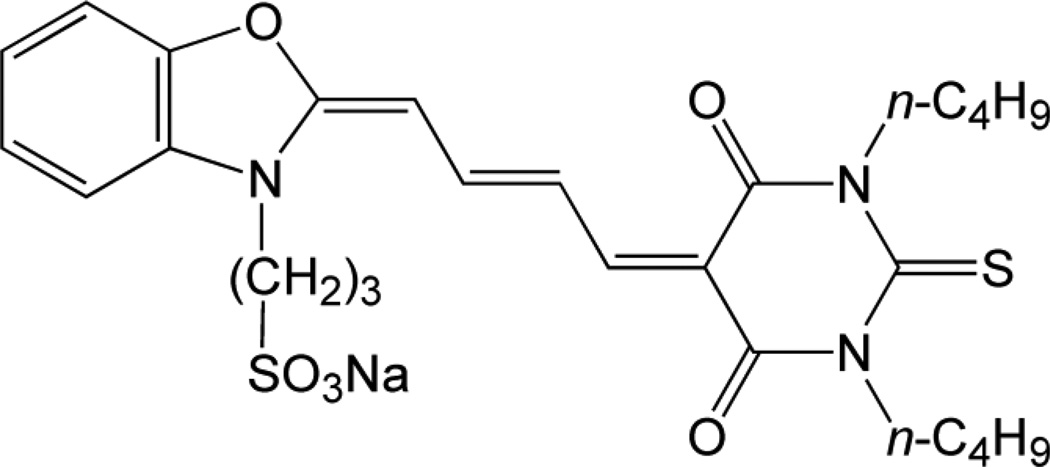
MC540 (5-[3-sulfopropyl-2(3H)-benzoxazolylidine)-2-butenylidene]-1,2-dibutyl-2-thiobarbituric acid) (Fig. 1) was from Kodak (Rochester, NY), fetal bovine serum from Irvine Scientific (Santa Ana, CA), N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES) from Research Organics (Cleveland, OH), Tris(hydroxymethyl)-aminomethane (Tris) from Boehringer Mannheim Biochemicals (Indianapolis, IN), anti-Thy 1.2 monoclonal antibody and Low-Tox-M® rabbit complement from Cedarlane (Hornby, Ontario, Canada), phytohemagglutinin HA 15 (PHA) from Wellcome Diagnostics (Dartford, England), lipopolysaccharide (LPS; E. coli) from Difco (Detroit, MI), recombinant human interleukin-2 (IL-2) from Amgen Biologicals, (Thousand Oaks, CA), tritiated thymidine (thymidine [methyl-3H]-; 2 Ci mmol−1) from New England Nuclear (Boston, MA), and 51Cr (sodium chromate; 250–500 mCi mg−1 Cr) from Amersham (Arlington Heights, IL). All other reagents were from Sigma Chemical Co. (St. Louis, MO).
Fig. 1.
Structure of Merocyanine 540
2.2. Animals and cells
Female B6D2F1/J (C57BL/6J × DBA/2J) (H-2b/d), C57BL/6J (H-2b), DBA/2J (H-2d), LP/J (H-2bc), AKR/J (H-2k), and B10.BR/SgSnJ (H-2k) mice (6–9 weeks old) were obtained from Jackson Laboratories (Bar Harbor, ME). Immunosuppressed animals were housed in autoclaved polycarbonate cages (≤5 animals per cage) on laminar flow racks in a facility that was fully accredited by the American Association for the Accreditation of Laboratory Animal Care. Autoclaved chow and acidified sterile water were provided ad libitum. YAC-1 lymphoma cells (H-2a) (ATCC TIB 160) and P815 mastocytoma cells (H-2d) (ATCC TIB 64) were from the American Type Culture Collection (Rockville, MD). Spleen cells were prepared as described by Mishell and Shiigi [21] and depleted of red cells by briefly suspending them in a hypotonic Tris-NH4Cl buffer. In vitro proliferation experiments were typically conducted with pooled spleen cells from 2–4 animals.
2.3. Transplantation experiments
Unless indicated otherwise, recipient mice received 11 Gy (single dose) of total body irradiation from an attenuated 137Cs source (Shepard Mark I; 89.6 R min−1; JL Shepard, San Fernando, CA) followed by the intravenous injection of spleen cells (2 × 107 or 5 × 107) or a mixture of bone marrow cells (107) and spleen cells (5 × 105 to 5 × 107). It is common practice to use spleen cells or mixtures of bone marrow and spleen cells in murine models of allogeneic bone marrow transplantation, as grafts consisting of bone marrow cells only would not elicit a significant graft-versus-host response [22]. However, there is no consensus as to which ratio of bone marrow-to-spleen cells most closely mimics a clinical allograft. We, therefore, included a series of experiments that explored how MC540-mediated PDT affected GVHD caused by allografts with different bone marrow-to-spleen cell ratios. The total number of bone marrow cells was chosen to insure hematopoietic reconstitution after marrow-ablative total body irradiation (TBI) even when grafts had been subjected to MC540-PDT prior to infusion into hosts [23]. The standard number of spleen cells was chosen to provoke robust (often lethal) graft-versus-host disease. Both the numbers of bone marrow cells and the numbers of spleen cell used for this study were identical or similar to the numbers used by other investigators [22].
All grafts were injected in 0.5 ml HEPES-buffered (10 mM, pH 7.4) alpha-medium supplemented with 5% fetal bovine serum. Unless indicated otherwise, treatment groups and control groups consisted of 10 animals each. Where indicated, allogeneic grafts were treated either with anti-Thy 1.2 antibody and complement or with MC540 and light prior to injection. Radiation controls received an infusion of HEPES-buffered alpha-medium containing 5% fetal bovine serum but no cells. Animals were monitored for ≥100 days for survival and obvious signs of GVHD (loss of body weight, dermatitis on tails and ears, chronic diarrhea). All animal experiments were conducted under protocols approved by the Institutional Animal Care and Use Committee.
2.4 T-cell depletion
T-cell depletions by complement-mediated immune lysis were performed as described by Korngold and Sprent [24]. In brief, mixtures of bone marrow and spleen cells were suspended at a density of 2 × 107 cells ml−1 in HEPES-buffered alpha-medium supplemented with 5% fetal bovine serum and anti-Thy 1.2 monoclonal antibody (diluted 1:500) and incubated on ice for 60 min. The cells were pelleted by low-speed centrifugation and resuspended in a 1:10 dilution of rabbit complement, incubated at 37 °C for 60 min, washed once, and then resuspended in the original volume of HEPES-buffered alpha-medium supplemented with 5% fetal bovine serum.
2.5 MC540-sensitized reactions
The MC540-sensitized photoirradiation of bone marrow cells, spleen cells, or mixtures of marrow and spleen cells was performed as described previously [20, 23, 25]. In brief, cells were suspended at a density of 107 cells ml−1 in HEPES-buffered alpha-medium supplemented with 12% fetal bovine serum. Dye was added from a 1-mg ml−1 stock solution in 50% ethanol to a final concentration of 15 µg ml−1. Clear polystyrene tubes (15 ml; Corning Glass Works, Corning, NY) containing the cell suspension were mounted on a Plexiglass disk that rotated at approximately 30 rpm between two banks of tubular fluorescent lights (5 lights per bank; F20T12.CW; General Electric, Cleveland, OH) and irradiated for up to 90 min. The fluence rate at the sample site was 35 W m−2 as determined by a United Detector Technology (Hawthorne, CA) power meter model S351A equipped with detector model 262 and radiometric filter number 1158. The reaction was terminated by transferring the tubes to the dark and by washing the cells once with HEPES-buffered alpha-medium supplemented with 5% fetal bovine serum. Cells that were exposed to dye in the dark, to light in the absence of dye, or to neither dye nor light served as controls. The highest doses of PDT used in this study were identical to the ones used previously for the ex vivo purging of murine bone marrow grafts contaminated with tumor cells [23, 25, 26]. They would have reduced the concentration of leukemia and lymphoma cells by ≥4 orders of magnitude while preserving enough pluripotent hematopoietic stem cells to insure hematopoietic reconstitution after marrow-ablative TBI.
2.6. Proliferation assays
MC540-treated or non-treated control cells were suspended at a density of 5 × 106 cells ml−1 in alpha-medium supplemented with 10% heat-inactivated fetal bovine serum and distributed in 0.1-ml aliquots into flat-bottom 96-well plates (Flow Laboratories, McLean, VA). Concanavalin A (ConA), PHA, LPS and IL-2 were dissolved in the same medium and added to the cells to final concentrations of 2.5 µg ml−1, 25 µg ml−1, 5 µg ml−1, and 25 U ml−1, respectively (pilot experiments had indicated that these concentrations were optimal for our application) and a final volume of 0.2 ml. Control wells received 0.1 ml of mitogen-free medium. After 48 hours at 37 °C in a humidified atmosphere of 5% CO2 in air, the cells in each well were pulsed with 50 µl of a 20 µC ml−1 stock solution of 3H-thymidine in alpha-medium supplemented with 10% fetal bovine serum. Cells were harvested 12 hours later and evaluated for 3H-thymidine incorporation.
2.7. Mixed lymphocyte reactions
Non-treated or photoinactivated spleen cells (5 × 106 ml−1) from C57BL/6J mice (responder cells) were suspended in Dulbecco's modified Eagle's medium (Dulbecco's medium) supplemented with sodium bicarbonate (44 mM), glucose (25 mM), sodium pyruvate (1 mM), L- arginine (0.55 mM), L-glutamine (2 mM), L-asparagine (0.24 mM), folic acid (13.6 µM), 2-mercaptoethanol (50 µM), and, where indicated, IL-2 (50 U ml−1) (all concentrations represent final concentrations), and mixed with an equal volume of irradiated (30 Gy) spleen cells (5 × 106 cells ml−1) from DBA/2J mice (stimulator cells) in Dulbecco's medium. The cells were subsequently aliquoted into 24-well plates (Costar, Cambridge, MA; 2 ml per well) and incubated at 37 °C in a humidified atmosphere of 5% CO2 in air. After 4 days of culture, cells were harvested and dispensed into flat bottom 96-well plates (0.2 ml per well), and pulsed with 3H-thymidine (20µCi in 50 µl of culture medium). Twelve hours later, cells were harvested and analyzed for 3H-thymidine incorporation. In some experiments, responder spleen cells were first stimulated (i.e. co-cultured with stimulator cells) for 4 days and subsequently exposed to MC540 and graded doses of light. Data are shown for one experiment that was representative of 3 replicate experiments.
2.8 Natural killer cells
Natural killer (NK) cells were assayed as described by Mishell and Shiigi [21] and Lewis et al. [27] using YAC-1 lymphoma cells as targets. YAC-1 cells (2.5 × 106 to 5 × 106) were pelleted and labeled with 51Cr (0.2 mCi) for 90 min at 37 °C in a humidified atmosphere of 5% CO2 in air, washed twice with HEPES-buffered alpha-medium (supplemented with 5% fetal bovine serum), and resuspended at a density of 5 × 104 cells ml−1 in Dulbecco's medium supplemented as indicated in the protocol for mixed lymphocyte reactions. One-hundred µl aliquots of the effector cell suspension (non-treated or photoinactivated splenocytes from B6D2F1/J mice) were dispensed into V-bottom 96-well microtiter plates (Flow Laboratories, McLean, VA) and mixed with 100 µl of target cell suspension to final ratios of 200:1, 100:1 and 50:1, respectively. Plates were centrifuged at 200 × g for 3 min, incubated at 37 °C for 3 hours, and then again centrifuged at 500 × g for 5 min. Supernatant (150 µl) was harvested from each well and analyzed for 51Cr release. Specific lysis (%) was defined as
In selected experiments, splenocytes were incubated with IL-2 (50 U ml−1) for 18 hours immediately preceding or following MC540-mediated PDT.
2.9. T-cell-mediated lysis
C57BL/6J spleen cells (effector cells) were co-cultured with irradiated (30 Gy) DBA/2J spleen cells (stimulator cells) in the presence or absence of IL-2 (50 U ml−1) in 24 well plates as described above for mixed lymphocyte reactions. Cells were harvested on day 5, and T-cell-mediated lysis was quantified by a 3-hour 51Cr release assay (analogous to the one described for the assay of NK cell activity) using P815 mastocytoma cells as targets. MC540-mediated PDT was applied either to effector cells immediately before initiation of the co-cultures or to mixtures of effector and stimulator cells at the completion of the co-culture period. Data are shown for one experiment that was representative of 5 replicate experiments.
2.10. Statistical analyses
The Prism software package (GraphPad Software, La Jolla, CA) was used for statistical analyses. Survival curves were compared using the log-rank (Mantel-Cox) and the Gehan-Breslow-Wilcoxon tests. The log-rank test gives all deaths equal weight and works best when the ratio of hazard functions is the same at all time points. The Gehan-Breslow-Wilcoxon test gives more weight to deaths at early time points, does not require a constant hazard ratio, but does require that one group consistently has a higher risk than the other. Analysis of variance (ANOVA) with the Tukey post hoc test was used to compare body weights. Seventy-six percent of data sets were normally distributed as judged by the Kolmogorov-Smirnov, the D’Agostino & Pearson (omnibus), and the Shapiro-Wilk tests. Two-tailed Student’s t-tests were used for the analysis of in vitro proliferation and cytotoxicity assays.
3. Results
3.1. Effect of MC540-mediated PDT on in vitro lymphocyte functions
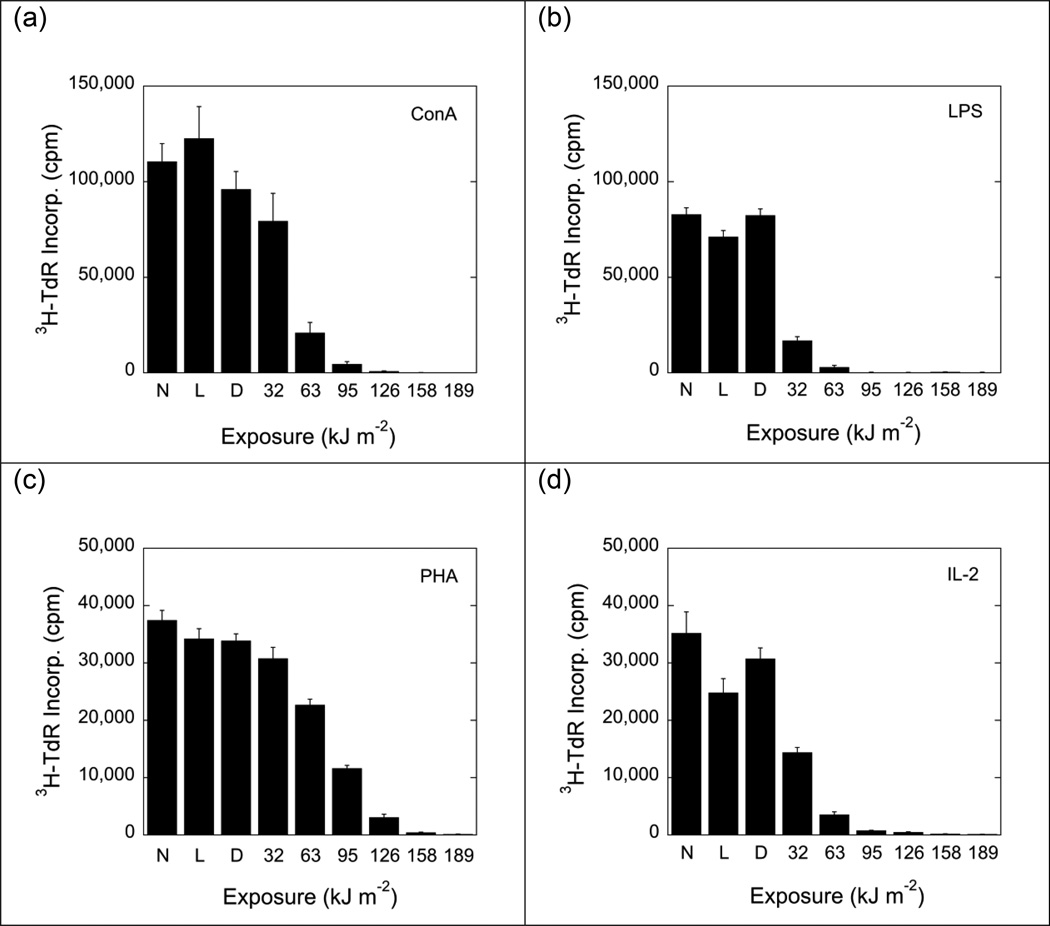
To determine the effect of MC540-mediated PDT on T- and B-lymphocyte functions, murine spleen cells were exposed to a fixed concentration of dye and graded doses of white light. Exposure to MC540 (15 µg ml−1) and white light reduced proliferative responses to ConA (mature/immature T-cell mitogen), PHA (mature T-cell mitogen), LPS (B-cell mitogen) and IL-2 in a dose-dependent fashion (Fig. 2), indicating that immature T-cells, mature T-cells, NK cells, and B-cells were susceptible to MC540-mediated PDT. The proliferative response to PHA was slightly less sensitive to MC540-mediated PDT than the proliferative responses to ConA, LPS and IL-2. At the highest dose of MC540-mediated PDT tested (dye: 15 µg ml−1; fluence: 189 kJ m−2), all proliferative responses were completely suppressed. Exposure of cells to dye in the dark or to light in the absence of dye had little or no effect, indicating that the observed inhibition of proliferation was indeed the result of a photosensitized reaction (Fig. 2).
Fig. 2.
Proliferative responses (incorporation of 3H-thymidine) of non-treated and photoinactivated B6D2F1/J spleen cells to Concanavalin A (ConA; 2.5 µg ml−1), phytohemagglutinin (PHA; 15 µg ml−1), lipopolysaccharide (LPS; 5 µg ml−1), and interleukin-2 (IL-2; 25 U ml−1). Data represent means ± standard errors (SE) of 9 determinations derived from 3 independent experiments. Background activity (incorporation of 3H-thymidine by non-stimulated cells) was 3500 to 4500 cpm. N: Cells exposed to neither dye nor light. L: Cells exposed to light (189 kJ m−2) but no dye. D: Cells exposed to dye (15 µg ml−1) but no light. Suppressions of proliferative responses were statistically significant (p<0.05; 2-tailed Student's t-test) for cells exposed to MC540 and light fluences of ≥63 kJ m−2 (panels a and c) and for cells exposed to MC540 and light fluences of ≥32 kJ m−2) (panels b and d).
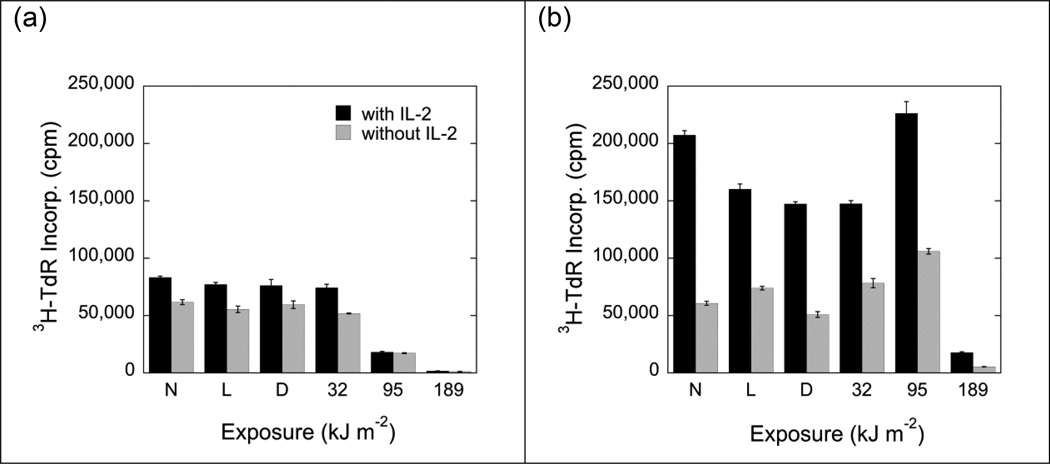
Mixed lymphocyte reactions were also sensitive to MC540-mediated PDT (Fig. 3), but the minimal doses of MC540-mediated PDT required to achieve partial or complete inhibition of mixed lymphocyte reactions were higher than the doses required to inhibit proliferative responses to lectins, LPS, or IL-2. If responder cells were first exposed to dye and light and subsequently co-cultured with stimulator cells, a fluence of 95 kJ m−2 was sufficient to achieve a significant inhibition of 3H-thymidine incorporation (Fig. 3a). By contrast, if responder cells were first co-cultured with stimulator cells and then exposed to MC540 (15 µg ml−1) and light, fluences in excess of 95 kJ m−2 were required to suppress the incorporation of 3H-thymidine (Fig. 3b). Responder cells that had been co-cultured with stimulator cells first and subsequently exposed to dye (15 µg ml−1) and a moderate dose (95 kJ m−2) of light incorporated slightly more 3H-thymidine than cells in any one of the three control groups (no dye, no light; dye, no light; light, no dye) or cells that had been exposed to dye and a low dose of light (32 kJ m−2) (Fig. 3b). These stimulatory effects of low doses of MC540-mediated PDT were small but reproducible. They were statistically significant (p<0.05) when comparisons were made between cells than had been exposed to fluences of 32 kJ m−2 and 95 kJ m−2, respectively, but no dye. The stimulatory effects were reminiscent of the small increases in plating efficiency that are commonly encountered when cytotoxic effects of MC540-PDT are quantified by in vitro clonal assay at the low end of the dose-response curve. It is possible that such minor stimulatory effects are the result of sub-lethal doses of reactive oxygen species (generated by MC540-PDT) that affect signal transduction pathways. A test of this hypothesis was beyond the scope of this study.
Fig. 3.
Proliferative responses of non-treated and photoinactivated C57BL/6J (H-2b) spleen cells to irradiated (30 Gy) DBA/2J (H-2d) spleen cells. Responder cells were either exposed to MC540-PDT and subsequently co-cultured with stimulator cells (panel a) or they were first co-cultured with stimulator cells and subsequently exposed to MC540-PDT (panel b). Black bars indicate the responses of cells that had been stimulated in the presence of IL-2 (50 U ml−1). Gray bars indicate the responses of cells stimulated in the absence of IL-2. Data represent means ± SE of 3 determinations derived from one representative experiment. Background activity (incorporation of 3H-thymidine by non-stimulated, non-photosensitized C57BL/6J spleen cells) was ≤4000 cpm. N: Cells exposed to neither dye nor light. L: Cells exposed to light (189 kJ m−2) but no dye. D: Cells exposed to dye (15 µg ml−1) but no light. Suppressions of proliferative responses were statistically significant (p<0.01; 2-tailed Student's t-test) for cells exposed to MC540 and light fluences of ≥95 kJ m−2 (panel a) and for cells exposed to MC540 and light fluences of 189 kJ m−2) (panel b).
Exposure of responder cells to dye in the dark or to light (189 kJ m−2) in the absence of dye had little or no effect, indicating that the inhibition of mixed lymphocyte reactions was indeed the result of a photosensitized reaction (Fig. 3).
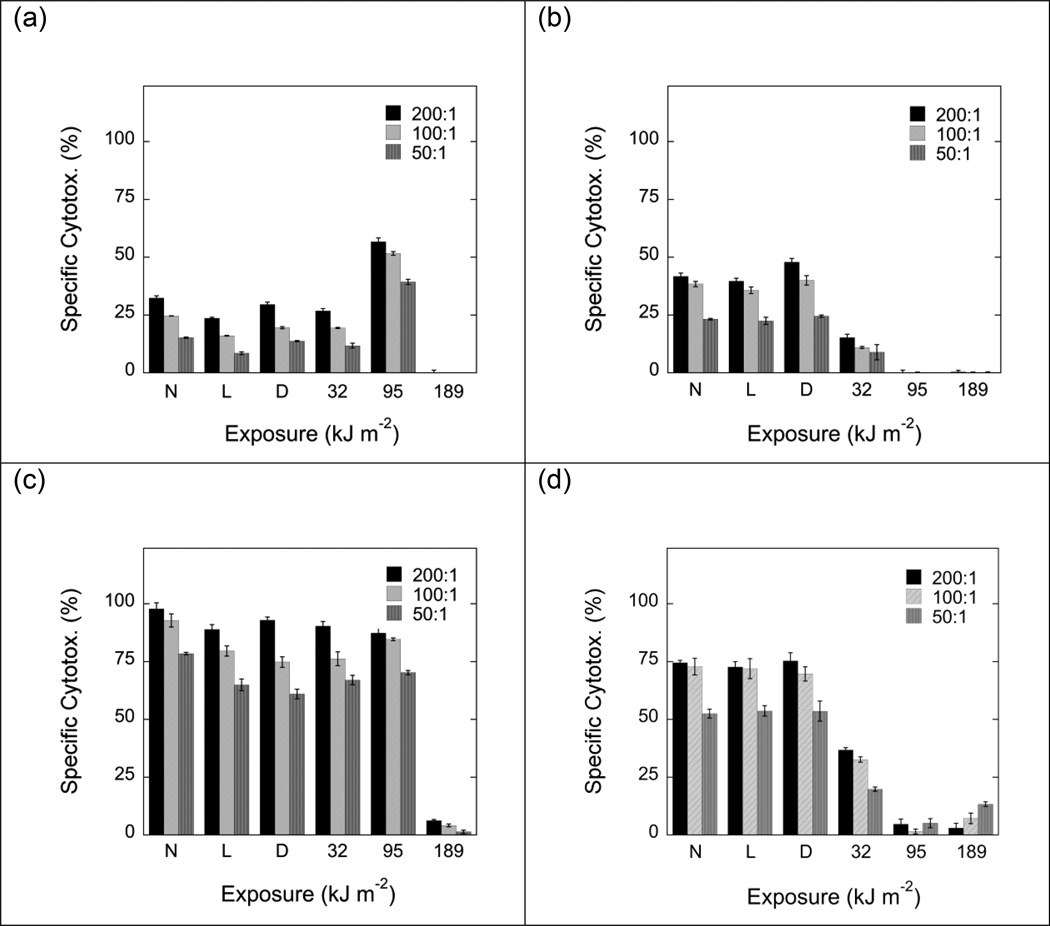
NK activity was markedly reduced after exposure to MC540 (15 µg ml−1) and light fluences as low as 32 kJ m−2 (Fig. 4). However, some residual NK activity remained detectable even after exposure to fluences as high as 189 kJ m−2. When cells were incubated with IL-2 before or after MC540-mediated PDT (Fig. 4b, c), NK activities were higher in both photoinactivated and non-treated samples. However, the minimum dose of MC540-mediated PDT required to cause a significant suppression of NK activity remained unchanged. Exposure to dye in the dark or to light in the absence of dye had little or no effect on NK activity (Fig. 4).
Fig. 4.
Natural killer (NK) cell activities of non-treated and photoinactivated B6D2F1/J (H-2b/d) spleen cells against YAC-1 (H-2a) target cells. Panel a shows data obtained with cells that had not been stimulated with IL-2. Panels b and c show data obtained with cells that had been stimulated with IL-2 (50 U ml−1) before (b) or after (c) MC540-mediated PDT. Effector:target cell ratios were 200:1 (black bars), 100:1 (light grey bars) and 50:1 (dark grey bars), respectively. Data represent means ± SE of ≥9 determinations derived from ≥3 independent experiments. N: Cells exposed to neither dye nor light. L: Cells exposed to light (189 kJ m−2) but no dye. D: Cells exposed to dye (15 µg ml−1) but no light. Suppressions of specific cytotoxicity were statistically significant (p<0.01; 2-tailed Student's t-test) for cells exposed to MC540 and light fluences of ≥32 kJ m−2) (panels b and c).
Exposure to MC540 and light also suppressed the T-cell-mediated lysis of MHC-incompatible cells (Fig. 5). When C57BL/6J (H-2b) responder cells were first co-cultured with DBA/2J (H-2d) stimulator cells in the absence (Fig. 5a) or presence (Fig. 5c) of IL-2 and subsequently exposed to MC540-PDT, light doses in excess of 95 kJ m−2 were required to inhibit T-cell-mediated lysis (Fig. 5a, c). When, however, C57BL/6J responder cells were first exposed to MC540-mediated PDT and subsequently co-cultered with DBA/2J stimulator cells in the absence (Fig. 5b) or presence (Fig. 5d) of IL-2, light doses as a low as 32 kJm−2 were sufficient to achieve a comparable suppression of T-cell-mediated lysis (Fig. 5b, d). Exposure to a standard concentration (15 µg ml−1) of dye and a moderate dose of light (95 kJ m−2) in the absence of IL-2 caused a moderate enhancement of cytotoxic activity (Fig. 5a), which was reminiscent of the previously described stimulatory effect of moderate doses of MC540-mediated PDT on mixed lymphocyte cultures (Fig. 3b). As in all previous experiments, exposure to dye in the dark or to light in the absence of dye had little or no effect on T cell-mediated cell lysis (Fig. 5).
Fig. 5.
T cell-mediated lysis of MHC-incompatible cells. C57BL/6J) (H-2b) responder cells were either co-cultured with irradiated (30 Gy) DBA/2J (H-2d) stimulator cells in the absence (panel a) or presence (panel c) of IL-2 (50 U ml−1) and subsequently exposed to MC540-PDT, or they were first exposed to MC540-PDT and subsequently co-cultured with stimulator cells in the absence (panel b) or presence (panel d) of IL-2. P815 mastocytoma cells (H-2d) served as targets. Effector:target cell ratios were 200:1 (black bars), 100:1 (light grey bars) and 50:1 (dark grey bars), respectively. Data represent means ± SE of 3 determinations derived from 1 representative experiment. N: Cells exposed to neither dye nor light. L: Cells exposed to light (189 kJ m−2) but no dye. D: Cells exposed to dye (15 µg ml−1) but no light. Suppressions of specific cytotoxicities were statistically significant (p<0.01; 2-tailed Student's t-test) for cells exposed to MC540 and light fluences of 189 kJ m−2 (panels a and c) and for cells exposed to MC540 and light fluences of ≥95 kJ m−2 (panels b and d).
Cell yields were always low when co-cultures were established with heavily photosensitized cells. In co-cultures set up with cells that had been exposed to a standard dose of MC 540 (15 µg ml−1) and a light dose of 189 kJ m−2, cell yields at the end of the co-culture period typically amounted to ≤20% of the initial inoculum. About 50% of cells were trypan blue-positive immediately following MC540-mediated PDT. The percentage of trypan blue positive cells rose to ≥80% when trypan blue exclusion assays were repeated 1 or 2 days later.
3.2. MC540-mediated PDT for the prophylaxis of GVHD in murine models of allogeneic bone marrow transplantation
The potential utility of MC540-mediated PDT for the prophylaxis of GVHD was evaluated in several murine models of allogeneic bone marrow transplantation in which donors and recipients were mismatched at minor or major histocompatibility antigens. Since murine bone marrow cells contain too few lymphocytes to cause lethal GVHD in mismatched recipients, we used - as is common practice - mixtures of bone marrow and spleen cells as allografts in most of our experiments.
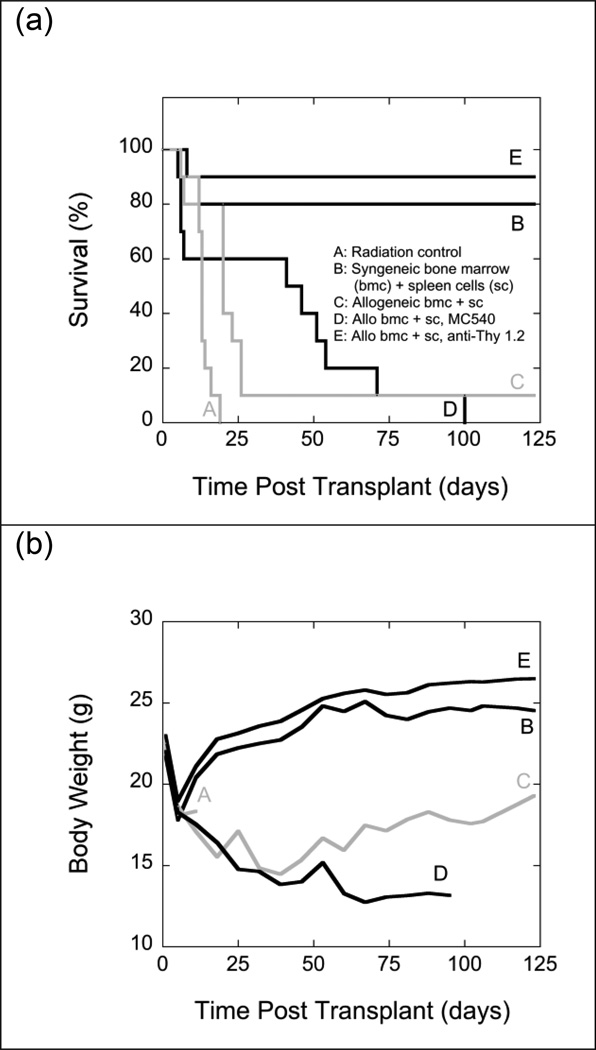
When mixtures of 107 bone marrow cells and 5 × 107 spleen cells from C57BL/6J (H-2b) mice were injected into irradiated (11 Gy) LP/J (H-2bc) mice, all recipients developed typical signs of severe GVHD: dermatitis, chronic diarrhea, alopecia, and weight loss. Ninety percent of recipients died during the 17-week observation period. As Fig. 6 shows, all animals lost about 20% of their body weights during the week immediately following TBI regardless of the origin of the graft. Recipients of syngeneic grafts subsequently returned to normal body weights relatively quickly. By contrast, recipients of allogeneic grafts that developed GVHD did not regain normal body weights for the duration of the experiment.
Fig. 6.
Survival (panel a) and mean body weights (panel b) of groups of 10 irradiated (11 Gy) LP/J (H-2bc) mice that were transplanted with mixtures of 107 bone marrow cells and 5 × 107 spleen cells from syngeneic LP/J (B) or allogeneic C57BL/6J (H-2b) (C, D, E) mice. Grafts transplanted into group D were exposed to MC540 (15 µg ml−1) and white light (35 W m−2) for 90 min (fluence: 189 kJ m−2) prior to infusion. Grafts transplanted into group E were treated with anti-Thy 1.2 antibody and complement. Radiation controls (A) received infusions of cell-free medium. Differences between the survival curves of groups C and D and B and E were statistically not significant (p>0.05). The difference between groups A and D was statistically significant (p<0.05) by the log-rank test but not significant (p>0.05) by the Gehan-Breslow-Wilcoxon test. All other differences between survival curves were statistically significant (p<0.05) by both tests. Differences in body weights for groups B and E between days 18 and 88 post TBI were statistically not significant (p>0.05). Differences between the body weights of all other groups were statistically significant (p<0.05).
Treating allogeneic marrow grafts with MC540 (15 µg ml−1) and light (189 kJ m−2) prior to infusion improved median survival times but had no beneficial effect on long-term survival (Fig. 6). In this particular case, the statistical analysis of early survival was complicated by the fact that the survival curves of interest crossed each other twice. Comparisons of mean body weights suggested that GVHD in recipients of MC540-treated grafts was more severe than in recipients of non-treated grafts. However, the comparison of body weights was mostly based on the body weights of very small numbers of survivors. In contrast to MC540-mediated PDT, treatment with anti-Thy 1.2 antibody and complement was highly effective in preventing GVHD as indicated by high (90%) survival rates and a rapid recovery of body weights (Fig. 6).
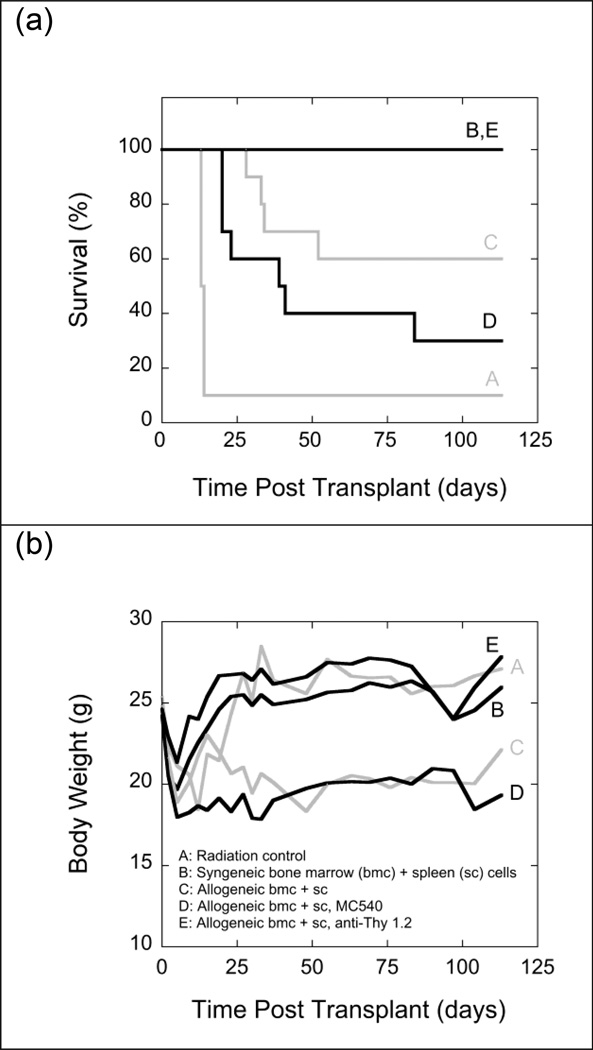
The experiment shown in Fig. 6 was repeated using a slightly reduced (10.75 Gy instead of 11 Gy) dose of TBI. With this less intensive conditioning regimen, survival of radiation controls increased from 0% to 30%, indicating that 10.75 Gy of TBI was not marrow-ablative in this mouse strain (Fig. 7). Long-term (4-month) survival of recipients of non-manipulated allogeneic grafts increased from 10% to 50%, and long-term survival of recipients of MC540-treated allogeneic grafts increased from 0% to 80%. Under this particular set of experimental conditions, MC540-PDT had a beneficial effect on survival (increase from 50% to 80%) but the difference did not reach statistical significance (p≥0.09). Comparisons of body weights showed no difference between recipients of non-manipulated allografts and recipients of MC540-treated allografts (Fig. 7).
Fig. 7.
Survival (panel a) and mean body weights (panel b) of groups of 10 irradiated (10.75 Gy) LP/J (H-2bc) mice that were transplanted with mixtures of 107 bone marrow cells and 5 × 107 spleen cells from syngeneic LP/J (B) or allogeneic C57BL/6J (H-2b) (C, D, E) mice. Grafts transplanted into group D were exposed to MC540 (15 µg ml−1) and white light (35 W m−2) for 90 min (fluence: 189 kJ m−2) prior to infusion. Grafts transplanted into group E were treated with anti-Thy 1.2 antibody and complement. Radiation controls (A) received infusions of cell-free medium. Differences between the survival curves of groups B and D, B and E, C and D, and D and E were statistically not significant (p>0.05). The difference between groups A and C was statistically significant by the the Gehan-Breslow-Wilcoxon test (p<0.05) but statistically not significant by the log-rank test (p>0.05). All other differences were significant (p<0.05) by both tests. Differences in body weights between days 20 and 88 post TBI were statistically not significant (p>0.05) between groups B and E and C and E. Differences between the mean body weights of all other groups were statistically significant (p<0.05). Body weights for radiation controls were not monitored past day 17 post TBI.
Results similar to the ones shown in Fig. 6 were obtained when mixtures of bone marrow cells (107) and spleen cells (5 × 107) from B10.BR/SgSNJ (H-2k) mice were injected into irradiated (11 Gy) AKR/J (H-2k) mice (Fig. 8). All recipients of MC540-treated allografts developed signs of severe GVHD. Survival among recipients of MC540-treated allografts was worse than among recipients of non-treated grafts, but the difference was not statistically significant. Comparisons of body weights showed no statistically significant differences. By contrast, treatment of the same allografts with anti-Thy 1.2-antibody and complement reliably prevented GVHD as indicated by a 100% survival rate and a rapid recovery of body weights that matched that seen in recipients of syngeneic grafts (Fig. 8).
Fig. 8.
Survival (panel a) and mean body weights (panel b) of groups of 10 irradiated (11 Gy) AKR/J (H-2k) mice that were transplanted with mixtures of 107 bone marrow cells and 5 × 107 spleen cells from syngeneic AKR/J (B) or allogeneic B10.BR/SgSnJ (H-2k) (C, D, E) mice. Grafts transplanted into group D were exposed to MC540 (15 µg ml−1) and white light (35 W m−2) for 90 min (fluence: 189 kJ m−2) prior to infusion. Grafts transplanted into group E were treated with anti-Thy 1.2 antibody and complement. Radiation controls (A) received infusions of cell-free medium. Differences between the survival curves of groups B and E and C and D were statistically not significant (p>0.05). Differences between all other survival curves were significant (p<0.05). Differences in body weights between days 23 and 90 post TBI were statistically not significant (p>0.05) between groups A and B, A and E, and C and D. Differences between the mean body weights of all other groups were statistically significant (p<0.05).
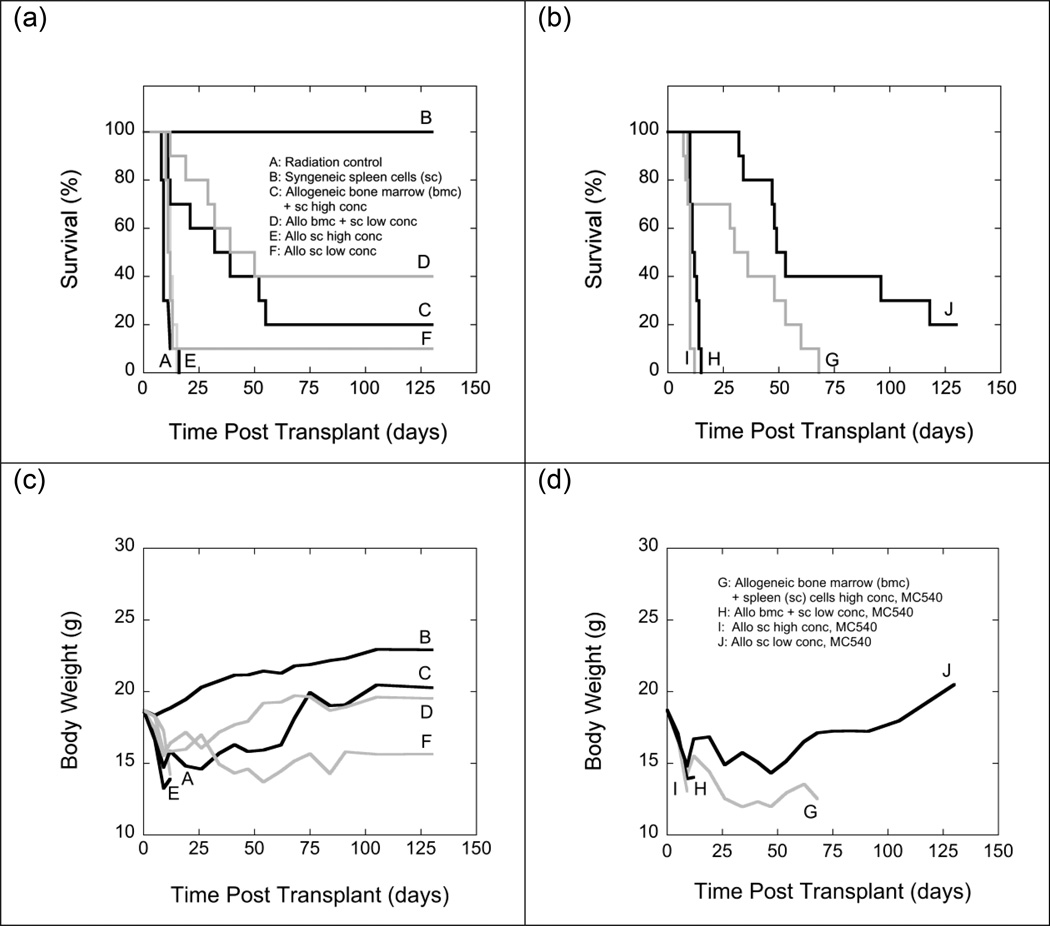
Experiments shown in Fig. 9 explored how MC540-PDT affected GVHD caused by allografts with different bone marrow-to-spleen cell ratios. For the first set of experiments (Fig. 9), donors and recipients were haplotype mismatched at the major histocompatibility complex. C57BL/6 (H-2b) mice served as donors and B6D2F1/J (H-2b/d) mice as recipients. If the allografts contained 107 bone marrow cells and a large number (5 × 107) of spleen cells, only 20% of recipients survived for the duration of the experiment. When the number of spleen cells was reduced to 2 × 107, 40% of recipients survived, but the improved survival was statistically not significant. Body weights were similar in long-term survivors of both groups, suggesting similar degrees of GVHD (Fig. 9). When grafts consisted exclusively of spleen cells, all recipients of large grafts (5 × 107 cells) died within less than 3 weeks of TBI with obvious signs of GVHD. When the graft size was reduced to 2 × 107 spleen cells, one single animal (10%) survived for the duration of the experiment but showed no weight gain during the post-transplant period (indicative of persistent GVHD).
Fig. 9.
Survival (panels a and b) and mean body weights (panels c and d) of groups of 10 irradiated (11 Gy) B6D2F1/J (H-2b/d) mice that were transplanted with spleen cells (2 × 107) from syngeneic B6D2F1/J mice (B), 5 × 107 (E, I) or 2 × 107 (F, J) spleen cells from haplotype mismatched C57BL6/J (H-2b) mice, or mixtures of 107 bone marrow cells and 5 × 107 (C, G) or 2 × 107 (D, H) spleen cells from haplotype mismatched C57BL6/J (H-2b) mice. Grafts injected into groups G, H, I and J were exposed to MC540 (15 µg ml−1) and white light (35 W m−2) for 90 min (fluence: 189 kJ m−2) prior to infusion. Radiation controls (A) received infusions of cell-free medium. Because of the large size of this experiment, groups A-F (panels a and c) and groups G-J (panels b and d) had to be processed on different days. Differences between the survival curves of groups A and E, A and I, C and D, C and G, C and J, D and G, D and J, E and I, F and G, and F and H were statistically not significant (p>0.05). Differences between groups C and F, E and F, and E and H were statistically significant by the Gehan-Breslow-Wilcoxon test (p<0.05) test but not by the log-rank test (p>0.05). The difference between groups G and H was significant by the log-rank test (p<0.05) but not by the Gehan-Breslow-Wilcoxon test (p>0.05). Differences between all other survival curves were statistically significant (p<0.05). Differences in body weights between days 19 and 91 post TBI were statistically not significant (p>0.05) between groups C and D, C and J, F and J. Differences between the body weights of all other groups were statistically significant (p<0.05).
Exposing mixtures of bone marrow and spleen cells to MC540-PDT prior to infusion did not result in any long-term survivors. Recipients of MC540-treated allografts containing low numbers of spleen cells showed shorter survival times than recipients of MC540-treated allografts containing high numbers of spleen cells (Fig. 9).
When grafts consisted exclusively of spleen cells, recipients of large grafts did not benefit from MC540-PDT. All animals died within two weeks with obvious signs of GVHD. However, MC540-PDT significantly improved survival among recipients of small spleen cell grafts. Furthermore, body weights of long-term survivors of MC540-treated small spleen cell transplants were recovering steadily and approaching normal body weights towards the end of the observation period, suggesting relatively mild GVHD or no GVHD in long-term survivors (Fig. 9).
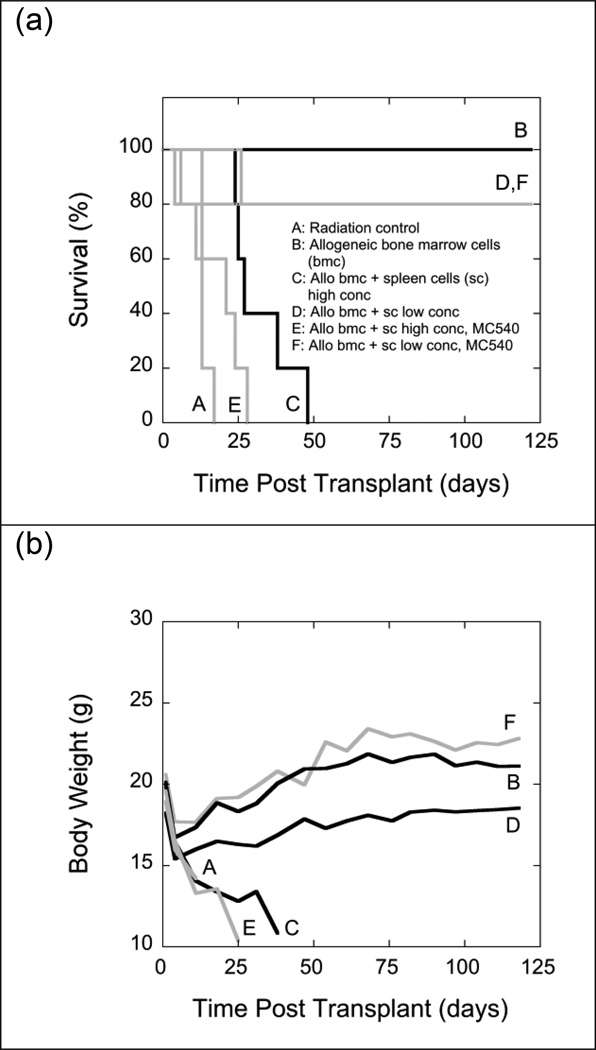
In a second set of experiments, irradiated LP/J (H-2bc) mice received allografts of 107 bone marrow cells from C57BL/6J (H-2b) donor mice or allografts consisting of mixtures of a fixed number (107) of C57BL/6J bone marrow cells and either high (5 × 107) or low (5 × 105) numbers of C57BL/6J spleen cells. Spleen cells were either left untreated or exposed to MC540-PDT prior to mixing them with bone marrow cells (Fig. 10). As expected, the two-log reduction of spleen cells in allografts did significantly reduce the incidence of fatal GVHD among allogeneic recipients (80% long-term survival). MC540-PDT of grafts containing low numbers of spleen cells did not improve long-term survival beyond 80%. However, the recovery of body weights was significantly improved and was indistinguishable from that of recipients of syngeneic grafts (Fig. 10).
Fig. 10.
Survival (panel a) and mean body weights (panel b) of groups of 5 irradiated (11 Gy) LP/J (H-2bc) mice that were transplanted with 107 bone marrow cells from allogeneic C57BL/6J (H-2b) mice (B) or mixtures of 107 marrow cells and 5 × 107 (C, E) or 5 × 105 (D, F) spleen cells from C57BL/6J (H-2b) mice. Spleen cells infused into groups E and F were exposed to MC540 (15 µg ml−1) and white light (35 W m−2) for 90 min (fluence: 189 kJ m−2). Radiation controls (A) received infusions of cell-free medium. Differences between the survival curves of groups A and E, B and D, B and F, and D and F were statistically not significant (p>0.05). Differences between all other survival curves were significant (p<0.05). Differences in body weights between days 18 and 90 post TBI were statistically not significant (p>0.05) between groups B and F and C and E. Differences between the body weights of all other groups were statistically significant (p<0.05).
4. Discussion
MC540-mediated PDT inhibited all in vitro T- and B-cell functions that were tested in the context of this study. The inhibitory patterns in our mouse models were similar to those observed previously in human peripheral blood lymphocytes [20], except that MC540-PDT caused more cell death (as indicated by trypan blue uptake) in murine cells than in human cells.
At the highest light dose used in our mouse models (189 kJ m−2), proliferative responses to ConA, LPS, PHA, and IL-2 were completely abrogated. Proliferative responses of mixed lymphocyte cultures were also completely abrogated provided responder cells were first exposed to MC540 and light and subsequently co-cultured with stimulator cells. When the sequence of treatments was reversed (i.e. responder cells were first co-cultured with stimulator cells and subsequently exposed to MC540-PDT) there was a small residual proliferative response.
At moderate doses of MC540-PDT (e.g. at fluences of 63 or 95 kJ m−2), proliferative responses to PHA (a mature T-cell mitogen) were less suppressed than proliferative responses to ConA, LPS, or IL-2. This indicated that not all pathways were equally sensitive to MC540-PDT. Dye binding is a major determinant of a cell’s sensitivity to MC540-PDT, and the binding of MC540 to lymphocytes is known to vary as a function of differentiation and proliferative status [28]. The reduced suppression of proliferative responses to PHA was thus consistent with the (known) reduced binding of MC540 to mature lymphocytes.
Natural killer (NK) activity and T-cell mediated cytotoxicity were strongly inhibited by MC540-PDT, but some residual activity was usually detected even after exposure to the highest doses of MC540-PDT when cells were cultured in the presence of IL-2.
In several experiments, the MC540-mediated inhibition of lymphocyte functions displayed pronounced threshold effects. That is, lymphocyte functions were not affected by MC540-PDT unless fluences exceeded a certain critical value (e.g. 95 kJ m−2 in the experiments shown in Fig. 4b or Fig. 6c). Threshold effects were probably the result of a competition between photodynamic damage and cellular repair mechanisms. That is, no net reductions of function were observed as long as photodynamic damage was inflicted at a rate that did not exceed the cells’ capacity to repair such damage. A remarkable capacity for repairing MC540-mediated photodynamic damage has previously been reported for experiments with tumor cell lines and freshly explanted granulocyte/macrophage progenitors [29–31].
Lymphocyte preparations that had been exposed to MC540 and high doses of light contained large numbers of trypan blue-positive cells, suggesting that the observed loss of lymphocyte functions was at least in part the result of a cytotoxic effect of MC540-PDT. While the explanation is plausible, a direct proof is difficult to obtain, 1) because splenocyte preparations are heterogeneous, and 2) because some lethally damaged MC540-treated cells are known to continue to exclude trypan blue for several hours or even a few days [32]. As a result, trypan blue exclusion assays tend to underestimate the cytotoxic effects of MC540-PDT, which makes quantitative correlations between trypan blue exclusion and loss of function problematic [32].
The same combinations of dye and light that were used in this study to inhibit lymphocyte functions have been used previously in murine models of autologous bone marrow transplantation for the inactivation of tumor cells in simulated autologous remission marrow grafts [23, 25, 26]. As these purging studies have shown, MC540-mediated PDT does not jeopardize hematopoietic rescue after marrow-ablative therapy as long as normal-sized grafts (about 5 × 106 nucleated bone marrow cells) are used [23, 25, 26]. However, a reduced capacity to rescue lethally irradiated recipients becomes obvious when recipients are transplanted with limiting numbers of bone marrow cells [23]. In other words, the dye and light combinations used to inhibit lymphocyte functions in this study are not entirely innocuous to hematopoietic stem cells, but they are reasonably safe under most circumstances.
The strong inhibition of a broad range of lymphocyte function by an apparently safe combination of MC540 and light raised the question whether MC540-PDT could be used to prevent GVHD in allogeneic hematopoietic stem cell transplantation. The second part of this study addressed this question in several mouse models of allogeneic transplantation.
Since murine bone marrow contains too few T cells to cause severe GVHD when transplanted into mismatched recipients, it is common practice to use spleen cells or – as we did – mixtures of bone marrow and spleen cells as allografts. For the majority of our experiments, we used mismatched grafts that consisted of 1:5 mixtures of bone marrow and spleen cells. The high spleen cell content of these grafts caused severe GVHD. Unlike treatment with anti-Thy 1.2-antibody and complement, treatment of such grafts with MC540-PDT had little or no beneficial effect. Neither the incidence nor the severity of GVHD was significantly reduced. However, when the spleen cell concentration was reduced 100-fold, most recipients survived with only mild symptoms of GVHD. When such grafts were exposed to MC540-PDT prior to infusion, there was no evidence of GVHD as indicated by a normal recovery of body weights after TBI. In other words, the MC540-PDT intervention was beneficial in situations that would typically lead to mild or moderate GVHD, but was of little or no benefit in situations that would typically lead to severe GVHD.
Lum et al. [33] also used MC540-PDT to prevent GVHD in a mouse model that typically caused severe GVHD. When they transplanted spleen cells from C3D2F1 (H-2d/H-2d) mice into B6D2F1 (H-2b/H-2d) mice, only one of 15 recipients of non-treated allografts survived. The remaining animals died with obvious signs of GVHD. However, when the mismatched allografts were treated with MC540 and light prior to infusion, 7 of 15 animals survived without developing obvious signs of GVHD. It is not clear what made MC540-PDT more effective for the prophylaxis of GVHD in this particular experimental setting. The transplant model was obviously different in the sense that Lum et al. [33] used a donor strain that was not included in our series. Furthermore, Lum et al. [33] used spleen cells rather than mixtures of bone marrow and spleen cells as allografts. On the other hand, photodynamic therapy regimens were identical using identical reagents and light sources. One potentially important difference, however, was the radiation dose used to condition recipients. We used a single dose of 11 Gy, whereas Lum et al. [33] used a single dose of only 9 Gy. Autologous recovery combined with graft rejection is more likely to occur after low doses of TBI. Mixed chimerism, which favors the development of tolerance, is also more likely to occur after low doses of TBI [34]. The identification of cells expressing donor-type antigens in randomly selected long-term survivors argued against autologous recovery and graft rejection being the main reason for the low incidence of fatal GVHD in Lum’s study [33]. However, mixed chimerism was not ruled out.
A comprehensive test of the hypothesis that GVHD prophylaxis with MC540-PDT works best if used under conditions that favor mixed chimerism was beyond the scope of this study. However, it is worth noting that one experiment performed as part of our series offered preliminary evidence in support of such a mechanism. The experiment shown in Fig. 7 was set up as an exact duplicate of the experiment shown in Fig. 6 (cells from C57BL/6J donors transplanted into LP/J recipients) except that the radiation dose was reduced from 11 Gy to 10.75 Gy. This minor change of the conditioning regimen had a surprisingly strong effect on survival. Long-term (4-month) survival of radiation controls increased from 0% to 30%. Survival of recipients of non-treated allografts increased from 10% to 50%, and survival of recipients of MC540-treated allografts increased from 0% to 80%. However, despite improved survival, long-term survivors of non-treated and MC540-treated allografts continued to show similar degrees of GVHD as indicated by reduced body weights. This suggested that improved survival was the result of tolerance rather than the result of an outright rejection of allografts followed by autologous reconstitution.
The improved survival of recipients of MC540-treated allografts is consistent with (but does not prove) the hypothesis that prophylaxis with MC540-PDT is most effective when used in situations that favor mixed chimerism. A more rigorous test of this hypothesis will require an extension of experiments to additional pairs of donors and recipients, a greater range of radiation doses, and a quantitative assessment of mixed chimerism in long-term survivors.
Mixed chimerism has become common in the clinical setting owing to the adoption of less ablative conditioning regimens. Methods that achieve stable mixed chimerism are actively pursued [34]. Therefore, a method that reduces the incidence and/or severity of GVHD most effectively in transplant settings that favor mixed chimerism might be of considerable value.
Highlights.
Extracorporeal PDT with MC540 inactivates broad range of lymphocyte functions.
MC540-PDT does not prevent GVHD in most murine models of allogeneic transplantation.
MC540-PDT may prevent GVHD in transplant regimens that encourage mixed chimerism.
Acknowledgements
We thank Drs. Charles C.-Y. Shih, Ann V. LeFever and Robert L. Truitt for helpful discussions and Ms. Laura J. McOlash for help with cytometric analyses.
Funding
This study was supported by grant CA42734 from the National Cancer Institute and the MACC Fund. The funding sources were not involved in study design, data collection, or analysis or interpretation of data.
Abbreviations
- ConA
concanavalin A
- GVHD
graft-versus-host disease
- IL-2
interleukin-2
- LPS
lipopolysaccharide
- MC540
Merocyanine 540
- NK cells
natural killer cells
- PDT
photodynamic therapy
- PHA
phytohemagglutinin
- TBI
total body irradiation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest statement
The authors have no conflicts of interest to declare.
References
- 1.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98:3192–3204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 2.Koreth J, Antin JH. Current and future approaches for control of graft-versus-host disease. Expert Rev. Hematol. 2008;1:111–128. doi: 10.1586/17474086.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rezvani AR, Storb RF. Prevention of graft-vs.-host disease. Expert Opin. Pharmacother. 2012;13:1737–1750. doi: 10.1517/14656566.2012.703652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett AJ, Le Blanc K. Prophylaxis of acute GVHD: manipulate the graft or the environment? Best Pract. Res. Clin. Haematol. 2008;21:165–176. doi: 10.1016/j.beha.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolb H-J. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–4383. doi: 10.1182/blood-2008-03-077974. [DOI] [PubMed] [Google Scholar]
- 6.Li JM, Giver CR, Lu Y, Hossain MS, Akhtari M, Waller EK. Separating graft-versus-leukemia from graft-versus-host disease in allogeneic hematopoietic stem cell transplantation. Immunotherapy. 2009;1:599–621. doi: 10.2217/imt.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landgren O, Gilbert ES, Rizzo JD, Socie G, Banks PM, Sobocinski KA, Horowitz MM, Jaffe ES, Kingma DW, Travis LB, Flowers ME, Martin PJ, Deeg HJ, Curtis RE. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001. doi: 10.1182/blood-2008-09-178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Müller AMS, Linderman JA, Florek M, Miklos D, Shizuru JA. Allogeneic T cells impair engraftment and hematopoiesis after stem cell transplantation. Proc. Natl. Acad. Sci. USA. 2010;107:14721–14726. doi: 10.1073/pnas.1009220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez L, Anasetti C, Pidala J. Have we improved in preventing and treating acute graft-versus-host disease? Curr. Opin. Hematol. 2011;18:408–413. doi: 10.1097/MOH.0b013e32834b6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hymes SR, Morison WL, Farmer ER, Walters LL, Tutschka P, Santos GW. Methoxalen and ultraviolet A radiation in the treatment of chronic cutaneous graft-versus-host reaction. J. Am. Acad. Dermatol. 1985;12:30–37. doi: 10.1016/s0190-9622(85)70005-9. [DOI] [PubMed] [Google Scholar]
- 11.Atkinson K, Weller P, Ryman W, Biggs J. PUVA therapy for drug resistant graft-vs-host disease. Bone Marrow Transplant. 1986;1:227–236. [PubMed] [Google Scholar]
- 12.Deeg HJ, Aprile J, Graham TC, Applebaum FR, Storb R. Ultraviolet irradiation of zlood prevents transfusion-induced sensitization and marrow graft rejection in dogs. Blood. 1986;67:537–539. [PubMed] [Google Scholar]
- 13.Cohn ML, Cahill RA, Deeg HJ. Hemopoietic reconstitution and prevention of graft-versus-host disease with UVB-irradiated haploidentical murine spleen and marrow cells. Blood. 1991;78:3317–3322. [PubMed] [Google Scholar]
- 14.Rook AH, Freundlich B, Jegasothy BV, Perez MI, Barr WG, Jimenez SA, Rietschel RL, Wintroub B, Kahaleh MB, Varga J, Heald PW, Steen V, Massa MC, Murphy GF, Perniciaro C, Istfan M, Ballas SK, Edelson RL. Treatment of systemic sclerosis with extracorporeal photochemotherapy. Results of a multicenter trial. Arch. Dermatol. 1992;128:337–346. [PubMed] [Google Scholar]
- 15.Hardy MA, Oluwole SF, Lau HT. Ultraviolet B-modified donor-specific blood transfusions and peritransplant cyclosporine in the induction of specific unresponsiveness to organ allografts. Transplant. Proc. 1988;20:1147–1150. [PubMed] [Google Scholar]
- 16.Chen BJ, Cui X, Liu C, Chao NJ. Prevention of graft-versus -host disease while preserving graft-versus-leukemia effect after selective depletion of host-reactive T cells by photodynamic cell purging process. Blood. 2002;99:3083–3088. doi: 10.1182/blood.v99.9.3083. [DOI] [PubMed] [Google Scholar]
- 17.Guimond M, Balassy A, Barrette M, Brochu S, Perreault C, Roy DC. P-glycoprotein targeting: a unique strategy to selectively eliminate immunoreactive T cells. Blood. 2002;100:375–382. doi: 10.1182/blood-2001-12-0353. [DOI] [PubMed] [Google Scholar]
- 18.McIver ZA, Melenhorst JJ, Grim A, Naguib N, Weber G, Fellowes V, Khuu H, Stroncek DS, Leitman SF, Battiwalla M, Barrett AJ. Immune reconstitution in recipients of photodepleted HLA-identical sibling donor stem cell transplantations: T cell subset frequencies predict outcome. Biol. Blood Marrow Transpl. 2011;17:1846–1854. doi: 10.1016/j.bbmt.2011.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sieber F. Extracorporeal purging of bone marrow grafts by dye-sensitized photoir-radiation. In: Gee AP, editor. Bone Marrow Processing and Purging: A Practical Guide. Boca Raton: CRC Press; 1991. pp. 263–280. [Google Scholar]
- 20.Lum LG, Yamagami M, Giddings BR, Joshi I, Schober SL, Sensenbrenner LL, Sieber F. The immunoregulatory effects of Merocyanine 540 on in vitro human T- and B-lymphocyte functions. Blood. 1991;77:2701–2706. [PubMed] [Google Scholar]
- 21.Mishell BB, Shiigi SM. Selected Methods in Cellular Immunology. San Francisco: Freeman; 1980. [Google Scholar]
- 22.Truitt RL, Atasoylu AA. Impact of pretransplant conditioning donor Tcells on Graft-versus-host disease chimerism graft-versus-leukemia reactivity, and tolerance after bone marrow transplantation. Blood. 1991;77:2515–2523. [PubMed] [Google Scholar]
- 23.Sieber F Sieber-Blum M. Dye-mediated photosensitization of murine neuroblastoma cells. Cancer Res. 1986;46:2072–2076. [PubMed] [Google Scholar]
- 24.Korngold R, Sprent J. Lethal graft-versus-host disease after bone marrow transplantation across minor histocompatibility barriers in mice. J. Exp. Med. 1978;148:1687–1698. doi: 10.1084/jem.148.6.1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sieber F, Spivak JL, Sutcliffe AM. Selective killing of leukemic cells by merocyanine 540-mediated photosensitization. Proc. Natl. Acad. Sci. USA. 1984;81:7584–7587. doi: 10.1073/pnas.81.23.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieber F. Photosensitizing agents for marrow purging in autologous bone marrow transplantation. In: Gorin NC, Douay L, editors. Experimental Hematology Today-1989. New York: Springer Verlag; 1990. pp. 10–15. [Google Scholar]
- 27.Lewis LL, Benike CJ, Phillips JH, Engleman EG. Recombinant interleukin 2 enhances natural killer cell-mediated cyctotoxicity in human lymphocyte subpopulations expressing the Leu 7 and Leu11 antigens. J. Immunol. 1985;134:794–801. [PubMed] [Google Scholar]
- 28.Del Buono BJ, Williamson PL, Schlegel RA. Alterations in plasma membrane lipid organization during lymphocyte differentiation. J. Cell. Physiol. 1986;126:379–388. doi: 10.1002/jcp.1041260308. [DOI] [PubMed] [Google Scholar]
- 29.Qiu K, Sieber F. Merocyanine 540-sensitized photoinactivation of leukemia cells: Effects of dose fractionation. Photochem. Photobiol. 1992;56:489–493. doi: 10.1111/j.1751-1097.1992.tb02192.x. [DOI] [PubMed] [Google Scholar]
- 30.Yamazaki T, Sato Y, Sieber F. Role of cytoprotective mechanisms in the photochemical purging of autologous bone marrow grafts. Exp. Hematol. 1997;25:629–637. [PubMed] [Google Scholar]
- 31.Yamazaki T, Sieber F. Effect of hypothermia on the Merocyanine 540-mediated purging of hematopoietic cells. J. Hematother. 1997;6:31–39. doi: 10.1089/scd.1.1997.6.31. [DOI] [PubMed] [Google Scholar]
- 32.Gaffney DK, Schober SL, Sieber F. Merocyanine 540-sensitized photoinactivation of leukemia cells: Role of oxygen and effects on plasma membrane integrity and mitochondrial respiration. Exp. Hematol. 1990;18:23–26. [PubMed] [Google Scholar]
- 33.Lum LG, Yamagami M, Sensenbrenner LL, Galoforo S. Merocyanine 540 dye-sensitized photoirradiation (DSP) of splenocytes prevents graft-versus-host disease (GVHD) in mismatched transplants from CD2F1 (d/d) mice into B6D2F1 (b/d) mice. Abstracts, 6th Annual Meeting of The Clinical Immunology Society; November 1–3, 1991; Arlington, VA. [Google Scholar]
- 34.Cosimi AB, Sachs DH, Sykes M, Kawai T. Clinical strategy for induction of transplantation tolerance through mixed chimerism. Clin. Transpl. 2003:127–134. [PubMed] [Google Scholar]