Abstract
Background
Athletes who return to sport after anterior cruciate ligament reconstruction are at increased risk of future ACL injury. Altered coordination of lower extremity motion may increase this risk. The purpose of this study was to prospectively determine if altered lower extremity coordination patterns exist in athletes who go on to sustain a 2nd anterior cruciate ligament injury.
Methods
Sixty-one female athletes who were medically cleared to return to sport after anterior cruciate ligament reconstruction were included. Hip-ankle coordination was assessed prior to return to sport with a dynamic postural coordination task. Within 12 months, 14 patients sustained a 2nd ACL injury. Fourteen matched subjects were selected for comparative analysis. Cross-recurrence quantification analysis characterized hip-ankle coordination patterns. A group × target speed (slow vs. fast) × leg (involved vs. uninvolved) analysis of variance was used to identify coordination differences.
Findings
A main effect of group (p = 0.02) indicated that the single injury group exhibited more stable hip-ankle coordination [166.2 (18.9)] compared to the 2nd injury group [108.4 (10.1)]. A leg × group interaction was also observed (p = .04). The affected leg of the single injury group exhibited more stable coordination [M = 187.1 (23.3)] compared to the affected leg of the 2nd injury group [M = 110.13 (9.8)], p = 0.03.
Interpretation
Hip-ankle coordination was altered in female athletes who sustained a 2nd anterior cruciate ligament injury after return to sport. Failure to coordinate lower extremity movement in the absence of normal knee proprioception may place the knee at high-risk.
Keywords: ACL Reconstruction, Postural Coordination, Second Injury, ACL, injury, recurrent injury
Introduction
An estimated 100,000 to 200,000 anterior cruciate ligament (ACL) injuries occur annually in the United States with the majority of patients electing to undergo ACL reconstruction[1] (ACLR). Athletes who attempt to return to sport (RTS) after ACLR are as much as 15 times more likely to sustain a second ACL injury to either knee in the first year after they return to sport.[2] Despite high second injury rates in ACLR, there is a lack of consensus regarding the underlying mechanism placing these athletes at increased risk for future injury. The current published literature has reported the relationship of various factors to second ACL injury rates, including demographic variables,[3] graft type, [4] biomechanical, neuromuscular and proprioceptive factors[5]. Furthermore, other impairments in strength[6], balance[7, 6], proprioception[6], and limb symmetry with dynamic tasks[8-10] may extend for over 2 years after RTS from ACLR. Despite this extensive research, the effect of ACL injury and ACLR on postural coordination remains unknown.
Postural coordination has been defined as the coordination between various body parts that underlies the integration of the maintenance of upright stance.[11] The absence of joint position awareness at the knee joint may result in impairments to postural coordination due to altered muscle recruitment with dynamic movements, deficits in joint stability, and decreased ability to control normal movement.[12-14] Coordinated movements of the hip and ankle are critical to optimally position the knee joint in the absence of normal knee proprioception during dynamic athletic movements. Failure to coordinate the movements of the joints proximal and distal to the knee has the potential to place the knee joint in high-risk positions during dynamic movements. The failure to optimally position the knee may make the passive structures susceptible to pathologic stresses that increase the risk of subsequent ligament or graft failure following ACLR.
Abnormal sagittal plane joint coupling patterns between the hip and ankle in patients following ACLR have been identified in the literature when compared to healthy control subjects.[15] Coupling refers to the synergistic movement of multiple segments to coordinate a gross movement. Despite identification of this difference between ACLR patients and controls, current research has yet to identify whether unique patterns of abnormal postural coordination and joint coupling are residual impairments associated with a high risk for future ACL injury.
The purpose of this prospective study was to determine if there are altered sagittal plane postural coordination patterns in female athletes who subsequently go on to suffer a second ACL injury to either limb after ACLR and RTS. The hypothesis tested was that athletes who subsequently sustained a second ACL injury would demonstrate altered sagittal plane, hip-ankle postural coordination patterns indicative of persistent sensorimotor deficits leading to abnormal joint coupling patterns at the time of RTS compared to female athletes who would not subsequently sustain a second ACL injury.
Methods
Participants
Sixty-one female pivoting and cutting athletes with a primary, unilateral ACLR were prospectively tested and then tracked for 12 months following RTS to identify those who went on to a second ACL injury. Testing occurred when the subject was medically cleared to return to pivoting and cutting sports after ACLR by their physician and rehabilitation specialist. All subjects returned to a Level I/II pivoting or cutting sport.[16] Within 12 months of RTS, 14 subjects (11 soccer players; 4 basketball players) subsequently sustained a second, non-contact ACL injury (N = 14). Those subjects formed a second injury (ACL2) group (Table 1)Four of these subjects sustained an ipsilateral graft re-tear and 10 suffered a contralateral ACL injury. The ACL2 group was matched in height, weight, BMI, and age (within two years) to 14 athletes (9 soccer, 3 basketball, 1 lacrosse, 1 tennis athlete) who did not sustain a second injury [ACL1 group) (Table 1). Mean time from surgical reconstruction to testing was 8.1(1.8) months, and all athletes participated in the experiment within four weeks of their RTS date following their initial injury. Exclusion criteria included history of an ACL injury prior to their first reported ACL injury in the present experiment, recent injury to the spine, hips, ankles or contralateral knee in the last 12 months, or failure to return to prior level of sport participation as measured by Tegner activity scale. Demographic data for each group are presented in Table 1. Sufficient sample size was estimated using historical data on comparable populations. These analyses estimated between 7-14 subjects per group were required to attain sufficient statistical power (.80). Therefore, 14 subjects were included per group to ensure sufficient power for all analyses.
Table 1.
Patient Demographics
| Subject Enrolled (n=28) | |||
|---|---|---|---|
| ACLR2 | ACLR1 | p-value | |
| Mean Age (SD) in years | 15.4 (0.5) | 17.2 (0.6) | 0.22 |
| Mean Height (SD) in meters | 1.65 (0.02) | 1.65 (0.01) | 0.98 |
| Mean Weight (SD) in kilograms | 56.8 (1.4) | 56.7 (1.5) | 0.75 |
| Mean BMI (SD) in kg/m2 | 20.8 (0.5) | 20.7 (0.6) | 0.77 |
| Tegner Activity Level at RTS | 8.8 (0.5) | 8.4 (1.6) | 0.26 |
Apparatus
Each participant was instrumented with 37 retro-reflective markers as previously described.[5](Figure 1) Three-dimensional motion capture was used to record kinematics at a sampling rate of 60 Hz via a ten-camera digital motion capture system (Motion Analysis Corp., Santa Rosa, CA) and post-processed with EVaRT (Version 4 Motion Analysis Corp., Santa Rosa, CA) and Matlab (Mathworks, Inc., Natick, MA) software.
Figure 1.
Sample marker set
Procedure
Participants provided informed consent prior to participation and the IRB approved all procedures. Each participant first completed a standing reference trial. The postural coordination task required participants to stand on a single leg and track the anterior-posterior (AP) movement of a square target (15.7 cm × 15.7 cm, subtending 8.92 × 8.92° visual angles) presented on a computer monitor at eye level, 1 m away. The target oscillated at one of two frequencies (0.2 Hz or 0.7 Hz). Participants tracked the motion of the target with their head so as to maintain a constant perceived distance between their head and the target by matching the amplitude of the target oscillations (apparent amplitude = 44 cm). (Figure 2) This amplitude was similar to that of previous work.[15] No explicit instructions were given to participants to produce oscillations about the ankle or hip, nor were they instructed to adopt a specific postural coordination pattern. Thus, the postural coordination patterns that participants adopted spontaneously emerged to subserve task performance. A trial was deemed successful and included in the final analysis if the participant completed 10 consecutive target oscillation cycles while maintaining the position of the foot on the floor. If the participant lost balance and their other foot touched the ground during the trial prior to the completion of 10 cycles, data collection continued until 10 consecutive, uninterrupted oscillation cycles were completed. Four trials (two frequencies on each leg) were performed in random order.
Figure 2.
Demonstration of testing procedure: The subject is instructed to track a moving square on a screen in front of them. As the square appears to move away from them they move forward and as the square appears to approach them the move backwards.
Data reduction and analysis
Kinematic data were filtered in Matlab using a low-pass Butterworth filter (5 Hz cut-off frequency) based on a residual analysis. Custom Matlab routines (modified from KineMat Toolbox[17] were used to quantify sagittal plane ankle and hip joint angles.
Summary measure of variability
The standard deviation of the ankle and hip angular position in the sagittal plane (SDankle and SDhip, respectively) was computed to investigate the variability of movement at each joint. Continuous relative phase takes into account positions at each sample in the calculation of M and SD relative phase. Thus, all summary measures are based on the continuous, sample-to-sample comparison of the two time series.
Cross-recurrence quantification
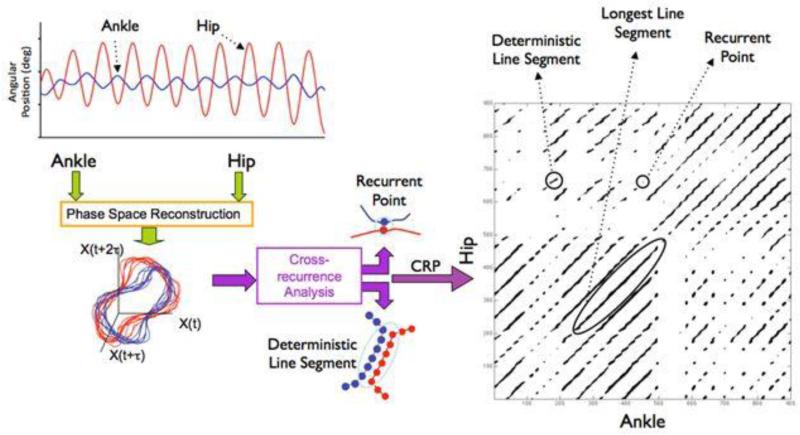
CRQ is a multivariate nonlinear analysis that, in the present experiment, indexed the repeated patterns of ankle and hip angular position over time for the examination of postural coordination patterns between the two joints. The methodological details of cross-recurrence quantification (CRQ) have been discussed in detail elsewhere[15, 18, 19] and the methodology used in the current experiment is similar to previous work in this domain.[15, 20] CRQ is useful in that it has the potential to elucidate potential underlying mechanisms that give rise to variations in postural coordination patterns (e.g., anatomical, neuromuscular, or physiological changes due to pathology). This analysis consists of plotting the ankle and hip kinematic time series data in the same reconstructed phase space, determining the instances where the movement of the two joints coordinate (i.e., recurrences—when the two joint time series overlap). This relationship is then quantified based on the frequency and organization of the coordinated patterns (Figure 3). These measures have previously been associated with degree of coordination, degree of neuromotor noise, and proprioceptive deficits.[21, 22]
Figure 3.
Schematic of the computational process of CRQ. First the ankle and hip time series are embedded in higher-dimensional space. Next, the trajectories are compared to determine where they recur, or repeat, within some tolerance. Each instance of intersection between the two trajectories is a single recurrence and depicted on the cross-recurrence plot (CRP) as a darkened pixel and make up the %CREC measure. Strings of these darkened pixels are consecutive time points over which the two signals co-evolve and make up the %CDET measure. The longest line segment, CML, is the longest number of consecutive time points over which the two signals evolved.
The analysis compares the trajectories of x (i.e., the ankle angular position time series) and y (i.e., the hip angular position time series) to determine where they intersect in space. A hyper-sphere with radius r is placed at each value of the x time series, and for each of those values of x any values of the y time series that fall within the hyper-sphere are determined. The radius values were determined by plotting the %CREC by radius and fitting a line to identify a stable region in the plot in which the change in %CREC stabilized, while keeping the radius around 5%. This is similar to other studies in the literature.[23, 24] The radius parameter is a sensitivity parameter that represents a cut-off limit thereby dictating which data points are considered recurrent. A radius size that is too large would saturate the recurrence plot, and the measures would lose all sensitivity. Thus, the consensus for the correct %CREC is approximately 5% to allow for recurrent patterns to be observed while not saturating the plot. Each instance of intersection within the radius is a single recurrence. The radius acts as a sensitivity parameter for identifying “neighboring” points in the reconstructed phase space (e.g., points where the actual hip and ankle angular positions might coarsely intersect). Specifically, the “neighborliness” of points in phase space indicates time points of common configurations between the hip and ankle joint time series. Ultimately, the identification of these states can illustrate patterns of postural coordination and are sensitive to subtle changes in the evolving time-specific properties of the ankle and hip joints. Additional CRQ parameters were determined as follows, consistent with standard practice using these methods[23, 24]. Embedding dimensions were selected based on the nearest neighbors analysis using the original signal and time delayed copies of it as dimensions x(t), x(t + delay), x(t + [2 × delay]), etc. Delay was selected that corresponded to the first local minimum average mutual information between points separated by that distance and, accordingly, results in nearly orthogonal dimensions in embedding space. Visually, the results of the recurrence calculations can be appreciated on a cross-recurrence plot, or CRP, which is a 2-D plot of time points of x vs. time points of y, with a darkened pixel at each (x, y) coordinate at which a recurrence in reconstructed phase space was identified (Figure 3, right).
The quantification of recurrences is computed from the darkened pixels in the CRP. The number and temporal distribution of recurrences indexes the coordination between the two signals. Three quantitative measures are of principal importance in the present study:
%CREC
The first is %CREC (%cross-recurrence), the percentage of darkened pixels in the plot. %CREC indicates the overall recurrences, which indicate the degree to which hip and ankle trajectories overlap as opposed to occupying distinct locations in the phase space. In other words, %CREC captures the number of shared joint configurations relative to all joint configurations, with a higher %CREC indicative of less noisy coordination.
%CDET
The second measure is %CDET (%cross-determinism), the percentage of darkened pixels that fall along diagonal lines in the CRP, which expresses how the trajectories of the two signals evolve together over time (i.e., the extent to which the two trajectories exhibit a common pattern). When more recurrent points fall along diagonal lines, this indicates that more segments of the trajectories of x and y are co-evolving. By quantifying the proportion of common patterns, %CDET indexes the regularity in the coordinated movement between x and y [24].
CML
CML[19] (cross-maxline) is the length of the longest diagonal line and is an indication of the coupling strength (i.e., a longer line indicates that the two signals were able to maintain a common pattern longer) as shown by simulations and empirical studies.[25, 22, 26] The CRQA measures are thus informative about the overall amount or degree of multi-joint postural coordination and how this might be affected by mechanistic factors such as neuromotor noise (%CREC) and the strength of joint coupling (CML).[25, 15] For example, if a subject produced postural coordination patterns indexed by lower %CREC but CML values comparable to other subjects, the subject's coordination patterns would be deemed comparatively noisy. If instead lower CML values characterized the subject's coordination patterns, this would indicate no change in neuromotor noise but instead a reduced strength of coupling between the ankle and hip joints. All CRQ results were computed using the cross recurrence plot toolbox.[18]
Statistical analysis
The dependent measures (SDankle, SDhip, %CREC, %CDET and CML) were submitted to a 2 × 2 × 2 mixed-model ANOVA with group (ACL1 vs. ACL2) as a between-subjects factor and stimulus frequency (low vs. high) and leg (injured vs. unaffected) as within-subjects factors (α = .05). All underlying assumptions of ANOVA were confirmed based on a descriptive exploratory analysis and distribution plotting. Only the variable CML violated normality and a data transformation was performed to ensure that normality assumptions were not violated. The data were transformed back for mean/SD reporting. Bonferroni-corrected paired-samples t-tests were used for follow-up comparisons, when appropriate.
Results
Summary measure of variability
A main effect of group was observed for SDankle, F(1,26) = 4.235, p = .05, η2 = 0.14, which indicated that the ACL2 group exhibited a decreased amount of variability, or more rigid posture, in overall ankle movement [0.001 (0.0001°)] compared to the ACL1 group [0.002 (0.0005°)] (Table 2). The interactions were not significant (p > .05). No significant differences were found for SDhip (p > .05).
Table 2.
Results of ACLR1 vs. ACLR2
| ACLR1 | ACLR2 | p-value | |
|---|---|---|---|
| Variability of Ankle Motion (SD) in degrees) | 0.002 (0.0005) | 0.001 (0.0001) | .05 |
| Variability of Hip Motion (SD) in degrees | 0.029 (0.012) | 0.028 (0.011) | >.05 |
| %CREC (SD) in % | 0.055 (0.016) | 0.067 (0.022) | .02 |
| %CDET (SD) in % | 0.987 (0.010) | 0.979 (0.010) | .01 |
| CML (SD) in no. of samples | 166.5 (102.7) | 108.4 (51.9) | .02 |
SD= Standard deviation; %CREC = percent cross recurrence; %CDET = percent cross determinism; CML = cross maxline
CRQ
CRP features
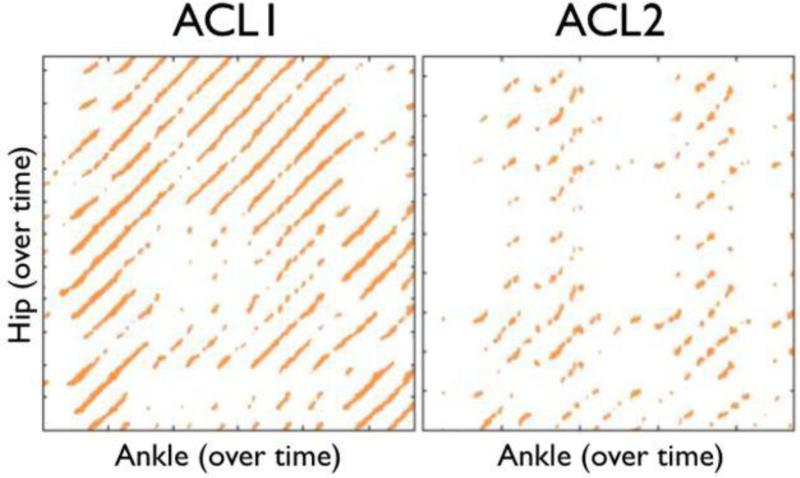
Figure 4 shows sample CRPs for an ACL1 athlete (left) and an ACL2 athlete (right). There are notable visual differences between the plots, with a striking degradation in the structure of the ACL2 plot.
Figure 4.
Sample cross-recurrence plots for a single ACLR athlete who did not go on to second injury (ACL1—left) and an ACLR athlete who did go on to a second injury (ACL2—right). Note the degradation in the structure of the darkened pixels in the ACL2 plot compared to the ACL1 plot. The ACL2 athlete exhibits less tightly coupled coordination patterns and a qualitative breakdown in coordination.
Transformation of Data
CML exhibited a moderate negative skew. Thus, prior to statistical analysis a square-root transform was applied such that
| (1) |
where k equates to a constant from which each score is subtracted so that the smallest score in the data set is equal to 1 (usually equal to the largest score + 1). All descriptive values of CML are presented in their actual, pre-transformed form.
%CREC
A group main effect was found for %CREC, F(1,26) = 5.73, p = .024, η2 = 0.18, and indicated that the ACL2 group exhibited a lower %CREC, or noisier pattern of coordination, compared to the ACL1 group. (Table 2) A main effect of frequency was also found, F(1,26) = 10.95, p = .003, η2 = 0.30; subjects in both groups exhibited noisier postural coordination in the low-frequency condition than when the stimulus oscillated at a high frequency. (Table 3)No other differences or interactions were found (p > .05).
Table 3.
Significant Results of Low Frequency vs. High Frequency
| Low | High | p-value | |
|---|---|---|---|
| %CREC (SD) in % | 0.057 (0.019) | 0.066 (0.020) | .003 |
| %CDET (SD) in % | 0.985 (0.010) | 0.981 (0.011) | .039 |
SD= Standard deviation; %CREC = percent cross recurrence; %CDET = percent cross determinism
%CDET
The analysis of %CDET indicated a significant main effect of group, F(1,26) = 8.94, p = .006, η2 = 0.26, indicating the ACL2 group's postural coordination patterns were less deterministic (i.e., the ankle and hip shared fewer patterns over time, or were less regular) than the ACL1 group's postural coordination patterns and ,respectively (Table 2). A main effect of frequency was also found, F(1,26) = 4.707, p = .039, η2 = 0.15; subjects exhibited a more deterministic pattern of coordination in the high-frequency condition than in the low-frequency condition. (Table 3)
%CML
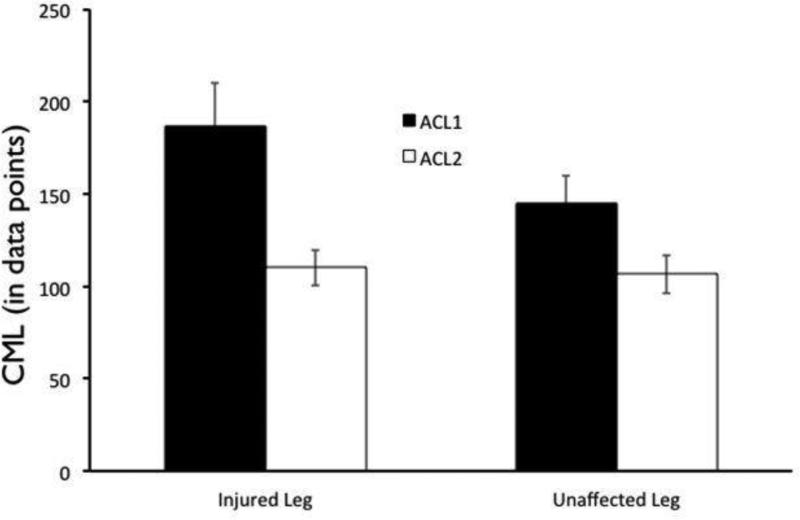
A main effect of group was found for CML, F(1,26) = 6.45, p = .017, η2 = 0.20; the ACL2 group exhibited significantly lower CML compared to the ACL1 group(Table 2). A leg × group interaction was also observed (see Figure 5), F(1,26) = 4.56, p = .042, η2 = 0.15. A Mann-Whitney test was employed due to unequal variances between groups, and indicated that the involved leg of the ACL1 group exhibited stronger joint coupling compared to the involved leg of the ACL2 group,p = .027. (Table 4) The involved limb of the ACL1 group also exhibited stronger joint coupling compared to their uninvolved limb [p = .029], whereas no limb difference was found for the ACL2 group [p > .05]. (Table 4) A main effect of leg, F(1,26) = 4.41, p = .046, η2 = .15, was also found, which indicated that the involved leg exhibited a stronger coupling [M = 148.59 (13.47)] compared to the uninvolved leg [M = 126.23 (9.00)], irrespective of group. No other comparisons were significant (p > .05).
Figure 5.
Significant Group × Leg interaction for CML in the comparison of ACLR athletes (ACL1) to second injury athletes (ACL2). Second injury athletes demonstrate a significantly weaker coupling between the ankle and the hip on both legs, compared to those who do not go on to second injury.
Table 4.
Cross Max Line Comparison [CML(SD) in number of samples] p=0.042
| ACLR1 | ACLR2 | p-value | |
|---|---|---|---|
| Involved Limb | 187.09(102.41) | 110.13(40.38) | .027 |
| Uninvolved Limb | 145.82(55.67) | 106.63(41.03) | >.05 |
| p-value | .029 | >.05 |
SD= Standard deviation; %CREC = percent cross recurrence; %CDET = percent cross determinism
Discussion
The results of this investigation support the stated hypothesis that female athletes who subsequently sustained a second ACL injury after ACLR and RTS would present with altered patterns of postural coordination at the time of RTS following initial ACLR. Specifically, females who subsequently sustained a second ACL injury demonstrated a more rigid posture (decreased variability of movement) at the ankle, less coordinated movements, and weaker coupling of movements between the hip and ankle joints. To the authors’ knowledge, this represents the first report in the published literature of the presence of identified postural coordination abnormalities in a population of athletes after ACLR who subsequently sustain a second ACL injury.
Variability in Magnitude of Movement
Alterations in the magnitude of variability of movement have been described in a variety of populations in the medical literature.[27, 28] Decker et al[28] suggested that kinematic movement patterns characterized either by too little or excessive variability can result in an increased vulnerability of the system. Some authors have noted that reductions in the magnitude of variability have been seen with postural sway measures in patients with cerebral palsy,[29] Parkinson's disease[30], cerebral vascular accidents[31], and after ACLR.[7] All of these studies suggest that a reduction in the magnitude of variability of movement, or a more rigid pattern of movement, is indicative of less flexibility of the neuromuscular system to adapt to perturbations. These studies indicate that a healthy level of variability in movement may afford patients who did not go on to sustain a second ACL injury a greater ability to adapt to their environment in the presence of perturbation.[27, 32, 7] The results of this study support the theory that with pathology or injury one may see a reduction in the magnitude of variability of movements as demonstrated by the reduction in variability of movement at the ankle. The population of females after ACLR who subsequently went onto suffer a second ACL injury demonstrated the greater reduction in variability in movement (i.e., a more rigid pattern of movement) at the ankle. This supports the theory that an over-constrained system may be at risk for injury when placed in an environment which requires dynamic movement, such as pivoting and cutting sports.
Excessive variability or random patterns of movement may create a high-risk situation for an athlete returning to a pivoting or cutting sport. The relationship between hip and ankle coordination in the group that suffered a second ACL injury tended to represent a less deterministic movement (i.e., more random) with more noise as compared to the population of patients after ACLR who did not suffer a second injury. Kiefer et al[15] compared a population of patients after ACLR to a healthy cohort and noted the patients after ACLR demonstrated a less deterministic pattern of movement when compared to controls. These data suggest the population of patients after ACLR who subsequently suffered a second ACL injury demonstrated less deterministic patterns of movement than patients after ACLR who did not suffer a second injury. Collectively, these data suggest the group who ultimately sustained a second ACL injury may present with the least deterministic (or most random) movement when compared to patients who only sustained one ACL injury and healthy controls. In addition, the CML results are indicative of weaker coupling of ankle and hip movements in the second injury group. Specifically, the patients who subsequently suffered a second ACL injury were less able to sustain coordinated movement between the hip and ankle. In this case, the coordination of movements between the hip and ankle appear to be more random and less coordinated in those who suffered additional injury. Anatomically and biomechanically, the knee must control significant forces generated both proximally and distally. Theoretically, the coordination of movements proximal and distal to the knee joint can optimize the position of the knee during dynamic activities and potentially control these forces across the knee joint. Proprioceptive deficits, which occur following ACL injury,[33] can also contribute to deficits with interjoint coordination.[34] A failure to optimally align the lower extremity may place the knee in a vulnerable position for future injury.
Current evidence regarding variability in movement during gait in patients after ACLR is consistent with these findings. Moraiti et al[35] reported on variability in knee flexion-extension kinematics during gait after ACLR. These authors reported a noisier and unpredictable movement pattern during gait in the involved limb of patients after ACLR when compared to healthy controls. In a follow-up study, this group reported similar findings in the contralateral limb of patients following ACLR.[36] Interestingly, this was the opposite of what was reported in a population of patients who were ACL deficient.[37] Those subjects presented with less variability in movement during gait. In theory, these subjects may have demonstrated a more rigid pattern of movement during gait as a means to create stability in a mechanically unstable knee.
Coupled Joint Movement in Patients with Musculoskeletal Injury
The concept of a dynamical systems approach to investigate coupled joint movements has been discussed in a variety of populations with musculoskeletal injury and pathology. Coupled joint movements can be defined as the coordinated movements of individual joints to create a dynamic movement. Coupling of foot and leg movements has been investigated extensively in the running population,[14, 38] and to a lesser extent with cutting tasks[39]. These studies identified altered variability in joint coupling in populations of patients with patellofemoral pain[14] and in high-risk populations such as female athletes.[39] Kiefer et al[15] also noted a decreased regularity of hip and ankle coupling in patients after ACLR when compared to controls. Similarly, this study found that patients who went on to suffer a second ACL injury demonstrated weaker coupling indicative of a decreased ability to coordinate movement between the hip and ankle than those patients after ACLR who did not suffer a second injury. These finding are consistent with the current published literature that indicates that, in the presence of injury and/or pathology, altered coupling patterns between lower extremity joints can occur and this can increase the potential for future injury or joint degeneration due to repetitive overload.
This study represents a novel approach that involves the use of a nonlinear analysis tool (CRQ) to determine if movement coordination patterns are unique in patients who go onto suffer subsequent ACL injury after ACLR and RTS. However, the study does present some limitations. The sample used in this population is relatively small and includes only female athletes. Future investigations should look to expand this sample to validate these findings in a larger sample in addition to determining if similar results would be seen in male subjects. Secondly, within the population of subjects who suffered a second ACL injury, 10 of the 14 subject suffered a contralateral injury. The primary aim of this study was to determine differences in populations who suffered any 2nd ACL injury (inclusive of ipsilateral and contralateral) compared to a group which did not suffer a 2nd ACL injury. This study was unable to determine if differences existed between the subjects who suffered ipsilateral versus contralateral 2nd injuries. Future studies with larger sample sizes should more specifically answer this question. Finally, the task employed was a novel, unilateral task. Such a task has been used in previous studies, and was selected here to isolate and identify specific differences between the injured and uninjured limbs. Prior studies of dynamic postural coordination have primarily utilized a bipedal task, although more recent work is progressing to the use of a more challenging, unilateral task.[20, 15] Future research should determine if similar findings would be observed with a bipedal postural coordination task. Concurrently, future research must validate the efficacy of current second injury prevention programs[40] on the presently identified deficits in postural coordination.
Conclusion
This study indicates that female patients who suffer a second ACL injury after ACLR and return to a pivoting and cutting sport present with altered hip-ankle coordination when compared to similar patients who did not suffer a second ACL injury. Failure to appropriately coordinate lower extremity movement between the adjoining proximal and distal hip and ankle in the absence of normal knee proprioception may place the knee in a high-risk position and increase the likelihood of a second ACL injury in this population. Future research is warranted to evaluate if neuromuscular training strategies focused on improved sensorimotor coordination reduce the risk of second ACL injury.
Highlights.
Coordination deficits exist in patients who sustain repeat knee injuries.
Altered postural coordination may lead to increased risk of future injury
Altered postural coordination may need to be addressed post-operatively.
Postural coordination may need to be included in return to play decision making.
Acknowledgements
The study was supported by the National Football League Charities and the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases grants R01-AR049735, R01-AR05563, R01-AR056259, and F32-AR055844.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The Cincinnati Children's Hospital Medical Center Institutional Review Board approved this study.
References
- 1.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. The Journal of bone and joint surgery American volume. 2011;93(11):994–1000. doi: 10.2106/JBJS.I.01618. doi:10.2106/JBJS.I.01618. [DOI] [PubMed] [Google Scholar]
- 2.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of Contralateral and Ipsilateral Anterior Cruciate Ligament (ACL) Injury After Primary ACL Reconstruction and Return to Sport. Clin J Sport Med. 2012;22(2):116–21. doi: 10.1097/JSM.0b013e318246ef9e. doi:10.1097/JSM.0b013e318246ef9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brophy RH, Schmitz L, Wright RW, Dunn WR, Parker RD, Andrish JT, et al. Return to play and future ACL injury risk after ACL reconstruction in soccer athletes from the Multicenter Orthopaedic Outcomes Network (MOON) group. The American journal of sports medicine. 2012;40(11):2517–22. doi: 10.1177/0363546512459476. doi:10.1177/0363546512459476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaeding CC, Aros B, Pedroza A, Pifel E, Amendola A, Andrish JT, et al. Allograft Versus Autograft Anterior Cruciate Ligament Reconstruction: Predictors of Failure From a MOON Prospective Longitudinal Cohort. Sports health. 2011;3(1):73–81. doi: 10.1177/1941738110386185. doi:10.1177/1941738110386185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Myer GD, Huang B, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. The American journal of sports medicine. 2010;38(10):1968–78. doi: 10.1177/0363546510376053. doi:0363546510376053 [pii] 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mattacola CG, Perrin DH, Gansneder BM, Gieck JH, Saliba EN, McCue FC., 3rd Strength, functional outcome, and postural stability after anterior cruciate ligament reconstruction. J Athl Train. 2002;37(3):262–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Hewett TE. Altered postural sway persists after anterior cruciate ligament reconstruction and return to sport. Gait & posture. 2013;38(1):136–40. doi: 10.1016/j.gaitpost.2012.11.001. doi:10.1016/j.gaitpost.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paterno MV, Ford KR, Myer GD, Heyl R, Hewett TE. Limb asymmetries in landing and jumping 2 years following anterior cruciate ligament reconstruction. Clin J Sport Med. 2007;17(4):258–62. doi: 10.1097/JSM.0b013e31804c77ea. doi:10.1097/JSM.0b013e31804c77ea 00042752-200707000-00005 [pii]. [DOI] [PubMed] [Google Scholar]
- 9.Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Myer GD, Hewett TE. Effects of sex on compensatory landing strategies upon return to sport after anterior cruciate ligament reconstruction. The Journal of orthopaedic and sports physical therapy. 2011;41(8):553–9. doi: 10.2519/jospt.2011.3591. doi:10.2519/jospt.2011.3591. [DOI] [PubMed] [Google Scholar]
- 10.Paterno MV, Schmitt LC, Ford KR, Rauh MJ, Myer GD, Hewett TE. Effects of Sex on Compensatory Landing Strategies Upon Return to Sport After Anterior Cruciate Ligament Reconstruction. J Orthop Sport Phys. 2011;41(8):553–9. doi: 10.2519/jospt.2011.3591. doi:DOI 10.2519/jospt.2011.3591. [DOI] [PubMed] [Google Scholar]
- 11.Bardy BG, Oullier O, Bootsma RJ, Stoffregen TA. Dynamics of human postural transitions. Journal of experimental psychology Human perception and performance. 2002;28(3):499–514. [PubMed] [Google Scholar]
- 12.Kiefer AW, Riley MA, Shockley K, Sitton CA, Hewett TE, Cummins-Sebree S, et al. Lower-limb proprioceptive awareness in professional ballet dancers. Journal of dance medicine & science : official publication of the International Association for Dance Medicine & Science. 2013;17(3):126–32. doi: 10.12678/1089-313x.17.3.126. [DOI] [PubMed] [Google Scholar]
- 13.Chiu SL, Chou LS. Variability in inter-joint coordination during walking of elderly adults and its association with clinical balance measures. Clinical biomechanics. 2013;28(4):454–8. doi: 10.1016/j.clinbiomech.2013.03.001. doi:10.1016/j.clinbiomech.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Hamill J, van Emmerik RE, Heiderscheit BC, Li L. A dynamical systems approach to lower extremity running injuries. Clinical biomechanics. 1999;14(5):297–308. doi: 10.1016/s0268-0033(98)90092-4. [DOI] [PubMed] [Google Scholar]
- 15.Kiefer AW, Ford KR, Paterno MV, Schmitt LC, Myer GD, Riley MA, et al. Inter-segmental postural coordination measures differentiate athletes with ACL reconstruction from uninjured athletes. Gait & posture. 2013;37(2):149–53. doi: 10.1016/j.gaitpost.2012.05.005. doi:10.1016/j.gaitpost.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632–44. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 17.Reinschmidt C, Van den Bogert AJ. KineMat. A MATLAB toolbox for the reconstruction of spatial marker positions and the analysis of three-dimensional joint movements. 1997.
- 18.Marwan N, Romano MC, Thiel M, Kurths J. Recurrence plots for the analysis of complex systems. Phys Rep. 2006;438(5-6):237–329. doi:DOI 10.1016/j.physrep.2006.11.001. [Google Scholar]
- 19.Webber Z. Recurrence Quantification Analysis of Nonlinear Dynamical Systems. Tutorials in Contemporary Nonlinear Methods for the Behanioral Sciences. 2005:33–101. [Google Scholar]
- 20.Kiefer AW, Riley MA, Shockley K, Sitton CA, Hewett TE, Cummins-Sebree S, et al. Multi-segmental postural coordination in professional ballet dancers. Gait & posture. 2011;34(1):76–80. doi: 10.1016/j.gaitpost.2011.03.016. doi:10.1016/j.gaitpost.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Pellecchia GL, Shockley K, Turvey MT. Concurrent cognitive task modulates coordination dynamics. Cognitive science. 2005;29(4):531–57. doi: 10.1207/s15516709cog0000_12. doi:10.1207/s15516709cog0000_12. [DOI] [PubMed] [Google Scholar]
- 22.Richardson MJ, Schmidt RC, Kay BA. Distinguishing the noise and attractor strength of coordinated limb movements using recurrence analysis. Biological cybernetics. 2007;96(1):59–78. doi: 10.1007/s00422-006-0104-6. doi:10.1007/s00422-006-0104-6. [DOI] [PubMed] [Google Scholar]
- 23.Riley MA, Balasubramaniam R, Turvey MT. Recurrence quantification analysis of postural fluctuations. Gait & posture. 1999;9(1):65–78. doi: 10.1016/s0966-6362(98)00044-7. [DOI] [PubMed] [Google Scholar]
- 24.Webber Zbilut. MA R, GC VO, editors. Recurrence Quantification Analysis of Nonlinear Dynamical Systems. Tutorials in Contemporary Nonlinear Methods for the Behavioral Sciences. :33–101. WWW.NSF.GOV/SBE/BCS/PAC/NMBS/NMBS.JSP2005.
- 25.Pellecchia GL, Shockley K, Turvey MT. Concurrent cognitive task modulates coordination dynamics. Cognitive Sci. 2005;29(4):531–57. doi: 10.1207/s15516709cog0000_12. doi:DOI 10.1207/s15516709cog0000_12. [DOI] [PubMed] [Google Scholar]
- 26.Shockley K, Butwill M, Zbilut JP, Webber CL. Cross recurrence quantification of coupled oscillators. Physics Letters A. 2002;305(1-2):59–69. doi:Pii S0375-9601(02)01411-1 Doi 10.1016/S0375-9601(02)01411-1. [Google Scholar]
- 27.Riley MA, Turvey MT. Variability and determinism in motor behavior. J Motor Behav. 2002;34(2):99–125. doi: 10.1080/00222890209601934. [DOI] [PubMed] [Google Scholar]
- 28.Decker LM, Moraiti C, Stergiou N, Georgoulis AD. New insights into anterior cruciate ligament deficiency and reconstruction through the assessment of knee kinematic variability in terms of nonlinear dynamics. Knee Surg Sport Tr A. 2011;19(10):1620–33. doi: 10.1007/s00167-011-1484-2. doi:DOI 10.1007/s00167-011-1484-2. [DOI] [PubMed] [Google Scholar]
- 29.Donker SF, Ledebt A, Roerdink M, Savelsbergh GJ, Beek PJ. Children with cerebral palsy exhibit greater and more regular postural sway than typically developing children. Experimental brain research. 2008;184(3):363–70. doi: 10.1007/s00221-007-1105-y. doi:10.1007/s00221-007-1105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmit JM, Riley MA, Dalvi A, Sahay A, Shear PK, Shockley KD, et al. Deterministic center of pressure patterns characterize postural instability in Parkinson's disease. Experimental brain research. 2006;168(3):357–67. doi: 10.1007/s00221-005-0094-y. doi:10.1007/s00221-005-0094-y. [DOI] [PubMed] [Google Scholar]
- 31.Roerdink M, De Haart M, Daffertshofer A, Donker SF, Geurts AC, Beek PJ. Dynamical structure of center-of-pressure trajectories in patients recovering from stroke. Experimental brain research. 2006;174(2):256–69. doi: 10.1007/s00221-006-0441-7. doi:10.1007/s00221-006-0441-7. [DOI] [PubMed] [Google Scholar]
- 32.Lamoth CJ, van Lummel RC, Beek PJ. Athletic skill level is reflected in body sway: a test case for accelometry in combination with stochastic dynamics. Gait & posture. 2009;29(4):546–51. doi: 10.1016/j.gaitpost.2008.12.006. doi:S0966-6362(08)00389-5 [pii] 10.1016/j.gaitpost.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. The American journal of sports medicine. 1982;10(6):329–35. doi: 10.1177/036354658201000601. [DOI] [PubMed] [Google Scholar]
- 34.Sainburg RL, Poizner H, Ghez C. Loss of proprioception produces deficits in interjoint coordination. Journal of neurophysiology. 1993;70(5):2136–47. doi: 10.1152/jn.1993.70.5.2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moraiti CO, Stergiou N, Ristanis S, Vasiliadis HS, Patras K, Lee C, et al. The effect of anterior cruciate ligament reconstruction on stride-to-stride variability. Arthroscopy : the journal of arthroscopic & related surgery : official publication of the Arthroscopy Association of North America and the International Arthroscopy Association. 2009;25(7):742–9. doi: 10.1016/j.arthro.2009.01.016. doi:10.1016/j.arthro.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 36.Moraiti CO, Stergiou N, Vasiliadis HS, Motsis E, Georgoulis A. Anterior cruciate ligament reconstruction results in alterations in gait variability. Gait & posture. 2010;32(2):169–75. doi: 10.1016/j.gaitpost.2010.04.008. doi:10.1016/j.gaitpost.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Stergiou N, Moraiti C, Giakas G, Ristanis S, Georgoulis AD. The effect of the walking speed on the stability of the anterior cruciate ligament deficient knee. Clinical biomechanics. 2004;19(9):957–63. doi: 10.1016/j.clinbiomech.2004.06.008. doi:10.1016/j.clinbiomech.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Heiderscheit BC, Hamill J, Van Emmerik RE. Variability of Stride Characteristics and Joint Coordination Among Individuals with Unilateral Patellofemoral Pain. J Appl Biomech. 2002;18(2):110–21. [Google Scholar]
- 39.Pollard CD, Heiderscheit BC, van Emmerik REA, Hamill J. Gender differences in lower extremity coupling variability during an unanticipated cutting maneuver. J Appl Biomech. 2005;21(2):143–52. doi: 10.1123/jab.21.2.143. [DOI] [PubMed] [Google Scholar]
- 40.Di Stasi S, Myer GD, Hewett TE. Neuromuscular training to target deficits associated with second anterior cruciate ligament injury. The Journal of orthopaedic and sports physical therapy. 2013;43(11):777–92, A1-11. doi: 10.2519/jospt.2013.4693. doi:10.2519/jospt.2013.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]