Abstract
Cytochrome P450 3A4 (CYP3A4) metabolizes 30–50% of clinically used drugs. Large interperson variability in CYP3A4 activity affects response to CYP3A4 substrate drugs. We had demonstrated that an intronic single nucleotide polymorphism (SNP) rs35599367 (CYP3A4*22, located in intron 6) reduces mRNA/protein expression; however, the underlying mechanism remained unknown. Here we show that CYP3A4*22 is associated with a twofold or greater increase in formation of a nonfunctional CYP3A4 alternative splice variant (aSV) with partial intron 6 retention in human liver (p=0.006), but not in small intestines. Consistent with this observation, in vitro transfection experiments with a CYP3A4 minigene (spanning from intron 5 to intron 7) demonstrated that plasmids carrying the rs35599367 minor T allele caused significantly greater intron 6 retention than the C allele in liver derived HepG2 cells, but not in intestine-derived LS-174T cells. These results indicate that tissue specific increased formation of nonfunctional aSV causes reduced CYP3A4 mRNA/protein expression in CYP3A4*22 carriers.
Keywords: CYP3A4, CYP3A4*22 allele, gene expression, polymorphism, alternative splicing
CYP3A4 is the main drug metabolizing enzymes in the liver, involved in metabolism of 30–50% of clinically used drugs [1]. CYP3A4 expression and enzyme activity are highly variable, in part as a result of substantial genetic factors [1]. However, the genetic factors contributing to CYP3A4 variability remained uncertain. While a single case of complete lack of CYP3A4 activity because of a homozygous exon 9 mutation was reported recently [2], the frequencies of single nucleotide polymorphisms (SNPs) in the coding region of CYP3A4 are rare [3, 4]. The proximal promoter variant CYP3A4*1B (rs2740574) is the most common and frequently studied variant; however, the functional effect of this variant remains controversial [1].
Recently, we have identified an intronic SNP (rs35599367, CYP3A4*22, frequency 0.04–0.08) that reduces CYP3A4 mRNA and protein expression and is associated with reduced statin dose requirement [5]. The effect of CYP3A4*22 on protein expression in livers was later replicated by other investigators (for example [6]). Moreover, CYP3A4*22 also displayed reduced CYP3A4 enzyme activity in vivo, revealed with midazolam and erythromycin as probes [7]. A number of clinical studies confirmed association of CYP3A4*22 with clearance or pharmacodynamics outcomes of multiple CYP3A substrate drugs, including statins, tacrolimus, cyclosporine, sunitinib, etc. [3]. Although some results remained inconclusive [8], CYP3A4*22 appears to be the most clinically relevant common variant in CYP3A4 [1, 3].
However, the molecular genetic mechanisms underlying CYP3A4*22 effects remained to be elucidated. Previously, using CYP3A4 minigenes and rs35599367 as marker, we showed that the minor rs35599367 T allele is associated with lower levels of heteronuclear RNA (hnRNA, RNA before splicing) than the C allele in HepG2 cells [5]. We proposed that rs35599367 may affect CYP3A4 nascent RNA elongation because rs35599367 appears to change the conformation of single stranded DNA [5], but alternative mechanisms are also feasible. SNP rs35599367 is located 192 bp upstream of exon 7, within several serine/arginine-rich protein-binding motifs. The substitution of C with T (CAGCGTA to CAGTGTA) deletes a predicted SF2/ASF-binding site (changing the score from 2.5 to −0.449, ESEfinder, http://rulai.cshl.edu/tools/ESE/), indicating a potential effect on splicing. However, we did not detect aberrant splice variants using primers located in exon 5 (forward) and exon 7 (reverse) in human liver samples and in CYP3A4 minigene transfected HepG2 cells [5].
Recently, an alternative CYP3A4 splice variant (aSV) with a 255bp partial intron 6 retention was identified by RNAseq [9], corresponding to transcript ENST00000480043 in Ensemble Genome Browser (Figure 1a). Because aSV terminates before exon 7, it escaped detection using our previous RT-PCR assays [5]. We hypothesized that rs35599367 may increase the formation of truncated aSV thereby decreasing the expression of full length CYP3A4 mRNA.
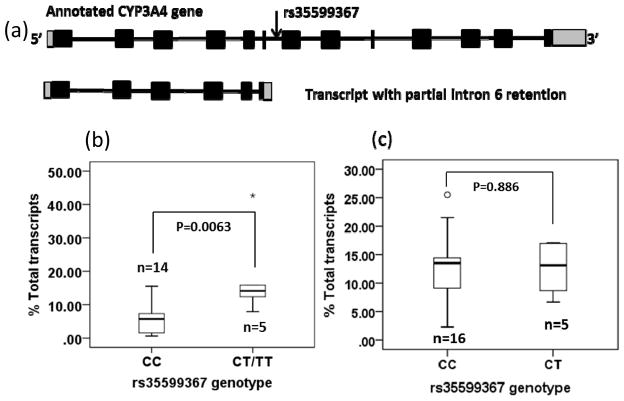
Figure 1.
CYP3A4*22 (rs35599367) genotype is associated with the expression of CYP3A4 splice variant with partial intron 6 retention (aSV) in human livers but not small intestines. Panel a. Genomic structure of CYP3A4 full length RNA and splice variant (aSV) with partial intron 6 retention. Panels b and c. Box plots of relative expression levels (% of total transcripts) of CYP3A4 alternative splice variant (aSV) in livers (b) and small intestines (c), grouped by CYP3A4*22 (rs35599367) genotype. Differences between groups were analyzed by Mann-whitney test. The box and horizontal line show the 25% and 75% percentiles and median, and whiskers show the minimum and maximum values. Small circle and star show outliers.
To test our hypothesis, we designed primers targeting exon 6 and intron 6 (E6F and I6R, Supplemental Table 1), and measured the expression level of aSV in samples with different rs35599367 genotypes. We also measured the level of full-length CYP3A4 mRNA using primers targeting exon 6 and exon7 (E6F and E7R, Supplemental Table 1). After normalization of both CYP3A4 aSV and full-length mRNA levels to the level of β-actin, we calculated the % of aSV over total CYP3A4 transcripts (% aSV=[aSV/(mRNA + aSV)]×100) [10]. As a control, we also measured hnRNA levels upstream of rs35599367 common to both aSV and full-length RNA using primers targeting intron 3 (I3F and I3R, Supplemental Table 1), normalizing the result to the level of full length CYP3A4 mRNA. Because rs35599367 caused allelic RNA expression imbalance only in liver but not in intestines, we tested the effect of rs35599367 on aSV formation in both liver and small intestine samples.
A total of 19 liver and 21 small intestine samples were obtained from The Cooperative Human Tissue Network Midwestern and Western Division under a protocol approved by Ohio State University Institutional Review Board. Genomic DNA and total RNA preparations, cDNA synthesis and rs35599367 genotyping were performed as described previously [5], with oligo-dT and gene specific primer used in cDNA synthesis (Supplemental Table 1). RNA expression levels were quantitated using real-time PCR with SYBR Green and specific primers in a 7500 Fast Real-time PCR System (Applied Biosystems). The average level of CYP3A4 hnRNA in liver and small intestine samples were 0.64% and 0.35% of mature mRNA, whereas the average levels of aSV were 8% and 12% of total CYP3A4 transcripts, indicating that only a small portion of the measured aSV represents hnRNA. Shown in Figure 1b&1c, livers carrying rs35599367 CT and TT genotypes displayed over 2-fold higher relative levels of aSV than samples with CC genotype (mean value 19% vs 5%; median value 15% vs 6% of total transcripts, , P=0.006, Mann-Whitney test)., while there were no differences in small intestines (mean value 12.5% vs 12.9%, P=0.89; median 13.1% vs 13.4%). The cycle threshold (Ct) values and the ratios of aSV/full-length RNA from each sample are provided in Supplemental Figure 1 and Supplemental Table 2. In contrast, expression levels of common hnRNA did not differ between genotypes in the same group of livers and small intestines (mean ± SD: 0.85 ± 0.63 for CT/TT, 0.60 ± 0.60 for CC, P=0.493 in livers; 0.21 ± 0.11 for CT/TT, 0.41 ± 0.27 for CC in small intestines, p=0.150).
To further test the effect of rs35599367 on CYP3A4 intron 6 splicing, we used a CYP3A4 minigene expression plasmid, which contains CYP3A4 gene fragments from intron 4 to intron 7 (~2300bp, from 75700 to 77994, AF280107) in a pcDNA3 vector, harboring either the C or T allele of rs35599367, as reported previously [5]. Transfection of the CYP3A4 minigene into liver derived HepG2 cells produced normally spliced mRNA that includes exon 5, exon 6 and exon7 adjoining the BGH polyadenylation signal sequence of the pcDNA3 vector [5]. To completely remove plasmid DNA contamination in total RNA prepared from transfected HepG2 cells, total RNA was digested with restriction enzymes (XbaI and DpnI) and DNaseI for 1 hour at 37°C as reported previously [5]. Further to avoid interference from endogenously expressed CYP3A4 mRNA, we used only gene-specific primer that target pcDNA3 vector and intron 6 for cDNA synthesis, without oligo-dT [11] (Supplemental Table 1). Cells transfected with the rs35599367 T allele yielded higher aSV levels than cells transfected with the C allele (18% vs 12% of total mRNA transcripts, p=0.003) in HepG2 cells (Figure 2a, Supplemental Figure 2b), while there is no difference in intestine-derived LS-174T cells (Figure 2b), consistent with the tissue specific effects of rs35599367 on aSV expression in this study, and results for CYP3A4 mRNA tissue expression reported previously [5]. These results indicate that rs35599367 increases the formation of splice variant with partial intron 6 retention.
Figure 2.
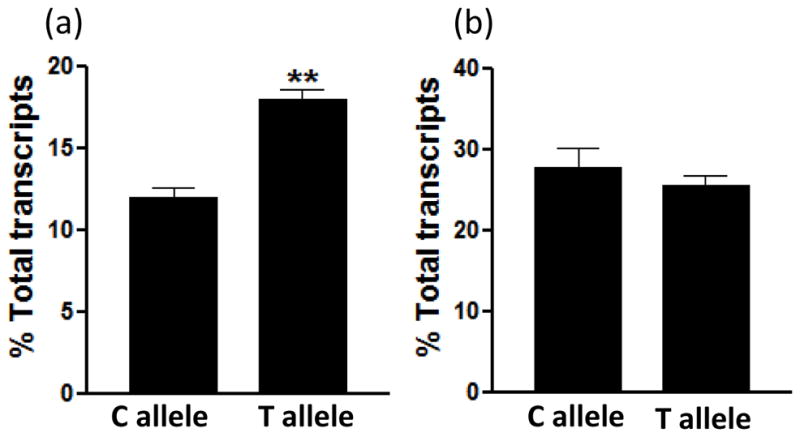
Relative expression levels (normalized by total CYP3A4 transcripts) of CYP3A4 splice variant aSV with partial intron 6 retention in HepG2 (a) or LS-174T (b) cells transfected with CYP3A4 minigene plasmids harboring rs35599367 C (wild-type) or T allele (variant). Data are mean ± SD, ** compared to C allele, P<0.05, t-test, n=4.
The CYP3A4 aSV with partial intron 6 retention is detectable in human livers, hepatocytes and HepRG cells, with increasing expression in developed livers, differentiated HepRG cells or after inducer treatment [9], indicating that aSV is an alternative splice variant subject to transcriptional regulation similar to full length CYP3A4 mRNA, even in the absence of cis-acting polymorphisms. This splice variant can potentially be translated into a protein with a shortened amino acid sequence, because of the presence of a translational stop codon (TAA) and a polyadenylation signal sequence (AATAAA) in intron 6 [9]. However, the absence of a heme-binding signature in the encoded polypeptide precludes a catalytically active protein.
The increased formation of aSV associated with SNP rs35599367 is supported by the prediction that the C to T substitution of rs35599367 may delete a splicing protein SF2/ASF binding site, potentially affecting exon 7 as a acceptor for correct splicing. Increased aSV formation parallels reduced formation of functional CYP3A4 mRNA, consistent with our previous results showing allelic expression imbalance of CYP3A4 mRNA in rs35599367 heterozygous samples and reduced CYP3A4 protein expression or enzyme activity in rs35599367 carriers [5]. The effect of rs35599367 is tissue specific, indicating the involvement of tissue specific splicing regulatory factors. It is noted that aSV levels are higher in small intestine than in liver samples (5.4% vs 12.9% of total CYP3A4 transcripts in non-variant allele carriers), possibly masking the genetic effect from rs35599367 in small intestine. Alternatively, additional factors or SNPs in linkage disequilibrium (LD) with rs35599367 may further modify the regulation of rs35599367 in different tissues, explaining the wide range (1.6 to 6.3 fold) of inter-person variability in allelic RNA imbalance of CYP3A4 in rs35599367 heterozygous livers. Moreover, next generation sequencing revealed a CYP3A4 antisense RNA, mapped to intron 8 of CYP3A4 (RP11-757A13.1-001, Ensemble Genome Browser). Whether this antisense RNA or other factors affect CYP3A4 expression remains to be determined.
In summary, we demonstrate that CYP3A4*22 (rs35599367) increases the formation of the nonfunctional CYP3A4 splice variant with partial intron 6 retention, thereby reducing the production of functional full-length CYP3A4 mRNA. We have repeatedly observed altered mRNA splicing caused by frequent intronic or exonic variants and reduced functional mRNA expression, for example in CYP2D6 [10], DRD2 [12] and CETP [13], indicating altered splicing is a common mechanism underlying reduced mRNA expression for structural RNA polymorphisms (srSNPs) [14]. The results with CYP3A4 are consistent with reduced mRNA expression/enzyme activity associated with CYP3A4*22 and provide a functional basis underlying CYP3A4*22 association studies, supporting the use of CYP3A4*22 as a biomarker predicting CYP3A4 activity.
Supplementary Material
Acknowledgments
Source of funding: This work was supported by National Institute of Health (NIH) grants U01 GM 092655, and partially by grant from the National Center for Research Resources (UL 1RR025755). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources of the National Institute of Health.
Footnotes
Conflicts of interest: none declared
References
- 1.Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Werk AN, Lefeldt S, Bruckmueller H, Hemmrich-Stanisak G, Franke A, Roos M, Kuchle C, Steubl D, Schmaderer C, Brasen JH, et al. Identification and characterization of a defective CYP3A4 genotype in a kidney transplant patient with severely diminished tacrolimus clearance. Clin Pharmacol Ther. 2014;95:416–422. doi: 10.1038/clpt.2013.210. [DOI] [PubMed] [Google Scholar]
- 3.Werk AN, Cascorbi I. Functional gene variants of CYP3A4. Clin Pharmacol Ther. 2014;96:340–348. doi: 10.1038/clpt.2014.129. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Sadee W. The Making of a CYP3A Biomarker Panel for Guiding Drug Therapy. J Pers Med. 2012;2:175–191. doi: 10.3390/jpm2040175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang D, Guo Y, Wrighton SA, Cooke GE, Sadee W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J. 2011;11:274–286. doi: 10.1038/tpj.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okubo M, Murayama N, Shimizu M, Shimada T, Guengerich FP, Yamazaki H. CYP3A4 intron 6 C>T polymorphism (CYP3A4*22) is associated with reduced CYP3A4 protein level and function in human liver microsomes. J Toxicol Sci. 2013;38:349–354. doi: 10.2131/jts.38.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elens L, Nieuweboer A, Clarke SJ, Charles KA, de Graan AJ, Haufroid V, Mathijssen RH, van Schaik RH. CYP3A4 intron 6 C>T SNP (CYP3A4*22) encodes lower CYP3A4 activity in cancer patients, as measured with probes midazolam and erythromycin. Pharmacogenomics. 2013;14:137–149. doi: 10.2217/pgs.12.202. [DOI] [PubMed] [Google Scholar]
- 8.Ragia G, Kolovou V, Tavridou A, Elens L, Tselepis AD, Elisaf M, Van Schaik RH, Kolovou G, Manolopoulos VG. No effect of CYP3A4 intron 6 C>T polymorphism (CYP3A4*22) on lipid-lowering response to statins in Greek patients with primary hypercholesterolemia. Drug Metabol Drug Interact. 2014 doi: 10.1515/dmdi-2014-0021. [DOI] [PubMed] [Google Scholar]
- 9.Li D, Gaedigk R, Hart SN, Leeder JS, Zhong XB. The role of CYP3A4 mRNA transcript with shortened 3′-untranslated region in hepatocyte differentiation, liver development, and response to drug induction. Mol Pharmacol. 2012;81:86–96. doi: 10.1124/mol.111.074393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Poi MJ, Sun X, Gaedigk A, Leeder JS, Sadee W. Common CYP2D6 polymorphisms affecting alternative splicing and transcription: long-range haplotypes with two regulatory variants modulate CYP2D6 activity. Hum Mol Genet. 2014;23:268–278. doi: 10.1093/hmg/ddt417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang D, Chen H, Momary KM, Cavallari LH, Johnson JA, Sadee W. Regulatory polymorphism in vitamin K epoxide reductase complex subunit 1 (VKORC1) affects gene expression and warfarin dose requirement. Blood. 2008;112:1013–1021. doi: 10.1182/blood-2008-03-144899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee ML, Xiao T, Papp A, Wang D, et al. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papp AC, Pinsonneault JK, Wang D, Newman LC, Gong Y, Johnson JA, Pepine CJ, Kumari M, Hingorani AD, Talmud PJ, et al. Cholesteryl Ester Transfer Protein (CETP) polymorphisms affect mRNA splicing, HDL levels, and sex-dependent cardiovascular risk. PLoS One. 2012;7:e31930. doi: 10.1371/journal.pone.0031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadee W, Wang D, Papp AC, Pinsonneault JK, Smith RM, Moyer RA, Johnson AD. Pharmacogenomics of the RNA world: structural RNA polymorphisms in drug therapy. Clin Pharmacol Ther. 2011;89:355–365. doi: 10.1038/clpt.2010.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.