Abstract
M1 profile macrophages exert a major influence on initial tissue repair process. Few days after the occurrence of injury, macrophages in the injured region exhibit a M2 profile, attenuate the effects of the M1 population, and stimulate the reconstruction of the damaged tissue. The different effects of macrophages in the healing process suggest that these cells could be the target of therapeutic interventions. Photobiomodulation has been used to accelerate tissue repair, but little is known regarding its effect on macrophages. In the present study, J774 macrophages were activated to simulate the M1 profile and irradiated with two different sets of laser parameters (780 nm, 70 mW, 2.6 J/cm2, 1.5 s and 660 nm, 15 mW, 7.5 J/cm2, 20 s). IL-6, TNF-α, iNOS and COX-2 gene and protein expression were analyzed by RT-qPCR and ELISA. Both lasers were able to reduce TNF-α and iNOS expression, and TNF-α and COX-2 production, although the parameters used for 780 nm laser provided an additional decrease. 660 nm laser parameters resulted in an up-regulation of IL-6 expression and production. These findings imply a distinct, time-dependent modulation by the two different sets of laser parameters, suggesting that the best modulation may involve more than one combination of parameters.
Keywords: Cytokines, Inflammatory markers, Photobiomodulation, LLLT, M1-activated macrophages, J774 cells
1. Introduction
Macrophages play a crucial role during the healing and remodeling process [1–3]. The diverse effects of these cells depend on the stimuli generated by the environment and on the time after injury. In the initial phases of the inflammatory process, macrophages normally adopt a pro-inflammatory or M1 profile and produce cytokines and inflammatory makers, such as interleukin 6 (IL-6), tumor necrosis factor alpha (TNF-α), cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS) [1–3].
Approximately four days after the occurrence of injury, macrophages in the injured region exhibit a different M2 profile. In this stage, these cells produce anti-inflammatory cytokines to attenuate the effects of the M1 population as well as enzymes and growth factors that stimulate the reconstruction of the damaged tissue [1–3]. The importance and complexity of the effects of different macrophage phenotypes in tissue repair process clearly suggests that these cells could be the target of therapeutic interventions involving many tissues, including skeletal muscle [4–8].
Photobiomodulation (also known as low-level laser therapy, LLLT) has been suggested to be a useful tool for accelerating the skeletal muscle repair process. LLLT has demonstrated ability to reduce pain, edema, leukocyte influx and myonecrosis as well as to alter the expression of inflammatory mediators and collagen remodeling [9–19]. However, the few studies evaluating the effect of LLLT on macrophages have significant methodological differences, especially with regard to dosimetric parameters and the particular cell activation state [20–27]. The activation state of macrophages drives phenotype transition and the orchestration of tissue healing [1–8]. Thus, understanding how photobiomodulation may influence macrophage function and/or activation is essential to choosing the best therapeutic parameters and regimen.
Choosing the best parameters in studies involving LLLT is a complex task. It is possible to vary many different parameters (wavelength, fluence, power density, mode of delivery, time of application, pulse) so that a large number of possible combinations exist, and furthermore, it is important to take into account the optical properties of the tissue, and the biphasic dose–response effect in photobiomodulation [28]. According to a recent review, the parameters to be tested in any proposed study should be based on previously published studies and the experience of the research groups in each specific therapeutic application of LLLT [29].
The aim of the present study was to evaluate the effect of photobiomodulation using two sets of parameters (already tested) on the expression of TNF-α, IL-6, iNOS and COX-2 by inflammatory macrophages (M1 profile) 24 and 72 h after irradiation. J774 mouse cells were used as a model of macrophages.
2. Methods
2.1. Cell Culture
J774 cells were derived from a BALB/c mouse (an albino, laboratory-bred strain of mouse) reticulum cell sarcoma [30], but have been widely used as an in vitro model of macrophages [31] and have been shown to express typical macrophage markers depending on their activation state [32]. J774 cells were grown in Dulbecco's modified Eagle medium (DMEM, Vitrocell, Campinas, Brazil) supplemented with 10% fetal bovine serum (FBS, Vitrocell), 1% antibiotic-antimycotic solution (Vitrocell) and 2 mM l-glutamine (Vitrocell) at 37 °C in a humidified atmosphere with 5% CO2. The cells were maintained at subconfluent densities and passaged every two to three days.
2.2. Spectroscopy of J774 Cells
J774 cells were washed two times with phosphate-buffered saline (PBS), detached using a cell-scraper, pelleted at 1200 rpm for 2 min at 10 °C and were re-suspended in PBS. The spectroscopic analysis was performed by placing cells in cuvettes. A spectrometer (Pico200, Picodrop Ltd., Saffron Walden, United Kingdom) was used to measure the absorbance of cells.
2.3. Macrophage Activation (M1 Phenotype)
The M1 macrophage profile was achieved by activation in vitro, as described elsewhere [1–4]. J774 macrophages were incubated with 0.2 µg/mL interferon-γ (IFN-γ, Sigma, St. Louis, MO, USA) and 1 µg/mL lipopolysaccharide (LPS; serotype O26:B6 from Escherichia coli) (Sigma) in DMEM 5% (FBS) for 24 h [24,26,33].
2.4. Laser Irradiation
Twenty-four hours following activation, the cells were washed two times with PBS, detached using a cell-scraper, counted and divided into tubes to form four experimental groups:
Activated = treated with LPS + IFN-γ for 24 h, no irradiation
Activated + 660 nm = treated with LPS + IFN-γ for 24 h and irradiated with 660 nm (InGaAlP diode) laser
Activated + 780 nm = treated with LPS + IFN-γ for 24 h and irradiated with 780 nm (GaAlAs diode) laser
Control = no activation, no irradiation.
J774 cells were pelleted at 1200 rpm for 2 min at 10 °C and irradiated using a Twin-Laser system (Twin-Laser, MMOptics Ltd., São Carlos, São Paulo, Brazil) with parameters described in Table 1.
Table 1.
Photobiomodulation dosimetry parameters.
| Parameter | Red laser | Infrared laser |
|---|---|---|
| Center wavelength [nm] | 660 | 780 |
| Spectral bandwidth [nm] | 10 | 10 |
| Operating mode | Continuous wave | Continuous wave |
| Average radiant power [mW] | 15 | 70 |
| Effective radiant power [mW] | 11.2 | 53.9 |
| Polarization | Random | Random |
| Aperture diameter [cm] | 0.23 | 0.23 |
| Irradiance at aperture [mW/cm2] | 375 | 1750 |
| Beam spot size at target [cm2] | 0.04 | 0.04 |
| Irradiance at target [mW/cm2] | 280 | 1347.5 |
| Exposure duration [s] | 20 | 1.5 (twice) |
| Radiant exposure [J/cm2] | 7.50 | 2.62 |
| Effective radiant exposure [J/cm2] | 5.60 | 2.02 |
| Radiant energy [J] | 0.22 | 0.08 |
| Number of points irradiated | 1 | 1 |
| Area irradiated [cm2] | 0.04 | 0.04 |
| Application technique | Contact | Contact |
| Number and frequency of treatment sessions | 1 | 2 |
| Total radiant energy [J] | 0.22 | 0.16 |
The laser spot was positioned at the bottom of 50 mL Falcon tubes (TPP, Trasadingen, Schaffhausen, Switzerland) to allow the beam to reach the cell pellet directly without going through the culture medium [26,34]. Throughout the exposure time, the laser remained fixed in the bottom of the Falcon tube.
The cells in the activated and control groups were subjected to the same experimental conditions as irradiated cells, but without irradiation. A laser-check power meter (Coherent, Santa Clara, CA, USA) was used to verify the output power. The experiments were performed in an environment with dim lighting to avoid the influence of external light.
Table 1 displays the effective energy (amount of energy that actually reached the cells after passing through the polypropylene tube), which was calculated based on Silva et al. [35]. In brief, for determination of effective transmission of radiation by Falcon tube (polypropylene thickness of 1 mm) the following equation was used:
| (1) |
where I0 is the photon flux normally incident on the sample, α is the absorption coefficient of polypropylene (α660 = 1.96 cm−1 and α780 = 1.79 cm−1) and z is the optical path (1 mm). R was obtained from the Fresnel equation simplified for normal incidence:
| (2) |
where n1 and n2 are the refraction indices of air and polypropylene, respectively.
The effective transmission is 75% for the λ = 660 nm and 77% for the λ = 780 nm. However, it is noteworthy that Table 1 shows the energy that effectively reached the cells, that is, the loss already deducted.
Following irradiation, the pellets were re-suspended, plated in Petri dishes (1 × 106 cells/dish) and incubated for further analysis.
2.5. Gene Expression
After 24 h incubation, homogenization was performed and total ribonucleic acid (RNA) was extracted using TRIzol reagent following the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). All samples were treated with DNase I (Invitrogen) to avoid deoxyribonucleic acid (DNA) contamination. First-strand complementary DNA (cDNA) synthesis was carried out using the High Capacity cDNA Reverse Transcription kit, following the manufacturer's instructions (Applied Biosystems, Carlsbad, CA, USA). The relative quantitation of messenger RNA (mRNA) was carried out using an Applied Biosystems 7500 Real-Time PCR System with SYBRGreen I dye reagent (Applied Biosystems). Specific primers for TNF-α, IL-6, COX-2, iNOS and β-actin were used (as described in Table 2). The data were normalized to the expression of β-actin (endogenous “housekeeping” gene) and analyzed according to Paffl mathematical model [36]. Melting curve analysis was performed in each run to confirm the specificity of amplification and lack of primer dimers.
Table 2.
Design of primer sequences.
| Primer | Forward (sense) | Reverse (antisense) |
|---|---|---|
| TNF-α | 5′-GCCCACGTTGTAGCCAATGTCAAA-3′ | 5′-GTTGTCTTTCAGCTTCAGGCCGTT-3′ |
| IL-6 | 5′-TCCAGTTGCCTTCTTGGGAC-3′ | 5′-GTGTAATTAAGCCTCCGACTTG-3′ |
| COX-2 | 5′-TGAGTACCGCAAACGCTTCTC-3′ | 5′-TGGACGAGGTTTTTCCACCAG-3′ |
| iNOS | 5′-GGCAGCCTGTGAGACCTTTG-3′ | 5′-GCATTGGAAGTGAAGCGTTTC-3′ |
| β-actin | 5′-AGGGTGTGATGGTGGGTATG-3′ | 5′-TGCCGTGTTCAATGGGGTAC-3′ |
2.6. Protein Concentration (Enzyme Linked Immunosorbent Assay, ELISA)
One and three days after irradiation, the amounts of IL-6, TNF-α and COX-2 protein in the macrophage cultures were determined using ELISA kits, following the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). IL-6 and TNF-α were measured in the supernatants of the J774 cell cultures and COX-2 was measured in the supernatant of the J774 cell lysates.
2.7. Experimental Design
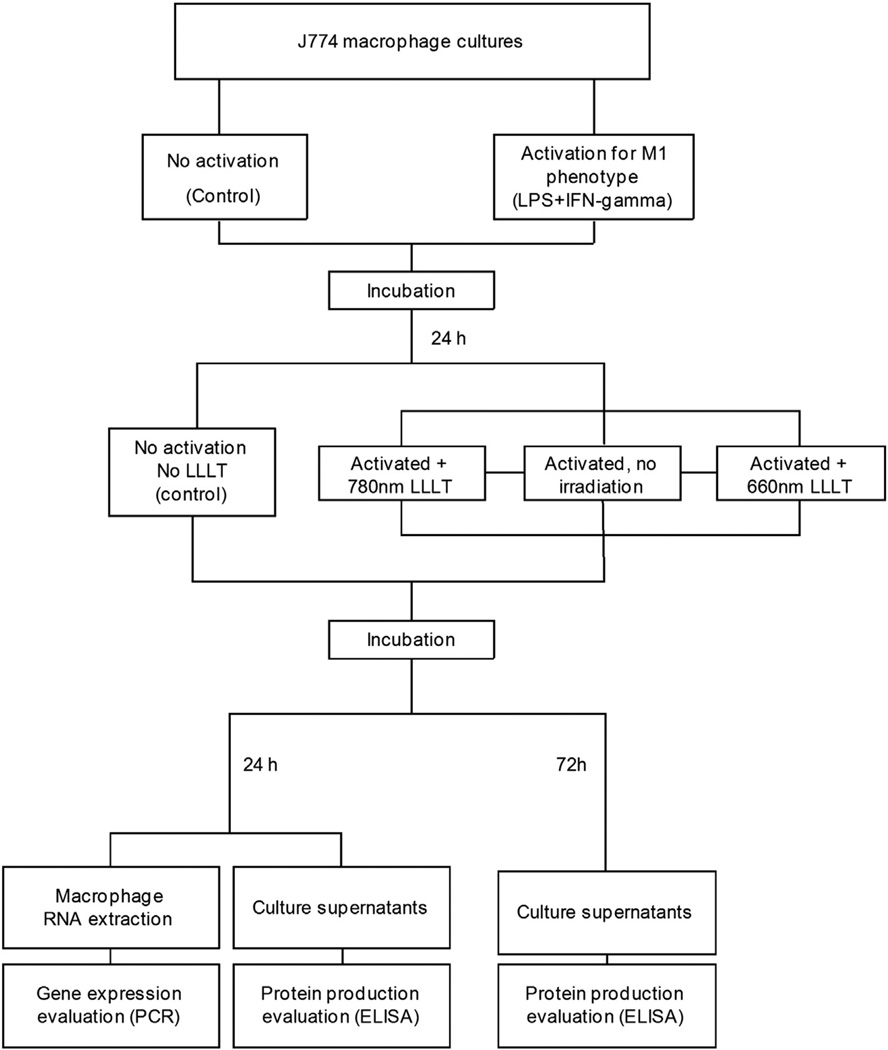
The experimental design is summarized in Fig. 1.
Fig. 1.
Flowchart showing experimental design.
2.8. Statistical Analysis
All experiments were performed in triplicate and analyzed using Kolmogorov–Smirnov normality test. The homogeneity of variance was tested with the Brow–Forsythe test. As the Brow–Forsythe test demonstrated different variances among mRNA groups, the T-test for independent samples was used for the comparisons, not assuming equal variances. As the Brow–Forsythe test demonstrated no differences in variance among the protein groups, one-way ANOVA, with the Tukey–Kramer test and Fisher's exact test were used for the comparisons. The significance level was set to 5% (p < 0.05) and all statistical analyses were performed with the aid of the OriginPro8® program (Origin Lab Corporation, Northampton, MA, USA).
3. Results
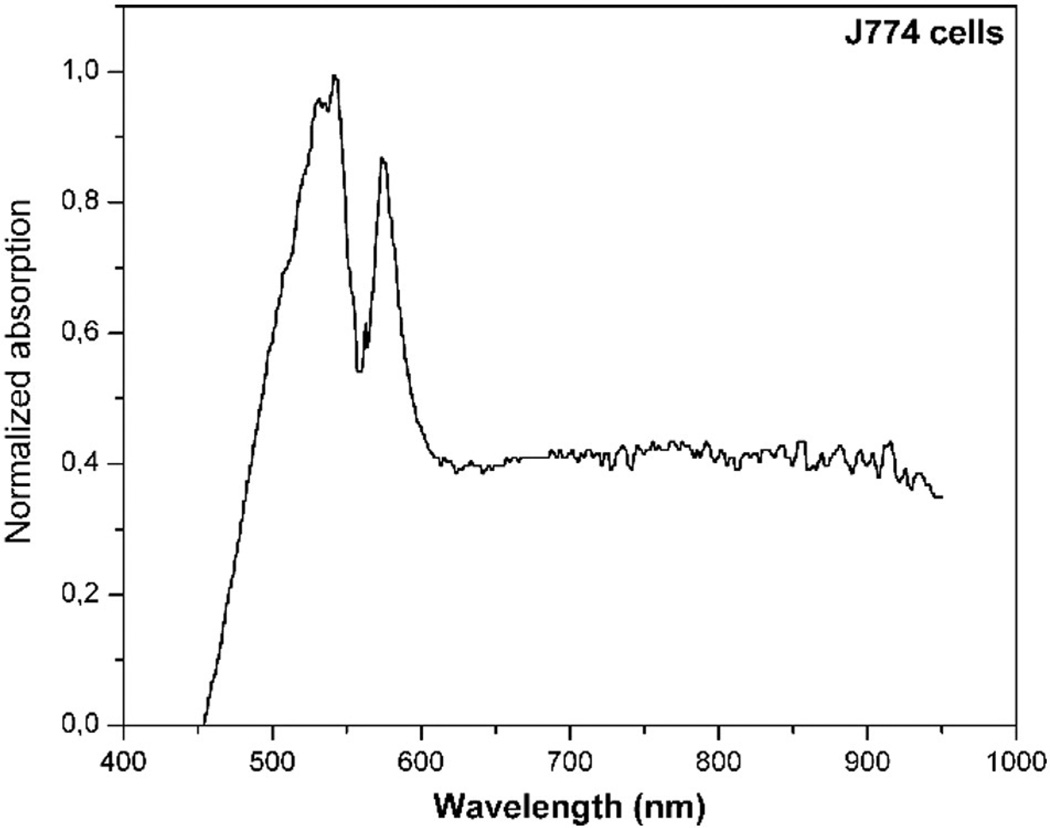
3.1. Spectroscopy Analysis
In the spectral range from 630 to 780 nm, the absorption of J774 cells was similar (Fig. 2). Studies report that cytochrome c oxidase (which is localized in the mitochondria) is the main cell photoacceptor [37,38].
Fig. 2.
Absorption spectrum of J774 cells.
3.2. IL-6 Expression
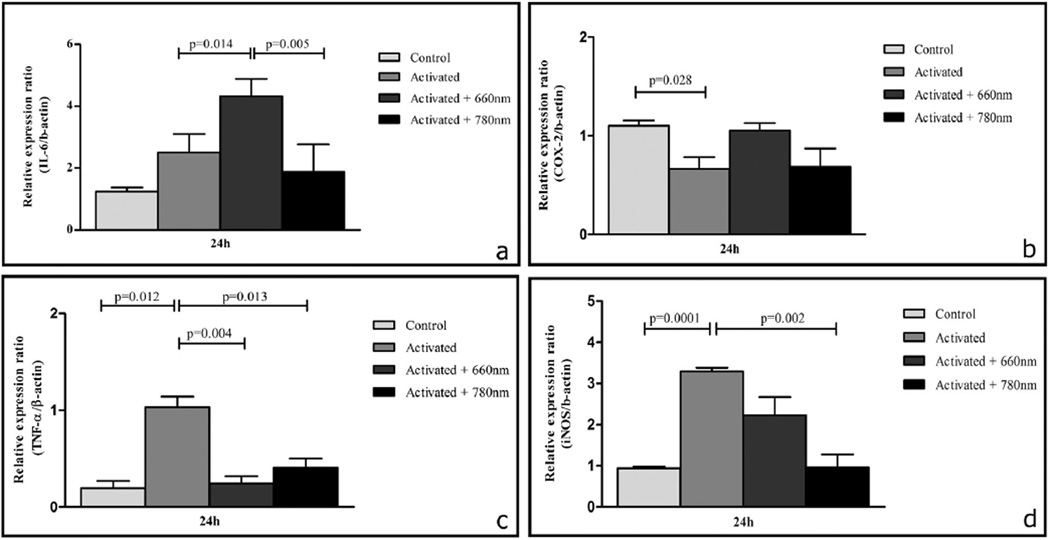
Twenty-four hours after the removal of LPS + IFN-γ (48 h since the onset of activation), treated cells had a higher mRNA expression of IL-6 in comparison to the control group, but the difference did not achieve statistical significance. In the same period (24 h after irradiation, i.e. 48 h since the onset of activation), the mRNA expression of IL-6 (p < 0.05) was higher in activated + 660 nm irradiated cells in comparison to non-irradiated activated cells. Nevertheless, the mRNA expression of IL-6 was lower in activated + 780 nm irradiated cells in comparison to activated cells (the difference did not achieve statistical significance). IL-6 mRNA expression was lower (p < 0.01) in activated + 780 nm irradiated cells than activated + 660 nm irradiated cells (as shown in Fig. 3a).
Fig. 3.
Effects of photobiomodulation on IL-6 (a), COX-2 (b), TNF-α (c) and iNOS (d) mRNA expression. The normalized mRNA levels for genes (24 h after irradiation procedures) are presented as the mean ± SE. Significances between groups were determined using T test. Control: no laser, no activation; activated: LPS + IFN-γ stimulation; activated + 660 nm: LPS + IFN-γ stimulation + 660 nm laser irradiation; activated + 780 nm: LPS + IFN-γ stimulation + 780 nm laser irradiation. n = 3 independent experiments for each group. Both lasers were able to reduce TNF-α and iNOS expression, 660 nm laser parameters resulted in an up-regulation of IL-6 expression.
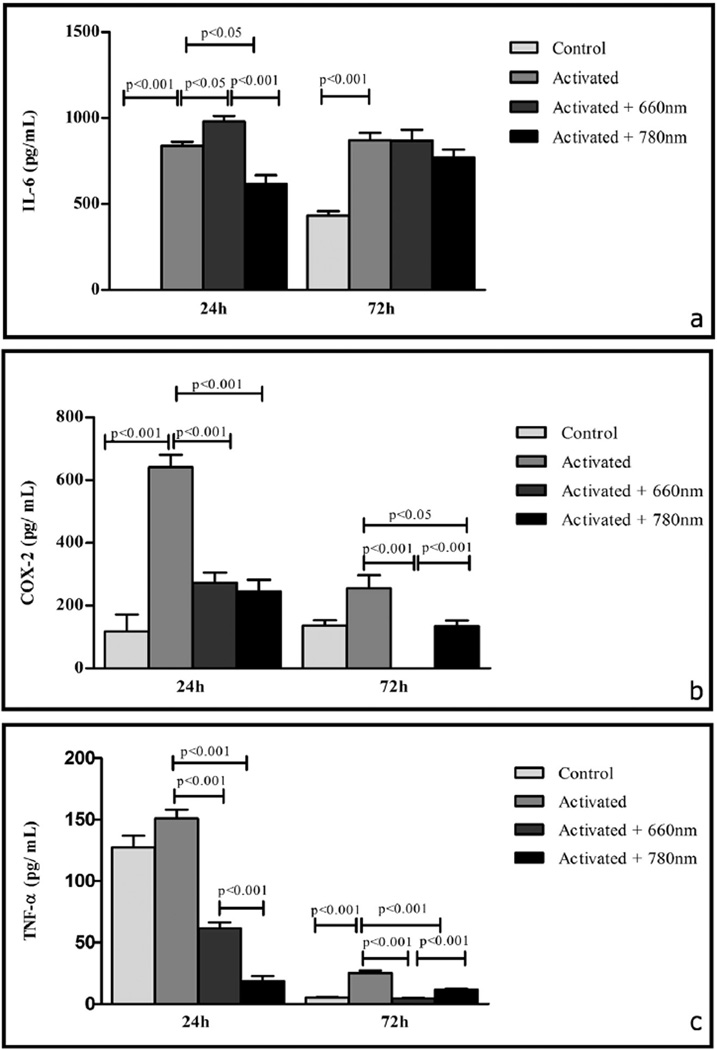
In the same period (48 h since the onset of activation, i.e. 24 h after irradiation), activated cells produced more IL-6 protein (p < 0.001) than control cells (there was no detectable production in this group). Activated + 660 nm irradiated cells produced more IL-6 protein (p < 0.05) than activated cells. On the other hand, activated + 780 nm irradiated cells produced significantly less IL-6 (p < 0.001) than activated cells. The amounts of IL-6 (p < 0.001)were lower in activated + 780 nm irradiated cells in comparison to activated + 660 nm irradiated cells (as shown in Fig. 4a).
Fig. 4.
Effects of photobiomodulation on IL-6 (a), COX-2 (b) and TNF-α(c) production. Protein concentration in the samples was quantified 24 h and 72 h after irradiation procedures and is presented as the mean ± SE. Significance between groups was determined using Fisher's exact test. Control: no laser, no activation; activated: LPS + IFN-γ stimulation; activated + 660 nm: LPS + IFN-γ stimulation + 660 nm laser irradiation; activated + 780 nm: LPS + IFN-γ stimulation + 780 nm laser irradiation. n = 3 independent experiments for each group. Both lasers were able to reduce TNF-α and COX-2 production, 660 nm laser parameters resulted in an up-regulation of IL-6 production.
Three days after the removal of LPS + IFN-γ (4 days since the onset of activation), the amounts of IL-6 (p < 0.001), were higher in the supernatants of activated cells in comparison to control cells. The production of IL-6 was similar between the supernatant of activated + 660 nm irradiated cells in comparison to activated cells as well as similar to the production found at 24 h. Smaller amounts of IL-6 protein were found in the supernatant of activated + 780 nm irradiated cells in comparison to activated cells, but the difference did not achieve statistical significance. Moreover, IL-6 production was higher in activated + 660 nm irradiated cells than in activated + 780 nm irradiated cells, but the difference was not statistically significant (as shown in Fig. 4a).
3.3. COX-2 Expression
Twenty-four hours after the removal of LPS + IFN-γ (48 h since the onset of activation), the mRNA expression of COX-2 was lower (p < 0.05) in activated cells in comparison to control cells. The mRNA expression of COX-2 was higher in activated + 660 nm irradiated cells in comparison to non-irradiated activated cells, but the difference did not achieve statistical significance. COX-2 mRNA expression was similar in activated and activated + 780 nm irradiated cells. COX-2 mRNA expression was lower in activated + 780 nm irradiated cells than activated + 660 nm irradiated cells, but the difference did not achieve statistical significance (as shown in Fig. 3b).
In the same period (48 h since the onset of activation), activated cells produced more COX-2 (p < 0.001) than control cells. Activated + 660 nm irradiated and activated + 780 nm irradiated cells produced less COX-2 (p < 0.001 for both comparisons) than activated cells. Additionally, COX-2 production was similar in activated + 660 nm irradiated cells and activated + 780 nm irradiated cells (as shown in Fig. 4b).
Three days after the removal of LPS + IFN-γ (4 days since the onset of activation), the amounts of COX-2 were higher in the supernatants of activated cells in comparison to control cells, but the difference did not achieve statistical significance. The amounts of COX-2 were undetectable in the supernatant of activated + 660 nm irradiated cells, therefore in comparison to activated cells, the difference was very significant (p < 0.001). Smaller amounts of COX-2 (p < 0.05) protein were found in the supernatant of activated + 780 nm irradiated cells in comparison to activated cells. Activated + 780 nm irradiated cells produced more COX-2 (p < 0.001) than activated + 660 nm irradiated cells, as there was no production of COX-2 in this group (as shown in Fig. 4b).
3.4. TNF-α Expression
Twenty-four hours after the removal of LPS + IFN-γ (48 h since the onset of activation), treated cells had a higher mRNA expression of TNF-α (p = 0.01) in comparison to the control group. The mRNA expression of TNF-α was lower in activated + 660 nm irradiated (p < 0.01) and in activated + 780 nm irradiated (p < 0.05) cells than in activated cells. TNF-α mRNA expression was higher in activated + 780 nm irradiated cells in comparison to activated + 660 nm irradiated cells, however, this difference was not statistically significant (as shown in Fig. 3c).
In the same period (48 h since the onset of activation), activated cells produced more TNF-α than control cells, but the difference did not achieve statistical significance. Activated + 660 nm irradiated cells (p < 0.001) and activated + 780 nm irradiated cells (p < 0.001) produced less TNF-α than activated cells. The amounts of TNF-α (p < 0.001) protein were lower in activated + 780 nm irradiated cells in comparison to activated + 660 nm irradiated cells (as shown in Fig. 4c).
Three days after the removal of LPS + IFN-γ (4 days since the onset of activation), the amounts of TNF-α (p < 0.001) were higher in the supernatants of activated cells in comparison to control cells. Lower amounts of TNF-α were found in the supernatant of activated + 660 nm irradiated cells (p < 0.001) and activated + 780 nm irradiated cells (p < 0.001) in comparison to activated cells. Activated + 780 nm irradiated cells produced more TNF-α (p < 0.001) than activated + 660 nm irradiated cells (as shown in Fig. 4c).
3.5. iNOS expression
Twenty-four hours after the removal of LPS + IFN-γ (48 h since the onset of activation), treated cells had a higher mRNA expression of iNOS (p < 0.001) in comparison to the control group. The mRNA expression of iNOS mRNA was lower in activated + 660 nm irradiated cells in comparison to activated cells, but the difference did not achieve statistical significance. The mRNA expression of iNOS (p < 0.01) was lower in activated + 780 nm irradiated cells in comparison to activated cells. iNOS mRNA expression was lower in activated + 780 nm irradiated cells than activated + 660 nm irradiated cells, but the difference did not achieve statistical significance (as shown in Fig. 3d).
4. Discussion
The results confirmed the up-regulation of M-1 related product expression in J774 cells in response to LPS + IFN-γ activation (as shown in Fig. 4), as already described [31,32] and showed that 660 nm and 780 nm lasers strongly reduced the mRNA expression of TNF-α and iNOS (as shown in Fig. 3) and down-regulated the production of TNF-α and COX-2 proteins in M1 J774 cells (as shown in Fig. 4). Cells irradiated with 660 nm laser expressed more IL-6 mRNA and produced more IL-6 protein than non-irradiated cells (as shown in Figs. 3 and 4).
Moreover, 24 h after irradiation, activated J774 cells irradiated with 780 nm laser produced less IL-6 and TNF-αproteins than cells irradiated with 660 nm laser (as shown in Fig. 4). Seventy-two hours after irradiation, the amount of TNF and COX-2 proteins was very low, but greater production was found when the M1 J774 cells were irradiated with 780 nm laser in comparison to 660 nm (as shown in Fig. 4). In general, both lasers were able to reduce M1 related markers, although the parameters used for 780 nm laser (2.6 J/cm2, 70 mW) provided an additional decrease when compared to the activated cells. Also, 660 nm laser parameters resulted in an up-regulation of IL-6 production (as shown in Fig. 4). These findings imply a distinct, time-dependent modulation by the two different sets of laser parameters, suggesting that the best therapeutic approach to the repair of tissue injury may involve more than one combination of laser parameters, each selected to treat a different phase of the regeneration process.
During tissue regeneration, M1-related products have time/concentration-dependent actions, such as stimulating the production of other pro-inflammatory molecules, inducing the migration of myeloid cells and carrying out functions related to tissue regeneration [1,2,7].
In muscle tissue, for example, TNF-α and IL-6 modulate the migration, proliferation and differentiation of myoblasts and satellite cells. Prostaglandins (derived from COX-2 enzyme activity) have modulating action on the proliferation and differentiation of muscle cells as well as the synthesis and degradation of muscle proteins [7]. Through the activation of the transcription factor NF-κβ (nuclear factor kappa-light-chain-enhancer of activated B cells), NO (nitric oxide) is produced from iNOS, and IL-1, TNF-α and IL-6 are up-regulated which can inhibit myoblast differentiation [4,7].
The ability of photobiomodulation to reduce inflammation is generally considered beneficial in cases of muscle injury [39]. A recent review concluded that phototherapy, although depending on the ideals dosimetric parameters, is an excellent resource for the treatment of muscle injuries [40].
In animal models, photobiomodulation has been found to reduce the expression of TNF-α [17,41,42], IL-1β [15,41,42], IL-6 [41,42], COX-2 [43,44], iNOS [45,46] and NFκβ [45]. As macrophages are the key cells in the muscle repair process, the results presented herein suggest that the in vivo effects of photobiomodulation may be largely dependent on these cells and could indicate new therapeutic possibilities, not only for muscle injuries [1–8].
The photobiomodulation effects on gene expression and the release of cytokines in some cells have also recently been reviewed [47]. However, only five studies published up to 2014 evaluated the effect of red light on macrophages or monocytes [21–23,25,33]. Three of these studies employed laser [22,23,33] and two employed a light emitting diode (LED) [21,25]. The power output ranged from 2.3 mW to 2.5 W and the fluence ranged from 0.01 to 10,714 J/cm2. The experimental models were also quite different. Only one used LPS to activate macrophages and found that LLLT (660 nm, 30 mW, 4.5 J/cm2, 252 s) attenuated the mRNA expression of MIP-2, the generation of intracellular radical oxygen species and the synthesis of the NF-kB in activated cells [22]. NF-kB is required for the transcription of many pro-inflammatory molecules, including TNF-α, IL-6, iNOS and COX-2.
There are only a few articles that consider the effective transmission of radiation in the discussion of results from in vitro studies [35,48]. In the present study, we took into consideration the loss due to the polypropylene wall of the Falcon tube in order to compare the dosimetry that actually reached the cells with previous studies that provided sufficient information to determine the dosimetry used. For example, the output parameters of the 660 nm laser employed in the present investigation, were similar to those used by Bolton et al. [21]. These authors used noncoherent light λ = 660 nm, spot size of 0.125 cm2, power output of 15 mW, irradiance of 120 mW/cm2, exposure time of 60 s and energy density of 7.2 J/cm2 directly at cells from the top of wells, without going through the wall of the culture plates. But even by irradiating the cells through the top of the plate, there was a loss of 7% due to the RPMI culture medium with a volume of 1 ml and a well diameter of 1.54 cm (obtained from the manufacturer). Applying equation 6 from Silva et al. [35], the optical path was 0.54 cm, which led to a transmission of 93% of the incident power. Therefore, that effective radiant exposure was 6.70 J/cm2, comparable to the present study using 5.60 J/cm2 with coherent laser radiation. Is noteworthy that in the present study, the cells were at the bottom of the Falcon tube, so the beam reached the cell pellet directly without going through the culture medium.
Bolton et al. [21] found that the supernatant of non-activated monocyte cultures following LED irradiation promoted fibroblast growth. Indeed, monocyte-derived and macrophage-derived IL-6 has been shown to enhance fibroblast growth [49].
All studies addressing the effect of infrared laser on macrophages and monocytes [17,21,23,24] used laser irradiation, but only two evaluated cytokine expression [24,26]. Gavish et al. [24] irradiated LPS-activated macrophages (from cell line RAW 264.7) with a 780 nm diode laser (2 mW/cm2, 2.2 J/cm2) and found reductions in the mRNA expression of MCP-1 (monocyte chemoattractant protein-1), IL-1α, IL-1β and IL-6 as well as reductions in the secretion of MCP-1 and IL-1β. The authors did not measure all proteins, but demonstrated the capacity of LLLT to down-regulate the mRNA expression of M1-related molecules.
Sousa et al. [26] irradiated the cells through the bottom of the microtiter plates, with λ = 780 nm, spot size of 0.04 cm2, power output of 70 mW, irradiance of 1750 mW/cm2, exposure time of 1.5 s (twice) and energy density of 2.6 J/cm2. Considering the 22% loss due to the polystyrene wall [35], the power decreased to 54.6 mW, irradiance to 1365.0 mW/cm2, and the energy density was 2.02 J/cm2, which was equal to the effective radiant exposure used in this study. These authors found significantly less TNF-α production in irradiated mouse peritoneal macrophages activated by IFN-γ (20 µg/mL) and LPS (10 ng/mL) in comparison to non-irradiated macrophages. The present results confirm and expand on these findings, demonstrating the capacity of 780-nm laser irradiation to regulate the production of other M1-related molecules.
When considering the parameters used in this study, 660 nm laser with low power density and a high energy density, and 780 nm laser with high power and a low energy density, achieved similar results (with the exception of IL-6 modulation), although an additional decrease was seen with 780 nm laser, suggesting this wavelength parameters returns the cells to a non stimulated state. Bolton et al. [20] described that low energy density combined with high power density, or vice-versa, produced similar results.
The increase in IL-6 seen here it is not easily explained at present. Numerous signaling pathways regulate M1 cytokine production, including p38 MAPK (P38 mitogen-activated protein kinases), ERK (extracellular signal-regulated kinases), and JNK (c-Jun. N-terminal kinases), and these kinases induce the activation of transcription factors, NFkB, ATF2 (activating transcription factor 2), AP1 (activator protein 1), and ELK1 (ETS domain-containing protein), respectively [50]. Conversely, it was shown that there is a parallel but distinct mechanism regulating IL-6 independently of both p38 MAPK and NF-kB activity [51]. IL-6 effects on cells vary, according to cell type, conditions, and so forth. IL-6 acts as a chemoattractant for myoblasts and myeloid cells, increases myoblast proliferation, inhibits myocyte fusion, and affects satellite cell differentiation [7].
Chen et al. [52] showed that LLLT using 810-nm (0.03 and 30 J/cm2) laser increased the activation of NF-kB in non-activated mouse embryonic fibroblasts via production of reactive oxygen species. However, these same authors [53] also showed that 810-nm laser (0.3, 3, and 30 J/cm2) reduced the level of expression of several inflammatory markers in bone marrow derived dendritic cells (a macrophage-lineage cell type) that had been pre-activated with CpG (a toll like receptor 9 agonist).
In another study by Chen et al. [54], the authors concluded that 660 nm laser irradiation could be used to enhance pro-inflammatory mediators by driving monocytes and macrophages to an M1 profile. The authors found that, after 24 h, laser irradiation at 660 nm (0.8 mW/cm2, 1–2 J/cm2, 7.5–15 J) and 808 nm (44.7 mW/cm2, 1–2 J/cm2, 3.8–7.6 J) enhanced M1-related chemokine CCL-2 (chemokine, C-C motif, ligand 2, also referred to as MCP-1)mRNA expression by THP-1 cells (human acute monocytic leukemia) treated with LPS. However, LLLT with 3 J/cm2 (660 nm, 22.5 J and 808 nm, 11.4 J) suppressed CCL-2 expression. CXCL-10 (C-X-C motif chemokine 10) and TNF-α mRNA expression were enhanced by LLLT at 660 nm (1 J/cm2, 7.5 J, 0.8 mW/cm2), but suppressed by LLLT at 808 nm (1 J/cm2, 3.8 J, 44.7 mW/cm2) in the same period. The analysis of protein expression (also 24 h after irradiation) revealed that 1 J/cm2 of 660 nm LLLT (i.e. 0.93 J/cm2 considering the data provided and using Eq. 1 from Silva et al. [35]) significantly induced CCL2, CXCL10 and TNF-α production in human monocytes, whereas 2 J/cm2 and 3 J/cm2 did not. The results of 808 nm laser on protein expression were not shown. In the present study, 660 nm laser (with a very similar energy density: 1.15 J/cm2, considering the effective energy density) also caused an increase in the expression of an M1-profile cytokine, but it was IL-6 rather than the TNF-α described by Chen et al. [54].
This difference may be explained by the activation of the cells (LPS vs. LPS + IFN-γ), the activation time point (2 h vs. 24 h), differences in species (human vs. mouse), differences in energy parameters (i.e. power density, total energy, etc.), and alternative pathways of inflammatory cytokine production [47,50–55]. But, the concept that photomodulation can drive macrophage phenotype in different ways was also confirmed by these authors.
It should be also stressed that the effects produced by various light sources on cells and tissues depend on the cell/tissue type and its particular biological state, on the dosimetric parameters (wavelength, fluence, power density, mode of delivery, time of application, pulse), on the tissue/cell optical properties, and is also affected by the biphasic/triphasic dose response curves that are still the subject of open discussion in the literature [29].
Considering the importance and complexity of different macrophage phenotypes in different disease states [1–4], along with the sometimes contradictory effects of LLLT on these cells [20–27,54], as well as the knowledge still to come on the different mechanisms that may operate during photobiomodulation (epigenetic, pre-transcriptional, posttranscriptional), there are exciting possibilities for further exploration to understand the effects of LLLT on macrophages and other cells. Combining different LLLT parameters could be useful in modulating different phases of the inflammatory process in the treatment of many injuries and conditions.
Acknowledgments
The authors would like to thank the Brazilian funding agencies São Paulo Research Foundation (FAPESP, grant n° 2011/14474-9 and grant n° 2013/23051-0) and Coordination for the Improvement of Higher Education Personnel (CAPES/PROSUP) (33092010004P5) for financial support. KPS Fernandes was supported by the National Council for Technological and Scientific Development (CNPq, grant n° 303662/2012-3). MR Hamblin was supported by US NIH grant R01AI050875.
Contributor Information
Nadhia Helena Costa Souza, Email: nadhiacosta@gmail.com.
Raquel Agnelli Mesquita-Ferrari, Email: raquel.mesquita@gmail.com.
Daniela de Fatima Teixeira da Silva, Email: fatesi@uol.com.br.
Lilia Alves Rocha, Email: lilia_mail81@yahoo.com.br.
Agnelo Neves Alves, Email: agnelonevesalves@gmail.com.
Kaline de Brito Sousa, Email: kaline.bsousa@gmail.com.
Sandra Kalil Bussadori, Email: sandra.skb@gmail.com.
Michael R. Hamblin, Email: hamblin@helix.mgh.harvard.edu.
Fábio Daumas Nunes, Email: fadnunes@usp.br.
References
- 1.Mokarram N, Bellamkonda RV. A perspective on immunomodulation and tissue repair. Ann. Biomed. Eng. 2014;42(2):338–351. doi: 10.1007/s10439-013-0941-0. http://dx.doi.org/10.1007/s10439-013-0941-0. [DOI] [PubMed] [Google Scholar]
- 2.Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit. Rev. Immunol. 2012;32(6):463–488. doi: 10.1615/critrevimmunol.v32.i6.10. http://dx.doi.org/10.1615/CritRevImmunol.v32.i6.10. [DOI] [PubMed] [Google Scholar]
- 3.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;3:6–13. doi: 10.12703/P6-13. http://dx.doi.org/10.12703/P6-13 (eCollection 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saclier M, Yacoub-Youssef H, Mackey AL, Arnold L, Ardjoune H, Magnan M, Sailhan F, Chelly J, Pavlath GK, Mounier R, Kjaer M, Chazaud B. Differentially activated macrophages orchestrate myogenic precursor cell fate during human skeletal muscle regeneration. Stem Cells. 2013;31(2):384–396. doi: 10.1002/stem.1288. http://dx.doi.org/10.1002/stem.1288. [DOI] [PubMed] [Google Scholar]
- 5.Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J. Leukoc. Biol. 2013;93(6):875–881. doi: 10.1189/jlb.1012512. http://dx.doi.org/10.1189/jlb.1012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weidenbusch M, Anders HJ. Tissue microenvironments define and get reinforced by macrophage phenotypes in homeostasis or during inflammation, repair and fibrosis. J. Innate Immun. 2012;4(5–6):463–477. doi: 10.1159/000336717. http://dx.doi.org/10.1159/000336717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tidball JG, Villalta SA. Regulatory interactions between muscle and the immune system during muscle regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298(5):R1173–R1187. doi: 10.1152/ajpregu.00735.2009. http://dx.doi.org/10.1152/ajpregu.00735.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosurgi L, Manfredi AA, Rovere-Querini P. Macrophages in injured skeletal muscle: a perpetuum mobile causing and limiting fibrosis, prompting or restricting resolution and regeneration. Front. Immunol. 2011;2:62. doi: 10.3389/fimmu.2011.00062. http://dx.doi.org/10.3389/fimmu.2011.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alves AN, Fernandes KP, Melo CA, Yamaguchi RY, França CM, Teixeira DF, Bussadori SK, Nunes FD, Mesquita-Ferrari RA. Modulating effect of low level-laser therapy on fibrosis in the repair process of the tibialis anterior muscle in rats. Lasers Med. Sci. 2014;29(2):813–821. doi: 10.1007/s10103-013-1428-9. http://dx.doi.org/10.1007/s10103-013-1428-9. [DOI] [PubMed] [Google Scholar]
- 10.Assis L, Moretti AI, Abrahão TB, Cury V, Souza HP, Hamblin MR, Parizotto NA. Low-level laser therapy (808 nm) reduces inflammatory response and oxidative stress in rat tibialis anterior muscle after cryolesion. Lasers Surg. Med. 2012;44(9):726–735. doi: 10.1002/lsm.22077. http://dx.doi.org/10.1002/lsm.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baptista J, Martins MD, Pavesi VC, Bussadori SK, Fernandes KP, Pinto Júnior DS, Ferrari RA. Influence of laser photobiomodulation on collagen IV during skeletal muscle tissue remodeling after injury in rats. Photomed. Laser Surg. 2011;29(1):11–17. doi: 10.1089/pho.2009.2737. http://dx.doi.org/10.1089/pho.2009.2737. [DOI] [PubMed] [Google Scholar]
- 12.Dawood MS, Al-Salihi AR, Qasim AW. Laser therapy of muscle injuries. Lasers Med. Sci. 2013;28(3):735–742. doi: 10.1007/s10103-012-1131-2. http://dx.doi.org/10.1007/s10103-012-1131-2. [DOI] [PubMed] [Google Scholar]
- 13.de Almeida P, Lopes-Martins RA, Tomazoni SS, Silva JA, Jr, de Carvalho PT, Bjordal JM, Leal Junior EC. Low-level laser therapy improves skeletal muscle performance, decreases skeletal muscle damage and modulates mRNA expression of COX-1 and COX-2 in a dose-dependent manner. Photochem. Photobiol. 2011;87(5):1159–1163. doi: 10.1111/j.1751-1097.2011.00968.x. http://dx.doi.org/10.1111/j.1751-1097.2011.00968.x. [DOI] [PubMed] [Google Scholar]
- 14.de Souza TO, Mesquita DA, Ferrari RA, Pinto Júnior DS, Correa L, Bussadori SK, Fernandes KP, Martins MD. Phototherapy with low-level laser affects the remodeling of types I and III collagen in skeletal muscle repair. Lasers Med. Sci. 2011;26(6):803–814. doi: 10.1007/s10103-011-0951-9. http://dx.doi.org/10.1007/s10103-011-0951-9. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes KP, Alves AN, Nunes FD, Souza NH, Silva JA, Jr, Bussadori SK, Ferrari RA. Effect of photobiomodulation on expression of IL-1beta in skeletal muscle following acute injury. Lasers Med. Sci. 2013;28(3):1043–1046. doi: 10.1007/s10103-012-1233-x. http://dx.doi.org/10.1007/s10103-012-1233-x. [DOI] [PubMed] [Google Scholar]
- 16.Franca CM, de Loura Santana C, Takahashi CB, Alves AN, De Souza Mernick AP, Fernandes KP, Silva DFT, Bussadori SK, Mesquita-Ferrari RA. Effect of laser therapy on skeletal muscle repair process in diabetic rats. Lasers Med. Sci. 2013;28(5):1331–1338. doi: 10.1007/s10103-012-1249-2. http://dx.doi.org/10.1007/s10103-012-1249-2. [DOI] [PubMed] [Google Scholar]
- 17.Mesquita-Ferrari RA, Martins MD, Silva JA, Jr, Silva TD, Piovesan RF, Pavesi VC, Bussadori SK, Fernandes KP. Effects of low-level laser therapy on expression of TNF-α and TGF-β in skeletal muscle during the repair process. Lasers Med. Sci. 2011;26(3):335–340. doi: 10.1007/s10103-010-0850-5. http://dx.doi.org/10.1007/s10103-010-0850-5. [DOI] [PubMed] [Google Scholar]
- 18.Oron U. Photoengineering of tissue repair in skeletal and cardiac muscles. Photomed. Laser Surg. 2006;24(2):111–120. doi: 10.1089/pho.2006.24.111. [DOI] [PubMed] [Google Scholar]
- 19.Rodrigues NC, Brunelli R, de Araújo HS, Parizotto NA, Renno AC. Low-level laser therapy (LLLT) (660 nm) alters gene expression during muscle healing in rats. J. Photochem. Photobiol. B. 2013;120:29–35. doi: 10.1016/j.jphotobiol.2013.01.002. http://dx.doi.org/10.1016/j.jphotobiol.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Bolton P, Young S, Dyson M. Macrophage responsiveness to light therapy with varying power and energy densities. Laser Ther. 1991;3(3):105–112. [Google Scholar]
- 21.Bolton P, Young S, Dyson M. Macrophage responsiveness to light therapy: a dose response study. Laser Ther. 1990;2:101–106. [Google Scholar]
- 22.de Lima FM, Villaverde AB, Albertini R, de Oliveira AP, Faria Neto HC, Aimbire F. Low-level laser therapy associated to N-acetylcysteine lowers macrophage inflammatory protein-2 (MIP-2) mRNA expression and generation of intracellular reactive oxygen species in alveolar macrophages. Photomed. Laser Surg. 2010;28(6):763–771. doi: 10.1089/pho.2009.2638. http://dx.doi.org/10.1089/pho.2009.2638. [DOI] [PubMed] [Google Scholar]
- 23.Dube A, Bansal H, Gupta PK. Modulation of macrophage structure and function by low level He–Ne laser irradiation. Photochem. Photobiol. Sci. 2003;2(8):851–855. doi: 10.1039/b301233f. [DOI] [PubMed] [Google Scholar]
- 24.Gavish L, Perez LS, Reissman P, Gertz SD. Irradiation with 780 nm diode laser attenuates inflammatory cytokines but upregulates nitric oxide in lipopolysaccharide-stimulated macrophages: implications for the prevention of aneurysm progression. Lasers Surg. Med. 2008;40(5):371–378. doi: 10.1002/lsm.20635. http://dx.doi.org/10.1002/lsm.20635. [DOI] [PubMed] [Google Scholar]
- 25.Lindgard A, Hultén LM, Svensson L, Soussi B. Irradiation at 634 nm releases nitric oxide from human monocytes. Lasers Med. Sci. 2007;22(1):30–36. doi: 10.1007/s10103-006-0419-5. [DOI] [PubMed] [Google Scholar]
- 26.Sousa LR, Cavalcanti BN, Marques MM. Effect of laser phototherapy on the release of TNF-alpha and MMP-1 by endodontic sealer-stimulated macrophages. Photomed. Laser Surg. 2009;27(1):37–42. doi: 10.1089/pho.2007.2220. http://dx.doi.org/10.1089/pho.2007.2220. [DOI] [PubMed] [Google Scholar]
- 27.Young S, Bolton P, Dyson M, Harvey W, Diamantopoulos C. Macrophage responsiveness to light therapy. Lasers Surg. Med. 1989;9(5):497–505. doi: 10.1002/lsm.1900090513. [DOI] [PubMed] [Google Scholar]
- 28.Huang YY, Sharma SK, Carroll J, Hamblin MR. Biphasic dose response in low level light therapy — an update. Dose-Response. 2011;9(4):602–618. doi: 10.2203/dose-response.11-009.Hamblin. http://dx.doi.org/10.2203/dose-response.11-009.Hamblin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann. Biomed. Eng. 2012;40(2):516–533. doi: 10.1007/s10439-011-0454-7. http://dx.doi.org/10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ralph P, Prichard J, Cohn M. Reticulum cell sarcoma: an effector cell in antibody-dependent cell-mediated immunity. J. Immunol. 1975;114(2):898–905. [PubMed] [Google Scholar]
- 31.Nathan CF, Brukner LH, Silverstein SC, Cohn ZA. Extracellular cytolysis by activated macrophages and granulocytes. 1. Pharmacologic triggering of effector cells and the release of hydrogen-peroxide. J. Exp. Med. 1979;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verma N, Chakrabarti R, Das RH, Gautam HK. Anti-inflammatory effects of shea butter through inhibition of iNOS, COX-2, and cytokines via the Nf-kB pathway in LPS-activated J774 macrophage cells. J. Complement. Integr. Med. 2012;9(1):1–11. doi: 10.1515/1553-3840.1574. http://dx.doi.org/10.1515/1553-3840.1574. [DOI] [PubMed] [Google Scholar]
- 33.Mehrsai A, Afsharpada M, Afsharpada M, Mohydinb M, Ansarib B, Pourmanda G, Nikbinb B. The effect of low-level helium–neon (HeNe) laser radiation on the secretion of cytokines that promote chronic graft rejection — an in vitro study. Med. Laser Appl. 2009;24(3):194–200. http://dx.doi.org/10.1016/j.mla.2009.03.001. [Google Scholar]
- 34.Nogueira GT, Mesquita-Ferrari RA, Souza NH, Artilheiro PP, Albertini R, Bussadori SK, Fernandes KP. Effect of low-level laser therapy on proliferation, differentiation, and adhesion of steroid-treated osteoblasts. Lasers Med. Sci. 2012;27(6):1189–1193. doi: 10.1007/s10103-011-1035-6. http://dx.doi.org/10.1007/s10103-011-1035-6. [DOI] [PubMed] [Google Scholar]
- 35.Silva DF, Mesquita-Ferrari RA, Fernandes KP, Raele MP, Wetter NU, Deana AM. Effective transmission of light for media culture, plates and tubes. Photochem. Photobiol. 2012;88(5):1211–1216. doi: 10.1111/j.1751-1097.2012.01166.x. http://dx.doi.org/10.1111/j.1751-1097.2012.01166.x. [DOI] [PubMed] [Google Scholar]
- 36.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Passarella S, Karu T. Absorption of monochromatic and narrow band radiation in the visible and near IR by both mitochondrial and non-mitochondrial photoacceptors results in photobiomodulation. J. Photochem. Photobiol. B. 2014;140:344–358. doi: 10.1016/j.jphotobiol.2014.07.021. http://dx.doi.org/10.1016/j.jphotobiol.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 38.Szundi I, Liao GL, Einarsdóttir O. Near-infrared time-resolved optical absorption studies of the reaction of fully reduced cytochrome c oxidase with dioxygen. Biochemistry. 2001;40(8):2332–2339. doi: 10.1021/bi002220v. [DOI] [PubMed] [Google Scholar]
- 39.Ferraresi C, Hamblin MR, Parizotto NA. Low-level laser (light) therapy (LLLT) on muscle tissue: performance, fatigue and repair benefited by the power of light. Photonics Lasers Med. 2012;1(4):267–286. doi: 10.1515/plm-2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alves AN, Fernandes KP, Deana AM, Bussadori SK, Mesquita-Ferrari RA. Effects of low-level laser therapy on skeletal muscle repair: a systematic review. Am. J. Phys. Med. Rehabil. 2014;93(12):1073–1085. doi: 10.1097/PHM.0000000000000158. http://dx.doi.org/10.1097/PHM.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 41.Albertini R, Villaverde AB, Aimbire F, Bjordal J, Brugnera A, Mittmann J, Silva JA, Costa M. Cytokine mRNA expression is decreased in the subplantar muscle of rat paw subjected to carrageenan-induced inflammation after low-level laser therapy. Photomed. Laser Surg. 2008;26(1):19–24. doi: 10.1089/pho.2007.2119. http://dx.doi.org/10.1089/pho.2007.2119. [DOI] [PubMed] [Google Scholar]
- 42.Almeida PD, Tomazoni SS, Frigo L, de Carvalho PT, Vanin AA, Santos LA, Albuquerque-Pontes GM, De Marchi T, Tairova O, Marcos RL, Lopes-Martins RÁ, Leal-Junior EC. What is the best treatment to decrease pro-inflammatory cytokine release in acute skeletal muscle injury induced by trauma in rats: low-level laser therapy, diclofenac, or cryotherapy? Lasers Med. Sci. 2014;29(2):653–658. doi: 10.1007/s10103-013-1377-3. http://dx.doi.org/10.1007/s10103-013-1377-3. [DOI] [PubMed] [Google Scholar]
- 43.Liu XG, Zhou YJ, Liu TC, Yuan JQ. Effects of low-level laser irradiation on rat skeletal muscle injury after eccentric exercise. Photomed. Laser Surg. 2009;27(6):863–869. doi: 10.1089/pho.2008.2443. http://dx.doi.org/10.1089/pho.2008.2443. [DOI] [PubMed] [Google Scholar]
- 44.Renno AC, Toma RL, Feitosa SM, Fernandes K, Bossini PS, de Oliveira P, Parizotto N, Ribeiro DA. Comparative effects of low-intensity pulsed ultrasound and low-level laser therapy on injured skeletal muscle. Photomed. Laser Surg. 2011;29(1):5–10. doi: 10.1089/pho.2009.2715. http://dx.doi.org/10.1089/pho.2009.2715. [DOI] [PubMed] [Google Scholar]
- 45.Rizzi CF, Mauriz JL, Freitas Corrêa DS, Moreira AJ, Zettler CG, Filippin LI, Marroni NP, González-Gallego J. Effects of low-level laser therapy (LLLT) on the nuclear factor (NF)-kappaB signaling pathway in traumatized muscle. Lasers Surg. Med. 2006;38(7):704–713. doi: 10.1002/lsm.20371. [DOI] [PubMed] [Google Scholar]
- 46.Silveira PC, da Silva LA, Pinho CA, De Souza PS, Ronsani MM, Scheffer DL, Pinho RA. Effects of low-level laser therapy (GaAs) in an animal model of muscular damage induced by trauma. Lasers Med. Sci. 2013;28(2):431–436. doi: 10.1007/s10103-012-1075-6. http://dx.doi.org/10.1007/s10103-012-1075-6. [DOI] [PubMed] [Google Scholar]
- 47.Peplow PV, Chung TY, Ryan B, Baxter GD. Laser photobiomodulation of gene expression and release of growth factors and cytokines from cells in culture: a review of human and animal studies. Photomed. Laser Surg. 2011;29(5):285–304. doi: 10.1089/pho.2010.2846. http://dx.doi.org/10.1089/pho.2010.2846. [DOI] [PubMed] [Google Scholar]
- 48.Damante CA, Marques MM. Laser power loss through polystyrene plates for cell culture. Lasers Med. Sci. 2014;29(1):373. doi: 10.1007/s10103-013-1271-z. http://dx.doi.org/10.1007/s10103-013-1271-z. [DOI] [PubMed] [Google Scholar]
- 49.Barnes TC, Anderson ME, Moots RJ. The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int. J. Rheum. Dis. 2011:721608. doi: 10.1155/2011/721608. http://dx.doi.org/10.1155/2011/721608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rossol M, Heine H, Meusch U, Quandt D, Klein C, Sweet MJ, Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011;31(5):379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 51.Palmer CD, Mutch BE, Workman S, McDaid JP, Horwood NJ, Foxwell BM. Bmx tyrosine kinase regulates TLR4-induced IL-6 production in human macrophages independently of p38 MAPK and NFkB activity. Blood. 2008;111(4):1781–1788. doi: 10.1182/blood-2007-07-102343. [DOI] [PubMed] [Google Scholar]
- 52.Chen AC, Arany PR, Huang YY, Tomkinson EM, Sharma SK, Kharkwal GB, Saleem T, Mooney D, Yull FE, Blackwell TS, Hamblin MR. Low-level laser therapy activates NF-kB via generation of reactive oxygen species in mouse embryonic fibroblasts. PLoS One. 2011;6(7):e22453. doi: 10.1371/journal.pone.0022453. http://dx.doi.org/10.1371/journal.pone.0022453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen AC, Huang YY, Sharma SK, Hamblin MR. Effects of 810 nm laser on murine bone-marrow-derived dendritic cells. Photomed. Laser Surg. 2011;29(6):383–389. doi: 10.1089/pho.2010.2837. http://dx.doi.org/10.1089/pho.2010.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen CH, Wang CZ, Wang YH, Liao WT, Chen YJ, Kuo CH, Kuo HF, Hung CH. Effects of low-level laser therapy on M1-related cytokine expression in monocytes via histone modification. Mediat. Inflamm. 2014:625048. doi: 10.1155/2014/625048. http://dx.doi.org/10.1155/2014/625048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Martin CA, Dorf ME. Interleukin-6 production by murine macrophage cell lines P388D1 and J774A.1: stimulation requirements and kinetics. Cell. Immunol. 1990;128(2):555–568. doi: 10.1016/0008-8749(90)90048-v. [DOI] [PubMed] [Google Scholar]