Abstract
There is growing recognition of the important role of metronomic chemotherapy in cancer treatment. On the basis of their unique anti-angiogenic effects, we tested the efficacy of nab-paclitaxel, which stimulates thrombospondin-1, and topotecan, which inhibits hypoxia-inducible factor 1-α, at metronomic dosing for the treatment of ovarian carcinoma. In vitro and in vivo SKOV3ip1, HeyA8, and HeyA8-MDR (taxane-resistant) orthotopic models were used to examine the effects of metronomic nab-paclitaxel and metronomic topotecan. We examined cell proliferation (Ki-67), apoptosis (cleaved caspase 3), and angiogenesis (microvessel density) in tumors obtained at necropsy. In vivo therapy experiments demonstrated treatment with metronomic nab-paclitaxel alone and in combination with metronomic topotecan resulted in significant reductions in tumor weight (62% in the SKOV3ip1 model, p < 0.01 and 96% in the HeyA8 model, p < 0.03) compared with vehicle (p < 0.01). In the HeyA8-MDR model, metronomic monotherapy with either cytotoxic agent had modest effects on tumor growth, but combination therapy decreased tumor burden by 61% compared with vehicle (p < 0.03). The greatest reduction in microvessel density (p <0.05) and proliferation was seen in combination metronomic therapy groups. Combination metronomic therapy resulted in prolonged overall survival in vivo compared to other groups (p < 0.001). Tube formation was significantly inhibited in RF-24 endothelial cells exposed to media conditioned with metronomic nab-paclitaxel alone and media conditioned with combination metronomic nab-paclitaxel and metronomic topotecan. The combination of metronomic nab-paclitaxel and metronomic topotecan offers a novel, highly effective therapeutic approach for ovarian carcinoma that merits further clinical development.
Keywords: nab-paclitaxel, topotecan, angiogenesis, metronomic, ovarian carcinoma
Introduction
Metronomic dosing is defined as the frequent administration of a chemotherapeutic agent at low, minimally toxic doses. Metronomic chemotherapy is believed to inhibit tumor growth primarily by targeting proliferating endothelial cells (1), with an expectation of reduced side effects. Preclinical and clinical data have strengthened the rationale for the use of metronomic chemotherapy in many malignancies, including ovarian cancer (2–8). Angiogenic pathways and the tumor microvasculature are attractive targets for cancer treatment, as supported by the robust number of ongoing clinical trials in this arena (6, 9–15) in a variety of solid malignancies including breast, prostate, neuroendocrine, hepatocellular, colorectal and ovarian cancer. The use of metronomic chemotherapy as a means of inhibiting angiogenesis is generating clinical interest (2, 5).
Paclitaxel plays a critical role in frontline adjuvant therapy for ovarian cancer and has been shown to result in increased thrombospondin-1 (TSP-1) in the tumor microenvironment (16). However, the cremophor diluent in paclitaxel may interfere with angiogenesis inhibition at metronomic doses (17, 18). Nab-paclitaxel is a cremophor-free compound that utilizes 130-nanometer albumin-bound (nab) technology to circumvent the need for solvents. Nab-paclitaxel achieves a larger volume of distribution and greater suppression of tumor growth at metronomic doses than cremophor paclitaxel in many preclinical tumor models (17, 19, 20). Nab-paclitaxel has demonstrated promising activity in patients with recurrent ovarian, fallopian tube, and primary peritoneal cancers (21).
Camptothecins such as topotecan, which are known to inhibit topoisomerase I, also have anti-angiogenic properties (22–26). One known mechanism of action of topotecan is its ability to suppress expression of hypoxia inducible factor (HIF)-1α, a key hypoxia-induced transcription factor that regulates the expression of several pro-angiogenic genes such as vascular endothelial growth factor (VEGF) (27, 28). Metronomic topotecan has been found effective in multiple preclinical and clinical models (29–31), including ovarian cancer (22, 32–37). We have previously shown that metronomic doses of topotecan decrease cell proliferation and angiogenesis while simultaneously increasing apoptosis in murine ovarian cancer models, effects likely attributable to reductions in VEGF and HIF-1α (22, 23).
Several preclinical studies in solid malignancies such as breast cancer and melanoma have previously evaluated the use of doublet combination metronomic therapy using metronomically dosed cyclophosphamide and uracil-tegafur (a fluorouracil oral prodrug) (38), metronomic low-dose metronomic vinblastine and low-dose metronomic oral cyclophosphamide (39), and low-dose metronomic vinblastine and low-dose metronomic cyclophosphamide (40). The effectiveness of dual metronomic therapy preclinically has prompted evaluation of combination metronomic chemotherapy in clinical trials (41–45).
Given the unique and non-overlapping mechanisms of action of nab-paclitaxel and topotecan (i.e., increasing secretion of TSP-1 and inhibiting HIF-1α), we investigated the effects of these agents combined at metronomic doses in preclinical models of ovarian carcinoma.
Materials and Methods
Ovarian Cancer Cell lines
Experiments were conducted using taxane-sensitive (HeyA8 and SKOV3ip1) and taxane-resistant (HeyA8-MDR) epithelial ovarian cancer cell lines. Descriptions of the derivation and maintenance of these cell lines have been reported previously (46–48). In brief, HeyA8 and SKOV3ip1 cells were maintained in RPMI-1640/15% fetal bovine serum (FBS) with 0.1% gentamicin sulfate (Gemini Bioproducts, Calabasas, CA). HeyA8-MDR cells were grown in the same media supplemented with 300 μg/mL paclitaxel. All experiments were performed with cells at 70–80% confluence. The tissue-specific microvascular endothelial cell line MOEC (murine ovarian endothelial cells) and immortalized human vascular endothelial cells (RF-24) were used for the in vitro experiments; their derivation and characterization have been previously reported (49, 50). Cells were maintained in Dulbecco’s modified Eagle medium (D-MEM) supplemented with pyruvate, amino acids, and penicillin/streptomycin. Cell lines were obtained from ATCC and routinely tested for absence of Mycoplasma using MycoAlert (Cambrex Bio Science, Rockland, ME) according to manufacturer instructions.
Nab-paclitaxel and topotecan reconstitution
Nab-paclitaxel was obtained from Abraxis (Costa Mesa, CA) (51). The nab-paclitaxel dosage was determined by the paclitaxel content of the albumin-bound formulation (51). According to manufacturer instructions, nab-paclitaxel was reconstituted in normal saline to a concentration of 5 mg/mL paclitaxel, prepared fresh each day, and administered within an hour of mixing. Topotecan was obtained from GlaxoSmithKline (Middlesex, UK) (25). According to manufacturer instructions, the lyophilized powder was reconstituted to 10 mM in Tri-HCl (pH 4.0), then diluted in sterile water for oral therapy prior to use.
Western blot analyses
HeyA8 cells were treated with nab-paclitaxel, metronomic topotecan or vehicle (normal saline) for 24 to 96 hours. Whole-cell lysates were prepared from both treated and untreated cells at 24-hour time points up to 96 hours using modified RIPA buffer. Forty micrograms of protein lysate was boiled for 5 minutes and then loaded onto a 8% polyacrylamide/SDS gel, transferred to nitrocellulose, and incubated with anti-human TSP-1 antibody overnight (1:100 dilution; Abcam, Cambridge, MA) and carbonic anhydrase IX (1:500; Novus Biologicals, Littleton, CO). Each Western blot was performed in triplicate. Equal loading was verified using vinculin (1:3000 dilution; Sigma-Aldrich, St. Louis, MO).
Immunohistochemistry
Immunostaining for Ki-67, cleaved caspase 3, CD31, and TSP-1 was performed on paraffin-embedded slides of ovarian cancer tumors from HeyA8 and HeyA8-MDR orthotopic models to determine the proliferative index of the cells (i.e., to determine the effects of treatment on tumor cell proliferation). Considering the proposed role of angiogenesis inhibition in the treatment of ovarian cancer, we also determined the microvessel density (MVD).
Slides were deparaffinized sequentially in xylene and declining grades of ethanol prior to rehydration. Antigen retrieval prior to Ki-67 and cleaved caspase 3 staining was performed by heating slides in a steam cooker for 10 minutes in 0.2M Tris buffer, pH 9.0, while antigen retrieval for CD31 and TSP-1 immunohistochemical analysis was accomplished using proteinase K (DakoCytomation, Carpinteria, CA) at room temperature. Endogenous peroxides were blocked with 3% H2O2 in methanol (Ki-67, cleaved caspase 3, and TSP-1) or phosphate-buffered saline (CD31). Nonspecific epitopes were blocked using 5% normal horse serum and 1% normal goat serum at room temperature. Slides were then incubated with primary antibody to Ki-67 (1:200; DakoCytomation); cleaved caspase 3-rabbit polyclonal anti-human, mouse, or rat (1:200; Biocare Medical, Concord, CA); TSP-1 (1:100; Abcam, Cambridge, MA); or CD31 (1:800; PharMingen, San Diego, CA) at 4°C overnight. After washing the slides with phosphate-buffered saline (PBS), we added the appropriate horseradish peroxidase–conjugated secondary antibody in blocking solution for one hour at room temperature. Slides were developed with 3, 3″-diaminobenzidine chromogen (Invitrogen, Carlsbad, CA) and counterstained with Gil No. 3 hematoxylin (Sigma-Aldrich).
The proliferative index was calculated by dividing the number of Ki-67–positive (brown) nuclei by the total number of cells for each of 5 randomly selected 200× high-power fields per tumor specimen for each treatment group. MVD was calculated by viewing 10 representative 200× fields per slide in each treatment group and counting the number of microvessels per field. A microvessel was defined as an open lumen with at least one CD31-positive cell immediately adjacent to it (52, 53). The apoptotic index for each treatment group was quantified as the number of apoptotic tumor cells in 10 randomly selected 200× high-power fields per slide, excluding areas of necrosis. TSP-1 expression was quantified using ImageJ software.
Hypoxic areas on tumor samples were then evaluated using immunohistochemical staining for pimonidazole protein adducts. Hypoxic areas were determined using the Hypoxyprobe staining kit (NPI, Inc.). Paraffin sections were used, and antigen retrieval was performed using a citrate solution in a microwave processor. Sections were probed with Hypoxyprobe Mab-1 (1:500) overnight at 4°C; after washing, slides were incubated with secondary antibody (1:500) for 1 hour at room temperature. The hypoxic area was measured using image J software.
Dose finding experiments using nab-paclitaxel and topotecan in orthotopic murine models
We injected SKOV3ip1 tumor cells intraperitoneally (106 cells) into female athymic mice. Fourteen days after tumor cell injection, mice were randomized into one of five treatment groups (n = 5 per group): vehicle alone, MTD paclitaxel (30 mg/kg daily for 5 consecutive days in a 28-day cycle), 2.5, 5, or 10 mg/kg metronomic (daily) nab-paclitaxel. Nab-paclitaxel was administered by intraperitoneal injection. All mice were collectively sacrificed and tumors were harvested when the control group became moribund. Blood samples were collected by intracardiac withdrawal prior to necropsy for hematological assessment. Mice were monitored daily for signs of toxic effects and intolerance to therapy. Tumor weights and nodule formation were recorded and analyzed. An additional dose finding experiment was then performed to ascertain whether greater distinction could be drawn between metronomic treatment groups. We conducted an additional dose-finding experiment using metronomic dosing of nab-paclitaxel at 2.5 mg/kg, 5 mg/kg, and 10 mg/kg every other day using HeyA8 cells (n = 5 per group).
To evaluate whether therapeutic effects could be augmented while maintaining treatment tolerability, we administered metronomic topotecan by oral gavage daily at dose levels previously identified and reported by our group (i.e., 0.5 mg/kg daily) (22). Nab-paclitaxel was administered intraperitoneally at metronomic doses every other day as determined by our dose-finding experiments. All treatments were started seven days after tumor cell inoculation, and mice were randomized into one of four treatment groups (n = 10). Mice were monitored daily for adverse effects and drug tolerance. All animals were sacrificed and tumors were harvested at necropsy when the control mice began to appear moribund, approximately three to four weeks after the initiation of therapy, depending on the cell line used (48). Mouse weight, tumor weight, tumor distribution, and ascites volume were recorded.
Animals and orthotopic model of ovarian cancer
In vivo studies used female athymic mice (NCr-nu) purchased from the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD). Housing and care in specific pathogen-free conditions were provided as stipulated by guidelines from the American Association for Accreditation of Laboratory Animal Care, the US Public Health Service Policy on Human Care and Use of Laboratory Animals, and the National Institutes of Health. All studies were approved and monitored by The University of Texas MD Anderson Cancer Center Institutional Animal Care and Use Committee.
The development of the orthotopic murine model of advanced ovarian cancer has been extensively reported by our laboratory (54). In brief, SKOV3ip1, HeyA8, and HeyA8-MDR cells were collected from 70–80% confluent cultures using either 0.25% Trypsin-EDTA (GIBCO, Carlsbad, CA) or 0.1% EDTA, depending on the cell line. Cells lifted with trypsin underwent trypsin-neutralization with FBS-containing medium before being centrifuged and resuspended in the appropriate volume of serum-free Hank’s balanced salt solution (HBSS; Invitrogen) for animal inoculation. Cell lines lifted with EDTA alone were directly centrifuged at 1,000 rotations per minute for 7 minutes at 4°C, washed with PBS, and then resuspended in serum-free HBSS at the appropriate concentrations for intraperitoneal inoculation: 2.5 × 105 cells/200 μL HBSS for HeyA8 cells or 1 × 106 cells/200 μL HBSS for SKOV3ip1 and HeyA8-MDR cells.
Therapy experiments were performed using all three cell lines. Mice were collectively sacrificed when mice in any group appeared near moribund, approximately 4 weeks after initiation of therapy, depending on the cell line. Tumors and ascites were harvested at the time of necropsy from the peritoneal cavities of mice. The number of tumor nodules were quantified, and total tumor weights were determined. Additional tumor tissue for hematoxylin-eosin staining and immunohistochemistry (see above) was fixed in formalin at the time of tumor collection and then embedded in paraffin. Paraffin sections were uniformly cut to 5 μm.
Endothelial cell tube formation assay
HeyA8 cells were seeded at a density of 100,000 per well in a 6-well plate and allowed to attach overnight. The culture medium was then aspirated and replaced with fresh culture medium containing 40nM MTD nab-paclitaxel, 25nM MTD topotecan, 20nM metronomic nab-paclitaxel, 25nM metronomic topotecan, or a combination of metronomic nab-paclitaxel and metronomic topotecan. In the control, MTD nab-paclitaxel, and MTD topotecan wells, the culture medium was aspirated every 24 hours and replaced with fresh culture medium containing no drug. In the metronomic nab-paclitaxel, metronomic topotecan, and metronomic combination wells, the culture medium was aspirated every 24 hours and replaced with a fresh preparation of medium containing treatment at the concentrations listed above. After 72 hours, the culture medium from each well was collected.
RF-24 cells were maintained in D-MEM supplemented with pyruvate, amino acids, and penicillin/streptomycin at 37°C. A 96-well plate was coated with 50 μL of Matrigel, which was allowed to solidify at 37°C for 10 minutes. Next, 20,000 cells per well were seeded on the Matrigel and cultured with conditioned media from wells treated with control (no treatment), MTD nab-paclitaxel, MTD topotecan, metronomic nab-paclitaxel, metronomic topotecan, or a combination of metronomic nab-paclitaxel and metronomic topotecan. The cells were incubated at 37°C for 16 hours. To assess tube formation, we counted and photographed complete tubes from randomly chosen fields using an Olympus inverted microscope connected to a digital camera.
Statistical analysis
In vivo therapy experiments were powered to detect a 50% difference in tumor weight (β error = 0.2). The Mann-Whitney rank-sum test was used to analyze nonparametric and non-normally distributed data sets. Survival experiments were analyzed by the Kaplan Meier method. Statistical analyses were performed using SPSS 12.0 for Windows (SPSS Inc, Chicago, IL), and significance was set at p ≤ 0.05 (2-tailed).
Results
Metronomic dosing of nab-paclitaxel
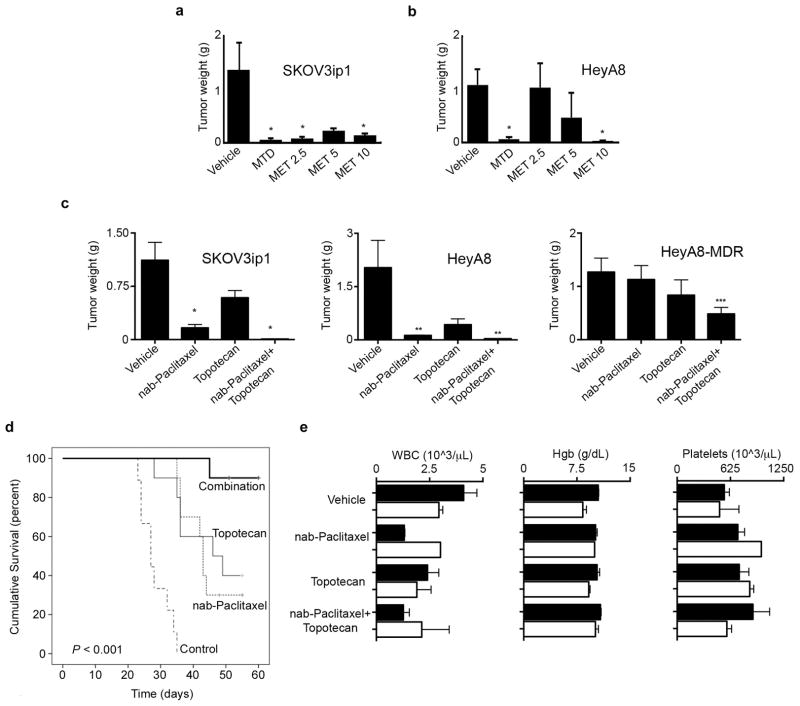
To determine the optimal dose and frequency of dosing to effectively inhibit tumor growth, we conducted a dose-finding experiment with nab-paclitaxel. Female nude mice (n = 5 per group) were injected with SKOV3ip1 cells intraperitoneally and were treated with vehicle, a single dose of MTD nab-paclitaxel (30 mg/kg intraperitoneally daily for 5 consecutive days in a 28-day cycle) or metronomic nab-paclitaxel at daily doses of 2.5 mg/kg, 5 mg/kg, or 10 mg/kg. All daily metronomic dosing levels of nab-paclitaxel produced profound inhibition of tumor growth (Figure 1a).
Figure 1. Tumor growth inhibition with metronomic nab-Paclitaxel alone and in combination with metronomic topotecan.
a) Nab-paclitaxel dose-finding experiment in SKOV3ip1 and b) HeyA8 tumor models of ovarian carcinoma in athymic mice. SKOV3ip1 or HeyA8 tumor bearing mice were given nab-paclitaxel at maximal tolerated dose (MTD) or metronomic (MET) doses of 2.5mg/kg, 5mg/kg or 10mg/kg. MET doses were administered daily for SKOV3ip1 and on alternating days for HeyA8. Mean tumor weights (±standard error [SE]; n = 5 per group) are shown. *p < 0.05 compared with vehicle. c) SKOV3ip1, HeyA8, and HeyA8-MDR tumor bearing mice were randomly allocated to four groups and treated with either: vehicle (control), metronomic nab-paclitaxel alone (2.5 mg/kg intraperitoneally every other day), metronomic topotecan alone (0.5 mg/kg orally every day), or metronomic nab-paclitaxel plus metronomic topotecan. Mean tumor weights (±standard error of the mean) are shown. *p < 0.01; **p <0.03; ***p < 0.05 compared with vehicle. d) Survival of mice treated with vehicle (control), metronomic nab-Paclitaxel or metronomic topotecan alone and combination metronomic nab-paclitaxel plus metronomic topotecan. Nude mice (n = 10 per group) were injected with HeyA8 cells and subsequently randomized into the different treatment regimens. Treatment was continued until mice were individually moribund, and the days of life were recorded. e) Mean (±SE) temporal complete blood count parameters in the SKOV3ip1 model from the therapy experiments at baseline (solid bars) and after three weeks of treatment (white bars). WBC: white blood count. Hgb: hemoglobin.
These findings led us to perform additional dose-finding experiments using an alternating day dosing frequency in the HeyA8 tumor model. This dosing schedule resulted in less tumor inhibition (Figure 1b). Moreover, pancytopenia was observed in mice that were treated with nab-paclitaxel doses of 5 and 10 mg/kg after two weeks, but not with the 2.5 mg/kg dose (Supplementary Figure 1). Therefore, we selected 2.5 mg/kg nab-paclitaxel intraperitoneally every other day as the optimal dose for subsequent combination experiments. MTD dosing and metronomic nab-paclitaxel at 10 mg/kg would abrogate tumor growth and prevent assessment of dual metronomic therapy. We had previously selected the dosing schedule for metronomic topotecan (22).
In vivo effects of metronomic nab-paclitaxel and topotecan on ovarian carcinoma
The next experiment had 4 treatment groups: 1) vehicle, 2) metronomic nab-paclitaxel (2.5 mg/kg intraperitoneally every other day), 3) metronomic topotecan (0.5 mg/kg daily via oral gavage), and 4) metronomic nab-paclitaxel every other day plus metronomic topotecan daily. Compared with vehicle, treatment with metronomic nab-paclitaxel alone resulted in a 62% reduction in tumor weight (p < 0.01), and treatment with the combination of metronomic nab-paclitaxel and metronomic topotecan resulted in a 96% reduction in tumor weight (p < 0.01) in the SKOV3ip1 tumor model (Figure 1c). Similar results were obtained in the HeyA8 tumor model: metronomic nab-paclitaxel alone and metronomic nab-paclitaxel combined with metronomic topotecan elicited >90% reductions in tumor weight (p < 0.03) compared with vehicle (Figure 1c).
Recognizing the high prevalence of taxane resistance in recurrent ovarian cancer, we also tested the therapeutic potential of metronomically dosed nab-paclitaxel and topotecan in the taxane-resistant HeyA8-MDR tumor model. Although metronomic monotherapy with either cytotoxic agent alone did not overcome taxane resistance, the combination of metronomic nab-paclitaxel and metronomic topotecan decreased tumor burden by 60% (p < 0.05) compared with vehicle, thereby supporting the use of a combination of two metronomic cytotoxic agents to augment the observed therapeutic effect (Figure 1c).
Based on these results with regard to inhibition of in vivo tumor growth using metronomic regimens, we next examined the effects of these regimens on survival using the HeyA8 model. Treatment with metronomic nab-Paclitaxel or metronomic topotecan prolonged survival; the most significant effect on survival time was in the combination arm with metronomic nab-paclitaxel and metronomic topotecan, where survival was significantly increased (p < 0.001; Figure 1d).
No differences in body weight were identified among treatment groups in any of the models. To assess hematologic tolerance of the combination therapy, complete blood counts were performed using blood drawn via tail vein phlebotomy just prior to initiating treatment and again after three weeks of treatment. In the complete blood count analysis, white blood cell and platelet counts did not differ over the course of treatment in animals treated with metronomic nab-paclitaxel alone or in those treated with metronomic nab-paclitaxel combined with metronomic topotecan (Figure 1e). Direct observation of the animals’ eating habits and daily veterinary care of other physical characteristics revealed no obvious deviation from typical bowel or feeding habits, posture, or mobility among the four treatment groups in any of the three models.
Biological effects of metronomic nab-paclitaxel and metronomic topotecan on proliferation, angiogenesis, and apoptosis
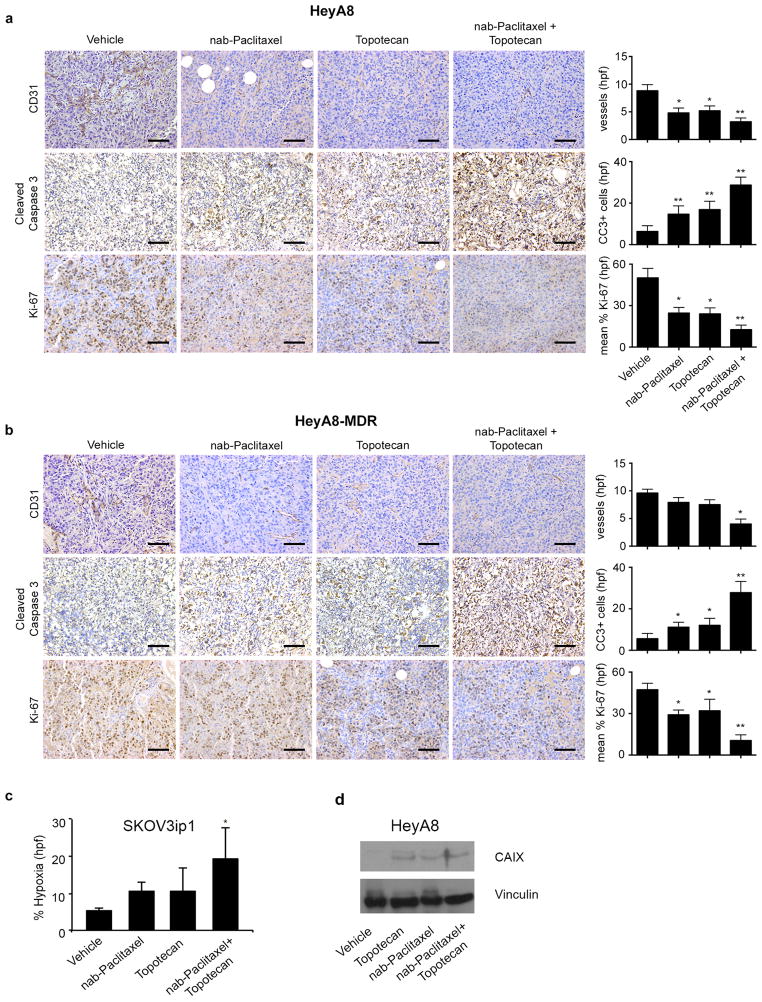
Given the proposed role of nab-paclitaxel on angiogenesis inhibition, we calculated the MVD of tumors harvested at the conclusion of the therapy experiments. In the HeyA8 tumor model, treatment with metronomic nab-paclitaxel or topotecan decreased MVD by 45% and 41%, respectively (p < 0.05), while the combination of metronomic nab-paclitaxel and metronomic topotecan resulted in a 64% reduction of MVD (p < 0.01) compared with vehicle (Figure 2a). In the HeyA8-MDR model, treatment with metronomic nab-paclitaxel or topotecan had no effect on MVD; however, treatment with the combination of metronomic nab-paclitaxel and metronomic topotecan resulted in a nearly 60% reduction of MVD compared with vehicle (p < 0.01; Figure 2b).
Figure 2. Biological effects of metronomic nab-Paclitaxel and metronomic topotecan.
a) Representative images of HeyA8 tumor samples immunohistochemically stained for CD31, cleaved caspase 3, and Ki-67 from mice treated with vehicle, metronomic nab-paclitaxel (2.5 mg/kg intraperitoneally every other day), metronomic topotecan (0.5 mg/kg orally every day), or a combination of metronomic nab-paclitaxel and metronomic topotecan. Error bars represent standard error of the mean (SEM). *p < 0.05 compared with vehicle. **p < 0.01 compared with vehicle. hpf: high-power field. b) Representative images of HeyA8-MDR samples immunohistochemically stained for CD31, cleaved caspase 3, and Ki-67 from mice treated with vehicle, metronomic nab-paclitaxel (2.5 mg/kg intraperitoneally every other day), metronomic topotecan (0.5 mg/kg orally every day), or a combination of metronomic nab-paclitaxel and metronomic topotecan. (original magnification ×100, scale bar represents 100 μM). Error bars represent SEM. *p < 0.05 compared with vehicle. **p < 0.01 compared with vehicle. hpf: high-power field. c) Percentage of tissue showing hypoxia in SKOV3ip1 tumor cells from each treatment group. Error bars represent standard error. *p < 0.05 compared with vehicle. d) Western blot images depicting the effect of vehicle, metronomic topotecan, maximally tolerated dose (MTD) nab-paclitaxel and combination on carbonic anhydrase IX (CAIX) expression from tumors in the HeyA8 orthotopic ovarian model.
To determine whether apoptosis contributed to the anti-tumor effects observed with metronomic nab-paclitaxel and metronomic topotecan, we conducted immunohistochemical staining for cleaved caspase 3 in tumors harvested after the therapy experiments. HeyA8 tumors from animals treated with metronomic nab-Paclitaxel or topotecan alone had a significant increase in apoptotic cells (2.3 and 2.68 fold respectively; p <0.01). Meanwhile the combination treatment had the most profound effect on apoptosis (4.5 fold; p < 0.01; Figure 2a). Furthermore, HeyA8-MDR tumors from mice treated with metronomic nab-paclitaxel or topotecan alone resulted in a 1.98 and 2.14 fold increase in apoptotic cells, while the combination therapy induced a 4.96 fold increase (Figure 2b; p < 0.01).
We examined treatment effects on tumor cell proliferation by calculating the proliferative index after immunohistochemical staining for Ki-67 in tumors collected at necropsy at the end of the therapy experiments. The proliferative index was significantly reduced in mice with HeyA8 tumors treated with either metronomic nab-paclitaxel (51%, p < 0.05) or metronomic topotecan alone (53%, p < 0.05) (Figure 2a). The combination treatment resulted in a 75% reduction (p < 0.01) in proliferative index. The proliferative index in HeyA8-MDR tumors treated with nab-paclitaxel or topotecan was reduced by 39% and 33%, respectively, while the combination treatment significantly reduced proliferation by 88% (p < 0.01; Figure 2b).
To further evaluate the contribution of metronomic therapy on angiogenesis inhibition and hypoxia, we treated three mice from each treatment arm in the SKOV3ip1 tumor model with a hypoxia marker, pimonidazole, prior to sacrificing them and stained the harvested tumor tissue for pimonidazole protein adducts, which represent areas of hypoxia within the solid tumor. Immunohistochemical staining with pimonidazole showed that treatment with metronomic nab-paclitaxel alone and metronomic topotecan alone resulted in hypoxic areas in approximately 10% of the tumor tissue, compared with only 5% after treatment with vehicle (Figure 2c). Treatment with the combination of metronomic nab-paclitaxel and metronomic topotecan resulted in nearly double the percentage of tissue showing hypoxia (19%, p = 0.04; Figure 2c), further supporting the observation that metronomic nab-paclitaxel and metronomic topotecan exert anti-angiogenic effects directly on tumors. Treatment with metronomic topotecan, nab-paclitaxel, or combination therapy led to an increase in carbonic anhydrase IX expression tumors from mice inoculated with HeyA8 cells (Figure 2d).
In vitro effects of metronomic nab-paclitaxel compared with MTD nab-paclitaxel
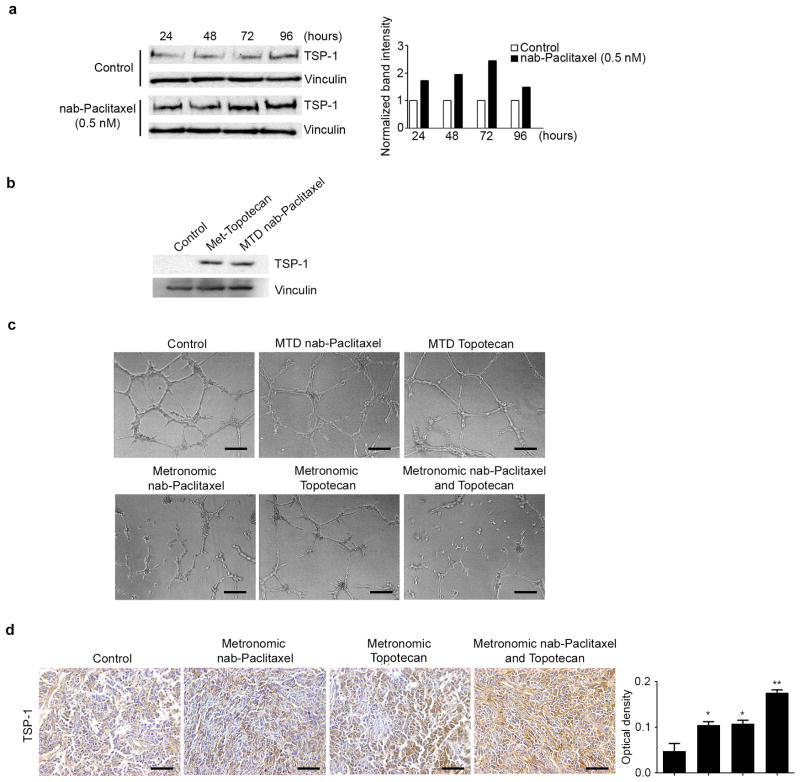
One possible mechanism underlying the anti-tumor effects of metronomic chemotherapy is increased tumor cell expression of anti-angiogenic factors such as TSP-1 in response to treatment. To test whether metronomic nab-paclitaxel induces TSP-1 expression in ovarian cancer cells, we treated HeyA8 cells with a sub-IC50 dose of nab-paclitaxel (0.5 nM) for up to 96 hours. In Western blot analysis, we found that protracted exposure of HeyA8 tumor cells to nab-paclitaxel resulted in a substantial and time-dependent induction of TSP-1 protein expression (Figure 3a). TSP-1 expression was maximally induced after 72 hours of treatment, corresponding to a 2.5 times increase in TSP-1 expression compared with vehicle. To determine if metronomic topotecan and MTD nab-paclitaxel could induce TSP-1, we treated HeyA8 cells with nab-paclitaxel at the beginning of the experiment and daily topotecan for 72 hours. Western blot analysis shows that both treatments induced TSP-1 expression (Figure 3b) treatment with daily topotecan, MTD nab-paclitaxel and combination treatments.
Figure 3. Effect of metronomic nab-Paclitaxel and topotecan on TSP-1 expression.
a) Western blot images depicting the effect of control (normal saline) and metronomic nab-paclitaxel on TSP-1 protein expression. HeyA8 cells were continuously treated with either vehicle or 0.5 nM nab-paclitaxel for up to 96 hours and cell lysates were collected at 24-hour time points. The graph shows the band intensity of TSP-1 normalized to the loading control, vinculin. b) Western blot depicting the effect of control (normal saline), metronomic topotecan (25 nM daily) and the maximally tolerated dose (MTD) of nab-paclitaxel (20 nM once) on TSP-1 expression. Cell lysates were collected after 72 hours. c) Tube formation of RF-24 cells after treatment with conditioned media of each treatment group, representative images shown. (original magnification ×100, scale bar represents 100 μM). d) Representative images of HeyA8 tumor samples immunohistochemically stained and quantified for TSP-1 (original magnification ×100, scale bar represents 100 μM). Error bars represent SEM. *p < 0.05 compared with vehicle. **p < 0.01 compared with vehicle.
Next, we performed tube formation assays utilizing RF-24 cells exposed to conditioned media from HeyA8 cells that were exposed to nab-paclitaxel or topotecan. Tube formation was inhibited in RF-24 endothelial cells exposed to conditioned media from HeyA8 cells exposed to metronomic nab-paclitaxel and topotecan while the combination treatment had the most robust effect (Figure 3c).
To investigate if metronomic dosing increased TSP-1 expression in vivo, we performed immunohistochemistry analyses on HeyA8 tumor samples. Our data show that metronomic nab-paclitaxel and topotecan increased TSP-1 expression while this effect was exacerbated in the combination group (Figure 3d).
Discussion
In this study we demonstrated marked anti-tumor activity in ovarian carcinoma models using combination metronomic chemotherapy. These effects were mediated, in part, through a significant reduction in tumor cell proliferation and increased apoptosis. We also demonstrated that metronomic administration of nab-paclitaxel increased levels of TSP-1, thereby disrupting angiogenesis and providing a mechanism by which this compound elicits its therapeutic effects. These anti-tumor and anti-angiogenic effects were validated in both taxane-sensitive and taxane-resistant tumor models.
Our results suggest that although apoptosis plays a role in eliciting the observed anti-tumor effects of treatment with metronomic topotecan, the reduction in tumor growth seen with metronomic nab-paclitaxel appears to be more attributable to the inhibition of angiogenic factors, resulting in large areas of hypoxia within the tumor. Additionally, a resultant increase in CAIX expression was observed in vitro treatment of cells with metronomic topotecan and nab-paclitaxel. The profound inhibition of endothelial tube formation by conditioned media from cells treated with metronomic nab-paclitaxel alone and in combination with metronomic topotecan supports existing evidence that metronomic dosing not only has direct cytotoxic effects on endothelial cells, but also indirectly inhibits angiogenesis through the release of mediators from the cancer cell into the tumor microenvironment (55).
Angiogenic pathways and the tumor microvasculature are attractive targets for cancer treatment, as supported by the robust number of ongoing clinical trials in this arena (6). Nevertheless, both intrinsic and acquired resistance to anti-angiogenic drugs are emerging as clinically relevant issues (56, 57). Mounting data suggest that administering traditional cytotoxic agents at metronomic doses exerts anti-angiogenic effects (6, 55, 58, 59). Metronomic paclitaxel has stronger anti-tumor activity in terms of suppressing primary and metastatic breast tumors, with fewer side effects and stronger anti-angiogenic and anti-lymphangiogenic activities, than MTD therapy. Moreover, metronomic paclitaxel chemotherapy causes a substantial increase in the expression of TSP-1. Metronomic dosing of chemotherapeutics has been shown to reduce normal tissue toxicity and minimize off-treatment exposure (60). Several clinical trials have shown that more frequent treatment with lower doses of chemotherapy leads to fewer toxic effects and improved pathologic response rates in patients with breast cancer (3, 60). However, a single metronomic regimen is unlikely to have universal efficacy, and novel combination regimens of metronomic chemotherapy are needed.
Our study provides evidence of potent anti-tumor activity in both taxane-sensitive and taxane-resistant orthotopic models of metastatic ovarian carcinoma after treatment with metronomic nab-paclitaxel alone and in combination with metronomic topotecan. With potent anti-tumor effects in the absence of overt toxic effects that occur with traditional chemotherapy, metronomic nab-paclitaxel alone or in combination with topotecan is a strategy worthy of further clinical investigation.
Supplementary Material
Acknowledgments
Financial Information
Financial support was provided by the National Institutes of Health (P50 CA098258, CA 109298, P50 CA083639, U54 CA151668, M. D. Anderson’s Cancer Center Support Grant CA016672), the Ovarian Cancer Research Fund, Inc. (Program Project Development Grant), the U.S. Department of Defense (OC073399, OC093146, BC085265), the Ann Rife Cox Chair in Gynecology, the Betty Anne Asche Murray Distinguished Professorship, the RGK Foundation, and the Gilder Foundation to A.K. Sood. R.A. Previs, J.M. Hansen and H.J. Dalton were supported by an NCI-DHHS-NIH T32 Training Grant (T32 CA101642).
The authors thank Nicholas Jennings, Donna Reynolds and Dr. Yun-Fang Wang for their insightful discussions and expertise.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors declare that there are no conflicts of interest.
References
- 1.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, et al. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell. 2009;15:220–31. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burstein HJ, Manola J, Younger J, Parker LM, Bunnell CA, Scheib R, et al. Docetaxel administered on a weekly basis for metastatic breast cancer. J Clin Oncol. 2000;18:1212–9. doi: 10.1200/JCO.2000.18.6.1212. [DOI] [PubMed] [Google Scholar]
- 3.Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23:5983–92. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 4.Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, et al. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008;26:1642–9. doi: 10.1200/JCO.2007.11.6699. [DOI] [PubMed] [Google Scholar]
- 5.Pasquier E, Kavallaris M, Andre N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7(8):455–65. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 6.Gasparini G. Metronomic scheduling: the future of chemotherapy? Lancet Oncol. 2001;2:733–40. doi: 10.1016/S1470-2045(01)00587-3. [DOI] [PubMed] [Google Scholar]
- 7.Burstein HJ, Manola J, Younger J, Parker LM, Bunnell CA, Scheib R, et al. Docetaxel administered on a weekly basis for metastatic breast cancer. J Clin Oncol. 2000;18:1212–9. doi: 10.1200/JCO.2000.18.6.1212. [DOI] [PubMed] [Google Scholar]
- 8.Green MC, Buzdar AU, Smith T, Ibrahim NK, Valero V, Rosales MF, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23:5983–92. doi: 10.1200/JCO.2005.06.232. [DOI] [PubMed] [Google Scholar]
- 9.Derosa L, Galli L, Orlandi P, Fioravanti A, Di Desidero T, Fontana A, et al. Docetaxel plus oral metronomic cyclophosphamide: a phase II study with pharmacodynamic and pharmacogenetic analyses in castration-resistant prostate cancer patients. Cancer. 2014;120:3923–31. doi: 10.1002/cncr.28953. [DOI] [PubMed] [Google Scholar]
- 10.Masuda N, Higaki K, Takano T, Matsunami N, Morimoto T, Ohtani S, et al. A phase II study of metronomic paclitaxel/cyclophosphamide/capecitabine followed by 5-fluorouracil/epirubicin/cyclophosphamide as preoperative chemotherapy for triple-negative or low hormone receptor expressing/HER2-negative primary breast cancer. Cancer Chemother Pharmacol. 2014;74:229–38. doi: 10.1007/s00280-014-2492-y. [DOI] [PubMed] [Google Scholar]
- 11.Berruti A, Fazio N, Ferrero A, Brizzi MP, Volante M, Nobili E, et al. Bevacizumab plus octreotide and metronomic capecitabine in patients with metastatic well-to-moderately differentiated neuroendocrine tumors: the XELBEVOCT study. BMC Cancer. 2014;14:184. doi: 10.1186/1471-2407-14-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brandi G, de Rosa F, Agostini V, di Girolamo S, Andreone P, Bolondi L, et al. Metronomic capecitabine in advanced hepatocellular carcinoma patients: a phase II study. The Oncologist. 2013;18:1256–7. doi: 10.1634/theoncologist.2013-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelley RK, Hwang J, Magbanua MJ, Watt L, Beumer JH, Christner SM, et al. A phase 1 trial of imatinib, bevacizumab, and metronomic cyclophosphamide in advanced colorectal cancer. Br J Cancer. 2013;109:1725–34. doi: 10.1038/bjc.2013.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eichbaum M, Mayer C, Eickhoff R, Bischofs E, Gebauer G, Fehm T, et al. The PACOVAR-trial: a phase I/II study of pazopanib (GW786034) and cyclophosphamide in patients with platinum-resistant recurrent, pre-treated ovarian cancer. BMC Cancer. 2011;11:453. doi: 10.1186/1471-2407-11-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia AA, Hirte H, Fleming G, Yang D, Tsao-Wei DD, Roman L, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26:76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 16.Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013;16:481–92. doi: 10.1007/s10456-013-9334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng SS, Sparreboom A, Shaked Y, Lee C, Man S, Desai N, et al. Influence of formulation vehicle on metronomic taxane chemotherapy: albumin-bound versus cremophor EL-based paclitaxel. Clin Cancer Res. 2006;12:4331–8. doi: 10.1158/1078-0432.CCR-05-2762. [DOI] [PubMed] [Google Scholar]
- 18.Bocci G, Falcone A, Fioravanti A, Orlandi P, Di Paolo A, Fanelli G, et al. Antiangiogenic and anticolorectal cancer effects of metronomic irinotecan chemotherapy alone and in combination with semaxinib. Br J Cancer. 2008;98:1619–29. doi: 10.1038/sj.bjc.6604352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparreboom A, Baker SD, Verweij J. Paclitaxel repackaged in an albumin-stabilized nanoparticle: handy or just a dandy? J Clin Oncol. 2005;23:7765–7. doi: 10.1200/JCO.2005.03.7135. [DOI] [PubMed] [Google Scholar]
- 20.Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in Cremophor (Taxol) Clin Cancer Res. 2005;11:4136–43. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 21.Teneriello MG, Tseng PC, Crozier M, Encarnacion C, Hancock K, Messing MJ, et al. Phase II evaluation of nanoparticle albumin-bound paclitaxel in platinum-sensitive patients with recurrent ovarian, peritoneal, or fallopian tube cancer. J Clin Oncol. 2009;20(27):1426–31. doi: 10.1200/JCO.2008.18.9548. [DOI] [PubMed] [Google Scholar]
- 22.Merritt WM, Danes CG, Shahzad MM, Lin YG, Kamat AA, Han LY, et al. Anti-angiogenic properties of metronomic topotecan in ovarian carcinoma. Cancer Biol Ther. 2009;8:1596–603. doi: 10.4161/cbt.8.16.9004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merritt WM, Nick AM, Carroll AR, Lu C, Matsuo K, Dumble M, et al. Bridging the gap between cytotoxic and biologic therapy with metronomic topotecan and pazopanib in ovarian cancer. Mol Cancer Ther. 2010;9:985–95. doi: 10.1158/1535-7163.MCT-09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mathijssen RH, Loos WJ, Verweij J, Sparreboom A. Pharmacology of topoisomerase I inhibitors irinotecan (CPT-11) and topotecan. Curr Cancer Drug Targets. 2002;2:103–23. doi: 10.2174/1568009023333890. [DOI] [PubMed] [Google Scholar]
- 25.Wethington SL, Wright JD, Herzog TJ. Key role of topoisomerase I inhibitors in the treatment of recurrent and refractory epithelial ovarian carcinoma. Expert Rev Anticancer Ther. 2008;8:819–31. doi: 10.1586/14737140.8.5.819. [DOI] [PubMed] [Google Scholar]
- 26.Puppo M, Battaglia F, Ottaviano C, Delfino S, Ribatti D, Varesio L, et al. Topotecan inhibits vascular endothelial growth factor production and angiogenic activity induced by hypoxia in human neuroblastoma by targeting hypoxia-inducible factor-1alpha and -2alpha. Mol Cancer Ther. 2008;7:1974–84. doi: 10.1158/1535-7163.MCT-07-2059. [DOI] [PubMed] [Google Scholar]
- 27.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rapisarda A, Uranchimeg B, Sordet O, Pommier Y, Shoemaker RH, Melillo G. Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer Res. 2004;64:1475–82. doi: 10.1158/0008-5472.can-03-3139. [DOI] [PubMed] [Google Scholar]
- 29.Kumar S, Mokhtari RB, Oliveira ID, Islam S, Toledo SR, Yeger H, et al. Tumor dynamics in response to antiangiogenic therapy with oral metronomic topotecan and pazopanib in neuroblastoma xenografts. Transl Oncol. 2013;6:493–503. doi: 10.1593/tlo.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hackl C, Man S, Francia G, Milsom C, Xu P, Kerbel RS. Metronomic oral topotecan prolongs survival and reduces liver metastasis in improved preclinical orthotopic and adjuvant therapy colon cancer models. Gut. 2013;62:259–71. doi: 10.1136/gutjnl-2011-301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aljuffali IA, Mock JN, Costyn LJ, Nguyen H, Nagy T, Cummings BS, et al. Enhanced antitumor activity of low-dose continuous administration schedules of topotecan in prostate cancer. Cancer Biol Ther. 2011;12:407–20. doi: 10.4161/cbt.12.5.15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cruz-Munoz W, Di Desidero T, Man S, Xu P, Jaramillo ML, Hashimoto K, et al. Analysis of acquired resistance to metronomic oral topotecan chemotherapy plus pazopanib after prolonged preclinical potent responsiveness in advanced ovarian cancer. Angiogenesis. 2014;17:661–73. doi: 10.1007/s10456-014-9422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez-Munoz A, Mendiola C, Perez-Ruiz E, Rodriguez-Sanchez CA, Jurado JM, Alonso-Carrion L, et al. Bevacizumab plus low-dose metronomic oral cyclophosphamide in heavily pretreated patients with recurrent ovarian cancer. Oncology. 2010;79:98–104. doi: 10.1159/000320602. [DOI] [PubMed] [Google Scholar]
- 34.Hashimoto K, Man S, Xu P, Cruz-Munoz W, Tang T, Kumar R, et al. Potent preclinical impact of metronomic low-dose oral topotecan combined with the antiangiogenic drug pazopanib for the treatment of ovarian cancer. Mol Cancer Ther. 2010;9:996–1006. doi: 10.1158/1535-7163.MCT-09-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merritt WM, Nick AM, Carroll AR, Lu C, Matsuo K, Dumble M, et al. Bridging the gap between cytotoxic and biologic therapy with metronomic topotecan and pazopanib in ovarian cancer. Mol Cancer Ther. 2010;9:985–95. doi: 10.1158/1535-7163.MCT-09-0967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurado JM, Sanchez A, Pajares B, Perez E, Alonso L, Alba E. Combined oral cyclophosphamide and bevacizumab in heavily pre-treated ovarian cancer. Clin Transl Oncol. 2008;10:583–6. doi: 10.1007/s12094-008-0254-7. [DOI] [PubMed] [Google Scholar]
- 37.Turner DC, Tillmanns TD, Harstead KE, Throm SL, Stewart CF. Combination metronomic oral topotecan and pazopanib: a pharmacokinetic study in patients with gynecological cancer. Anticancer Res. 2013;33:3823–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Munoz R, Man S, Shaked Y, Lee CR, Wong J, Francia G, et al. Highly efficacious nontoxic preclinical treatment for advanced metastatic breast cancer using combination oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res. 2006;66:3386–91. doi: 10.1158/0008-5472.CAN-05-4411. [DOI] [PubMed] [Google Scholar]
- 39.Cruz-Munoz W, Man S, Xu P, Kerbel RS. Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 2008;68:4500–5. doi: 10.1158/0008-5472.CAN-08-0041. [DOI] [PubMed] [Google Scholar]
- 40.Cruz-Munoz W, Man S, Kerbel RS. Effective treatment of advanced human melanoma metastasis in immunodeficient mice using combination metronomic chemotherapy regimens. Clin Cancer Res. 2009;15:4867–74. doi: 10.1158/1078-0432.CCR-08-3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dellapasqua S, Bertolini F, Bagnardi V, Campagnoli E, Scarano E, Torrisi R, et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J Clin Oncol. 2008;26:4899–905. doi: 10.1200/JCO.2008.17.4789. [DOI] [PubMed] [Google Scholar]
- 42.Montagna E, Cancello G, Bagnardi V, Pastrello D, Dellapasqua S, Perri G, et al. Metronomic chemotherapy combined with bevacizumab and erlotinib in patients with metastatic HER2-negative breast cancer: clinical and biological activity. Clin Breast Cancer. 2012;12:207–14. doi: 10.1016/j.clbc.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimoto M, Takao S, Hirata M, Okamoto Y, Yamashita S, Kawaguchi Y, et al. Metronomic oral combination chemotherapy with capecitabine and cyclophosphamide: a phase II study in patients with HER2-negative metastatic breast cancer. Cancer Chemother Pharmacol. 2012;70:331–8. doi: 10.1007/s00280-012-1826-x. [DOI] [PubMed] [Google Scholar]
- 44.Orlando L, Cardillo A, Ghisini R, Rocca A, Balduzzi A, Torrisi R, et al. Trastuzumab in combination with metronomic cyclophosphamide and methotrexate in patients with HER-2 positive metastatic breast cancer. BMC Cancer. 2006;6:225. doi: 10.1186/1471-2407-6-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wong NS, Buckman RA, Clemons M, Verma S, Dent S, Trudeau ME, et al. Phase I/II trial of metronomic chemotherapy with daily dalteparin and cyclophosphamide, twice-weekly methotrexate, and daily prednisone as therapy for metastatic breast cancer using vascular endothelial growth factor and soluble vascular endothelial growth factor receptor levels as markers of response. J Clin Oncol. 2010;28:723–30. doi: 10.1200/JCO.2009.24.0143. [DOI] [PubMed] [Google Scholar]
- 46.Yu D, Wolf JK, Scanlon M, Price JE, Hung MC. Enhanced c-erbB-2/neu expression in human ovarian cancer cells correlates with more severe malignancy that can be suppressed by E1A. Cancer Res. 1993;53:891–8. [PubMed] [Google Scholar]
- 47.Buick RN, Pullano R, Trent JM. Comparative properties of five human ovarian adenocarcinoma cell lines. Cancer Res. 1985;45:3668–76. [PubMed] [Google Scholar]
- 48.Yoneda J, Kuniyasu H, Crispens MA, Price JE, Bucana CD, Fidler IJ. Expression of angiogenesis-related genes and progression of human ovarian carcinomas in nude mice. J Natl Cancer Inst. 1998;90:447–54. doi: 10.1093/jnci/90.6.447. [DOI] [PubMed] [Google Scholar]
- 49.Langley RR, Ramirez KM, Tsan RZ, Van Arsdall M, Nilsson MB, Fidler IJ. Tissue-specific microvascular endothelial cell lines from H-2K(b)-tsA58 mice for studies of angiogenesis and metastasis. Cancer Res. 2003;63:2971–6. [PubMed] [Google Scholar]
- 50.Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8:1038–44. [PubMed] [Google Scholar]
- 52.Lin YG, Han LY, Kamat AA, Merritt WM, Landen CN, Deavers MT, et al. EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer. 2007;109:332–40. doi: 10.1002/cncr.22415. [DOI] [PubMed] [Google Scholar]
- 53.Lin YG, Kunnumakkara AB, Nair A, Merritt WM, Han LY, Armaiz-Pena GN, et al. Curcumin Inhibits Tumor Growth and Angiogenesis in Ovarian Carcinoma by Targeting the Nuclear Factor-{kappa}B Pathway. Clin Caner Res. 2007;13:3423–30. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 54.Lin YG, Immaneni A, Merritt WM, Mangala LS, Kim SW, Shahzad MM, et al. Targeting aurora kinase with MK-0457 inhibits ovarian cancer growth. Clin Cancer Res. 2008;14:5437–46. doi: 10.1158/1078-0432.CCR-07-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- 56.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jain RK, Duda DG, Willett CG, Sahani DV, Zhu AX, Loeffler JS, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–38. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Drevs J, Fakler J, Eisele S, Medinger M, Bing G, Esser N, et al. Antiangiogenic potency of various chemotherapeutic drugs for metronomic chemotherapy. Anticancer Res. 2004;24:1759–63. [PubMed] [Google Scholar]
- 59.Gately S, Kerbel R. Antiangiogenic scheduling of lower dose cancer chemotherapy. Cancer J. 2001;7:427–36. [PubMed] [Google Scholar]
- 60.Nardi M, Azzarello D, Maisano R, Del Medico P, Giannicola R, Raffaele M, et al. FOLFOX-4 regimen as fist-line chemotherapy in elderly patients with advanced gastric cancer: a safety study. J Chemother. 2007;19:85–9. doi: 10.1179/joc.2007.19.1.85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.