Abstract
Background
Offspring of parents with BD (BO) are at higher risk of bipolar disorder (BD) than offspring of parents with non-BD psychopathology (NBO), although both groups are at higher risk than offspring of psychiatrically healthy parents (HC) for other affective and psychiatric disorders. Abnormal functioning in reward circuitry has been demonstrated previously in individuals with BD. We aimed to determine whether activation and functional connectivity in this circuitry during risky decision-making differentiated BO, NBO and HC.
Methods
BO (n=29;mean age=13.8 years;14 female), NBO (n=28;mean age=13.9 years;12 female) and HC (n=23;mean age=13.7 years;11 female) were scanned while performing a number guessing reward task. 11 BO and 12 NBO had current non-BD psychopathology; 5 BO and 4 NBO were taking psychotropic medications.
Results
A 3(Group) x 2(Conditions:Win-Control/Loss-Control) ANOVA revealed a main effect of Group on right frontal pole activation: BO showed significantly greater activation than HC. There was a significant main effect of Group on functional connectivity between bilateral ventral striatum (VS) and right ventrolateral prefrontal cortex (Z>3.09, cluster-p<0.05): BO showed significantly greater negative functional connectivity than other participants. These between-group differences remained after removing youth with psychiatric disorders and psychotropic medications from analyses.
Conclusions
This is the first study to demonstrate that reward circuitry activation and functional connectivity distinguish BO from NBO and HC. The fact that the pattern of findings remained when comparing healthy BO vs. healthy NBO vs. HC suggests that these neuroimaging measures may represent trait-level neurobiological markers conferring either risk for, or protection against, BD in youth.
Keywords: youth offspring of parents with bipolar disorder, risk of bipolar disorder, fMRI, decision-making, frontal pole, biological markers
Introduction
It is well established that offspring of parents with bipolar disorder (BD) are at increased risk of developing psychiatric disorders and BD (Birmaher et al. 2009, 2010; Goldstein et al. 2010). Like offspring of parents with BD (BO), offspring of parents with non-BD psychopathology (NBO) are at future risk of a range of non-BD disorders, but are at lower risk of future BD than offspring of parents with BD (Birmaher et al 2009). In order to understand neural mechanisms of risk for BD, previous studies compared BO with healthy offspring of psychiatrically healthy parents (HC) (Singh et al. 2014). Given that having a parent with BD not only confers risk for BD, but also confers risk for non-BD psychopathology, it is difficult to determine whether findings from previous studies in BO were due to risk in these offspring for BD specifically, or for other psychopathology in general.
Little is known regarding the neurophysiological processes that predispose to, or protect from, risk for BD versus risk for other psychiatric disorders in these offspring, however, given that no study has directly compared neurophysiological processes in BO, NBO and HC. Critically, studies examining these processes have potential to help identify biomarkers denoting which at-risk offspring are most likely to develop which specific psychiatric disorders in the future, and ultimately provide biological targets to guide early interventions for these individuals. Neuroimaging studies are appropriate as a way forward in this research field, as they can determine the extent to which BO and NBO show abnormal functioning in neural circuitries supporting information processing domains known to be aberrant in individuals with established BD. One such information processing domain is reward processing, as an increasing number of studies reported abnormally heightened reward sensitivity in individuals with BD (Alloy et al. 2012; Ibanez et al. 2012; Mason et al. 2012). Specifically, youth with BD, relative to healthy youth, show impaired reward learning (Gorrindo et al. 2005) and greater reward-related arousal (Ernst et al., 2004; Rich et al., 2005).
Reward circuitry comprises a complex, highly-interconnected network of fronto-subcortical regions (Haber & Knutson 2010; Russo & Nestler 2013). The ventral striatum (VS) supports reward anticipation and prediction error (Schultz et al. 2000; Knutson et al., 2001; Pagnoni et al. 2002; O’Doherty 2004; Tanaka et al. 2004), the pallidum encodes expected reward value (Tachibana & Hikosaka, 2012), the amygdala, stimulus-value associations (Baxter & Murray 2002), and the putamen, action-specific value signals (FitzGerald et al. 2012) and effort costs (Kurniawan et al. 2012). Different prefrontal cortical (PFC) regions contribute differently to reward processing and decision-making (Rushworth et al. 2011). The ventromedial prefrontal (vmPFC) and the orbitofrontal (OFC) cortices encode reward values and compare values of different options (Boorman et al. 2009). The ventrolateral PFC (vlPFC) encodes the value of choice/decision-making options and is important for credit assignment (Walton et al. 2011). The frontal polar (FP) region encodes the value of a non-chosen option during decision-making (Boorman et al. 2009), responds in situations of uncertainty (Yoshida & Ishii 2006), and maintains possible behavioral choices (Koechlin & Hyafil 2007) and intentions (Burgess et al. 2007) in memory for future use. The anterior cingulate cortex (ACC) is involved in cost-benefit decision-making (Croxson et al. 2009; Walton et al. 2006) and action-reward associations (Rushworth et al. 2011).
Neuroimaging studies report abnormal functioning in reward circuitry in individuals with BD during reward and loss expectation and processing, in particular, elevated activity in vmPFC, OFC, vlPFC, FP and striatum (Bermpohl et al. 2010; Nusslock et al. 2012; Cardoso de Almeida & Phillips, 2013; Caseras et al. 2013; Chase et al. 2013; Phillips & Kupfer 2013; Singh et al. 2013) and reduced negative VS-vlPFC functional connectivity (Trost et al. 2014). Findings in individuals with other, non-BD disorders indicate different patterns of abnormal reward circuitry functioning, including abnormally reduced or altered VS and ACC activity in individuals with major depressive disorders (e.g., Kumar et al. 2008; Chase et al. 2013; Greenberg et al. 2015, in press), abnormally reduced striatal activation in individuals with posttraumatic stress disorder (Elman et al 2009), and increased striatal activation (Guyer et al., 2012) and striatal-ACC negative functional connectivity in individuals with anxiety disorders (Cremers et al., 2014).
Only one previous study compared neural underpinnings of reward processing in BO vs. HC (Singh et al. 2014). This study showed elevated vlPFC activity and altered vlPFC-ACC functional connectivity during reward processing in healthy BO vs. HC (Singh et al. 2014). No study to date compared reward circuitry functioning in BO and NBO who have not as yet developed BD, and HC. In the present study, we thus aimed to identify the effect of familial genetic risk for BD on activation and functional connectivity in reward circuitry by comparing these neural measures in BO vs. NBO vs. HC during a number-guessing reward task (Forbes et al. 2009). By including NBO, we were able to control for the impact of risk for non-BD psychopathology upon neuroimaging measures in BO, and for any potential effects on neuroimaging measures in BO of their living with parents with comorbid non-BD psychopathology. We thereby aimed to address the limitation of previous studies that did not include NBO as a comparison group. Choice of regions in reward circuitry was determined from previous neuroimaging findings (Schultz et al. 2000; Knutson et al., 2001; Baxter & Murray 2002; Pagnoni et al. 2002; O’Doherty 2004; Tanaka et al. 2004; Yoshida & Ishii 2006; Walton et al. 2006, 2011; Burgess et al. 2007; Koechlin & Hyafil 2007; Boorman et al. 2009; Croxson et al. 2009; Haber & Knutson 2010; Rushworth et al. 2011; FitzGerald et al. 2012; Kurniawan et al. 2012; Tachibana & Hikosaka, 2012; Russo & Nestler 2013). BO and NBO included youth with and without current non-BD psychopathology, some of whom were treated with psychotropic medications. This allowed us to identify the extent to which trait-level neuroimaging markers of risk for, or resilience against, BD were evident in all BO, regardless of the presence of current psychopathology or psychotropic medication.
Based on the above neuroimaging findings in adults and youth with BD, we hypothesized the following:
Given previous findings showing abnormally elevated fronto-striatal activity in individuals with BD (Bermpohl et al. 2010; Nusslock et al. 2012; Caseras et al. 2013; Chase et al. 2013; Singh et al. 2013), and abnormally elevated prefrontal cortical activation in BO vs. HC (Singh et al. 2014), we hypothesized greater prefrontal activation to reward in both healthy BO and in BO with non-BD psychopathology than either NBO or HC, representing increased risk for, or resilience against, development of future BD in BO.
Given a key role of the VS in reward processing (e.g., Schultz et al. 2000; Knutson et al., 2001; Pagnoni et al. 2002; O’Doherty 2004), and previous findings showing altered functional connectivity between VS and anterioventral prefrontal cortex in individuals with BD versus HC (Trost et al. 2014), we hypothesized that BO, but not NBO, would show significantly altered functional connectivity between these regions during reward processing compared with HC.
This differential pattern of reward circuitry functioning between BO and other participants would be present in BO with and without current psychiatric diagnoses and in both medicated and unmedicated BO.
We also noted, however, that if patterns of abnormal reward circuitry functioning, relative to HC, were comparable in healthy BO and NBO and BO and NBO with non-BD psychopathology, these neuroimaging findings may represent markers of increased risk for, or resilience against, future development of more severe psychopathology in general, rather than BD specifically, in both BO and NBO.
Methods and Materials
The Bipolar Offspring Study (BIOS) is an ongoing longitudinal study examining psychiatric symptomatology in youth offspring of parents with BD (Birmaher et al. 2009) and functioning in neural circuitries underlying information processing domains implicated in the pathogenesis of BD, including reward circuitry. The study was approved by the Institutional Review Board of the University of Pittsburgh. Prior to study participation, parents/guardians provided written informed consent, and children provided written informed assent. Participants received monetary compensation for their participation.
Participants
Three groups of participants aged 7–17 years (matched in age) who were not affected with BD took part in this study: youth offspring of parent(s) with BD (BO;n=35), youth offspring of parent(s) with non-BD psychopathology (NBO;n=37) and psychiatrically healthy youth offspring of psychiatrically healthy parents (HC;n=25) without family history of any psychiatric disorders (including first-degree relatives). Importantly, both parents with BD and parents with non-BD psychopathology had a range of non-BD psychiatric diagnoses. This allowed us to control in BO for risk for future non-BD psychopathology, and for any potential effects of living with parents with a range of comorbid non-BD psychopathology. Twenty-four HC were recruited from the healthy comparison youth group of the Longitudinal Assessment of Manic Symptoms (LAMS) study (Findling et al. 2010; Horwitz et al., 2010) at the University of Pittsburgh Medical Center/Western Psychiatric Institute and Clinic, a parallel study examining neural circuitry functioning in youth with behavioral and emotional dysregulation. One HC was recruited from BIOS (Birmaher et al. 2009). Most BO and NBO were recruited from BIOS, with the exception of 2 BO and 5 NBO, who were recruited from LAMS.
Exclusion criteria for all participants were: systemic medical illness, neurological disorders, head trauma, alcohol or illicit substance use, standard exclusion criteria for MRI research (metal in the head or body, claustrophobia, etc), IQ<70(using the Weschler Abbreviated Scale of Intelligence[Wechsler 1999]), unable to read and write in standard English, and corrected far visual acuity worse than 20/40 on the Snellen visual acuity test. Six BO, 9 NBO, and 2 HC were excluded from data analysis due to inability to complete the scanning session or due to excessive motion in the scanner (translation ≥4mm in any direction). The total numbers of participants with usable fMRI data were: 29 BO, 28 NBO, and 23 HC. Eleven BO and 12 NBO had current non-BD psychopathology. Five BO and 4 NBO were taking one class of psychotropic medications (Table 1). Given ethical concerns with stopping medication for research participation, participants were permitted to use prescribed medication(s) before, and on the day of, scanning.
Table 1.
Demographic and clinical variables
| BO n=29 | NBO n=28 | HC n=23 | Statistics | p-value | |
|---|---|---|---|---|---|
| Number of youth without psychiatric diagnoses | 18 (62%) | 16 (57%) | 23 (100%) | BO vs. NBO χ2(2)<1 |
ns |
| Number of youth untreated with psychotropic medications | 24(83%) | 24(86%) | 23(100%) | BO vs. NBO χ2(2)<1 |
ns |
| Age at scan | 13.81(2.45) | 13.93(2.38) | 13.74(1.80) | F(2,77)<1 | ns |
| Gender (female) | 14 | 12 | 11 | χ2(2)<1 | ns |
| Handedness (right hand) | 26 | 26 | 21 | Yates' χ2(2)<1 | ns |
| IQ (WASI) | 103.21(14.51) | 102.86(14.33) | 105.78(13.7 9) | F(2,77)<1 | ns |
| SES based on parental education | 5.48(0.95) | 5.54(0.96) | 5.30(1.02) | F(2,77)<1 | ns |
|
| |||||
| Medications | |||||
|
| |||||
| antidepressants | na | sertraline HCI: n=1 | na | ||
| antipsychotics | Risperidone: n=1 Quetiapine Fumarate: n=1 |
na | na | ||
| mood stabilizers | na | na | na | ||
| stimulants | amphetamine, dextroamphetamine mixed salts: n=1 | methylphenidate: n=1 amphetamine, dextroamphetamine mixed salts: n=2 |
na | ||
| non-stimulants | Atomoxetine HCl: n=2 | na | na | ||
| benzodiazepines | na | na | na | ||
|
| |||||
| Youth offspring current psychiatric diagnoses | |||||
|
| |||||
| Number of youth with more than 1 diagnosis | 6 | 7 | na | ||
| MDD/DDNOS | 3 | 2 | na | ||
| Attention deficit hyperactivity disorder | 6 | 5 | na | ||
| Anxiety disorders | 2 | 2 | na | ||
| Oppositional Defiant Disorder | 1 | 2 | na | ||
| Phobias | 2 | 2 | na | ||
| Tourette's Disorder | 1 | 0 | na | ||
| Obsessive compulsive disorder | 0 | 2 | na | ||
| Eating disorder | 2 | 0 | na | ||
|
| |||||
| Symptom Assessment Scale Scores administered on the day of scan | |||||
|
| |||||
| SCARED Parent Total | 9.45(6.86) | 10.85(11.70) | 4.17(4.32) | F(2,76)=4.35 | 0.02 |
| SCARED Child Total | 11.66(8.61) | 10.79(13.80) | 9.33(11.42) | F(2,77)<1 | ns |
| MFQ Parent | 5.90(8.97) | 5.42(9.09) | 1.57(2.09) | F(2,75)=2.4 | ns |
| MFQ Child | 8.86(10.73) | 10.18(10.97) | 5.09(10.57) | F(2,77)=1.5 | ns |
| CALS Parent Total | 7.97(10.26) | 5.07( 7.65) | 1.78( 2.59) | F(2,76)=4.04 | 0.02 |
| CALS Child Total | 10.52(12.22) | 8.79(11.28) | 5.96(13.39) | F(2,77)<1 | ns |
Note: Standard deviations (SD) are reported in parentheses. BO – youth offspring of parents with bipolar disorder; NBO – youth offspring of parents with non-bipolar psychopathology; HC – healthy offspring of psychiatrically healthy parents. MDD – major depressive disorder; SCARED - Self-Report for Childhood Anxiety Related Emotional Disorders; MFQ - Mood and Feelings Questionnaire; CALS - The Children’s Affective Lability scale; na – not applicable; ns – not significant.
Assessment procedures
Parental psychopathology was ascertained by a trained clinician using the Structural Clinical Interview for DSM-IV (SCID-I;First et al. 2002) for BIOS youth, and using detailed clinical assessment for LAMS youth. Another trained clinician, blind to the parent’s condition, interviewed the parents about their children, and also interviewed their children, using the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime version (KSADS-PL;Kaufman et al., 1997). All cases were supervised by a “blind” child psychiatrist who was responsible for the ultimate parental and children diagnoses. Inter-rater reliability for all psychiatric diagnoses ascertained through the KSADS>0.8.
On the day of scan, parents/guardians of youth participants completed the PGBI-10M (Parent Version, General Behavior Inventory (Youngstrom et al. 2008), to assess the severity of behavioral and emotional dysregulation in their offspring during the last 6 months; only parents of BO and NBO completed this questionnaire); the SCARED-P (Self-Report for Childhood Anxiety Related Emotional Disorders, parent version, to assess offspring anxiety over last 2 weeks;Birmaher et al. 1997); the CALS-P (The Children’s Affective Lability scale, parent version; Gerson et al. 1996); the MFQ-P (Mood and Feelings Questionnaire, parent version, to assess the severity of depression during the last 2 weeks;Angold et al. 1995); and a questionnaire to assess sociodemographic status represented by parental education (Hollingshead 1975). Youth participants completed child report versions of affective symptomatology scales: the CALS-C, SCARED-C, MFQ-C. All participants completed medication forms that documented psychotropic medications used that day, during the last 24 hours, and those used on regular basis; drug/alcohol/pregnancy screens; the Edinburgh Handedness Inventory (EHI;Oldfield 1971) and the Snellen visual acuity test. Table 1 reports demographic and clinical variables. Table S1 reports demographic and clinical variables for youth without psychopathology and youth untreated with psychotropic medications. Table 2 reports lifetime psychiatric diagnoses in parents.
Table 2.
Lifetime psychiatric diagnoses in parents
| BO n=29 | NBO n=28 | Statistics | p-value | |
|---|---|---|---|---|
| BD-I | 23 | 0 | χ2(1)=37.2 | p<0.001 |
| BD-II | 6 | 0 | χ2(1)=6.5 | p=0.01 |
| BD-NOS | 0 | 0 | ||
| Major depressive disorder/Depressive disorder NOS | 1 | 20 | χ2(1)=28.3 | p<0.001 |
| Generalized Anxiety | 16 | 8 | χ2(1)=4.1 | p=0.04 |
| Disorder/Anxiety disorders NOS Phobias | 21 | 14 | χ2(1)<3.0 | ns |
| Alcohol/Drug abuse/dependence | 23 | 13 | χ2(1)=6.6 | p=0.01 |
| Post-traumatic stress disorder | 12 | 4 | χ2(1)=5.2 | p=0.02 |
| Panic disorder | 16 | 6 | χ2(1)=6.8 | p<0.01 |
| Eating disorder | 4 | 1 | χ2(1)=1.8 | ns |
| Obsessive-compulsive disorder | 10 | 0 | χ2(1)=11.7 | p<0.001 |
| Attention deficit hyperactivity disorder | 4 | 2 | χ2(1)<1 | ns |
Note: Standard deviations (SD) are reported in parentheses. BO – youth offspring of parents with bipolar disorder; NBO – youth offspring of parents with non-bipolar psychopathology; BD – bipolar disorderl MDD – major depressive disorder; na – not applicable; ns – not significant.
Reward task
Participants were scanned while performing a number guessing reward task (Forbes et al. 2009) that reliably activates fronto-striatal reward circuitry, and has been used previously in neuroimaging studies of adults and youth with mood disorders (Forbes et al. 2009; Bebko et al. 2014). The task required participants to guess whether the upcoming number were smaller or greater than 5. Participants were told that they would receive $1 if they were correct and lose $0.50 if they were incorrect, and that they could win up to $10 in the game. If a participant correctly guessed the number, a green upward arrow appeared (Figure 1). If an incorrect guess were made, a red downward arrow appeared. There were fifteen 7-sec Win trials, fifteen 7-sec Loss trials, and eighteen 6-sec Control trials. Control trials did not involve any guessing (and, consequently, no winning or losing) and required pressing a button when an asterisk appeared on the screen. Further in the text, we refer to Win and Loss trials as decision-making trials and Control trials as non-decision-making trials. Please note that decision-making trials included a decision component (when participants decided which button to press) and a reward component (when participants received feedback about winning or losing on that specific trial). Non-decision-making trials did not include either of these decision and reward components. Win and Loss trial comprised a 3-sec guessing period when participants decided whether the upcoming number were greater/lower than 5. The actual number was then presented for 500ms, followed by a 500ms green upward arrow (for positive feedback) or 500ms red downward arrow (for negative feedback), and then a 3,000ms inter-stimulus interval fixation cross. Outcome was fixed for all participants so that each participant won $10. Previous studies using this task showed that participants were unaware of the fixed outcome of the task and believed that their performance was due to chance (Forbes et al. 2009).
Figure 1.
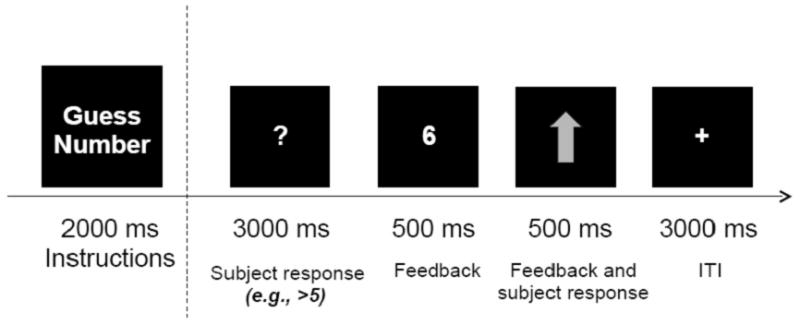
An example of a Win trial in the reward task. An upward arrow shows that a subject correctly guessed that the number was greater than 5.
fMRI data acquisition and analysis
fMRI data were acquired using a Siemens MAGNETOM TrioTim 3T MR system. A high-resolution structural image (1x1x1mm) was acquired using MPRAGE (TR=2300msec, TE=3.93msec, FOV=256 , FA=9°, 192 slices). Functional data were collected using a gradient-echo, echo-planar sequence (voxel size: 3.2x3.2x3.1mm, TR=2000msec, TE=28msec, FOV=205, FA=90°, 38 slices). These data comprised 178 volumes (TRs). Field maps were collected at the 4x4x4 mm resolution using a gradient echo sequence (TR=488msec, TE1=4.92msec, TE2=7.38msec, FOV=256, FA=60°, 32 slices).
Data preprocessing and analyses were done using FSL5.0.2 (www.fmrib.ox.ac.uk/fsl). Preprocessing included motion correction with MCFLIRT (Jenkinson et al. 2002), non-brain removal using BET (Smith 2002), fieldmap-based EPI unwarping using PRELUDE+FUGUE (Jenkinson 2003), spatial smoothing with a Gaussian kernel of FWHM 6mm, grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor, high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=50.0s). A field map image was prepared using the fsl_prepare_fieldmap script. The high-resolution structural images were segmented to separate white matter, grey matter and cerebrospinal fluid (CSF). The white matter and CSF masks were then coregistered with functional images, and their timecourses were extracted from the preprocessed functional data for further analyses. Motion outliers (time points where the fMRI signal was corrupted due to subject motion) were identified using the fsl_motion_outliers script (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLMotionOutliers). A confound matrix from this analysis was then combined with the white matter and CSF time courses and used as a confound variable of no interest in the first-level analyses.
Co-registration was carried out using FLIRT (FMRIB's Linear Image Registration Tool (Jenkinson & Smith 2001;Jenkinson et al. 2002) and FNIRT (FMRIB's Non-linear Image Registration Tool; Andersson et al. 2007). BOLD images were registered to the high-resolution structural images using FLIRT; the high-resolution images were registered to the MNI152_T1_2mm template, as in previous neuroimaging studies of youth with age ranges comparable to that in the present study (Burgund et al. 2002;Kang et al. 2003;Singh et al. 2013, 2014;Bebko et al. 2014;Olsavsky et al. 2014), using FNIRT, and the two resulting transformations were concatenated and applied to the original BOLD image to transform it to MNI space. The registration quality was checked for each subject. In rare cases FNIRT was substituted with FLIRT to obtain a better quality registration.
A first-level GLM analysis was implemented using FEAT (FMRI Expert Analysis Tool,v6.0). The model included four regressors (Win, Loss, and Control trials, and Instructions). The magnitude of activation was examined for each of these conditions and to the Win-Control, Loss-Control, and Win-Loss contrasts. All group-level analyses were conducted using FLAME1 (FMRIB's Local Analysis of Mixed Effects). Gender, age, IQ, and presence/absence of psychopathology were used as covariates in the group-level analyses in order to factor out the effects of these variables. Multiple comparisons correction (p-corrected<0.05) was performed using Gaussian Random Field theory. Significant clusters of activation were determined by thresholding Z-statistic images in the reward circuitry mask at z>3.09 (uncorrected voxel-wise p<0.001) and a FWE (family-wise error) corrected cluster significance threshold of p<0.05 (Worsley 2001).
Hypothesis 1 testing
Activity in the reward circuitry ROI
Brain activation in the reward circuitry ROI was analyzed using a 3(Group: BO/NBO/HC)x2(Condition:Win-Control/Loss-Control) ANOVA where Win-Control and Loss-Control were the contrast images from the first-level analysis. The reward circuitry ROI mask was the anatomical mask used in a previous study (Bebko et al. 2014) that examined reward circuitry function in emotionally dysregulated youth, using the same reward task. The mask included key neural regions implicated in reward processing: bilateral dorsal ACC(BA24/32), medial and lateral FP(BA10), OFC(BA11), vlPFC(BA47), and ventral striatum (VS; spherical ROIs with radius of 8mm centered [±9,9, 8]).
Hypothesis 2 testing
Functional connectivity between bilateral VS and the reward circuitry mask
Functional connectivity was examined using psychophysiological interaction (PPI) analysis (Friston et al. 1997), in FEAT. The bilateral VS served as a seed region and the reward circuitry mask served as a target region. The PPI first-level analysis model included four psychological regressors (Win, Loss, and Control trials, and Instructions), one physiological regressor—a mean time course extracted from the seed region, and three interaction terms between the physiological and Win, Loss and Control regressors. To parallel the activation analysis, the group-level connectivity analysis was conducted using a 3(Group)x2(Condition) ANOVA.
Post-hoc tests
To determine the direction of the between- or within-group effects, post-hoc t-tests of activation and connectivity values (parameter estimates extracted from the significant activation and connectivity clusters) were conducted using SPSS and Bonferroni corrected to control for multiple t-tests.
Hypothesis 3 testing
Here, we examined the effect of diagnosis and medications on activation and connectivity in the brain regions identified in the previous analyses. For this purpose, we first extracted activation and connectivity values from the significant clusters using the Featquery tool in FSL. Then, we conducted two 3x2 ANOVAs, using SPSS, on 1) participants without diagnoses; 2) unmedicated participants.
Results
Behavioral data
There were no between-group differences in decision-making reaction time across all trials or across Reward and Loss trials separately.
Neuroimaging
Hypothesis 1
Activation
A 3(Group:BO/NBO/HC)x2(Condition:Win-Control/Loss-Control) ANOVA revealed main effects of Group (Figure 2A) and Condition (Table S2), but no Group x Condition interaction, on brain activation. A main effect of Group was found in the right frontal pole (RFP; nvox=66, z-max=4.0, [24,64,6], p-corrected<0.05). Follow up t-tests conducted on RFP activation values revealed that activation was significantly greater in BO than HC (t(50)=3.7,p<0.001), and just missed significance in BO vs. NBO (t(55)=2.3,p=0.02), using a Bonferroni-corrected statistical threshold of p=0.05/3 for between group tests p=0.017. NBO did not differ from BO.
Figure 2.
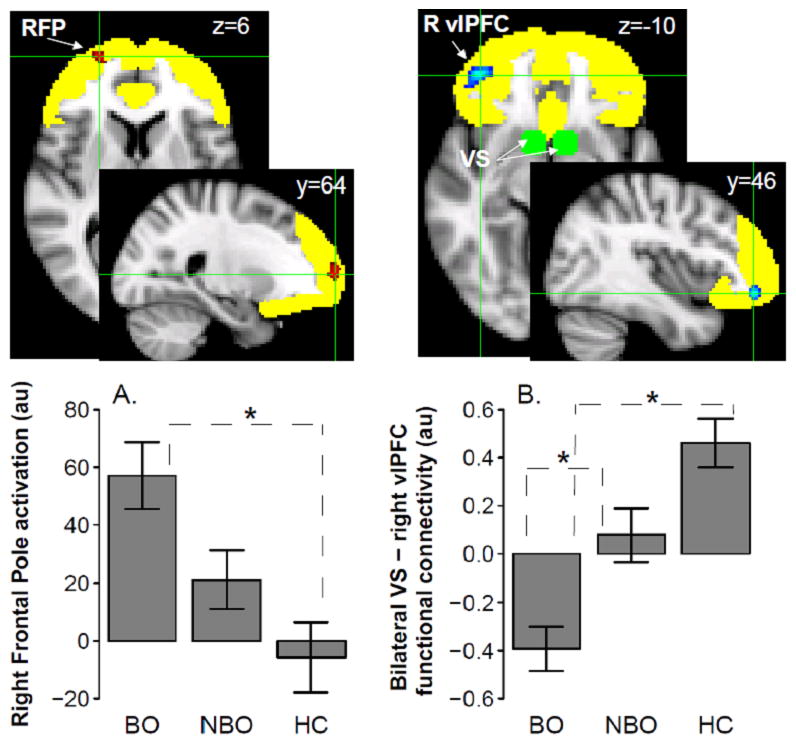
A main effect of Group on activation in the right frontal pole (RFP [24 64 6]; shown in red) and functional connectivity between the bilateral VS (centered 8 mm around [±9 9 –8]; shown in green) and the right vlPFC ( [40 46 –10]; shown in blue) for decision-making trials (i.e., Win and Loss trials) vs. non-decision-making trials (i.e., Control trials). The reward circuitry ROI mask is shown in yellow. “au” stands for arbitrary units. ‘*’ indicates significant t-test results. BO – offspring of parents with bipolar disorder, NBO – offspring of parents with psychiatric disorders other than bipolar disorder, HC – healthy offspring of psychiatrically healthy parents.
Hypothesis 2
Connectivity
The PPI analyses conducted with the bilateral VS as a seed region and the reward circuitry mask as a target region revealed a main effect of Group (Figure 2B), but no main effect of Condition or Group x Condition interaction, on functional connectivity. A main effect of Group was found in the right vlPFC( nvox=96,z-max=4.7, [40,46,–10], p-corrected<0.05). Follow up t-tests revealed that functional connectivity between bilateral VS and right vlPFC was significantly more negative, reflecting the fact that VS-right vlPFC connectivity was more positive for Control than for Win and Loss trials, in BO than NBO (t(55)=−3.3,p=0.002) and in BO than HC (t(50)=−6.2,p<0.001), using a Bonferroni-corrected threshold of p=0.017, as above.
Hypothesis 3
Activation and connectivity
The results of RFP activation and bilateral VS-right vlPFC connectivity analyses in unmedicated participants and those without psychopathology were consistent with the results of the full-sample analyses testing Hypotheses 1–2. There was a significant effect of Group on RFP activation (participants without psychopathology: F(2,54)=11.1,p<0.001; unmedicated participants: F(2,68)=7.3,p=0.001; Figure S1), and on bilateral VS-right vlPFC functional connectivity (participants without psychopathology: F(2,54)=14.4,p<0.001; unmedicated participants: F(2,68)=16.1,p<0.001; Figure S2).
The results of post-hoc comparisons paralleled main findings from Hypotheses 1–2. RFP activation was significantly greater in BO than in HC (participants without psychopathology: t(39)=4.3,p<0.001; unmedicated participants: t(45)=3.4,p=0.001) and was also significantly greater in NBO than in HC (participants without psychopathology: t(37)=2.9,p=0.006; unmedicated participants: t(45)=2.5, p=0.01). Bilateral VS-right vlPFC functional connectivity was significantly more negative in BO vs. NBO (participants without medications: t(46)=−3.3,p=0.002; the comparison just missed significance in BO and NBO without psychopathology: t(32)=−2.6,p=0.02); and in BO vs. HC (participants without psychopathology: t(39)=−5.8,p<0.001; unmedicated participants: t(45)=−6.0,p<0.001).
Exploratory analyses
Across all participants, RFP activation positively correlated with CALS-P (r=0.34,p=0.002) and MFQ-P (r=0.23,p=0.047). In BO, RFP activation positively correlated with CALS-P (r=0.37,p<0.05).
Discussion
The goal of the present study was to determine the extent to which alterations in reward circuitry function characterized at-risk youth offspring of parents with BD (BO) relative to offspring of non-bipolar parents (NBO) and youth offspring of psychiatrically healthy parents (HC). Main findings supported all three hypotheses. RFP activation for decision-making (Win and Loss) trials, relative to non-decision making (Control) trials, was significantly greater in BO than in HC, but did not differ significantly for BO vs. NBO, or for NBO vs. HC. Bilateral VS-right vlPFC functional connectivity was significantly more negative in BO than in NBO and HC, reflecting a pattern of greater positive connectivity to Control than either Win or Loss trials in BO than other groups. These patterns of activation and functional connectivity remained for umedicated participants and those without psychopathology, supporting our third hypothesis. These findings suggest that while elevated RFP activation may reflect risk for, or resilience against, future development of worsening psychopathology in general, abnormally decreased bilateral VS-right vlPFC functional connectivity to Win or Loss versus Control trials may reflect risk for, or resilience against, future development of BD specifically, in BO.
The FP is involved in decision-making and prospective memory by supporting the maintenance of delayed intentions and representations (Burgess et al. 2007; Koechlin & Hyafil 2007) and integrating outcomes of previous trials (Ramnani & Owen 2004) for potential use in future trials. The magnitude of FP activation positively correlated with amount of uncertainty remaining between multiple choices (Yoshida & Ishii 2006), and tracked unchosen options (Boorman et al. 2009). A recent study of adolescents with BD demonstrated significantly greater activation in the right frontal cortex [x=11,y=55,z=14] during reward anticipation in those with BD vs. HC (Singh et al. 2013). In our study, abnormally elevated RFP activation during decision-making trials in BO suggests that they may have experienced abnormal levels of uncertainty during these trials, and maintained non-chosen option-outcome contingencies in memory to predict (i.e., make more certain) response-outcome mapping for future trials. Furthermore, similar patterns of significantly elevated RFP activation during decision-making trials were present in unmedicated BO and NBO vs. HC, and in BO and NBO without psychopathology vs. HC. Given that BO were not different from NBO in their RFP activation, abnormally elevated RFP activation may represent a vulnerability marker for future development of, or resilience against future worsening of psychopathology in general, but not BD specifically.
The VS supports reward anticipation, reward evaluation and prediction error (Schultz et al. 2000; Knutson et al., 2001; Pagnoni et al. 2002; O’Doherty 2004; Tanaka et al. 2004). The vlPFC is implicated in learning the value of different options and formation of associations between visual stimuli and reward values (Rushworth et al. 2011). Functional interaction between prefrontal cortex and VS influences guided behavior and may be modulated by the environment (Del Arco & Mora 2009). In HC, an increase in bilateral VS activation was associated with increase in right vlPFC activation during decision-making (Win, Loss) vs. non-decision-making (Control) trials, highlighting a positive relationship between encoding stimulus-outcome associations and reward evaluation. In BO, an increase in bilateral VS activation was associated with decrease in right vlPFC activation, and vice versa, during decision-making vs. non-decision-making trials. Such an inverse relationship between activation in bilateral VS and right vlPFC may suggest that in BO, evaluation of reward and loss encoded by the vlPFC may be inhibited by formation of associations between reward values and corresponding visual stimuli in the VS, and vice versa.
Given that BO not only differed from HC, but also differed from NBO on the direction of VS-vlPFC functional connectivity, and that this pattern remained significant even for unmedicated participants and those without psychopathology, a pattern of aberrant negative bilateral VS-right vlPFC functional connectivity during decision-making trials may reflect a vulnerability marker for, or resilience against, BD, rather than for psychopathology in general. Because the between-group differences in functional connectivity were independent of the fact that BO, NBO and HC did not differ significantly in magnitude of activation in these regions, our findings may provide further support for dysfunctional coupling between the vlPFC and subcortical regions during emotionally-salient processing as a pathophysiological process underlying vulnerability to, or protection against, future BD (Altshuler et al. 2008; Phillips & Swartz 2014).
Exploratory analyses showed that greater RFP activation was associated with higher mood dysregulation scores (CALS-P) and higher MFQ across all participants, and with higher CALS-P in BO. Higher RFP activation during decision-making trials may thus be a precursor for development of mood dysregulation and depression. Future studies need to examine these exploratory findings.
The fact that findings remained significant even after approximately 40% of participants were removed for comparisons of healthy BO vs. healthy NBO vs. HC suggests that the pattern of findings was robust, at least with regard to the general BO and NBO populations. Given that only a small number of youth were taking medications, there was insufficient statistical power to directly compare unmedicated BO vs. medicated BO vs. unmedicated NBO vs. medicated NBO. Further studies should compare larger samples of medicated and unmedicated BO and NBO, and BO and NBO with and without current diagnoses. Additionally, future studies can also directly compare youth with established BD, and genetically and symptomatically at-risk youth, to determine the degree of similarity between neural measures of risk status and neural measures of BD.
In summary, our findings demonstrate, for the first time, that youth offspring, as yet unaffected with BD, of parents with BD exhibit altered patterns of frontal activation and vlPFC-striatal functional connectivity, that distinguish these youth from youth offspring of parents unaffected with BD. These activation and connectivity differences remained significant for participants without current psychopathology and medication history and may represent neurobiological markers conferring either risk for, or protection against, BD in youth. Future, longitudinal follow-up studies in youth at-risk for BD should aim to distinguish between these two possibilities, by determining the extent to which abnormal patterns of reward circuitry functioning predict, or protect against, development of BD, and development/worsening of affective pathology in general.
Supplementary Material
Acknowledgments
The authors thank the families for participating in this research study.
Financial support: This work was supported by grants from the National Institute of Health 2 R01 MH060952-12S1 to Birmaher, Axelson, Phillips (MPIs); R01 MH073953 to Birmaher and Phillips (MPIs); K01 MH104348 to Manelis; and Pittsburgh Foundation to Phillips.
Footnotes
Conflict of Interest Disclosures: None reported.
Ethical standards: The authors assert that all procedures contributing to this work comply with the ethical standards of the University of Pittsburgh Institutional Review Board and with the Helsinki Declaration of 1975, as revised in 2008.
Financial Disclosures: The authors report no financial relationships with commercial interests. Dr. Phillips was supported by R01MH100041, U01 MH092221, R01 MH073953, R01 MH060952. Dr. Birmaher has or will receive royalties for publications from Random House, Inc. (New Hope for Children and Teens with Bipolar Disorder), Lippincott Williams & Wilkins (Treating Child and Adolescent Depression). Dr. T. Goldstein has received research support from NIMH, NIDA, NICHD, The Fine Foundation, The Ryan Licht Sang Foundation, and The Pittsburgh Foundation, and royalties from Guilford Press. Dr. B. Goldstein is supported by a New Investigator Award from the Canadian Institutes for Health Research (CIHR). Other authors report no biomedical financial interests or potential conflicts of interest.
References
- Alloy LB, Bender RE, Whitehouse WG, Wagner CA, Liu RT, Grant DA, Jager-Hyman S, Molz A, Choi JY, Harmon-Jones E, Abramson LY. High Behavioral Approach System (BAS) sensitivity, reward responsiveness, and goal-striving predict first onset of bipolar spectrum disorders: a prospective behavioral high-risk design. Journal of Abnormal Psychology. 2012;121(2):339–351. doi: 10.1037/a0025877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler L, Bookheimer S, Townsend J, Proenza MA, Sabb F, Mintz J, Cohen MS. Regional brain changes in bipolar I depression: a functional magnetic resonance imaging study. Bipolar Disorders. 2008;10(6):708–717. doi: 10.1111/j.1399-5618.2008.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Jenkinson M, Smith S. Technical Report FMRIB Technical Report TR07JA2. Oxford: FMRIB Centre; 2007. Non-linear registration aka spatial normalisation. [Google Scholar]
- Angold A, Prendergast M, Cox A, Harrington R, Simonoff E, Rutter M. The Child and Adolescent Psychiatric Assessment (CAPA) Psychological Medicine. 1995;25(4):739–753. doi: 10.1017/s003329170003498x. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3(7):563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Bebko G, Bertocci MA, Fournier JC, Hinze AK, Bonar L, Almeida JRC, Perlman SB, Versace A, Schirda C, Travis M, Gill MK, Demeter C, Diwadkar VA, Ciuffetelli G, Rodriguez E, Olino T, Forbes E, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson D, Horwitz SM, Arnold LE, Fristad MA, Youngstrom EA, Findling RL, Phillips ML. Parsing Dimensional vs Diagnostic Category-Related Patterns of Reward Circuitry Function in Behaviorally and Emotionally Dysregulated Youth in the Longitudinal Assessment of Manic Symptoms Study. JAMA Psychiatry. 2014;71:71–80. doi: 10.1001/jamapsychiatry.2013.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermpohl F, Kahnt T, Dalanay U, Hgele C, Sajonz B, Wegner T, Stoy M, Adli M, Krger S, Wrase J, Strhle A, Bauer M, Heinz A. Altered representation of expected value in the orbitofrontal cortex in mania. Human Brain Mapping. 2010;31(7):958–969. doi: 10.1002/hbm.20909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Goldstein B, Monk K, Kalas C, Obreja M, Hickey MB, Iyengar S, Brent D, Shamseddeen W, Diler R, Kupfer D. Psychiatric disorders in preschool offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study (BIOS) American Journal of Psychiatry. 2010;167(3):321–330. doi: 10.1176/appi.ajp.2009.09070977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Axelson D, Monk K, Kalas C, Goldstein B, Hickey MB, Obreja M, Ehmann M, Iyengar S, Shamseddeen W, Kupfer D, Brent D. Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring study. Archives of General Psychiatry. 2009;66(3):287–296. doi: 10.1001/archgenpsychiatry.2008.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, Neer SM. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(4):545–553. doi: 10.1097/00004583-199704000-00018. [DOI] [PubMed] [Google Scholar]
- Boorman ED, Behrens TEJ, Woolrich MW, Rushworth MFS. How green is the grass on the other side? Frontopolar cortex and the evidence in favor of alternative courses of action. Neuron. 2009;62(5):733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Burgess PW, Gilbert SJ, Dumontheil I. Function and localization within rostral prefrontal cortex (area 10) Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2007;362(1481):887–899. doi: 10.1098/rstb.2007.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, Buckner RL, Snyder AZ, Petersen SE, Schlaggar BL. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Cardoso de Almeida JR, Phillips ML. Distinguishing between unipolar depression and bipolar depression: current and future clinical and neuroimaging perspectives. Biological Psychiatry. 2013;73(2):111–118. doi: 10.1016/j.biopsych.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. American Journal of Psychiatry. 2013;170(5):533–541. doi: 10.1176/appi.ajp.2012.12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Nusslock R, Almeida JR, Forbes EE, Labarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disorders. 2013;15(8):839–854. doi: 10.1111/bdi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremers HR, Veer IM, Spinhoven P, Rombouts SARB, Roelofs K. Neural sensitivity to social reward and punishment anticipation in social anxiety disorder. Frontier in Behavioral Neuroscience. 2014;8:439. doi: 10.3389/fnbeh.2014.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxson PL, Walton ME, O’Reilly JX, Behrens TEJ, Rushworth MFS. Effort-based cost-benefit valuation and the human brain. Journal of Neuroscience. 2009;29(14):4531–4541. doi: 10.1523/JNEUROSCI.4515-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Arco A, Mora F. Neurotransmitters and prefrontal cortex- limbic system interactions: implications for plasticity and psychiatric disorders. Journal of Neural Transmission. 2009;116(8):941–952. doi: 10.1007/s00702-009-0243-8. [DOI] [PubMed] [Google Scholar]
- Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biological Psychiatry. 2009;66(12):1083–1090. doi: 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Dickstein DP, Munson S, Eshel N, Pradella A, Jazbec S, Pine DS, Leibenluft E. Reward-related processes in pediatric bipolar disorder: a pilot study. Journal of Affective Disorders. 2004; 82(Suppl 1):S89–S101. doi: 10.1016/j.jad.2004.05.022. [DOI] [PubMed] [Google Scholar]
- Findling RL, Youngstrom EA, Fristad MA, Birmaher B, Kowatch RA, Arnold LE, Frazier TW, Axelson D, Ryan N, Demeter CA, Gill MK, Fields B, Depew J, Kennedy SM, Marsh L, Rowles BM, Horwitz SM. Characteristics of children with elevated symptoms of mania: the Longitudinal Assessment of Manic Symptoms (LAMS) study. Journal of Clinical Psychiatry. 2010;71(12):1664–1672. doi: 10.4088/JCP.09m05859yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. (SCID-I/NP) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- FitzGerald THB, Friston KJ, Dolan RJ. Action-specific value signals in reward-related regions of the human brain. Journal of Neuroscience. 2012;32(46):16417–1623a. doi: 10.1523/JNEUROSCI.3254-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2009;14(1):60–70. doi: 10.1038/sj.mp.4002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gerson AC, Gerring JP, Freund L, Joshi PT, Capozzoli J, Brady K, Denckla MB. The Children’s Affective Lability Scale: a psychometric evaluation of reliability. Psychiatry Research. 1996;65(3):189–198. doi: 10.1016/s0165-1781(96)02851-x. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Shamseddeen W, Axelson DA, Kalas C, Monk K, Brent DA, Kupfer DJ, Birmaher B. Clinical, demographic, and familial correlates of bipolar spectrum disorders among offspring of parents with bipolar disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2010;49(4):388–396. [PMC free article] [PubMed] [Google Scholar]
- Gorrindo T, Blair R, JR, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. American Journal of Psychiatry. 2005;162(10):1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- Greenberg T, Chase HW, Almeida JR, Stiffler R, Zevallos CR, Aslam H, Deckersbach T, Weyandt S, Cooper C, Toups M, Carmody T, Kurian B, Peltier S, Adams P, McInnis MG, Oquendo MA, McGrath PJ, Fava M, Weissman M, Parsey R, Trivedi MH, Phillips ML. Anhedonia moderates the relationship between reward expectancy and prediction error-related ventral striatal reactivity in unmedicated major depressive disorder: Findings from the EMBARC study. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2015.14050594. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Detloff A, Benson B, Nelson EE, Perez-Edgar K, Fox NA, Pine DS, Ernst M. Striatal functional alteration during incentive anticipation in pediatric anxiety disorders. American Journal of Psychiatry. 2012;169(2):205–212. doi: 10.1176/appi.ajp.2011.11010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AA. Four Factor Index of Social Status. Yale University Department of Sociology; New Haven, CT: 1975. Unpublished manuscript. [Google Scholar]
- Horwitz SM, Demeter CA, Pagano ME, Youngstrom EA, Fristad MA, Arnold LE, Birmaher B, Gill MK, Axelson D, Kowatch RA, Frazier TW, Findling RL. Longitudinal Assessment of Manic Symptoms (LAMS) study: background, design, and initial screening results. Journal of Clinical Psychiatry. 2010;71(11):1511–1517. doi: 10.4088/JCP.09m05835yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez A, Cetkovich M, Petroni A, Urquina H, Baez S, Gonzalez-Gadea ML, Kamienkowski JE, Torralva T, Torrente F, Strejilevich S, Teitelbaum J, Hurtado E, Guex R, Melloni M, Lischinsky A, Sigman M, Manes F. The neural basis of decision-making and reward processing in adults with euthymic bipolar disorder or attention-deficit/hyperactivity disorder (ADHD) PLoS One. 2012;7(5):e37306. doi: 10.1371/journal.pone.0037306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magnetic Resonance in Medicine. 2003;49(1):193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kang HC, Burgund ED, Lugar HM, Petersen SE, Schlaggar BL. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2003;19(1):16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child & Adolescent Psychiatry. 1997;36(7):980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21(16):RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E, Hyafil A. Anterior prefrontal function and the limits of human decision-making. Science. 2007;318(5850):594–598. doi: 10.1126/science.1142995. [DOI] [PubMed] [Google Scholar]
- Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain. 2008;131(Pt 8):2084–2093. doi: 10.1093/brain/awn136. [DOI] [PubMed] [Google Scholar]
- Kurniawan IT, Seymour B, Talmi D, Yoshida W, Chater N, Dolan RJ. Choosing to make an effort: the role of striatum in signaling physical effort of a chosen action. Journal of Neurophysiology. 2010;104(1):313–321. doi: 10.1152/jn.00027.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason L, O’Sullivan N, Blackburn M, Bentall R, El-Deredy W. I want it now! Neural correlates of hypersensitivity to immediate reward in hypomania. Biological Psychiatry. 2012;71(6):530–537. doi: 10.1016/j.biopsych.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Nusslock R, Almeida JR, Forbes EE, Versace A, Frank E, Labarbara EJ, Klein CR, Phillips ML. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disorders. 2012;14(3):249–260. doi: 10.1111/j.1399-5618.2012.01012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current Opinion in Neurobiology. 2004;14(6):769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olsavsky AK, Brotman MA, Rutenberg JG, Muhrer EJ, Deveney CM, Fromm SJ, Towbin K, Pine DS, Leibenluft E. Amygdala hyperactivation during face emotion processing in unaffected youth at risk for bipolar disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(3):294–303. doi: 10.1016/j.jaac.2011.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnoni G, Zink CF, Montague PR, Berns GS. Activity in human ventral striatum locked to errors of reward prediction. Nature Neuroscience. 2002;5(2):97–98. doi: 10.1038/nn802. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. American Journal of Psychiatry. 2014;171(8):829–843. doi: 10.1176/appi.ajp.2014.13081008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips ML, Kupfer DJ. Bipolar disorder diagnosis: challenges and future directions. Lancet. 2013;381(9878):1663–1671. doi: 10.1016/S0140-6736(13)60989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nature Reviews Neuroscience. 2004;5(3):184–194. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- Rich BA, Schmajuk M, Perez-Edgar KE, Pine DS, Fox NA, Leibenluft E. The impact of reward, punishment, and frustration on attention in pediatric bipolar disorder. Biological Psychiatry. 2005;58(7):532–539. doi: 10.1016/j.biopsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Rushworth MFS, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70(6):1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nature Reviews Neuroscience. 2013;14(9):609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10(3):272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Singh MK, Chang KD, Kelley RG, Cui X, Sherdell L, Howe ME, Gotlib IH, Reiss AL. Reward processing in adolescents with bipolar I disorder. Journal of the American Academy of Child & Adolescent Psychiatry. 2013;52(1):68–83. doi: 10.1016/j.jaac.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh MK, Kelley RG, Howe ME, Reiss AL, Gotlib IH, Chang KD. Reward processing in healthy offspring of parents with bipolar disorder. JAMA Psychiatry. 2014;71(10):1148–1156. doi: 10.1001/jamapsychiatry.2014.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana Y, Hikosaka O. The primate ventral pallidum encodes expected reward value and regulates motor action. Neuron. 2012;76(4):826–837. doi: 10.1016/j.neuron.2012.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nature Neuroscience. 2004;7(8):887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- Trost S, Diekhof EK, Zvonik K, Lewandowski M, Usher J, Keil M, Zilles D, Falkai P, Dechent P, Gruber O. Disturbed anterior prefrontal control of the mesolimbic reward system and increased impulsivity in bipolar disorder. Neuropsychopharmacology. 2014;39(8):1914–1923. doi: 10.1038/npp.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Behrens TEJ, Noonan MP, Rushworth MFS. Giving credit where credit is due: orbitofrontal cortex and valuation in an uncertain world. Annals of the New York Academy of Sciences. 2011;1239:14–24. doi: 10.1111/j.1749-6632.2011.06257.x. [DOI] [PubMed] [Google Scholar]
- Walton ME, Kennerley SW, Bannerman DM, Phillips PEM, Rushworth MFS. Weighing up the benefits of work: behavioral and neural analyses of effort-related decision making. Neural Networks. 2006;19(8):1302–1314. doi: 10.1016/j.neunet.2006.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. The Psychological Corporation; San Antonio, TX: 1999. [Google Scholar]
- Worsley K. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. Oxford University Press; 2001. pp. 251–270. [Google Scholar]
- Yoshida W, Ishii S. Resolution of uncertainty in prefrontal cortex. Neuron. 2006;50(5):781–789. doi: 10.1016/j.neuron.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA, Frazier TW, Demeter C, Calabrese JR, Findling RL. Developing a 10- item mania scale from the Parent General Behavior Inventory for children and adolescents. Journal of Clinical Psychiatry. 2008;69(5):831–839. doi: 10.4088/jcp.v69n0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


