Abstract
This study is to determine the therapeutic effects of Panax notoginseng saponins (PNS) on coxsackievirus B3 (CVB3)-induced myocarditis, and whether cystathionine-γ-lyase (CSE)/hydrogen sulfide (H2S) pathway is involved. Mouse model of myocarditis was induced by CVB3 infection and the mice were subjected to vehicle (saline) or drug treatments (sodium bisulfide (NaHS), propargylglycine (PAG) or PNS). The results showed that there were inflammatory cell infiltrations, interstitial edemas, as well as elevated inflammatory cytokines, in CVB3-induced myocarditis. PAG administration increased, whereas NaHS treatment decreased the severity of the myocarditis. PNS treatment dramatically alleviated these myocardial injuries and decreased the viral mRNA expression by the enhanced expression of CSE/H2S pathway. Moreover, the therapeutic effects of PNS on myocarditis were stronger than NaHS. Finally, the effect of PNS on CSE/H2S pathway and cardiac cell protection were verified in cultured cardiac cells. PNS may be a promising medication for viral myocarditis therapy.
Keywords: Myocarditis, Panax notoginseng saponins, hydrogen sulfide, cystathionine-γ-lyase
Introduction
Viral myocarditis is a main cause of cardiomyopathy, which may lead to heart failure, arrhythmia, and even sudden death[1]. Many supportive therapeutic strategies such as anti-virus, immune suppression, and anti-oxidation therapies, have been tried to reverse the underlying active myocardial inflammation; however, they are not efficient enough to improve the patient survival in clinical applications [1–3]. Viral myocarditis remains a challenging disease, and effective therapies are still needed in practice. Therefore, elucidation of the fundamental mechanisms responsible for the development of myocarditis is very important [4–6].
Recently, hydrogen sulfide (H2S) has been shown to play key roles in physiological and/or pathological processes including inflammation, oxidative stress, apoptosis, and vaso-relaxation [7]. Moreover, high levels of H2S, as well as the activation of cystathionine-γ-lyase (CSE)/H2S pathway, have also been found in myocardial tissues, making this pathway a potential target in the treatment of inflammatory heart diseases [8,9]. Indeed, activation of CSE/H2S pathway could effectively protect hearts against ischemic injuries [8–11]. In addition, CSE/H2S pathway is also involved in viral myocarditis [12].
Panax notoginseng saponins (PNS) is the major active ingredient in the traditional Chinese herb Panax notoginseng, which has been used to treat cardiovascular diseases for more than 1000 years. PNS has been found to have extensive effects on the cardiovascular system by regulating multiple signaling pathways [13]. For example, PNS could attenuate atherosclerosis via reciprocal regulation of lipid metabolism and inflammation [14], and inhibit ischemia-induced apoptosis in cardio-myocytes by the activation of the phosphoinositide Kinase-3 (PI3K)/Akt pathway [15]. In vivo, injection of PNS is able to reduce myocardial injuries induced by ischemia [16].
Although PNS has a strong protective effect on myocardial injures, the therapeutic effects of PNS on viral myocarditis have not been reported. In addition, the effect of PNS on CSE/H2S pathway in hearts with viral myocarditis is still unclear. In the present study, we try to investigate the effects of PNS on myocarditis, and to determine whether the CSE/H2S pathway is involved in PNS-mediated therapeutic effect on myocarditis in CVB3-infected mice.
Methods
Reagents and kits
CVB3 Nancy strains and Hela cells were purchased from ATCC (Rockville, MD, USA). PNS was purchased from Guangxi Wuzhou Pharmaceutical (Group) Co., Ltd. (Wuzhou, Guangxi, China). DL-propargylglycine (PAG) and sodium bisulfides (NaHS) were purchased from Sigma (St. Louis, MO, USA). DMEM, FBS, and Penicillin/Streptomycin were purchased from Gibco (Grand Island, NY, USA). BCA kit was from Thermo Scientific (Rockford, IL, USA). IL-6 and TNF-α ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA). H2S ELISA kit was purchased from Shanghai Biological Technology Co., Ltd. (Shanghai, China). The cDNA synthesis kit and SYBR green were purchased from Boston Biomedica Inc. (West Bridgewater, Mass., USA). GAPDH and CSE antibodies were purchased from GeneTex (San Antonio, TX, USA). ECL kit was purchased from Advanstar (Cleveland, Ohio, USA). Anti-rabbit IgG was purchased from Millipore (Bedford, MA, USA). The primers for CVB3 (F-5′-GTATGCTGCGACTAGACGTTGT-3′, R-5′-TTCTCTTCTCTGCGTTTCCTGT-3′), CSE (F-5′-CTGATACGACTTTCTGTGGGC-3′, R-5′-AGTTCTGCGTATGCTCCGTAA-3′), and GAPDH (F-5′-GGTTGTCTCCTGCGACTTCA-3′, R-5′-TGGTCCAGGGTTTCTTACTCC-3′) were synthesized by Sangon Biotech (Shanghai, China). Lipofectamine™ 2000 transfection reagents were obtained from Invitrogen (Carlsbad, CA).
Cell culture and detection of apoptosis
H9c2 cells (ATCC CRL1446, a cardiac cell line) were grown at a density of about 105 cells/cm2 and cultured as monolayers in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum, glutamine (2 mM), nonessential amino acids (1%), penicillin (100 IU), and streptomycin (100 j,g/ml) under an atmosphere of 5% CO2 in air saturated with water vapor at 37°C. The medium was replaced by fresh medium every 2 days. Cell apoptosis was induced by H2O2 (200 μM) for 6 h and was measured by terminal deoxynucleotide transferase dUTP nick end labeling (TUNEL) staining as described in our previous study [17]. Briefly, cardiac myocytes cultured on coverslips in 24-well plates were fixed in 4% paraformaldehyde. The TUNEL staining was done using the in situ cell death detection kit (Roche) according to the manufacturer’s protocol. The numbers of TUNEL-positive cells and the total cells were counted under a fluorescence microscope.
RNA Interference
siRNA for CSE (siRNA-CSE) and control siRNA (siRNA-control) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Lipofectamin 2000 and siRNAs (50 nM) were mixed and kept still for 20 min at room temperature, and then the mixtures were added to cardio-myocytes cultured in 6-well plates for transfection. Knockdown of CSE was confirmed by using Western blotting.
Virus propagation
Hela cells were maintained in DMEM medium, supplemented with 10% FBS at 37°C in 5% CO2. After reaching at least 80% confluence, CVB3 was added. When cytopathic affection (CPE) was evident, the virus was released from these cells by three freeze-thaw cycles. Samples were centrifuged at 3000 rpm at 4 °C for 10 min, and the supernatant was collected. Then, the virus titer was calculated by Reed-Muench formula.
Animals
All animal protocols were approved by the Animal Care and Use Committee of Wenzhou Medical University and were consistent with the Guide for the Care and Use of Laboratory Animals (updated (2011) version of the NIH guidelines). A total of 140 Balb/c male mice from two repeated experiments (five-weeks-old, weighing 20.9 g ± 2 g) obtained from the Experimental Animal Research Center (Zhejiang, China), were randomly assigned into 5 groups: control group (n=20), viral myocarditis model group (n=30), NaHS-treated group (n=30), PAG-treated group (n=30), and PNS-treated group (n=30). Mice in the control group were injected daily with phosphate-buffered saline. All the other groups were infected by injecting 0.1 ml CVB3 (10−6.5TCID50), and then the mice received intraperitoneal injections of PBS, NaHS (50 μmol/kg), PNS (100 mg/kg) [18], or PAG (40 mg/kg), for 10 consecutive days. The mice were sacrificed on day 4 and day 10 respectively after infection. Blood specimens and heart tissue were collected from these mice for following assays.
Histopathology
Histopathological analysis was performed as described [19]. In brief, hearts were isolated after perfusion to remove blood. The ratio of heart weight to tibia length (HW/TL) was calculated to assess the degree of edema. The heart tissue was then fixed in 10% paraformaldehyde, and embedded in paraffin. Then, the tissue was sectioned, stained with hematoxylin and eosin and were sealed with neutral balsam. % inflamed lesion areas of heart tissue were measured in these heart sections.
Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) kits were used to measure the levels of IL-6, TNF-α, H2S in serum, cardiac homogenate, and cell culture supernatant according to the manufacturers’ instructions. The concentrations were calculated with reference to the standard curves.
RNA extraction and RT-PCR
Total RNA from heart tissues and cardiac cells was extracted with Trizol reagent, according to the manufacturer’s instructions. After denaturing at 95°C for 5 min, reverse transcription was performed with a total volume of 20 μL, at 70°C for 5min, 37°C for 5 min, 42°C for 60 min, and 70°C for 10 min, followed by 40 cycles of PCR reaction consisting of 95°C (10s) and 60°C (40s). The PCR reaction was directly monitored by ABI StepOne Plus Sequence Detection System (Applied Biosystems), and all the results were normalized against GAPDH.
Western blot analysis
The proteins were extracted from the cardiac tissue and cardiac cells. The concentrations were determined by the BCA protein assay kit. Then an equal amount of protein extract (50μg) was separated by 10% SDS-PAGE and stacked by 5% SDS-PAGE. They were then transferred onto polyvinylidene difluoride (PVDF) membrane. Then the blots were probed with primary antibody, followed by secondary antibody. Bands were visualized by using enhanced-chemiluminescence (ECL) kit, and then quantified by densitometry using AlphaEaseFC software.
Statistical analysis
Data was expressed as mean ± SE. Statistical analysis was performed with SPSS 19 software (SPSS Inc., Chicago, IL, USA). Three or more groups were compared by one-way ANOVA and post-hoc analysis with the least significant difference, Student-Newman-Keuls test and Dunnett’s test. Statistical significance was defined as P < 0.05.
Results
Animals
There were no deaths in control group. Eight out of 30 mice were died in viral myocarditis model group (Mortality rate: 26.67%); Six mice out of 30 mice were died in NaHS-treated group (Mortality rate: 20.00%); Ten mice out of 30 mice were died in PAG-treated group (Mortality rate: 33.33%); Five mice out of 30 mice were died in PNS-treated group (Mortality rate: 16.67%). The sacrificed animals of each group at two time points are: day 4: control group (n=10), viral myocarditis model group (n=10), NaHS-treated group (n=10), PAG-treated group (n=10), and PNS-treated group (n=10); day 10: control group (n=10), viral myocarditis model group (n=12), NaHS-treated group (n=14), PAG-treated group (n=10), and PNS-treated group (n=15).
PNS alleviates myocardial injuries in CVB3-induced myocarditis
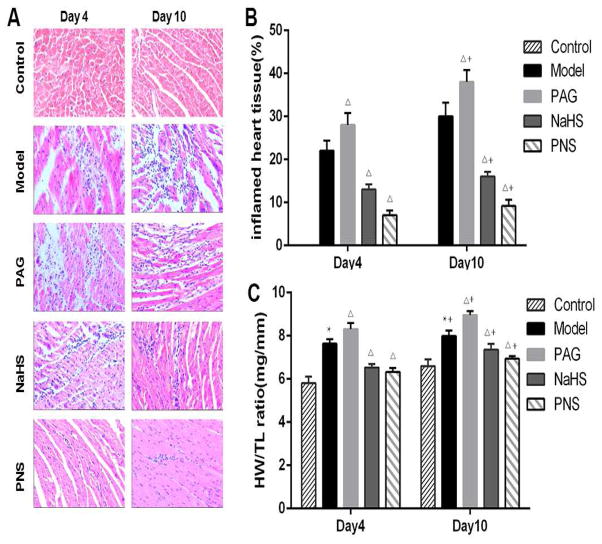
Histopathological analysis demonstrated that, compared with the control group, there were obvious myocardial injuries in the CVB3-induced model group on day 4 after infection, which became more severe on day 10, in terms of the inflamed area in heart tissue sections, and interstitial edema (HW/TL ratios) (Fig. 1). PAG treatment aggravated these injuries in myocarditis models. In contrast, the treatment with NaHS or PNS significantly alleviated these myocardial injuries, with a greater extent in the PNS-treated group (Fig. 1).
Fig. 1. Histological analysis of the infected hearts.
(A) Representative H&E staining images (×400) of heart sections from normal control mice (control, n=10 on day 4 and 10 on day10), mice with viral myocarditis (model, n=10 on day 4 and 12 on day10), mice with myocarditis and PAG treatment (PAG, n=10 on day 4 and 10 on day10), mice with myocarditis and NaHS treatment (NaHS, n=10 on day 4 and 14 on day10), and mice with myocarditis and PNS treatment (PNS, n=10 on day 4 and 15 on day10; (B) % inflamed heart tissue in heart sections from different groups; (C) The ratio of heart weight to tibia length (HW/TL) from different groups. * P<0.05 compared with the control group; ΔP < 0.05 compared with the model group; +P < 0.05 within the same group compared with the time point day 4.
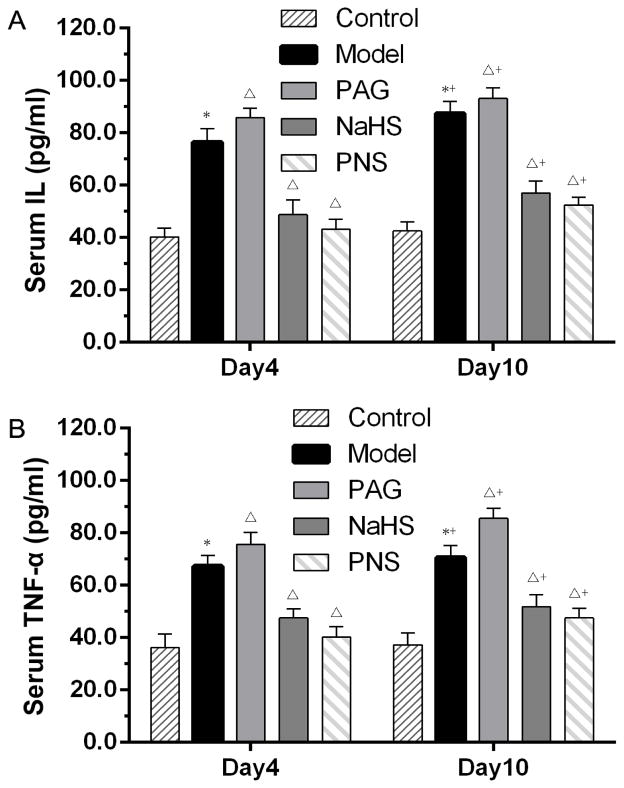
The concentrations of serum IL-6 and TNF-a were utilized as inflammatory markers for the severity of myocarditis. As shown in Fig. 2, compared with basal levels, IL-6 and TNF-a increased two-fold in the CVB3 group on day 4 and day 10 following infection. Administration of PAG increased the serum levels of IL-6 and TNF-α, while PNS and NaHS reduced these inflammatory cytokines in serum (Fig. 2).
Fig. 2. Levels of proinflammatory cytokines in serum in CVB3-induced myocarditis.
ELISA analysis was used to detect the IL-6 (A) and TNF-α (B) in serum in each group. *P < 0.05 compared with the control group. The animal numbers of each group are described in Fig. 1. ΔP < 0.05 compared with the model group; +P < 0.05 within the same group compared with the time point day 4. Note: control, n=10 on day 4 and 10 on day10; mice with viral myocarditis (model), n=10 on day 4 and 12 on day10; mice with myocarditis and PAG treatment (PAG), n=10 on day 4 and 10 on day10; mice with myocarditis and NaHS treatment (NaHS), n=10 on day 4 and 14 on day10; and mice with myocarditis and PNS treatment (PNS), n=10 on day 4 and 15 on day10.
These results suggest that there were obvious inflammatory cell infiltration, interstitial edema, as well as elevated inflammatory cytokines in CVB3-induced myocarditis. PAG administration increased, while NaHS treatment decreased the severity of the myocarditis and its blood inflammatory biomarkers. Interestingly, treatment with PNS could alleviate these myocardial injuries, even to a greater extent than the NaHS treatment.
PNS activates the CSE/H2S pathway in CVB3-induced myocarditis
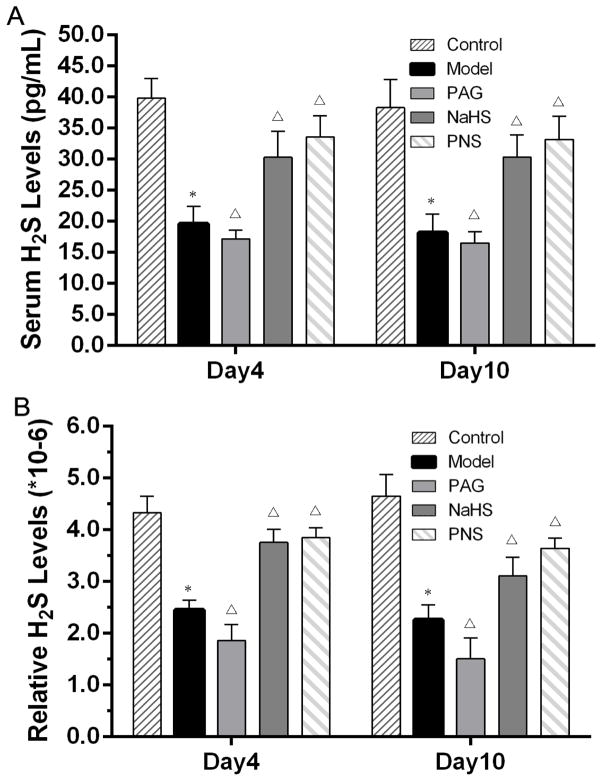
To find out whether the CSE/H2S pathway was involved in the therapeutic effects of PNS on CVB3-induced myocarditis, the H2S levels in serum and heart tissue were detected by ELISA, and the mRNA and protein expression levels of CSE in heart tissues were assessed with qRT-PCR and Western blot, respectively. Our results indicated that CVB3-infection reduced the levels of H2S in both serum and heart tissue (Fig. 3). The administration of PAG, which inhibited CSE activity, resulted in even lower levels of H2S both in serum and heart tissue. The treatment of NaHS, the H2S donor, increased H2S levels in myocarditis models, which was further increased by PNS treatment (Fig. 3).
Fig.3. Serum H2S levels in CVB3-induced myocarditis.
(A) ELISA was performed to detect the H2S levels in serum; (B) Serum H2S levels expressed relative to total protein contents. *P<0.05 compared with the control group. ΔP<0.05 compared with the model group; +P< 0.05 within the same group compared with the time point day 4. Note: control, n=10 on day 4 and 10 on day10; mice with viral myocarditis (model), n=10 on day 4 and 12 on day10; mice with myocarditis and PAG treatment (PAG), n=10 on day 4 and 10 on day10; mice with myocarditis and NaHS treatment (NaHS), n=10 on day 4 and 14 on day10; and mice with myocarditis and PNS treatment (PNS), n=10 on day 4 and 15 on day10.
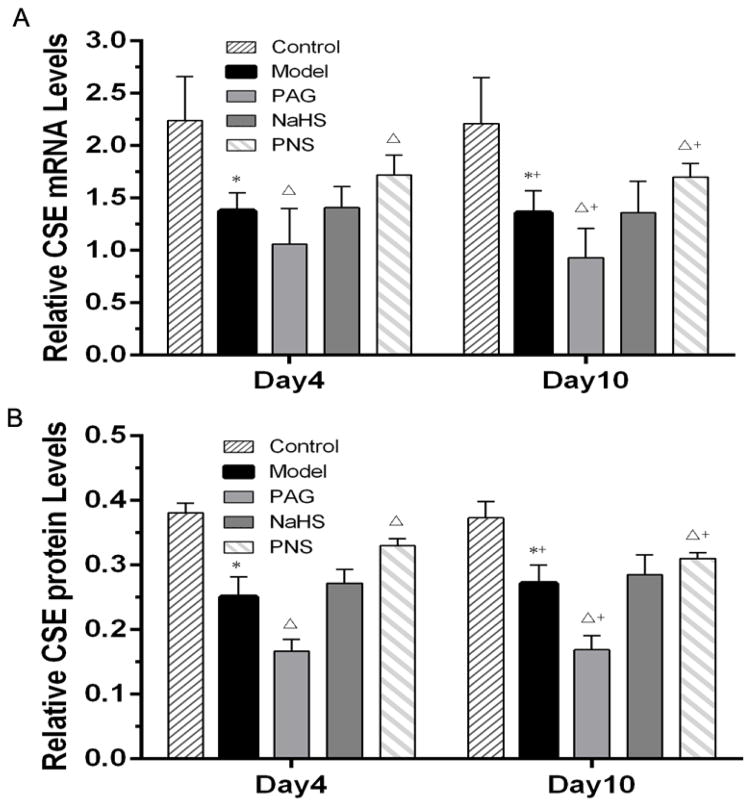
The detection of CSE expression indicated that CVB3-infection decreased the expressions of CSE at both mRNA and protein levels, compared to the control group on both the time points, which were further declined by the PAG treatment (Fig. 4). However, both CSE mRNA level and CSE protein level in PNS-treated group were higher than those in viral myocarditis model group. For the treatment of NaHS, no effects on CSE expression at either mRNA or protein level were observed in the myocarditis models. Moreover, for either H2S or CSE expression, there were no significant differences between data on day 4 and day 10 after infection (Fig. 3 and 4). These results suggested that PNS treatment could enhance the expression of H2S and CSE in CVB3-induced myocarditis, which might contribute to its therapeutic effects in these models.
Fig. 4. CSE mRNA and protein levels in CVB3-induced myocarditis.
(A) CSE mRNA level was measured by qRT-PCR; (B) Western blot analysis of CSE; *P<0.05 compared with the control group; ΔP<0.05 compared with the model group; +P < 0.05 within the same group compared with the time point day 4. Note: control, n=10 on day 4 and 10 on day10; mice with viral myocarditis (model), n=10 on day 4 and 12 on day10; mice with myocarditis and PAG treatment (PAG), n=10 on day 4 and 10 on day10; mice with myocarditis and NaHS treatment (NaHS), n=10 on day 4 and 14 on day10; and mice with myocarditis and PNS treatment (PNS), n=10 on day 4 and 15 on day10.
PNS activates the CSE/H2S pathway in the cultured cardiac cell line
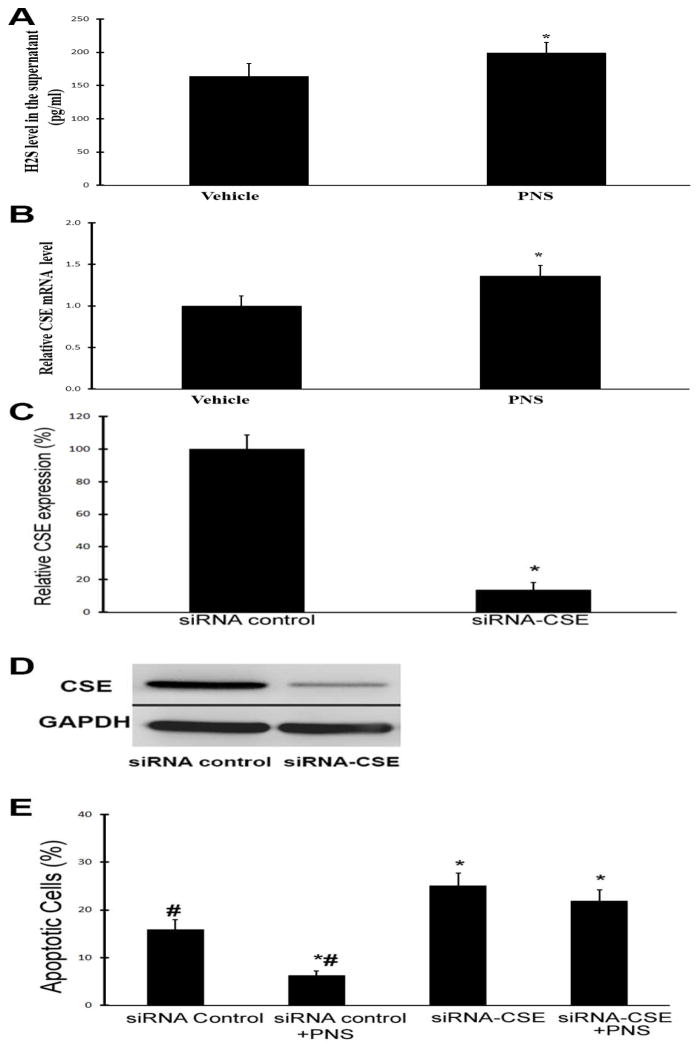
To provide direct evidence that PNS could enhance the CSE/H2S pathway in cardiac cells, the cardiac cell line H9c2 cells were applied. The cells were treated with vehicle (saline) or PNS (10 μg/ml). At 4h after treatments, cell culture supernatants were collected to determine the levels of H2S by ELISA. Then, the RNAs of H9c2 cells were isolated and CSE mRNA was measured by qRT-PCR. As shown in Fig. 5, PNS treatment significantly increased the levels of H2S and CSE in cultured H9c2 cells.
Fig. 5. PNS activates the CSE/H2S pathway in cultured cardiac cell line and protect the cardiac cells from apoptosis via CSE.
A &B: Cultured cardiac cell line H9c2 cells were treated with vehicle (saline) or PNS (10 μg/ml). At 4h after treatments, cell culture supernatants were collected to determine the levels of H2S by ELISA. Then, the RNAs of H9c2 cells were isolated and CSE mRNA was measured by qRT-PCR. A. PNS increases the level of H2S in the supernatant of cultured H9c2 cells. B. PNS increases the level of CSE mRNA in cultured H9c2 cells. n=6; *P<0.05 compared with vehicle group. C. CSE is knocked down by its siRNA (siRNA-CSE, 50 nM) in cardiac cell. n=6; *P<0.05 compared with siRNA control group (siRNA control). D. Representative Western blots of CSE. E. H9c2 cells were treated with siRNA control (50 nM), siRNA control plus PNS (10 μg/ml) (siRNA control+PNS), siRNA-CSE (50 nM), and siRNA-CSE plus PNS (10 μg/ml). Cell apoptosis was induced by H2O2 (100 μM) for 6 h and was measured by TUNEL. n=6, *p<0.05 compared with siRNA control; # p<0.05 compared with siRNA-CSE group.
PNS mediated cardiac cell protection is related to CSE
To test whether PNS-mediated protective on cardiac cells was related to CSE, the expression of CSE was knocked down as shown in Fig. 5C and 5D. H2O2-induced cardiac cell apoptosis was significantly inhibited by PNS (Fig. 5E). Interestingly, PNS-mediated protective effect on cardiac apoptosis was blocked in CSE-knock-downed cells (Fig. 5E).
PNS decreases the viral mRNA expressions in CVB3-induced myocarditis
To determine whether PNS has effects on virus propagation in CVB3-induced myocarditis, the mRNA levels of CVB3 were determined by qRT-PCR. Our data showed that, compared with the control group, the expression of CVB3 mRNA was increased by the treatment of PAG on both day 4 and day 10 after infections (Fig. 6). When treated with H2S, the virus replication was significantly inhibited, as indicated by the obviously decreased CVB3 mRNA level. Furthermore, PNS treatment resulted in an even more dramatic reduction of the CVB3 mRNA expression in the myocarditis model, at the two time points (Fig. 6). These results suggested that PNS could significantly decrease the viral mRNA expressions in CVB3-induced myocarditis.
Fig. 6. CVB3 mRNA levels in CVB3-induced myocarditis measured by qRT-PCR.
ΔP< 0.05 compared with the model group; +P<0.05 within the same group compared with the time point day 4. Note: mice with viral myocarditis (model), n=10 on day 4 and 12 on day10; mice with myocarditis and PAG treatment (PAG), n=10 on day 4 and 10 on day10; mice with myocarditis and NaHS treatment (NaHS), n=10 on day 4 and 14 on day10; and mice with myocarditis and PNS treatment (PNS), n=10 on day 4 and 15 on day10.
Discussion
Myocarditis is defined as inflammation of the myocardium, always followed by cardiomyocyte necrosis and/or degeneration [20, 21]. Viral myocarditis is a common cause of acute heart failure, and CVB3 is the most common virus in the disease etiology [22]. H2S, the third novel gasotransmitter in addition to NO and CO, has been considered as a biologically relevant signaling molecule with potentials in the development of various diseases [23–28]. Previous studies have shown that H2S plays key roles in some biological processes, including inflammation, oxidative stress, apoptosis, and vaso-relaxation [7, 29]. Recently, several researchers have found that CSE, cystathionine-β-synthase (CBS), and 3-mercaptopyruvate sulfurtransferase (3-MST) are the key enzymes to produce H2S, using L-cysteine as substrate [30, 31]. Moreover, CSE is the major H2S-forming enzyme in myocardial tissues [8]. The predominant role of CSE/H2S pathway has also been demonstrated by the high levels of CSE mRNA expression and H2S concentration in rat myocardium [9]. Our study provides several lines of evidence indicating the CSE/H2S pathway is involved in the pathogenesis of viral myocarditis.
H2S has been thought of as toxic gas with a foul odor for a long time; however its role in viral myocarditis is still unclear. H2S has been demonstrated to exert significant cardio-protective effects through its anti-apoptotic, anti-inflammatory, and antioxidant effects [32]. Fox et al. found that H2S might represent a novel endogenous mechanism of cytoprotection in inflamed joints, suggesting a potential anti-inflammation therapy for viral myocarditis [33].
CSE is the major H2S-forming enzyme in myocardial tissues [8, 9, 34, 35]. Several researchers found that the increased CSE expressions could reduce inflammation and/or oxidative stress in the myocardium through hydrogen sulfide generation [36, 37]. In our study, the mice received intraperitoneal injections of a high dosage of CVB3 (0.1 ml/TCID5010−6.5) would induce a large area of inflammatory cell infiltration, myocardial necrosis, and interstitial edema. In addition, recent studies have shown that intermittent hypoxia would induce cellular damages, decrease endothelial CSE expression and reduce endogenous H2S production [38]. Accordingly, we hypothesized that lower expression of CSE in infected myocardium might be due to the protein degradation and cell damage. In line with our results, Hua et al. demonstrated that the exogenous administration of H2S was protective to the infected myocardium, while the inhibition of endogenous H2S exerted harmful effects (39).
Our data also showed that the administration of NaHS alleviated myocardial injuries, inflammatory cell infiltration, as well as interstitial edema, and inhibited virus replication, while PAG treatment aggravated myocardial injuries and enhanced the virus replication as shown by the viral mRNA expression. Virus titers should be detected to further verify the discovery in future studies. NaHS is a H2S donor, which would exert a protective effect on myocardial injuries through increasing H2S levels. Conversely, PAG is an irreversible CSE inhibitor, which could aggravate myocardial injuries through inhibiting the expressions of CSE, as well as H2S. Our findings give strong evidence indicating that the CSE/H2S pathway plays a significant role in viral myocarditis and up-regulating H2S production has a protective effect at the early stage of the disease.
PNS have been demonstrated to have extensive effects on the cardiovascular system [19]. Our results showed that PNS activated CSE expression at both mRNA and protein levels, along with the increased H2S levels in our animal model in vivo. To provide direct evidence that PNS could enhance the CSE/H2S pathway in cardiac cells, the cardiac cell line H9c2 cells were applied. We found that PNS treatment could indeed increase the levels of H2S and CSE in cultured H9c2 cells. In addition, PNS could protect the cardiac cells from H2O2-mediated apoptosis. It should be noted the concentration of H2O2 (200 μM) we used is only for the apoptosis cell model. It may not be related to the real the concentration of H2O2 (200 μM) in hearts with virus myocarditis. The protective effect of PNS is related to CSE as the effect was inhibited in CSE-knock-downed cells. Moreover, PNS effectively ameliorated myocardial injuries, inhibited inflammatory cell infiltration and reduced interstitial edema. These observations indicated that PNS could ameliorate viral myocarditis by activating CSE/H2S pathway. Compared with the H2S donor, NaSH, PNS was more effective to activate the restrained expression of CSE and H2S in these models. Furthermore, our data showed that both PNS and NaHS could reduce CVB3 mRNA levels, while PAG resulted in enhanced viral levels. Therefore, we conclude that PNS may influence virus propagation in the early stage. Luo and Esfandiarei found that CVB3 replication could be reduced by inhibition of the PI3K/Akt and ERK1/2 pathways [40, 41]. Moreover, these pathways have been found to be related to the effects of PNS [15, 42–44]. In addition, H2S could also influence these pathways in biological processes [45–48]. But whether or not H2S and/or these pathways participate in the viral proliferation-inhibiting effects of PNS still merits in-depth studies.
In conclusion, our results show that PNS could ameliorate myocardial injuries, inflammatory cell infiltration, and interstitial edema by activating the CSE/H2S pathway, and could inhibit CVB3 replication during the early stage of CVB3-induced myocarditis. Based on these results, PNS would be a potential medication for viral myocarditis treatment.
There are some limitations in this study. First, in this study, the treatment of infected mice began the day of virus infection. Although it was good for a pre-clinical study to test the therapeutic effect of PNS and the potential mechanisms involved, clinically, the treatments for myocarditis are regularly performed after the viral myocarditis has become established. Thus, additional set of studies should be performed to determine the therapeutic effect of PNS on myocarditis after virus infection. Second, the dose-response and the side effects should be determined before it could be used in clinical. Third, the myocarditis was induced in mice that were 5 weeks old. The mouse immune system may not be mature until 7–8 weeks of age. Thus, the study and the discoveries should be repeated using adult mice. In addition, although we found that CSE and H2S may be involved in PNS-mediated cardiac protection, other mechanisms should also be identified in future studies.
Clinical relevance of the study
Viral myocarditis remains a challenging disease, and novel effective therapies based on fundamental mechanisms are needed in clinical practice. In this study, we have identified that CSE/H2S pathway may be a critical molecular mechanism in the development of viral myocarditis. PNS could ameliorate myocardial injuries, inflammatory cell infiltration, and interstitial edema and inhibit CVB3 replication by activating of the CSE/H2S pathway in CVB3-induced myocarditis. Thus, PNS would be a new therapeutic medication for viral myocarditis treatment.
Acknowledgments
Sources of Funding
This study was supported by Zhejiang Provincial Natural Science Foundation of China (LY12H02003), Zhejiang Provincial Medical and Health Science and Technology plan (2010KYA138, 2012ZDA035), Zhejiang Province TCM Science and Technology plan (2010ZA089), Scientific Research Foundation of Wenzhou (Y20140051), Project of Science and Technology Department of Zhejiang Province (2015C33163). This study was also supported in part by the National Institutes of Health, HL109656, and NR013876.
Abbreviations
- PNS
Panax notoginseng saponins
- CVB3
coxsackievirus B3
- CSE
cystathionine-γ-lyase
- H2S
hydrogen sulfide
- NaHS
sodium bisulfide
- PAG
propargylglycine
- PI3K
Phosphoinositide Kinase-3
- qRT-PCR
Quantitative real-time reverse transcription polymerase chain reaction
Footnotes
Disclosures
None
Human subjects/informed consent statement
No human studies were carried out by the authors for this article
Animal Studies
The work was conducted with the approval and in accordance with the guidelines of the Wenzhou Medical University. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the university institutional committees.
References
- 1.Maisch B, Pankuweit S. Current treatment options in (peri)myocarditis and inflammatory cardiomyopathy. Herz. 2012;37:644–656. doi: 10.1007/s00059-012-3679-9. [DOI] [PubMed] [Google Scholar]
- 2.Hendry RG, Bilawchuk LM, Marchant DJ. Targeting matrix metalloproteinase activity and expression for the treatment of viral myocarditis. J Cardiovasc Transl Res. 2014;7:212–225. doi: 10.1007/s12265-013-9528-2. [DOI] [PubMed] [Google Scholar]
- 3.Mody KP, Takayama H, Landes E, Yuzefpolskaya M, Colombo PC, Naka Y, Jorde UP, Uriel N. Acute mechanical circulatory support for fulminant myocarditis complicated by cardiogenic shock. J Cardiovasc Transl Res. 2014;7:156–164. doi: 10.1007/s12265-013-9521-9. [DOI] [PubMed] [Google Scholar]
- 4.Massilamany C, Huber SA, Cunningham MW, Reddy J. Relevance of molecular mimicry in the mediation of infectious myocarditis. J Cardiovasc Transl Res. 2014;7:165–171. doi: 10.1007/s12265-013-9519-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koenig A, Sateriale A, Budd RC, Huber SA, Buskiewicz IA. The role of sex differences in autophagy in the heart during coxsackievirus B3-induced myocarditis. J Cardiovasc Transl Res. 2014;7:182–191. doi: 10.1007/s12265-013-9525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoniak S, Mackman N. Coagulation, protease-activated receptors, and viral myocarditis. J Cardiovasc Transl Res. 2014;7:203–211. doi: 10.1007/s12265-013-9515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng D, Zhu C, Cao J, Jiang W. The protective effects of polyphenols from jujube peel (Ziziphus Jujube Mill) on isoproterenol-induced myocardial ischemia and aluminum-induced oxidative damage in rats. Food Chem Toxicol. 2012;50:1302–1308. doi: 10.1016/j.fct.2012.01.026. [DOI] [PubMed] [Google Scholar]
- 8.Elsey DJ, Fowkes RC, Baxter GF. L-cysteine stimulates hydrogen sulfide synthesis in myocardium associated with attenuation of ischemia-reperfusion injury. J Cardiovasc Pharmacol Ther. 2010;15:53–59. doi: 10.1177/1074248409357743. [DOI] [PubMed] [Google Scholar]
- 9.Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun. 2004;313:362–368. doi: 10.1016/j.bbrc.2003.11.130. [DOI] [PubMed] [Google Scholar]
- 10.Xie YH, Zhang N, Li LF, Zhang QZ, Xie LJ, Jiang H, Li LP, Hao N, Zhang JX. Hydrogen sulfide reduces regional myocardial ischemia injury through protection of mitochondrial function. Mol Med Rep. 2014;10:1907–1914. doi: 10.3892/mmr.2014.2391. [DOI] [PubMed] [Google Scholar]
- 11.Chang L, Geng B, Yu F, Zhao J, Jiang H, Du J, Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids. 2008;34:573–585. doi: 10.1007/s00726-007-0011-8. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Peng H, Du Q, Lin W, Liu Y. GYY4137, a hydrogen sulfide-releasing molecule, inhibits the inflammatory response by suppressing the activation of nuclear factor-kappa B and mitogen-activated protein kinases in Coxsackie virus B3-infected rat cardiomyocytes. Mol Med Rep. 2015;11:1837–1844. doi: 10.3892/mmr.2014.2901. [DOI] [PubMed] [Google Scholar]
- 13.Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58:1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- 14.Fan JS, Liu DN, Huang G, Xu ZZ, Jia Y, Zhang HG, Li XH, He FT. Panax notoginseng saponins attenuate atherosclerosis via reciprocal regulation of lipid metabolism and inflammation. 2012 doi: 10.1016/j.jep.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Liu J, Liu X, Fu Y, Zhang M, Lin Q, Zhu J, Mai L, Shan Z, Yu X, Yang M, Lin S. Panax notoginseng saponins inhibit ischemia-induced apoptosis by activating PI3K/Akt pathway in cardiomyocytes. J Ethnopharmacol. 2011;137:263–270. doi: 10.1016/j.jep.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Yang Q, Wang X, Cui J, Wang P, Xiong M, Jia C, Liu L, Ning B, Li L, Wang W, Chen Y, Zhang T. Bidirectional regulation of angiogenesis and miR-18a expression by PNS in the mouse model of tumor complicated by myocardial ischemia. BMC Complement Altern Med. 2014;14:183. doi: 10.1186/1472-6882-14-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. MicroRNA expression signature and the role of microRNA-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding RB, Tian K, Cao YW, Bao JL, Wang M, He C, Hu Y, Su H, Wan JB. Protective effect of panax notoginseng saponins on acute ethanol-induced liver injury is associated with ameliorating hepatic lipid accumulation and reducing ethanol-mediated oxidative stress. J Agric Food Chem. 2015;63:2413–2422. doi: 10.1021/jf502990n. [DOI] [PubMed] [Google Scholar]
- 19.Kong Q, Xue Y, Wu W, Yang F, Liu Y, Gao M, Lai W, Pan X. IL-22 exacerbates the severity of CVB3-induced acute viral myocarditis in IL-17A-deficient mice. Mol Med Rep. 2013;7:1329–1335. doi: 10.3892/mmr.2013.1323. [DOI] [PubMed] [Google Scholar]
- 20.Tang-Feldman YJ, Lochhead SR, Lochhead GR, Yu C, George M, Villablanca AC, Pomeroy C. Murine cytomegalovirus (MCMV) infection upregulates P38 MAP kinase in aortas of Apo E KO mice: a molecular mechanism for MCMV-induced acceleration of atherosclerosis. J Cardiovasc Transl Res. 2013;6:54–64. doi: 10.1007/s12265-012-9428-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirvan CA, Galvin JE, Hilt S, Kosanke S, Cunningham MW. Identification of streptococcal m-protein cardiopathogenic epitopes in experimental autoimmune valvulitis. J Cardiovasc Transl Res. 2014;7:172–181. doi: 10.1007/s12265-013-9526-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seong IW, Choe SC, Jeon ES. Fulminant coxsackieviral myocarditis. N Engl J Med. 2001;345:379. doi: 10.1056/NEJM200108023450518. [DOI] [PubMed] [Google Scholar]
- 23.Wallace JL, Caliendo G, Santagada V, Cirino G, Fiorucci S. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 2007;132:261–271. doi: 10.1053/j.gastro.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 24.Wallace JL, Caliendo G, Santagada V, Cirino G. Markedly reduced toxicity of a hydrogen sulphide-releasing derivative of naproxen (ATB-346) Br J Pharmacol. 2010;159:1236–1246. doi: 10.1111/j.1476-5381.2009.00611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang R. Hydrogen sulfide: the third gasotransmitter in biology and medicine. Antioxid Redox Signal. 2010;12:1061–1064. doi: 10.1089/ars.2009.2938. [DOI] [PubMed] [Google Scholar]
- 26.Wang MJ, Cai WJ, Zhu YC. Mechanisms of angiogenesis: role of hydrogen sulphide. Clin Exp Pharmacol Physiol. 2010;37:764–771. doi: 10.1111/j.1440-1681.2010.05371.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Tang ZH, Ren Z, Qu SL, Liu MH, Liu LS, Jiang ZS. Hydrogen sulfide, the next potent preventive and therapeutic agent in aging and age-associated diseases. Mol Cell Biol. 2013;33:1104–1113. doi: 10.1128/MCB.01215-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faller S, Ryter SW, Choi AM, Loop T, Schmidt R, Hoetzel A. Inhaled hydrogen sulfide protects against ventilator-induced lung injury. Anesthesiology. 2010;113:104–115. doi: 10.1097/ALN.0b013e3181de7107. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson CK, Calvert JW. Hydrogen sulfide and ischemia-reperfusion injury. Pharmacol Res. 2010;62:289–297. doi: 10.1016/j.phrs.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura H. Hydrogen sulfide: its production, release and functions. Amino Acids. 2011;41:113–121. doi: 10.1007/s00726-010-0510-x. [DOI] [PubMed] [Google Scholar]
- 31.Kimura H. Production and Physiological Effects of Hydrogen Sulfide. Antioxid Redox Signal. 2014;20:783–793. doi: 10.1089/ars.2013.5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lavu M, Bhushan S, Lefer DJ. Hydrogen sulfide-mediated cardioprotection: mechanisms and therapeutic potential. Clin Sci (Lond) 2011;120:219–229. doi: 10.1042/CS20100462. [DOI] [PubMed] [Google Scholar]
- 33.Fox B, Schantz JT, HaighRWood ME, Moore PK, Viner N, Spencer JP, Winyard PG, Whiteman M. Inducible hydrogen sulfide synthesis in chondrocytes and mesenchymal progenitor cells: is H2S a novel cytoprotective mediator in the inflamed joint? J Cell Mol Med. 2012;16:896–910. doi: 10.1111/j.1582-4934.2011.01357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsieh YS, Kuo WH, Lin TW, Chang HR, Lin TH, Chen PN, Chu SC. Protective effects of berberine against low-density lipoprotein (LDL) oxidation and oxidized LDL-induced cytotoxicity on endothelial cells. J Agric Food Chem. 2007;55:10437–10445. doi: 10.1021/jf071868c. [DOI] [PubMed] [Google Scholar]
- 36.Zhu X, Tang Z, Cong B, Du J, Wang C, Wang L, Ni X, Lu J. Estrogens increase cystathionine-gamma-lyase expression and decrease inflammation and oxidative stress in the myocardium of ovariectomized rats. Menopause. 2013;20:1084–1091. doi: 10.1097/GME.0b013e3182874732. [DOI] [PubMed] [Google Scholar]
- 37.Sen U, Givvimani S, Abe OA, Lederer ED, Tyagi SC. Cystathionine beta-synthase and cystathionine gamma-lyase double gene transfer ameliorate homocysteine-mediated mesangial inflammation through hydrogen sulfide generation. Am J Physiol Cell Physiol. 2011;300:C155–163. doi: 10.1152/ajpcell.00143.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jackson-Weaver O, Paredes DA, Gonzalez Bosc LV, Walker BR, Kanagy NL. Intermittent hypoxia in rats increases myogenic tone through loss of hydrogen sulfide activation of large-conductance Ca(2+)-activated potassium channels. Circ Res. 2011;108:1439–1447. doi: 10.1161/CIRCRESAHA.110.228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hua W, Chen Q, Gong F, Xie C, Zhou S, Gao L. Cardioprotection of H2S by downregulating iNOS and upregulating HO-1 expression in mice with CVB3-induced myocarditis. Life Sci. 2013;93:949–954. doi: 10.1016/j.lfs.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 40.Luo H, Yanagawa B, Zhang J, Luo Z, Zhang M, Esfandiarei M, Carthy C, Wilson JE, Yang D, McManus BM. Coxsackievirus B3 replication is reduced by inhibition of the extracellular signal-regulated kinase (ERK) signaling pathway. J Virol. 2002;76:3365–3373. doi: 10.1128/JVI.76.7.3365-3373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esfandiarei M, Luo H, Yanagawa B, Suarez A, Dabiri D, Zhang JH, McManus BM. Protein kinase B/Akt regulates coxsackievirus B3 replication through a mechanism which is not caspase dependent. J Virol. 2004;78:4289–4298. doi: 10.1128/JVI.78.8.4289-4298.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu L, Liu JT, Liu N, Lu PP, Pang XM. Effects of Panax notoginseng saponins on proliferation and apoptosis of vascular smooth muscle cells. J Ethnopharmacol. 2011;137:226–230. doi: 10.1016/j.jep.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 43.Yuan Z, Liao Y, Tian G, Li H, Jia Y, Zhang H, Tan Z, Li X, Deng W, Liu K, Zhang Y. Panax notoginseng saponins inhibit Zymosan A induced atherosclerosis by suppressing integrin expression, FAK activation and NF-kappaB translocation. J Ethnopharmacol. 2011;138:150–155. doi: 10.1016/j.jep.2011.08.066. [DOI] [PubMed] [Google Scholar]
- 44.Dou L, Lu Y, Shen T, Huang X, Man Y, Wang S, Li J. Panax notogingseng saponins suppress RAGE/MAPK signaling and NF-kappaB activation in apolipoprotein-E-deficient atherosclerosis-prone mice. Cell Physiol Biochem. 2012;29:875–882. doi: 10.1159/000315061. [DOI] [PubMed] [Google Scholar]
- 45.Luan HF, Zhao ZB, Zhao QH, Zhu P, Xiu MY, Ji Y. Hydrogen sulfide postconditioning protects isolated rat hearts against ischemia and reperfusion injury mediated by the JAK2/STAT3 survival pathway. Braz J Med Biol Res. 2012;45:898–905. doi: 10.1590/S0100-879X2012007500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Y, Chen X, Pan TT, Neo KL, Lee SW, Khin ES, Moore PK, Bian JS. Cardioprotection induced by hydrogen sulfide preconditioning involves activation of ERK and PI3K/Akt pathways. Pflugers Arch. 2008;455:607–616. doi: 10.1007/s00424-007-0321-4. [DOI] [PubMed] [Google Scholar]
- 47.Hu LF, Wong PT, Moore PK, Bian JS. Hydrogen sulfide attenuates lipopolysaccharide-induced inflammation by inhibition of p38 mitogen-activated protein kinase in microglia. J Neurochem. 2007;100:1121–1128. doi: 10.1111/j.1471-4159.2006.04283.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang T, Wang L, Zaidi SR, Sammani S, Siegler J, Moreno-Vinasco L, Mathew B, Natarajan V, Garcia JG. Hydrogen Sulfide Attenuates Particulate Matter-Induced Human Lung Endothelial Barrier Disruption via Combined Reactive Oxygen Species Scavenging and Akt Activation. Am J Respir Cell Mol Biol. 2012;47:491–496. doi: 10.1165/rcmb.2011-0248OC. [DOI] [PMC free article] [PubMed] [Google Scholar]