Abstract
Diminished glucose metabolism accompanies many neurodegenerative diseases including Alzheimer’s disease. An understanding of the relation of these metabolic changes to the disease will enable development of novel therapeutic strategies. Following a metabolic challenge, cells generally conserve energy to preserve viability. This requires activation of many cellular repair/regenerative processes such as mitophagy/autophagy and fusion/fission. These responses may diminish cell function in the long term. Prolonged fission induces mitophagy/autophagy which promotes repair but if prolonged progresses to mitochondrial degradation. Abnormal glucose metabolism alters protein signaling including the release of proteins from the mitochondria or migration of proteins from the cytosol to the mitochondria or nucleus. This overview provides an insight into the different mechanisms of autophagy/mitophagy and mitochondrial dynamics in response to the diminished metabolism that occurs with diseases, especially neurodegenerative diseases such as Alzheimer's disease. The review discusses multiple aspects of mitochondrial responses including different signaling proteins and pathways of mitophagy and mitochondrial biogenesis. Improving cellular bioenergetics and mitochondrial dynamics will alter protein signaling and improve cellular/mitochondrial repair and regeneration. An understanding of these changes will suggest new therapeutic strategies.
Keywords: Mitophagy/autophagy, fission, fusion, glucose metabolism, neurodegeneration
1. Glucose metabolism and brain function
Normal brain function requires a large and continuous supply of glucose and oxygen. The brain represents only 2% of the body’s mass and consumes 20% of the body’s oxygen. Further, since the energy reserves in the brain are small compared to high demand, reduced oxygen or glucose availability diminishes brain function. The high energy demand of synaptic functions links glucose consumption closely to neuronal firing. Thus, measures of glucose metabolism provide detailed anatomical maps of neuronal activation in the brains of animals and in humans. These maps of glucose utilization provide powerful tools to observe changes in brain activation during various diseases including neurodegeneration as the disease progresses.
Glucose metabolism is essential as a source of energy and as a carbon source of many key molecules. The brain, like other tissues, shifts metabolism in order to conserve the intracellular energy adenosine triphosphate (ATP), and preserve viability of the living cells. Although this shift in metabolism is benefical in the short-term, the long-term consequences may diminish brain function and lead to neurodegeneration. Glucose is metabolized in the brain by the same classical pathways as in every other tissue including glycolysis, hexose mono-phosphate (HMP) shunt, tricarboxylic acid cycle (TCA) and electron transport chain (ETC). The glucose-linked metabolic pathways in brain provide many products (e.g., riboses for synthesis of DNA and RNA, the neurotransmitters acetylcholine, glutamate, gamma-amino butyric acid (GABA), serine) that are necessary for normal brain functions or can be damaging (e.g., excess lactate). Therefore, as metabolic pathways of glucose shift to maintain intracellular ATP, the resulting perturbations of glucose metabolism alter the ability of the brain to respond to further insults. This concept is very important in understanding the importance of changes in glucose metabolism in the pathobiology of neurodegenerative disorders.
The shift in metabolism can be readily modeled in animals and in cell-based experimental systems. Metabolic shift has been demonstrated by diminishing the activity of alpha-ketoglutarate dehydrogenase complex (KGDHC), a key enzyme of the TCA cycle by one-half. This reflects the KGDHC reduction in brains from AD patients. In cultured neurons, neuronal cell-lines or living mice, the reduction is associated with decreased in vivo and in vitro overall glucose metabolism and activation of the GABA shunt [1–3]. Under such circumstances, there is little overt pathology except for a dramatic decrease in the number of neuroprogenitor cells in the hippocampal zone. On the other hand, the response to neurotoxins is greatly exaggerated in these mice. The lesions from 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), malonate or 3-nitroproprionic acid (3-NP) are 3–10 times greater [4]. The importance of the shift in metabolism being benefical in the short-term and damaging in the long-term has been demonstrated directly in culture of neuronal cell lines and/or primary cultures of neurons. Short-term reduction of KGDHC activity helps to facilitate the abilities of the cells to diminish external oxidative stress. On the other hand, more prolonged reduction of KGDHC impairs the ability of neurons to diminish oxidative stress. Similarly, long-term reduction in KGDHC causes Alzheimer's disease (AD) - like changes in calcium regulation whereas acute reductions do not mimic features of AD [5].
An inefficient interface between glycolysis and the mitochondrial pathways is also potentially damaging. For example, if the mitochondria does not consume pyruvate as fast as it is produced by glycolysis, lactate accumulates and the corresponding acidosisis can be very toxic. If glucose is administered to delirious patients one must also give thiamine to shift metabolism to promote the mitochondria’s ability to use pyruvate. Impaired transport of pyruvate into the mitochondria may be important in cancer cells which are very glycolytic and in AD, where aerobic glycolysis has been proposed as an early and defining feature.
Although estimations of human brain glucose metabolism are very sophisiticated in measuring regional utilization of glucose, they are very limited in their capacities to identify the underlying mechanism(s). Positron emission tomography (PET) with 2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG PET) provides detailed morphological map of brain glucose uptake and even temporal changes with the disease. However, 18F-FDG PET only measures the first step of glucose metabolism. One can also measure the last step of metabolism by measuring oxygen consumption. The current methods fail to distinguish where the decline in synaptic activities cause or are a result of the abnormalities in glucose metabolism.
1.1 Glucose metabolism in patients with Alzheimer’s disease
Glucose metabolism has been studied extensively in AD. Reduced glucose metabolism is an invariant feature and the best marker of cognitive state of this disease. The decline in glucose metabolism is highly correlated with changes in cognitive measures whereas the correlation of these measures to plaques is very poor [6]. Reduction in glucose metabolism occurs at early stages of AD. In patients with a genetic predisposition to developing AD, the decline in glucose utilization can occur when the person is in their twenties (i.e., long before the onset of the disease) [7]. However, the consequences of these reductions in glucose metabolism, in absence of overt energy failure, remains elusive. As mentioned previously, the shift in metabolism occuring in AD reflects changes in the interface between glycolysis and the mitochondrial TCA cycle. Aerobic metabolism indicates an increase in glycolysis without a corresponding increase in pyruvate oxidation by the mitochondria. Aerobic glycolysis, which can be measured in humans by PET scan, is a good predictor of the regions to be damaged in AD and the progression of the disease [8]. Although the cause of the reduced glucose utilization with AD has not been established, one possibility is a reduction in activities of the key enzymes of the TCA cycle. Both the pyruvate dehydrogenase complex (PDHC), which provides acetyl CoA, and the KGDHC are diminished in AD. The reductions in the activitites of these enzymes are highly correlated to a decline in a cognitive rating scale in the patients, whereas plaques and tangles are poorly correlated to these measures [9].
The mild reduction in glucose metabolism that occur in AD due to altered mitochondrial function can be modeled by making animals thiamine (vitamin B1) deficient, by reducing E2k (the second protein of the KGDHC complex), or diminishing E3 (a protein common to KGDHC and PDHC). Although the homozygous mutation is lethal, the activity in heterozygotes declines by one-half. The E2k deficiency reduces KGDHC activity by half, and the E3 mutation diminishes activities of KGDHC and PDHC by one-half. Deficiencies of thiamine, E2k or E3 reduce neurogenesis in the hippocampal zone, but have surprisingly little effect on other phenotypes [10].
Multiple preclinical models demonstrate that the interuption of energy metabolism can be linked to the development of the pathologies that define AD, namely the plaques and tangles.Thiamine (vitamin B1) deficiency leads to a reduction in transketolase and KGDHC and causes memory deficits as well as plaque and tangle formation [11]. Reducing E2k exaggerates plaque formation and memory deficits in animal models of AD. In cell-based models, interruption of metabolism leads to hyperphosporylated tau and to production of amyloid-beta (Aβ)1–42. In animal models, reduction of glucose metabolism leads to activation of β-secretase which is critical to plaque formation. Glycolysis in astrocytes may have a role in amyloid accumulation and cytotoxicity. Inhibiting astrocytic glycolysis results in an accumulation of amyloid protein and vulnerability to amyloid Aβ cytotoxicity [12].
2. Decreased glucose metabolism and the ability of cells to repair themselves
An understanding of the link between these changes in metabolism to cell repair and cell death will enable the development of new therapeutic strategies. The above discussion details that the changes in glucose metabolism are critical in AD pathology, and suggests involvement of mitochondrial alterations to these changes. The reduction in KGDHC activity may be particularly critical in this respect. Experiments that tested the effects of reduced KGDHC on the ability of cells to buffer oxidants and on calcium regulation suggest that reductions in KGDHC may initially be protective, but with time cause AD-like changes [5]. Mild reduction in metabolism may alter mitochondrial repair, and inadequate mitochondrial repair may make neurons vulnerable to cellular insults. Removal of damaged mitochondria is critical for maintaining cellular homeostasis. If damaged mitochondria fuse with other existing healthy mitochondria, the resulting mitochondria can produce more reactive oxygen species (ROS) and release proteins that induce apoptosis. Thus, an understanding of mitochondria in cell repair and cell death is important in understanding their role in neurodegeneration and the link to altered brain metabolism.
2.1 Mitochondrial degradation by autophagy/mitophagy
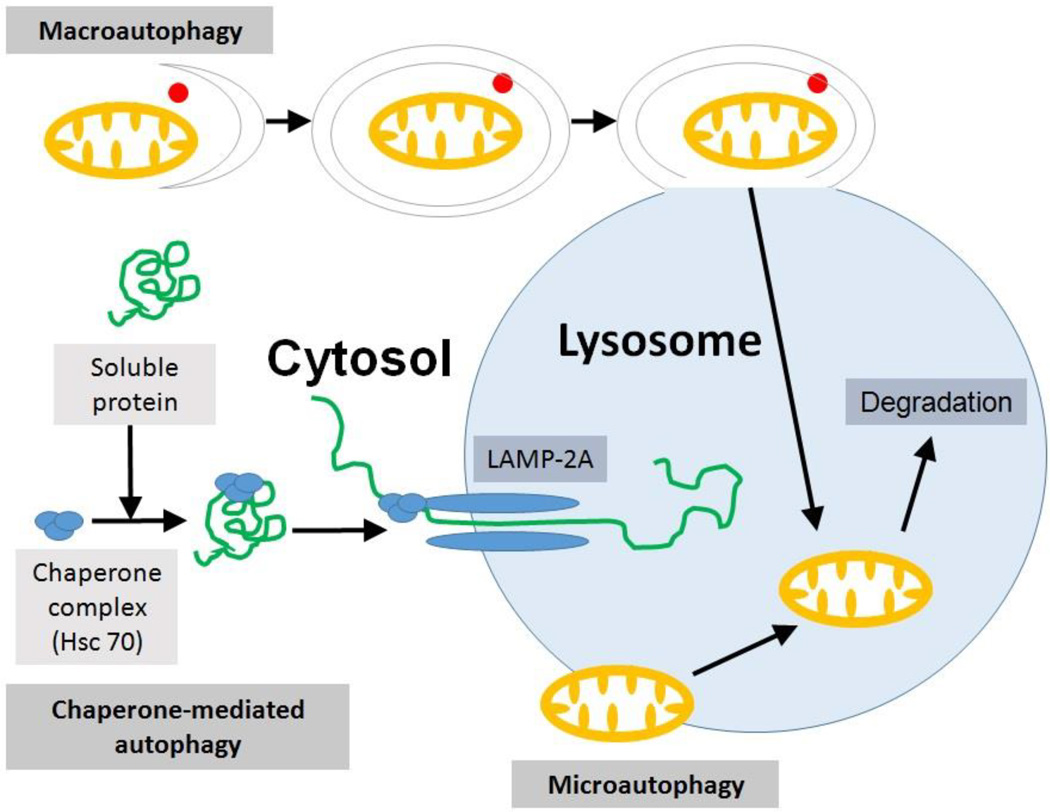
Autophagy is the process of degradation of cellular components using the cells' own enzymes that is generally restorative and protective in nature. Autophagy (auto: self, phagy: eating, self-eating) is a lysosomal degradation process to recycle cellular constituents to eliminate or re-model damaged organelles. During autophagy, autophagic vacuoles/autophagosomes engulf cytoplasmic components, such as cytosolic proteins and sub-cellular organelles including mitochondria or endoplasmic reticulum (ER). When the components targeted for degradation are only mitochondria, the process is known as mitophagy (mitochondrial-specific autophagy). There are three basic types of autophagy / mitophagy: macroautophagy, microautophagy and chaperone-mediated autophagy (Fig. 1).
Fig. 1. Types of autophagy/mitophagy.
Macroautophagy involves the formation of an autophagosome containing a portion of cytosol along with the damaged organelle (here mitochondria) within it. LC3-I is activated to LC3-II and binds to the autophagosomal membrane eventually causing it to fuse with lysosome utilizing proteins such as PI3 kinase and Atg by ubiquitin-like conjugation reactions. Microautophagy involves the direct engulfment of cytoplasmic material with the organelle using autophagic tubes or by simple invaginations. Chaperone-mediated autophagy involves the translocation of certain proteins complexed with chaperone proteins (like Hsc 70) into the lysosome by lysosomal integral membrane protein LAMP-2A (elongated oval). Lysosomal enzymes degrade the engulfed organelle and other components that need to be eliminated by the cell.
2.1.1 Macroautophagy
is the formation of a double-membrane around part of cytoplasm followed by the formation of an autophagosome. This leads to engulfment of that part of cytoplasm along with the damaged organelle by the lysosomes for degradation. Upon autophagic activation, a cytoplasmic 18 kDa protein microtubule-associated protein 1A/1B-light chain (LC3-I) is processed and conjugated with phosphatidylethanolamine (PE) to form 16 kDa LC3-PE conjugate (LC3-II), which in turn is recruited to autophagosomal membranes [13]. Following autophagosome formation with the binding of LC3-II protein, engulfment of the specific cytoplasmic compartment and the fusion with lysosome for the degradation of the engulfed cytoplasmic content occurs. Macroautophagy involves phosphoinositide-3-kinase (PI3 kinase, which helps in autophagic vesicle nucleation), the AuTophaGy-related (ATG) proteins (which help to recycle membrane and to form complex with PE for membrane expansion) and ubiquitin-like conjugation reactions that leads to the formation of an isolation membrane from ER-mitochondria contact sites following the formation of autophagic vacuole and fusion with lysosome (Fig. 1) [14, 15].
2.1.2 Microautophagy
is a nonselective lysosomal degradation process that involves direct engulfment of cytoplasmic cargo at a boundary membrane by tube-like structures called autophagic tubes or by simply tubular like invaginations (Fig.1) [16].
2.1.3 Chaperone-mediated autophagy
targets proteins or organelles. For example, the huntingtin protein Htt has a pentapeptide KFERQ-like sequence that is recognized by the Hsc70 chaperone, which then associates with the integral lysosome membrane protein LAMP-2A [14, 17]. The association promotes LAMP-2A multimerization to form a translocation complex. The substrates then cross the lysosomal membrane by a luminal chaperone and enter into the lysosome. The lysosomal enzymes then degrade those substrates (Fig. 1) [18].
3. Mitochondrial fission, fusion and autophagy (mitophagy)
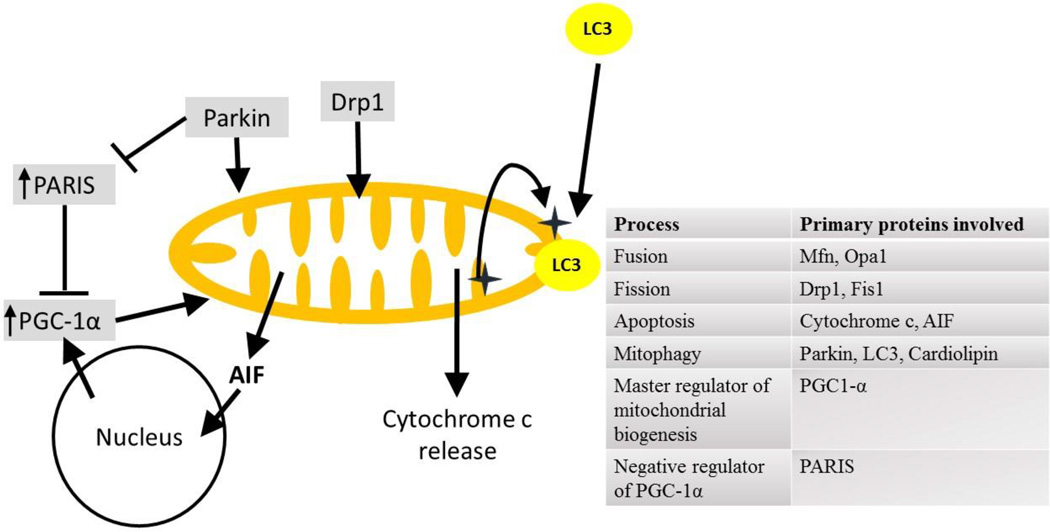
Healthy mitochondria are important for normal glucose metabolism and cellular health. Mitochondrial health in cells depends on their formation / rebuilding (biogenesis), their degradation (mitophagy) and remodelling (fission/fusion) [19, 20]. These processes are mediated by a variety of complex protein signaling pathways (Fig. 2). Mitochondria respond to different metabolic conditions by either repairing themselves (fission / fusion) or by degradation (mitophagy) or by increasing their mass (mitochondrial biogenesis). Depending on the cellular metabolic requirements and energy supplies, mitochondria fuse (fusion), divide (fission), fragment, swell, extend, and are recycled constantly in a regulated fashion [21]. Fission and fusion control the mitochondrial structure and function. More fission leads to more fragmented and smaller mitochondria, which are easily detected as damaged mitochondria and are degraded by mitophagy. Lower mitochondrial membrane potential diminishes fusion and promotes fission and mitophagy [22]. Unbalanced fusion leads to mitochondrial elongation while unbalanced fission leads to excessive mitochondrial fragmentation, both of which can lead to impaired mitochondrial functions (Fig. 2).
Fig. 2. Mitochondrial remodeling by fission/fusion and the proteins involved.
Disruption to the balance of mitochondrial fission/fusion can lead to increased mitochondrial mass, increased mitochondrial number or induce mitochondrial degradation. Fission/fusion is controlled by the translocation of several proteins, both into and from the mitochondria. Some of the most important proteins are mentioned in the chart.
3.1. Fission
Fission is controlled by several mitochondrial and cytosolic proteins. Outer mitochondrial membrane (OMM) proteins including mitochondrial fission protein 1 (Fis1), mitochondrial fission factor (Mff), mitochondrial dynamics proteins (MiD49 and MiD51) are involved in fission. They recruit the cytosolic dynamin-related protein 1 (Drp1) to the mitochondria, which is the critical step to initiate mitochondrial fission [23, 24]. Drp1 is a guanosine triphosphatase (GTPase) that controls mitochondrial fission. It is mainly cytoplasmic and can be activated by modification at distinct sites. Phosphorylation, SUMOylation, ubiquitination, S-nitrosylation and O-linked-N-acetyl glucosamine glycosylation promote Drp1 recruitment to mitochondria, thereby increasing fission. Activated Drp1 translocates to mitochondria and assembles into helical structures that encircle and penetrate the mitochondria which induces fragmentation or fission of the organelle. Overexpression of a dominant negative form of Drp1 (Drp1K38E) prevents mitochondrial fission [25]. Parkin ubiquitinates Drp1 and promotes its proteasome-dependent degradation [26]. Thus a loss of parkin from cytosol, as happens during parkin translocation to mitochondria, induces accumulation of Drp1 in the cytosol and increases mitochondrial fission. Drp1 is also involved in mitophagy and in cytochrome c release [27, 28], but is not involved in release of other protein signals from the mitochondria such as Smac/Diablo and Tim8a (Fig 2) [29, 30].
3.2 Fusion
Fusion is mediated by the proteins optic atrophy 1 (Opa1), mitofusins 1 and 2 (Mfn1 and Mfn2) [24]. Mitofusins are present in the OMM and Opa 1 is in mitochondrial intermembrane space [23]. Mfn1 is required for the first step of fusion, the initiation of tethering of fusing mitochondria. The second step of mitochondrial fusion requires the physical interaction between Opa1 and the two inner mitochondrial membranes (IMMs) to be fused by the hydrolysis of guanosine triphosphate (GTP). An intact mitochondrial membrane potential is required for mitochondrial fusion, and diminished mitochondrial membrane potential selectively inhibits fusion [31]. Another IMM enzyme, ATPases Associated with diverse cellular Activities (AAA) protease controls Opa 1 processing and mitochondrial protein degradation [32, 33]. Degradation or loss of Opa 1 from mitochondria inhibits the fusion of mitochondria and can alter mitochondrial structure. An increase in fusion is associated with neuroprotection (Fig. 2) [34, 35].
3.3 Mitophagy
Mitophagy protects cells from damaged mitochondria. Removal of damaged mitochondria prevent them from fusing with healthy mitochondria and from releasing proapoptotic proteins such as apoptosis-inducing factor (AIF) or cytochrome c. When the health of mitochondria cannot be restored by fusion, the unhealthy mitochondria are removed by mitochondria-specific autophagy referred to as mitophagy. Specific protein signal including LC3 are required to induce mitophagy. Depolarized mitochondria are not able to fuse and are selected for mitophagy [19]. Increased fission makes mitochondria more fragmented which are specifically designated as damaged mitochondria and are selected for mitophagy. The fragmented, small mitochondria can be easily engulfed by autophagosome/lysosome [36]. Mitophagy can be parkin-dependent or parkin-independent (Fig. 3).
Fig. 3. Mechanisms of mitophagy induction.
(a) In Parkin-dependent mitophagy, parkin translocates to damaged mitochondria with decreased membrane potential and ubiquitinates Mfn2 (diamond) in the mitochondrial membrane, prevents fusion with healthy mitochondria and facilitates binding with LC3 via P62, thereby inducing mitophagy of the damaged organelle. (b) In the BNIP/NIX-mediated parkin-independent pathway, the cytosolic protein NIX (rectangle) translocates to the mitochondria and binds with BNIP inducing mitophagy. (c) In the cardiolipin (CL)-dependent parkin-independent pathway, the phospholipid CL (star-shape) "flips" to the exterior surface of outer membrane of stressed mitochondria with the help of flippase/scramblase 3 and binds with LC3 (circle) after recruiting the latter and induces mitophagy.
3.3.1 Parkin-dependent mitophagy
Parkin is a cytosolic E3 ubiquitin ligase which plays an important role in mitochondrial degradation. Decreased mitochondrial membrane potential can trigger translocation of parkin to mitochondria which is the initiating step for mitophagy [16, 37]. Many studies suggest that parkin translocation to damaged mitochondria is responsible for inducing mitophagy in neurodegenerative diseases. Parkin induces ubiquitination and proteasomal degradation of selected mitochondrial fusion proteins [38]. It ubiquitinates Mfn2 protein present in the mitochondrial membrane to prevent fusion of that damaged mitochondria with other healthy mitochondria, and this ultimately leads to binding of mitochondria with LC3 via a protein called p62, followed by mitochondrial degradation (Fig. 3). Parkin translocation to mitochondria may reduce proteasomal activity in cytosol and that can lead to accumulaton of several proteins in the cytosol (i.e. Parkin interacting substrate (PARIS), α-synuclein, p-tau, Aβ etc.) [39, 40]. Parkin is involved in mitophagy following treatment with the mitochondrial uncoupler, carbonylcyanide-3-chlorophenylhydrazone (CCCP) [16, 41].
3.3.2 NIX-mediated parkin-independent mitophagy
Non-parkin mediated mitophagy mainly occurs during reticulocyte maturation through the involvement of the cytosolic NIP3-like protein X (NIX) protein [16]. In the process of erythrocyte maturation, NIX protein is upregulated and translocates to the mitochondria. At the mitochondria, NIX binds with BCL2/adenovirus E1B 19 kd-interacting protein (BNIP) and induces mitophagy, possibly through its ability to directly bind the autophagy protein LC3 [42].
3.3.3 Cardiolipin-mediated parkin-independent mitophagy
Mitophagy can also be induced by conditions that alter cardiolipin (CL) localization in mitochondria. CL is a phospholipid which is normally present only in the IMM. In compromised mitochondria, it “flips” to the outer side of the OMM. A mitochondria specific enzyme flippase/scramblase 3 is responsible for this CL movement in mitochondria. CL has specific binding site for LC3 protein. Externalized CL recruits LC3 to the OMM and induces mitophagy (Fig. 3). The increased level of LC3-II is a common marker for monitoring autophagic activity including mitophagy. RNA interference (RNAi) causing knockdown of cardiolipin synthase or of phospholipid scramblase-3 decreases mitophagy following mitochondrial damage [43]. CL flip and mitophagy can occur without increasing the Drp1 dependent-fission in a Drp1-independent manner. Mitophagy is inhibited in cells overexpressing Drp1 if mitochondrial scramblase 3 is knocked out. This supports the suggestion that CL externalization by scramblase 3 causes mitophagy and this is downstream of Drp1 [43].
3.3.4 Other possible mechanisms of mitophagy
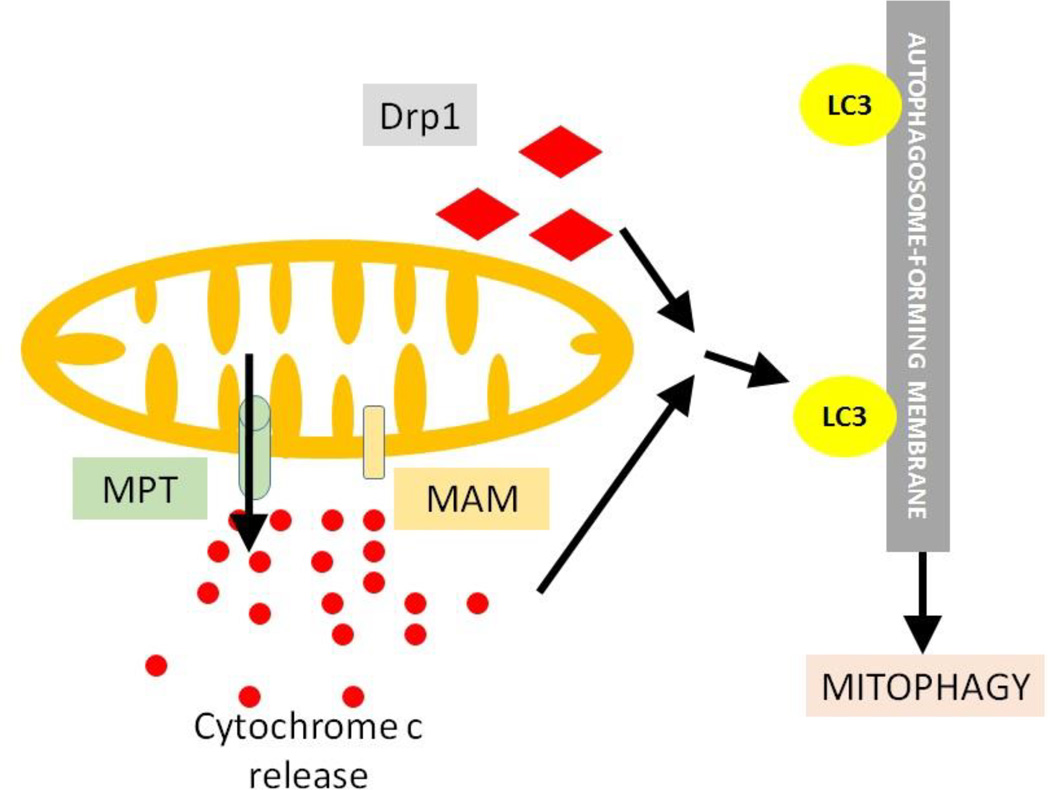
3.3.4.1 MPT pore
The mitochondrial permeabilization transition (MPT) pore is a non-specific pore in the inner mitochondrial membrane. MPT causes mitochondria to become permeable to molecules smaller than 1.5 kDa, which, once inside, draw water in by increasing the organelle's osmolar load. This causes mitochondria to swell and may cause the outer membrane to rupture, which leads to release of cytochrome c [44, 45] (Fig. 4). Composition of phospholipids in the mitochondrial membrane also affects the MPT pore opening. Fatty acids have a role in regulating the activity of MPT pore which links lipids with mitochondrial dysfunction as well as with mitochondrial degradation (Fig. 4) [22]. Mitophagy mediated by MPT pore can also be induced by Drp1 translocation from cytosol to mitochondria. Downregulation of phosphatase and tensin homolog (PTEN) induced putative kinase 1 (PINK1) can also induce mitophagy through the MPT pore complex, though the detailed mechanism is still not very clear [46].
Fig. 4. Probable novel mechanism of mitophagy induction.
Besides the pathways mentioned previously in fig. 3, there is the possibility of other pathways by different mitochondrial insults that may induce mitophagy. These could be Drp1 (diamond) translocation to mitochondria, or by the action of MPT pore (cylinder) or MAM (rectangle) or by cytochrome c (small circle) release.
3.3.4.2 Mitochondria-associated ER membrane (MAM)
The MAMs are a sub-compartment of the ER involved in mitochondrial function and dynamics. They are important for induction of mitophagy and autophagy, as the isolation membrane formation begins from this site [47]. In AD, there is a significant increase in both MAM function and the connections between the ER and the mitochondria and could be associated with mitochondrial degradation (Fig. 4). The proteins involved in processing the amyloid precursor protein (APP) for the generation of Aβ are also located in the MAM [48].
3.3.5 Mitochondrial-cytosolic-nuclear crosstalk to apoptosis and mitogenesis/mitophagy
3.3.5.1 Release of cytochrome c
Cytochrome c is small mitochondrial protein (12 kDa) that is released to the cytosol from compromised and stressed mitochondria. Although it can induce caspase-dependent apoptosis, cytochrome c release also occurs in the absence of apoptosis [49]. Release of cytochrome c from mitochondria to cytosol can induce non-specific autophagy within the cell. Several different mechanisms can trigger the release of cytochrome c: CL peroxidation, leakage through MPT pore complex, Bax and Bac interactions with voltage-dependent anion channel (VDAC), and high K+ level in extra-mitochondrial matrix [50, 51].
3.3.5.2 Suppression of nuclear transcription
Mitochondria can also control the migration of proteins from the cytosol to the nucleus where they regulate transcription. Migration of parkin to the mitochondria promotes accumulation of the protein PARIS in the cytosol, which in turn promotes its translocation to the nucleus where it suppresses the expression of genes important in mitochondrial biogenesis, including peroxisome proliferator-activated receptor gamma coactivator (PGC-1α) and its target gene, nuclear respiratory factor (NRF) - 1, as well as the nicotinamide adenine dinucleotide (NAD+) - dependent deacetylase sirtuin (silent mating type information regulation 2 homolog) 1 (S. cerevisiae) (SIRT1). PARIS mediates this action by binding to the insulin response sequences in the PGC-1α promoter [39, 52]. P53 can translocate from the nucleus to mitochondria [53]. Mitochondrial stress changes expression of several genes in the nucleus (like PGC-1α, cathespin L) [54]. Oxidative stess can damage mitochondrial DNA (mtDNA) and that can alter cellular signaling by changing nuclear gene expression [54–56].
3.3.5.3 Apoptosis-inducing factor (AIF) and other proteins
The flavoprotein AIF is normally present within mitochondria. Following mitochondrial damage, it is released from mitochondria and translocates to the nucleus [57]. AIF translocation can take place even before the release of cytochrome c or before an alteration in the mitochondrial membrane potential. Poly (ADP-ribose) polymerase 1 (PARP-1) activation is responsible for AIF release from mitochondria [58]. Mitochondria specific enzymes, including those in the matrix, like the PDHC can also translocate from mitochondria to nucleus under stress and regulate gene expression as a transacetylase [59].
3.3.5.4 Other cellular signaling pathways
Apoptosis and mitophagy/autophagy are life and death partners that control each other. Generally autophagy blocks the induction of apoptosis, and apoptosis-associated caspase activation shuts off the autophagic process [60]. Autophagy depends on several signaling pathways, most important of which is the mammalian target of rapamycin (mTOR) pathway. Glucose metabolism and the mitochondrial signal are closely related with autophagy. Intact mitochondrial membrane potential is a necessary factor for fusion following fission. Low ATP generation from mitochondria induces adenosine monophosphate (AMP) - activated protein kinase (AMPK) signaling pathway and inhibits mTOR [61]. Several mitochondrial proteins that go between mitochondria and cytoplasm are also connected with cellular death signal (apoptosis) activation. These vary from those involved in mitophagy and include cytochrome c, Smac/Diablo, HtrA2/Omi, Endo G, VDAC, AIF [62, 63].
3.4 Relation of mitochondrial dynamics with diseases
Alterations in mitochondrial dynamics may lead to several diseases including neurodegeneration [64, 65]. In several models of neurodegenerative diseases, changing mitochondrial fission or fusion can modify disease phenotypes [66]. Thus, a better understanding of mitochondrial biogenesis and repair and their relation to autophagy and cell death should enable development of better therapies in diseases that are accompanied by mild impairment of oxidative metabolism.
3.4.1 Autophagy / mitophagy in diseases
Autophagy has been implicated in multiple diseases including neurodegenerative diseases, cardiovascular diseases, ischemia, traumatic brain injury, pulmonary diseases and cancer [61, 67, 68]. Mitophagy has been related to AD, Parkinson's disease (PD) and Huntington disease (HD) [69]. Autophagy protects against tumors, necrosis and inflammation, and mitigate genome damage in cancer cells in response to metabolic stress [70]. The autophagy related gene, Beclin 1, is deleted in several cancers [71]. Loss of atg5 in cardiac tissue leads to more cardiac hypertrophy, abnormal contractile dysfunction, and accumulation of ubiquitinated proteins with abnormal mitochondrial structure [69]. Mice with knockout autophagy genes like atg5 or atg7 show more neurodegeneration and deficits in more functions as well as more accumulation of inclusion bodies [72]. Inducing autophagy by rapamycin removes toxic proteins from cells and is protective. In a HD model, the mitochondrial TCA cycle inhibitor 3-NP elevates apoptotis and reduces autophagic proteins LC3-II. LC3-II are significantly reduced by a p53 specific inhibitor which is protective [73].
3.4.2 PGC-1α in diseases
Mitochondrial biogenesis is regulated by PGC-1α - NRF - mitochondrial transcription factor A (TFAM) pathway as well as by Drp1 [74, 75]. Peroxisome proliferator-activated receptor (PPAR) is a transcriptional coactivator that interacts with a broad range of transcription factors including PGC-1α which is involved in a wide variety of biological processes including mitochondrial biogenesis, oxidative phosphorylation, antioxidant defense, adaptive thermogenesis, glucose/fatty acid metabolism, fiber type switching in skeletal muscle, and heart development [76–78]. PGC-1α does not bind to DNA directly, but forms heteromeric complexes with transcription factors, including NRF-1 and NRF-2, and the nuclear receptors, PPARα, PPARδ, PPARγ, and estrogen related receptor α [79]. Expression levels of PGC-1α, NRF 1, NRF 2, and TFAM are decreased in both AD hippocampal tissues and APPswe M17 cells, suggesting reduced mitochondrial biogenesis. In APPswe M17 cells diminished mitochondrial DNA/nuclear DNA ratio correlates with reduced ATP content, and decreased cytochrome c oxidase activity [80]. Thus, impaired mitochondrial biogenesis can induce mitochondrial dysfunction in AD [81].
3.4.3 Drp1 in diseases
Alterations in Drp1 are implicated in different diseases. Impaired mitochondrial biogenesis has been observed with the abnormal expression of Drp1 in postmortem AD brains, as well as in AD mouse models, and APP cell lines [82]. Inhibition of Drp1 is beneficial in cardiac dysfunction [83]. p-Tau and Aβ can increase Drp1 phosphorylation and Drp1 translocation to mitochondria to increase fission, resulting in more fragmented mitochondria, and mitophagy [16, 27, 84, 85]. Even mild oxidative stress can induce a Drp1-dependent increased fission which promotes mitophagy [20]. For example, in HD models, mutant Htt interacts with Drp1, elevates its GTPase activity, increases abnormal mitochondrial dynamics, and results in defective anterograde mitochondrial movement and synaptic deficiencies [86]. In systemic lupus erythematous (SLE), HRES-1/Rab4 mediated Drp1 depletion and endocytic control is targeted in treatment [87]. Mitochondrial fission defects related to Drp1 contribute to apoptotic resistance in cancers [88]. In fibroblasts, Drp1 contributes to ABT-737-induced respiratory changes and the kinetics of cytochrome c release [89]. Thus, drugs targeted to the interaction between Drp1 and other proteins and mitochondria could be important for therapeutics.
4. Mitochondria as a therapeutic target
Mitochondrial dysfunction occurs with several diseases, thus this organelle has been suggested as a therapeutic target in several diseases including neurodegenerative diseases, heart failure, diabetes and cancer [90, 91].
4.1 Mitophagy as a therapeutic target
Treatment with a mitophagic/autophagic inducer rapamycin improves neurological outcome and mitochondrial function. These protective effects of rapamycin are reversed by treatment with an inhibitor of autophagy [13]. Benfotiamine, a thiamine derivative, largely prevents the AD pathology in a mouse model by enhancing the mitochondrial KGDHC enzyme activity [92].
4.2 Bioenergetics as a therapeutic target
Another approach to improving mitochondrial function is to enhance its bioenergetics. A shift from carbohydrate glycolytic metabolism to mitochondrial fatty acid and ketone oxidative metabolism has potential for therapeutics by modulating metabolism and signal transduction pathways [93]. Several compounds including creatine, coenzyme Q10, nicotinamide, riboflavin and lipoic acid have been proposed for use [94–97].
4.3 Activation of transcriptional pathways as a therapeutic target
Another way to improve mitochondrial function is by the activation of transcriptional pathways pathways [98]. PGC-1α and the other transcription factors associated with it (NRF-1, NRF-2, and the nuclear receptors, PPARα, PPARδ, PPARγ) can regulate the expression of many nuclear encoded mitochondrial proteins. Therefore, pharmacological activation of the PGC-1α pathway should be neuroprotective. One approach to activate PGC-1α pathway is by activation of the PPARs. The PPARs are a subfamily of nuclear receptors that regulate gene expression programs of metabolic pathways. PPAR γ agonists that have been proven beneficial in in vivo and in vitro models include thiazolidinediones like rosiglitazone. Bezafibrate is a pan-PPAR agonist that is beneficial in multiple cellular and animal models [99]. Over-expression of PGC-1α completely rescues impaired mitochondrial biogenesis and mitochondrial deficits in APPswe M17 cells, while knockdown of PGC-1α exaggerates deficits [80]. Cyclic AMP (cAMP) in a dose-dependent manner rescues the reduced expression of phospho-cAMP response element-binding protein (p-CREB) and PGC-1α in APPswe M17 cells, and the effect can be diminished by the protein kinase A (PKA) inhibitor H89. Thus, the PKA/CREB pathway can play a significant role in the regulation of PGC-1α expression in APPswe M17 cells. Resveratol can induce biogenesis and control the interplay between apoptosis, mitophagy and biogenesis [100].
4.4 Activation of sirtuins as a therapeutic target
Another way to activate mitochondria is by activation of sirtuins, NAD+ dependent deacetylases. Activation of SIRT-1 activates PGC-1α, which reduces plaque burden in plaque competent mice [101]. Resveratol is a potent activator of SIRT-1, at least outside the central nervous system. SIRT-3 is primarily mitochondrial and is also activated by increasing NAD+ and it deacetylates several mitochondrial proteins, increases fatty acid oxidation, superoxide dismutase (SOD) 2 and levels of glutathione [102]. SIRT5 leads to desuccinylation in the mitochondria [103].
4.5 Activation of AMP-activated protein kinsase also promotes mitogenesis
Mitogenesis is activated by increased cAMP and produces a cascade of phosphorylation dependent modification of several factors including PGC-1α to switch on catabolic pathways to produce ATP, while simultaneously shutting down energy consuming anabolic pathways. 5-Aminoimidazole-4-carboxamide ribonucleoside (AICAR) and metformin are two compounds that activate AMP-activated protein kinase [104].
4.6. Activation by Nrf2/antioxidant response element (ARE) pathway can overcome mitochondrial deficits
After activation, nuclear factor erythroid-2 related factor 2 (Nrf 2) dissociates from Kelch-like ECH-associating protein 1 (KEAP1), translocate to the nucleus and binds to the antioxidant response promoter sequences leading to coordinated induction of a battery of antioxidant and anti-inflammatory genes known as antioxidant response element (ARE) dependent genes. These mechanisms have been extensively reviewed elsewhere [58]. Synthetic triterpenoids such as 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid (CDDO) and its derivatives like CDDO-methyl ester (CDDO-Me), CDDO-methyl amide (CDDO-Ma), CDDO-ethyl amide (CDDO-Ea), and CDDO-imidazolide (CDDO-Im) are powerful inhibitors of oxidative stress and cellular inflammatory processes, and CDDO-Me (or RTA402) is very potent in activating KEAP1/Nrf 2/ARE pathway [105]. CDDO-Me has been tested in Phase III clinical trials for its therapeutic benefits in chronic kidney disease (CKD) and malignancies having inflammatory and oxidative components. It acts by activating Nrf2 and upregulating the antioxidant response while suppressing the nuclear factor (NF) κB and proinflammatory signaling [105]. Thus it plays a promising role as a candidate drug for the diseases with defects in mitochondrial dynamics and biogenesis.
5. Conclusion and future directions
Impaired metabolism occurs in many neurodegenerative diseases. This causes changes in protein signaling that may be protective in the short-term, but are damaging in the long-term. Even mild reductions in metabolism lead to imbalanced mitochondrial fusion and fission. With more severe and prolonged impairment, these processes progress to mitophagy/autophagy which is restorative, while failure of these events lead to cell death. Thus, targeting mitochondrial changes is a potential effective therapeutic modality for many diseases including neurodegenerative disorders like AD.
Ackowledgements
The work was supported by the National Institutes of Health (NIH) grant AG14930 and the Burke Medical Research Institute. Dr. Dienel has made immense contributions to neurochemistry and has had a large impact on how I (we) view the brain’s use of glucose. His intellectual contribution to our understanding of how different cell types in the brain interact has changed the field and my own research. I am very grateful for many contributions and his friendship.
References
- 1.Santos SS, Gibson GE, Cooper AJL, Denton TT, Thompson CM, Bunik VI, Alves PM, Sonnewald U. Inhibitors of the α-ketoglutarate dehydrogenase complex alter [1–13C]glucose and [U-13C]glutamate metabolism in cerebellar granule neurons. Journal of Neuroscience Research. 2006;83:450–458. doi: 10.1002/jnr.20749. [DOI] [PubMed] [Google Scholar]
- 2.Shi Q, Risa Ø, Sonnewald U, Gibson GE. Mild reduction in the activity of the α-ketoglutarate dehydrogenase complex elevates GABA shunt and glycolysis. Journal of Neurochemistry. 2009;109:214–221. doi: 10.1111/j.1471-4159.2009.05955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nilsen LH, Shi Q, Gibson GE, Sonnewald U. Brain [U-13C]glucose metabolism in mice with decreased α-ketoglutarate dehydrogenase complex activity. Journal of Neuroscience Research. 2011;89:1997–2007. doi: 10.1002/jnr.22606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klivenyi P, Starkov AA, Calingasan NY, Gardian G, Browne SE, Yang L, Bubber P, Gibson GE, Patel MS, Beal MF. Mice deficient in dihydrolipoamide dehydrogenase show increased vulnerability to MPTP, malonate and 3-nitropropionic acid neurotoxicity. Journal of Neurochemistry. 2004;88:1352–1360. doi: 10.1046/j.1471-4159.2003.02263.x. [DOI] [PubMed] [Google Scholar]
- 5.Gibson GE, Chen H-L, Xu H, Qiu L, Xu Z, Denton TT, Shi Q. Deficits in the mitochondrial enzyme α-ketoglutarate dehydrogenase lead to Alzheimer's disease-like calcium dysregulation. Neurobiology of Aging. 2012;33:1121.e1113–1121.e1124. doi: 10.1016/j.neurobiolaging.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furst AJ, Rabinovici GD, Rostomian AH, Steed T, Alkalay A, Racine C, Miller BL, Jagust WJ. Cognition, glucose metabolism and amyloid burden in Alzheimer's disease. Neurobiology of Aging. 2012;33:215–225. doi: 10.1016/j.neurobiolaging.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarawneh R, Holtzman DM. Biomarkers in translational research of Alzheimer’s Disease. Neuropharmacology. 2010;59:310–322. doi: 10.1016/j.neuropharm.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-β (Aβ) deposition. Proceedings of the National Academy of Sciences. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: Mechanistic implications. Annals of Neurology. 2005;57:695–703. doi: 10.1002/ana.20474. [DOI] [PubMed] [Google Scholar]
- 10.Calingasan NY, Ho DJ, Wille EJ, Campagna MV, Ruan J, Dumont M, Yang L, Shi Q, Gibson GE, Beal MF. Influence of mitochondrial enzyme deficiency on adult neurogenesis in mouse models of neurodegenerative diseases. Neuroscience. 2008;153:986–996. doi: 10.1016/j.neuroscience.2008.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karuppagounder SS, Xu H, Shi Q, Chen LH, Pedrini S, Pechman D, Baker H, Beal MF, Gandy SE, Gibson GE. Thiamine deficiency induces oxidative stress and exacerbates the plaque pathology in Alzheimer's mouse model. Neurobiology of Aging. 2009;30:1587–1600. doi: 10.1016/j.neurobiolaging.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fu W, Shi D, Westaway D, Jhamandas JH. Bioenergetic mechanisms in astrocytes may contribute to amyloid plaque deposition and toxicity. Journal of Biological Chemistry. 2015 doi: 10.1074/jbc.M114.618157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Q, Zhang T, Wang J, Zhang Z, Zhai Y, Yang GY, Sun X. Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke. Biochemical and biophysical research communications. 2014;444:182–188. doi: 10.1016/j.bbrc.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 14.Pyo J-O, Nah J, Jung Y-K. Molecules and their functions in autophagy. Exp Mol Med. 2012;44:73–80. doi: 10.3858/emm.2012.44.2.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Esclatine A, Chaumorcel M, Codogno P. Macroautophagy signaling and regulation. Current topics in microbiology and immunology. 2009;335:33–70. doi: 10.1007/978-3-642-00302-8_2. [DOI] [PubMed] [Google Scholar]
- 16.Ashrafi G, Schwarz TL. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013;20:31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi L, Zhang X-D, Wu J-C, Lin F, Wang J, DiFiglia M, Qin Z-H. The Role of Chaperone-Mediated Autophagy in Huntingtin Degradation. PLoS ONE. 2012;7:e46834. doi: 10.1371/journal.pone.0046834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24:92–104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Twig G, Shirihai O. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal. 2011;14:1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank M, Duvezin-Caubet S, Koob S, Occhipinti A, Jagasia R, Petcherski A, Ruonala MO, Priault M, Salin B, Reichert AS. Mitophagy is triggered by mild oxidative stress in a mitochondrial fission dependent manner. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2012;1823:2297–2310. doi: 10.1016/j.bbamcr.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 22.Barbour JA, Turner N. Mitochondrial Stress Signaling Promotes Cellular Adaptations. International Journal of Cell Biology. 2014;2014:12. doi: 10.1155/2014/156020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan DC. Mitochondrial fusion and fission in mammals. Annual review of cell and developmental biology. 2006;22:79–99. doi: 10.1146/annurev.cellbio.22.010305.104638. [DOI] [PubMed] [Google Scholar]
- 24.Berman SB, Pineda FJ, Hardwick JM. Mitochondrial fission and fusion dynamics: the long and short of it. Cell Death Differ. 2008;15:1147–1152. doi: 10.1038/cdd.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee Y, Lee H-Y, Hanna RA, Gustafsson ÅB. Mitochondrial autophagy by Bnip3 involves Drp1-mediated mitochondrial fission and recruitment of Parkin in cardiac myocytes. 2011 doi: 10.1152/ajpheart.00368.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Song P, Du L, Tian W, Yue W, Liu M, Li D, Wang B, Zhu Y, Cao C, Zhou J, Chen Q. Parkin Ubiquitinates Drp1 for Proteasome-dependent Degradation: IMPLICATION OF DYSREGULATED MITOCHONDRIAL DYNAMICS IN PARKINSON DISEASE. Journal of Biological Chemistry. 2011;286:11649–11658. doi: 10.1074/jbc.M110.144238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. Embo j. 2014;33:2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estaquier J, Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 2007;14:1086–1094. doi: 10.1038/sj.cdd.4402107. [DOI] [PubMed] [Google Scholar]
- 29.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y-i, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 30.Babbar M, Sheikh MS. Metabolic Stress and Disorders Related to Alterations in Mitochondrial Fission or Fusion. Molecular and cellular pharmacology. 2013;5:109–133. [PMC free article] [PubMed] [Google Scholar]
- 31.Benard G, Karbowski M. Mitochondrial fusion and division: Regulation and role in cell viability. Seminars in cell & developmental biology. 2009;20:365–374. doi: 10.1016/j.semcdb.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: The bioenergetic view. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Langer T, Kaser M, Klanner C, Leonhard K. AAA proteases of mitochondria: quality control of membrane proteins and regulatory functions during mitochondrial biogenesis. Biochemical Society transactions. 2001;29:431–436. doi: 10.1042/bst0290431. [DOI] [PubMed] [Google Scholar]
- 34.Hoekstra J, Montine K, Zhang J, Montine T. Mitochondrial therapeutics in Alzheimer's disease and Parkinson's disease. Alzheimer's Research & Therapy. 2011;3:21. doi: 10.1186/alzrt83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehses S, Raschke I, Mancuso G, Bernacchia A, Geimer S, Tondera D, Martinou JC, Westermann B, Rugarli EI, Langer T. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. The Journal of cell biology. 2009;187:1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomes LC, Scorrano L. Mitochondrial morphology in mitophagy and macroautophagy. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2013;1833:205–212. doi: 10.1016/j.bbamcr.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Scarffe LA, Stevens DA, Dawson VL, Dawson TM. Parkin and PINK1: much more than mitophagy. Trends in Neurosciences. 2014;37:315–324. doi: 10.1016/j.tins.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RLJ, Hess S, Chan DC. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Human Molecular Genetics. 2011 doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shin J-H, Ko Han S, Kang H, Lee Y, Lee Y-I, Pletinkova O, Troconso Juan C, Dawson Valina L, Dawson Ted M. PARIS (ZNF746) Repression of PGC-1α Contributes to Neurodegeneration in Parkinson's Disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banerjee K, Munshi S, Sen O, Pramanik V, Roy Mukherjee T, Chakrabarti S. Dopamine Cytotoxicity Involves Both Oxidative and Nonoxidative Pathways in SH-SY5Y Cells: Potential Role of Alpha-Synuclein Overexpression and Proteasomal Inhibition in the Etiopathogenesis of Parkinson's Disease. Parkinson’s Disease. 2014;2014:12. doi: 10.1155/2014/878935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vives-Bauza C, Zhou C, Huang Y, Cui M, de Vries RLA, Kim J, May J, Tocilescu MA, Liu W, Ko HS, Magrané J, Moore DJ, Dawson VL, Grailhe R, Dawson TM, Li C, Tieu K, Przedborski S. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proceedings of the National Academy of Sciences. 2010;107:378–383. doi: 10.1073/pnas.0911187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, Ney PA. ROLE OF BNIP3 AND NIX IN CELL DEATH, AUTOPHAGY, AND MITOPHAGY. Cell death and differentiation. 2009;16:939–946. doi: 10.1038/cdd.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang T-S, Cho C-S, Park S, Yu S, Kang SW, Rhee SG. Peroxiredoxin III, a Mitochondrion-specific Peroxidase, Regulates Apoptotic Signaling by Mitochondria. Journal of Biological Chemistry. 2004;279:41975–41984. doi: 10.1074/jbc.M407707200. [DOI] [PubMed] [Google Scholar]
- 44.Büki A, Okonkwo DO, Wang KKW, Povlishock JT. Cytochrome c Release and Caspase Activation in Traumatic Axonal Injury. The Journal of Neuroscience. 2000;20:2825–2834. doi: 10.1523/JNEUROSCI.20-08-02825.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- 46.Cui T, Fan C, Gu L, Gao H, Liu Q, Zhang T, Qi Z, Zhao C, Zhao H, Cai Q, Yang H. Silencing of PINK1 induces mitophagy via mitochondrial permeability transition in dopaminergic MN9D cells. Brain Research. 2011;1394:1–13. doi: 10.1016/j.brainres.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 47.Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, Amano A, Yoshimori T. Autophagosomes form at ER-mitochondria contact sites. Nature. 2013;495:389–393. doi: 10.1038/nature11910. [DOI] [PubMed] [Google Scholar]
- 48.Schon EA, Area-Gomez E. Mitochondria-associated ER membranes in Alzheimer disease. Molecular and cellular neurosciences. 2013;55:26–36. doi: 10.1016/j.mcn.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Ghibelli L, Coppola S, Fanelli C, Rotilio G, Civitareale P, Scovassi AI, Ciriolo MR. Glutathione depletion causes cytochrome c release even in the absence of cell commitment to apoptosis. Faseb j. 1999;13:2031–2036. doi: 10.1096/fasebj.13.14.2031. [DOI] [PubMed] [Google Scholar]
- 50.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochimica et Biophysica Acta (BBA) - Bioenergetics. 2006;1757:639–647. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Borutaite V, Jekabsone A, Morkuniene R, Brown GC. Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. Journal of molecular and cellular cardiology. 2003;35:357–366. doi: 10.1016/s0022-2828(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 52.Aquilano K, Vigilanza P, Baldelli S, Pagliei B, Rotilio G, Ciriolo MR. Peroxisome Proliferator-activated Receptor γ Co-activator 1α (PGC-1α) and Sirtuin 1 (SIRT1) Reside in Mitochondria: POSSIBLE DIRECT FUNCTION IN MITOCHONDRIAL BIOGENESIS. Journal of Biological Chemistry. 2010;285:21590–21599. doi: 10.1074/jbc.M109.070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marchenko ND, Zaika A, Moll UM. Death Signal-induced Localization of p53 Protein to Mitochondria: A POTENTIAL ROLE IN APOPTOTIC SIGNALING. Journal of Biological Chemistry. 2000;275:16202–16212. doi: 10.1074/jbc.275.21.16202. [DOI] [PubMed] [Google Scholar]
- 54.Amuthan G, Biswas G, Zhang S-Y, Klein-Szanto A, Vijayasarathy C, Avadhani NG. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. The EMBO Journal. 2001;20:1910–1920. doi: 10.1093/emboj/20.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biswas G, Guha M, Avadhani NG. Mitochondria-to-nucleus stress signaling in mammalian cells: nature of nuclear gene targets, transcription regulation, and induced resistance to apoptosis. Gene. 2005;354:132–139. doi: 10.1016/j.gene.2005.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chakrabarti S, Munshi S, Banerjee K, Thakurta IG, Sinha M, Bagh MB. Mitochondrial Dysfunction during Brain Aging: Role of Oxidative Stress and Modulation by Antioxidant Supplementation. Aging and Disease. 2011;2:242–256. [PMC free article] [PubMed] [Google Scholar]
- 57.Maiuri MC, Zalckvar E, Kimchi A, Kroemer G. Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol. 2007;8:741–752. doi: 10.1038/nrm2239. [DOI] [PubMed] [Google Scholar]
- 58.Hong SJ, Dawson TM, Dawson VL. Nuclear and mitochondrial conversations in cell death: PARP-1 and AIF signaling. Trends in pharmacological sciences. 2004;25:259–264. doi: 10.1016/j.tips.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 59.Sutendra G, Kinnaird A, Dromparis P, Paulin R, Stenson TH, Haromy A, Hashimoto K, Zhang N, Flaim E, Michelakis ED. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell. 2014;158:84–97. doi: 10.1016/j.cell.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 60.Marino G, Niso-Santano M, Baehrecke EH, Kroemer G. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. doi: 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tait SW, Green DR. Mitochondria and cell signalling. Journal of cell science. 2012;125:807–815. doi: 10.1242/jcs.099234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang C, Youle RJ. The Role of Mitochondria in Apoptosis*. Annual Review of Genetics. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang H-M, Ou H-C, Xu H, Chen H-L, Fowler C, Gibson GE. Inhibition of α-ketoglutarate dehydrogenase complex promotes cytochrome c release from mitochondria, caspase-3 activation, and necrotic cell death. Journal of Neuroscience Research. 2003;74:309–317. doi: 10.1002/jnr.10756. [DOI] [PubMed] [Google Scholar]
- 64.Banerjee K, Sinha M, Pham Cle L, Jana S, Chanda D, Cappai R, Chakrabarti S. Alpha-synuclein induced membrane depolarization and loss of phosphorylation capacity of isolated rat brain mitochondria: implications in Parkinson's disease. FEBS letters. 2010;584:1571–1576. doi: 10.1016/j.febslet.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 65.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 66.Chen H, Chan DC. Mitochondrial dynamics–fusion, fission, movement, and mitophagy–in neurodegenerative diseases. Human Molecular Genetics. 2009;18:R169–R176. doi: 10.1093/hmg/ddp326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leclere L, Fransolet M, Cote F, Cambier P, Arnould T, Van Cutsem P, Michiels C. Heat-Modified Citrus Pectin Induces Apoptosis-Like Cell Death and Autophagy in HepG2 and A549 Cancer Cells. PLoS One. 2015;10:e0115831. doi: 10.1371/journal.pone.0115831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan CH, Li Y, Tian XX, Zhu N, Song HX, Zhang J, Sun MY, Han YL. CREG1 ameliorates myocardial fibrosis associated with autophagy activation and Rab7 expression. Biochim Biophys Acta. 2015;1852:353–364. doi: 10.1016/j.bbadis.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 69.Meijer AJ, Codogno P. Autophagy: Regulation and role in disease. Critical Reviews in Clinical Laboratory Sciences. 2009;46:210–240. doi: 10.1080/10408360903044068. [DOI] [PubMed] [Google Scholar]
- 70.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967. doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niu YN, Liu QQ, Zhang SP, Yuan N, Cao Y, Cai JY, Lin WW, Xu F, Wang ZJ, Chen B, Wang JR. Alternative messenger RNA splicing of autophagic gene Beclin 1 in human B-cell acute lymphoblastic leukemia cells. Asian Pacific journal of cancer prevention : APJCP. 2014;15:2153–2158. doi: 10.7314/apjcp.2014.15.5.2153. [DOI] [PubMed] [Google Scholar]
- 72.Kaushal GP. Autophagy protects proximal tubular cells from injury and apoptosis. Kidney international. 2012;82:1250–1253. doi: 10.1038/ki.2012.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang XD, Wang Y, Wang Y, Zhang X, Han R, Wu JC, Liang ZQ, Gu ZL, Han F, Fukunaga K, Qin ZH. p53 mediates mitochondria dysfunction-triggered autophagy activation and cell death in rat striatum. Autophagy. 2009;5:339–350. doi: 10.4161/auto.5.3.8174. [DOI] [PubMed] [Google Scholar]
- 74.Smirnova E, Griparic L, Shurland D-L, van der Bliek AM. Dynamin-related Protein Drp1 Is Required for Mitochondrial Division in Mammalian Cells. Molecular Biology of the Cell. 2001;12:2245–2256. doi: 10.1091/mbc.12.8.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Martin OJ, Lai L, Soundarapandian MM, Leone TC, Zorzano A, Keller MP, Attie AD, Muoio DM, Kelly DP. A role for peroxisome proliferator-activated receptor gamma coactivator-1 in the control of mitochondrial dynamics during postnatal cardiac growth. Circulation research. 2014;114:626–636. doi: 10.1161/CIRCRESAHA.114.302562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Puigserver P, Spiegelman BM. Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α (PGC-1α): Transcriptional Coactivator and Metabolic Regulator. Endocrine Reviews. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 77.Liang H, Ward WF. PGC-1α: a key regulator of energy metabolism. 2006 doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 78.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 79.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metabolism. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 80.Sheng B, Wang X, Su B, Lee HG, Casadesus G, Perry G, Zhu X. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer's disease. J Neurochem. 2012;120:419–429. doi: 10.1111/j.1471-4159.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sheng B, Wang X, Su B, Lee H-g, Casadesus G, Perry G, Zhu X. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. Journal of Neurochemistry. 2012;120:419–429. doi: 10.1111/j.1471-4159.2011.07581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cho D-H, Nakamura T, Fang J, Cieplak P, Godzik A, Gu Z, Lipton SA. S-Nitrosylation of Drp1 Mediates β-Amyloid-Related Mitochondrial Fission and Neuronal Injury. Science. 2009;324:102–105. doi: 10.1126/science.1171091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ, Archer SL. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. Faseb j. 2014;28:316–326. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Manczak M, Calkins MJ, Reddy PH. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer's disease: implications for neuronal damage. Human Molecular Genetics. 2011;20:2495–2509. doi: 10.1093/hmg/ddr139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Manczak M, Reddy PH. Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer's disease neurons: implications for mitochondrial dysfunction and neuronal damage. Human Molecular Genetics. 2012;21:2538–2547. doi: 10.1093/hmg/dds072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shirendeb UP, Calkins MJ, Manczak M, Anekonda V, Dufour B, McBride JL, Mao P, Hemachandra Reddy P. Mutant huntingtin's interaction with mitochondrial protein Drp1 impairs mitochondrial biogenesis and causes defective axonal transport and synaptic degeneration in Huntington's disease. Human Molecular Genetics. 2011 doi: 10.1093/hmg/ddr475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Caza TN, Fernandez DR, Talaber G, Oaks Z, Haas M, Madaio MP, Lai Z-w, Miklossy G, Singh RR, Chudakov DM, Malorni W, Middleton F, Banki K, Perl A. HRES-1/Rab4-mediated depletion of Drp1 impairs mitochondrial homeostasis and represents a target for treatment in SLE. Annals of the Rheumatic Diseases. 2013 doi: 10.1136/annrheumdis-2013-203794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thomas KJ, Jacobson MR. Defects in Mitochondrial Fission Protein Dynamin-Related Protein 1 Are Linked to Apoptotic Resistance and Autophagy in a Lung Cancer Model. PLoS ONE. 2012;7:e45319. doi: 10.1371/journal.pone.0045319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Clerc P, Ge SX, Hwang H, Waddell J, Roelofs BA, Karbowski M, Sesaki H, Polster BM. Drp1 is dispensable for apoptotic cytochrome c release in primed MCF10A and fibroblast cells but affects Bcl-2 antagonist-induced respiratory changes. British journal of pharmacology. 2014;171:1988–1999. doi: 10.1111/bph.12515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Galluzzi L, Larochette N, Zamzami N, Kroemer G. Mitochondria as therapeutic targets for cancer chemotherapy. Oncogene. 0000;25:4812–4830. doi: 10.1038/sj.onc.1209598. [DOI] [PubMed] [Google Scholar]
- 91.Sorriento D, Pascale AV, Finelli R, Carillo AL, Annunziata R, Trimarco B, Iaccarino G. Targeting Mitochondria as Therapeutic Strategy for Metabolic Disorders. The Scientific World Journal. 2014;2014:9. doi: 10.1155/2014/604685. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 92.Pan X, Gong N, Zhao J, Yu Z, Gu F, Chen J, Sun X, Zhao L, Yu M, Xu Z, Dong W, Qin Y, Fei G, Zhong C, Xu TL. Powerful beneficial effects of benfotiamine on cognitive impairment and beta-amyloid deposition in amyloid precursor protein/presenilin-1 transgenic mice. Brain : a journal of neurology. 2010;133:1342–1351. doi: 10.1093/brain/awq069. [DOI] [PubMed] [Google Scholar]
- 93.Wallace DC, Fan W, Procaccio V. Mitochondrial energetics and therapeutics. Annual review of pathology. 2010;5:297–348. doi: 10.1146/annurev.pathol.4.110807.092314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moreira PI, Zhu X, Wang X, Lee H-g, Nunomura A, Petersen RB, Perry G, Smith MA. Mitochondria: A therapeutic target in neurodegeneration. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2010;1802:212–220. doi: 10.1016/j.bbadis.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Szeto HH, James LP, Atkinson AJ. Mitochondrial Pharmacology: Its Future Is Now. Clinical Pharmacology & Therapeutics. 2014;96:629–633. doi: 10.1038/clpt.2014.177. [DOI] [PubMed] [Google Scholar]
- 96.Beal MF. Therapeutic approaches to mitochondrial dysfunction in Parkinson's disease. Parkinsonism & Related Disorders. 15:S189–S194. doi: 10.1016/S1353-8020(09)70812-0. [DOI] [PubMed] [Google Scholar]
- 97.Chaturvedi RK, Beal MF. Mitochondria targeted therapeutic approaches in Parkinson's and Huntington's diseases. Molecular and cellular neurosciences. 2013;55:101–114. doi: 10.1016/j.mcn.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 98.Johri A, Beal MF. Mitochondrial Dysfunction in Neurodegenerative Diseases. Journal of Pharmacology and Experimental Therapeutics. 2012;342:619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fernandes-Santos C, Carneiro RE, de Souza Mendonca L, Aguila MB, Mandarim-de-Lacerda CA. Pan-PPAR agonist beneficial effects in overweight mice fed a high-fat high-sucrose diet. Nutrition. 2009;25:818–827. doi: 10.1016/j.nut.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 100.Meira Martins L, Vieira M, Ilha M, de Vasconcelos M, Biehl H, Lima D, Schein V, Barbé-Tuana F, Borojevic R, Guma F. The Interplay Between Apoptosis, Mitophagy and Mitochondrial Biogenesis Induced by Resveratrol Can Determine Activated Hepatic Stellate Cells Death or Survival. Cell Biochem Biophys. 2015;71:657–672. doi: 10.1007/s12013-014-0245-5. [DOI] [PubMed] [Google Scholar]
- 101.Karuppagounder SS, Pinto JT, Xu H, Chen LH, Beal MF, Gibson GE. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s Disease. Neurochemistry international. 2009;54:111–118. doi: 10.1016/j.neuint.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging (Albany NY) 2013;5:144–150. doi: 10.18632/aging.100544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Park J, Chen Y, Tishkoff DX, Peng C, Tan M, Dai L, Xie Z, Zhang Y, Zwaans BM, Skinner ME, Lombard DB, Zhao Y. SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Molecular cell. 2013;50:919–930. doi: 10.1016/j.molcel.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Johri A, Beal MF. Mitochondrial Dysfunction in Neurodegenerative Diseases. The Journal of Pharmacology and Experimental Therapeutics. 2012;342:619–630. doi: 10.1124/jpet.112.192138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang YY, Yang YX, Zhe H, He ZX, Zhou SF. Bardoxolone methyl (CDDO-Me) as a therapeutic agent: an update on its pharmacokinetic and pharmacodynamic properties. Drug design, development and therapy. 2014;8:2075–2088. doi: 10.2147/DDDT.S68872. [DOI] [PMC free article] [PubMed] [Google Scholar]