Abstract
Background
Patients with acetabular cartilage defects reported increased pain and disability compared to those without acetabular cartilage defects. The specific effects of acetabular cartilage defects on lower extremity coordination patterns are unclear. The purpose of this study was to determine hip and knee joint coordination variability during gait in those with and without acetabular cartilage defects.
Methods
A combined approach, consisting of a semi-quantitative MRI-based quantification method and vector coding, was used to assess hip and knee joint coordination variability during gait in those with and without acetabular cartilage lesions.
Findings
The coordination variability of the hip flexion-extension/knee rotation, hip abduction-adduction/knee rotation and hip rotation/knee rotation joint couplings were reduced in the acetabular lesion group compared to the control group during loading response of the gait cycle. The lesion group demonstrated increased variability in the hip flexion-extension/knee rotation and hip abduction-adduction/knee rotation joint couplings, compared to the control group, during the terminal stance/pre-swing phase of gait.
Interpretation
Reduced variability during loading response in the lesion group may suggest reduced movement strategies and a possible compensation mechanism for lower extremity instability during this phase of the gait cycle. During terminal stance/pre-swing, a larger variability in the lesion group may suggest increased movement strategies and represent a compensation or pain avoidance mechanism caused by the load applied to the hip joint.
Keywords: Acetabular Cartilage Lesions, Vector Coding, Joint Coordination, Gait, Lower Extremity, MRI
1. Introduction
Altered gait patterns have been observed in individuals with hip osteoarthritis (OA) (Eitzen et al., 2012; Foucher et al., 2012; Hurwitz et al., 1998; Hurwitz et al., 1997; Watelain et al., 2001). Traditional methods of analyzing kinematics (i.e. Euler angles, inverse kinematics, etc.) are widely used in understanding the effects of hip joint OA on gait patterns (Eitzen et al., 2012; Foucher et al., 2012; Hurwitz et al., 1998; Hurwitz et al., 1997; Watelain et al., 2001). Specifically, hip joint sagittal plane range of motion was reduced in those with hip OA when compared to healthy controls (Eitzen et al., 2012; Hurwitz et al., 1998; Hurwitz et al., 1997; Kumar et al., 2015; Zeni et al., 2014). Those with hip OA also demonstrated reduced hip extension in late stance (Eitzen et al., 2012; Watelain et al., 2001; Zeni et al., 2014). The average standard deviation, used to quantify variability during the entire gait cycle, was reduced for hip and knee sagittal plane angles in those with severe hip OA (Kiss, 2010) and may indicate less flexibility (mobility) in movement strategies between the hip and knee joints (Newel and Corcos, 1993). In addition, those with hip OA demonstrated significantly greater hip internal rotation and adduction compared to healthy controls during gait (Watelain et al., 2001).
Although valuable, these traditional kinematic analyses in isolation do not allow for an understanding of the joint coupling mechanisms that are involved in healthy or pathological gait. Vector coding is a simple method of assessing position data and quantifies the variability in the coordinative structures within or between joints or segments during performance of a dynamic task (Sparrow et al., 1987). Modified versions of the vector coding method have been used to study coordination patterns in walking (Chang et al., 2008) and running (Heiderscheit et al., 2002). Vector coding was suggested to be a clinically useful tool that is able to analyze and draw conclusions on joint position data (Miller et al., 2010). In addition, analysis of joint coordination patterns may be more sensitive to subtle changes in motion patterns, compared to traditional joint kinematic analyses (Armour Smith et al., 2014). Joint coordination variability has been studied in gait of healthy individuals (Armour Smith et al., 2014; Barrett et al., 2008; Chang et al., 2008) yet this type of analysis has not been used to understand the gait patterns of those with hip joint OA.
Assessment of hip joint OA using magnetic resonance imaging (MRI) provides significant additional information beyond that provided by standard radiographs, particularly in the early phases of the disease. MRI allows for direct visualization of cartilage and other joint structures that are critical in understanding the OA disease process (Kumar et al., 2013; Morgenroth et al., 2014). Using a semi-quantitative MRI-based quantification method (Lee et al., 2014), it was shown that acetabular cartilage lesions in those with hip OA were associated with greater pain and disability, compared to those with other types of hip joint abnormalities (Kumar et al., 2013). This suggests that acetabular cartilage lesions hold greater clinical significance than other hip joint abnormalities such as femoral cartilage lesions, bone marrow edema and labral tears (Kumar et al., 2013). Despite a greater amount of pain and disability, measures of physical performance did not demonstrate any significant relationships with MRI-based findings and suggests that further analysis is needed to evaluate the relationships between hip MRI abnormalities and hip joint movement patterns (Kumar et al., 2013). Also, weak associations between acetabular cartilage lesions and gait parameters were observed and suggest that a study with a larger sample size is needed to confirm these findings (Kumar et al., 2015). Therefore, it may be possible that traditional planar kinematic analyses may not be sensitive enough to detect changes that may occur with pathologies such as acetabular cartilage lesions.
An analysis combining MRI based determination of acetabular cartilage lesions and coordination variability may help researchers and clinicians to better understand the pathophysiology of hip OA and potentially how this could be modified. Therefore, the purpose of this study was to use vector coding to examine lower extremity hip and knee joint coordination variability during gait between individuals with and without acetabular cartilage lesions. It was hypothesized that individuals with acetabular cartilage lesions would demonstrate decreased joint coordination variability during the stance phase of gait compared to those without acetabular cartilage lesions.
2. Methods
2.1 Participants
These are retrospective analyses from the baseline data of an ongoing longitudinal observational study on hip OA. Participants in this study were recruited from the community using flyers and advertisements. Weight bearing anterior-posterior radiographs of each participant were analyzed using the Kellgren-Lawrence (KL) score (Kellgren and Lawrence, 1957) and a KL score was determined for each hip joint. The hip joint with the higher KL score was selected as the “index” hip and was used for testing in this study. Participants were classified as those with mild-moderate radiographic hip OA (KL grade 2 or 3) and without radiographic hip OA (KL grade 0 or 1). A total of 93 participants were recruited for this study. Since previous studies using vector coding to analyze the effects of radiographic hip OA have not been performed, previously published data on hip sagittal plane excursion in those with (KL grade 2 or 3) and without (KL grade 0 or 1) radiographic hip OA (Kumar et al., 2015) was used as inputs into a power analysis. The power analysis performed in the current study used an alpha and a power of 0.05 and 95%, respectively. The power analysis indicated a minimum of 38 participants per group were needed. Participants were excluded if they presented with any of the following: contraindication to MRI imaging, hip KL grade greater than 3, total joint replacement of any lower extremity joint, previous hip joint trauma, pain at any other lower extremity joint, any spine or lower extremity conditions that would limit or affect their ability to perform the dynamic tasks of the data collection and any radiographic indications of knee or ankle joint OA. All participants provided informed consent prior to participation in the study. This study was approved by the university committee on human research.
In addition, each participant's self-reported measure of pain and activities of daily living (ADL) were assessed using the Hip disability and Osteoarthritis Outcome Score (HOOS) (Nilsdotter et al., 2003). The HOOS was demonstrated to be a reliable and valid measure of overall hip joint function in people with OA (Nilsdotter et al., 2003). These scores were based on a scale of 0 to 100, with 0 representing worse hip pain or function and 100 representing no hip pain or function issues.
2.2 MRI Acquisition and Analysis
MRI acquisition was performed using a 3 Tesla MRI scanner (GE MR750, GE Healthcare, Waukesha, WI, USA) and an eight channel cardiac coil (GE Healthcare, Waukesha, WI, USA). Participants were positioned supine and immobilized using straps in order to ensure a consistent and comfortable position (Supplementary Figure 1). The participants’ feet were stabilized in order to prevent any type of movement during the scan. The imaging parameters and protocol used in this study to obtain MR images of the hip joint have been previously described (Kumar et al., 2013; Lee et al., 2014) and are explained here briefly. Sagittal, oblique coronal and oblique axial orientated images using an intermediate-weighted, fat-suppressed, fast spin-echo (FSE) sequence were obtained with a repetition time (TR) of 2400-3700ms, echo time (TE) of 60ms, field of view of 14 – 20cm, matrix size of 288×224 and slice thickness of 3 – 4mm.
Using the semi-quantitative MRI-based SHOMRI quantification method (Lee et al., 2014), the acetabular cartilage layer was divided into 4 sub-regions and scored using a 3-point scale where a score of 0, 1 and 2 indicate no loss, partial thickness loss and full thickness loss, respectively. The four sub-regions of the acetabulum included the anterior, posterior, superomedial and superolateral portions of the acetabulum cartilage layer (supplementary figure 2). The anterior and posterior regions of the acetabulum were defined as the anterior and posterior 1cm of the femoral head using the sagittal plane images. The mid-portion of the acetabular region was defined using the sagittal plane image and divided into a superolateral and superomedial region, moving from the lateral to medial direction, using the coronal plane images. The anterior and posterior acetabulum regions were scored on the sagittal plane images while the superolateral and superomedial regions were scored using the coronal plane images. Participants with an acetabular cartilage score of greater than 0 in any of the 4 acetabular sub-regions was considered to have an acetabular cartilage lesion and were included into the acetabular lesion group (LG) while participants with no acetabular cartilage lesions were included into the non-acetabular lesion group (NG).
2.3 Gait Data Acquisition and Processing
Three dimensional position data were collected at 250Hz using a 10-camera motion capture system (VICON, Oxford, UK) and kinetic data were collected synchronously at 1000Hz using force platforms (AMTI, Watertown, MA, USA). A marker set consisting of 41 retro-reflective markers was used to collect three dimensional position data (Kumar et al., 2014; Kumar et al., 2015). Specifically, fifteen retro-reflective markers placed at anatomical landmarks on both lower extremities were used to define joint centers. Ten of the fifteen markers were used for identification of joint centers and were placed at the greater trochanters, medial and lateral femoral epicondyles, medial and lateral malleoli, the first and fifth metatarsal heads. The remaining five markers were used to track pelvis motion and were placed at the anterior superior iliac spines, iliac crests and at the L5/S1 joint. Additional segment tracking was performed using marker clusters, each consisting of four retro-reflective markers, placed on the lateral thighs and shanks. A cluster of three retro-reflective markers placed on the heel show counters and a marker on the fifth metatarsal head were used for tracking of the foot. After a standing calibration trial was performed, the calibration markers were removed from the participant.
Each participant was asked to perform 4 successful gait trials at a controlled speed of 1.35 m·s−1. This speed was chosen as it is the mean of the average walking speeds of female and male adults on a smooth surface (Perry and Burnfield, 2010). A trial was considered successful if the participant's entire foot made a clean strike on one of the two force plates and the participant maintained the prescribed speed within 5% (0.7 m·s−1) of the controlled speed. All raw marker position and ground reaction force data were filtered using a 4th order, Butterworth filter at 6Hz and 50Hz, respectively. The standing calibration trial was used to create a seven segment (i.e. pelvis, bilateral thighs, shanks and feet) kinematic model in Visual3D (v5.00.16, C-Motion, Germantown, MD, USA). Local joint coordinate systems were created and an unweighted least squares method (Spoor and Veldpaus, 1980) was used to describe position and orientation of each segment. Joint rotations were solved using a Cardan sequence of X-Y’-Z”, representing the medial-lateral, anterior-posterior and superior-inferior directions, respectively. Joint angles were normalized to the standing calibration trial. Initial contact was defined when the foot struck the force plate and the vertical ground reaction force exceeded a 20N threshold. The stance phase of gait was defined as initial contact to toe-off.
2.4 Vector Coding Analysis
A vector coding technique (Chang et al., 2008; Hamill et al., 2000; Heiderscheit et al., 2002; Sparrow et al., 1987), was used to analyze hip and knee joint coordination variability as an effect of acetabular cartilage lesions. A custom written MATLAB (The Mathworks, Natick, MA, USA) program was used to perform the vector coding calculations. The vector coding algorithm provided a coupling angle, which is a quantitative description of the coordinative patterns between two joints (i.e. hip and knee). The coupling angle, reported in degrees (0 - 360°), is calculated as the angle from the horizontal axis of a vector connecting two successive time points of the stance phase and is repeated for each trial being analyzed (Eq. 1). The coupling angles calculated for each trial are directional in nature and therefore, circular statistics (Batschelet, 1981) were used to calculate the mean coupling angle. Nine pairs of joint couplings were chosen for the analysis in this study and include: hip flexion-extension/knee flexion-extension, hip flexion-extension/knee abduction-adduction, hip flexion-extension/knee rotation, hip abduction-adduction/knee flexion-extension, hip abduction-adduction/knee abduction-adduction, hip abduction-adduction/knee rotation, hip rotation/knee flexion-extension, hip rotation/knee abduction-adduction and hip rotation/knee rotation. These couplings were chosen as many studies have demonstrated hip and knee sagittal plane differences as an effect of hip OA (Eitzen et al., 2012; Hurwitz et al., 1998; Hurwitz et al., 1997; Kiss, 2010; Watelain et al., 2001; Zeni et al., 2014) yet only a few of these studies analyzed frontal and transverse plane rotations of the hip (Watelain et al., 2001; Zeni et al., 2014).
| (1) |
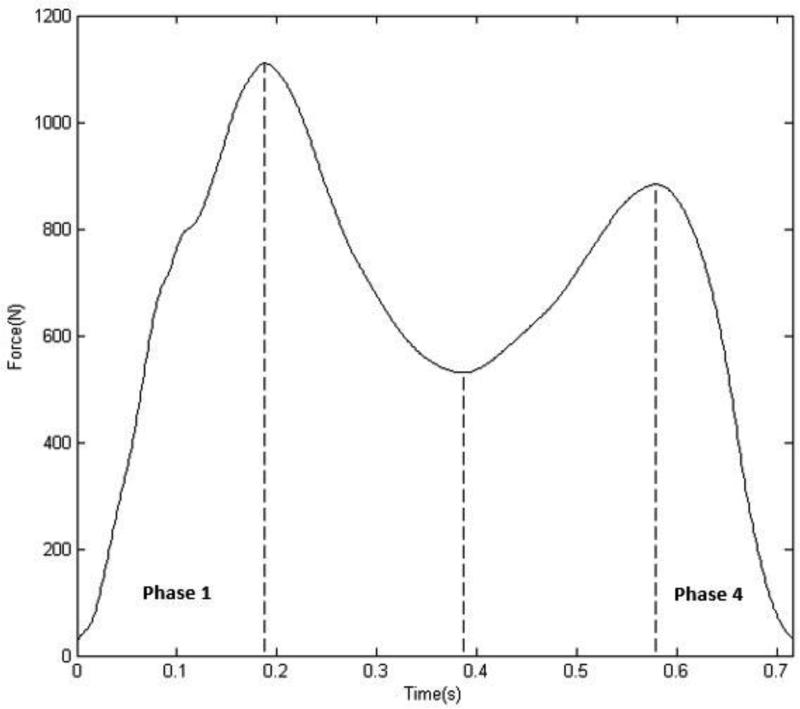
Therefore, analysis of coordination variability was performed during pre-determined phases of the entire stance phase of gait as has been done in previous work (Heiderscheit et al., 2002). The vertical ground reaction force (GRF) profile was used to divide the stance phase of gait into four phases (Fig. 1). The four phases were defined using three discrete time points of the vertical GRF profile. These time points were the first peak vertical GRF (P1), the minimum vertical GRF (M1) after P1 and the second peak vertical GRF (P2). Phases 1 – 4 consisted of the time points between initial contact and P1, P1 and M1, M1 and P2, P2 and toe-off, respectively. For this study, the loading response (phase 1) and terminal stance/pre-swing (phase 4) phases of the gait cycle were analyzed as previous research has demonstrated differences in hip joint kinematics due to hip OA during these phases of the gait cycle (Eitzen et al., 2012; Hurwitz et al., 1997; Watelain et al., 2001; Zeni et al., 2014). In addition, phases 1 and 4 represent subdivisions of the stance phase that reflect joint loading patterns during walking (Chang et al., 2008). All four gait trials from each participant were used in the vector coding analysis. A minimum of 3 trials was suggested to provide valid data in various biomechanical analyses (Mullineaux et al., 2001) and therefore, 4 trials should provide sufficiently accurate data for use in this study. Hip and knee joint kinematic data, within the loading response and terminal stance phases, were time normalized to 101 points and used as inputs into the vector coding algorithm. The average standard deviation of each joint coupling angle was determined and used as a measure of the between trial, within participant variability (Heiderscheit et al., 2002). This process was repeated for each participant during both phases 1 and 4.
Fig. 1.
A representative example of a participant's vertical ground reaction force (GRF) profile during gait. The vertical lines (left to right) indicate the location of the first vertical GRF peak (P1), the minimum vertical GRF (M1) and the second vertical GRF peak (P2), which were used to divide the entire stance phase into four discrete regions. Phases 1 – 4 were defined as the time points between initial contact to P1, P1 to M1, M1 to P2 and P2 to toe-off, respectively.
2.5 Statistical Analysis
Independent t-tests were performed in order to determine group differences of age, height, mass, BMI and HOOS scores, where significance was set a priori at the 0.05 level. Mean variability (degrees) for all of the hip and knee joint couplings in the loading response and terminal stance/pre-swing phases were analyzed using an analysis of covariance (ANCOVA) with a covariate of age. Significance level of the ANCOVA was set a priori at the 0.05 level. All statistical analyses were performed using SPSS statistics (v21, IBM Corporation, Armonk, NY, USA).
In addition, the minimum, maximum and range of motion of each degree of freedom during phases 1 and 4 were assessed using an ANCOVA with a covariate of age. This analysis was performed in order to determine whether or not traditional kinematic analysis would provide information on the effects of acetabular cartilage lesions on walking during phases 1 and 4.
3. Results
Participants with acetabular lesions were significantly older (p < 0.001) than the participants in the control group (Table 1). In addition, participants with acetabular lesions demonstrated significantly worse self-reported pain and increased limitations in the ADL.
Table 1.
The mean and standard deviation of the demographics, pain and activities of daily living (ADL) scores of the non-acetabulum (NG) and acetabulum cartilage lesions (LG) groups, where an * indicates statistically significant differences (p<0.05). In addition, the percentage of participants with a particular Kellgren-Lawrence (KL) score of the tested limb are listed.
| NG (n=54) | LG (n=39) | p-value | |
|---|---|---|---|
| Age (years)* | 40.7(13.1) | 50.8(11.4) | <0.001 |
| Mass (kg) | 67.1(12.4) | 71.7(14.1) | 0.10 |
| Height (m) | 1.69(0.10) | 1.71(0.12) | 0.514 |
| BMI (kg/m2) | 23.4(3.09) | 24.5(3.05) | 0.09 |
| HOOS (Pain)* | 93.7(12.3) | 84.8(20.8) | 0.01 |
| HOOS (ADL)* | 96.3(9.71) | 88.6(18.8) | 0.02 |
| KL < 2 | 82% | 46% | |
| KL ≥ 2 | 18% | 54% |
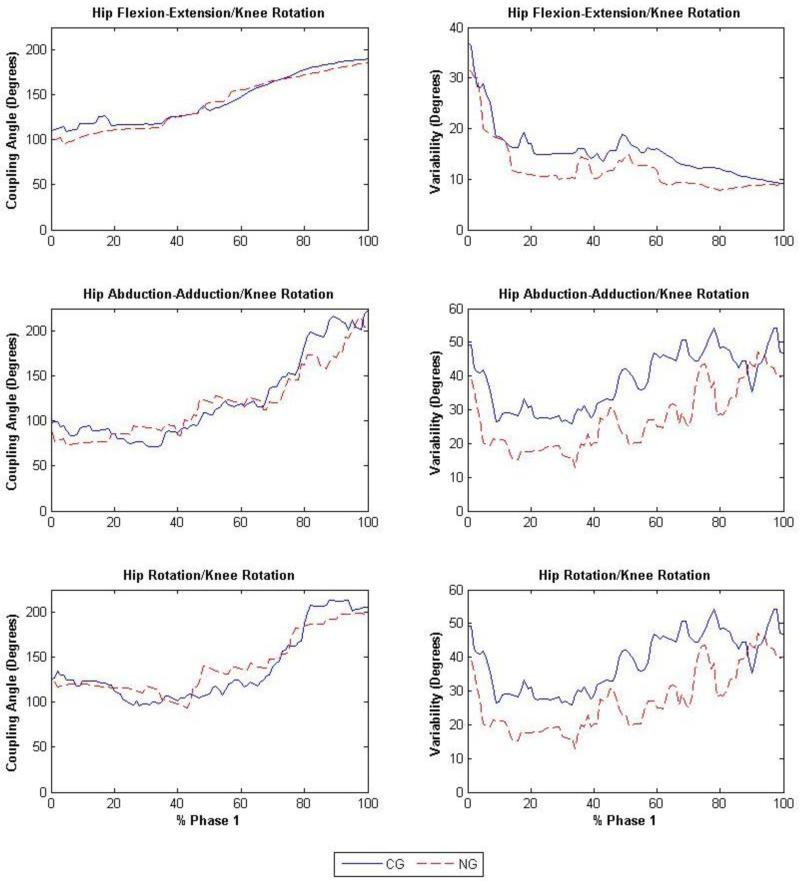
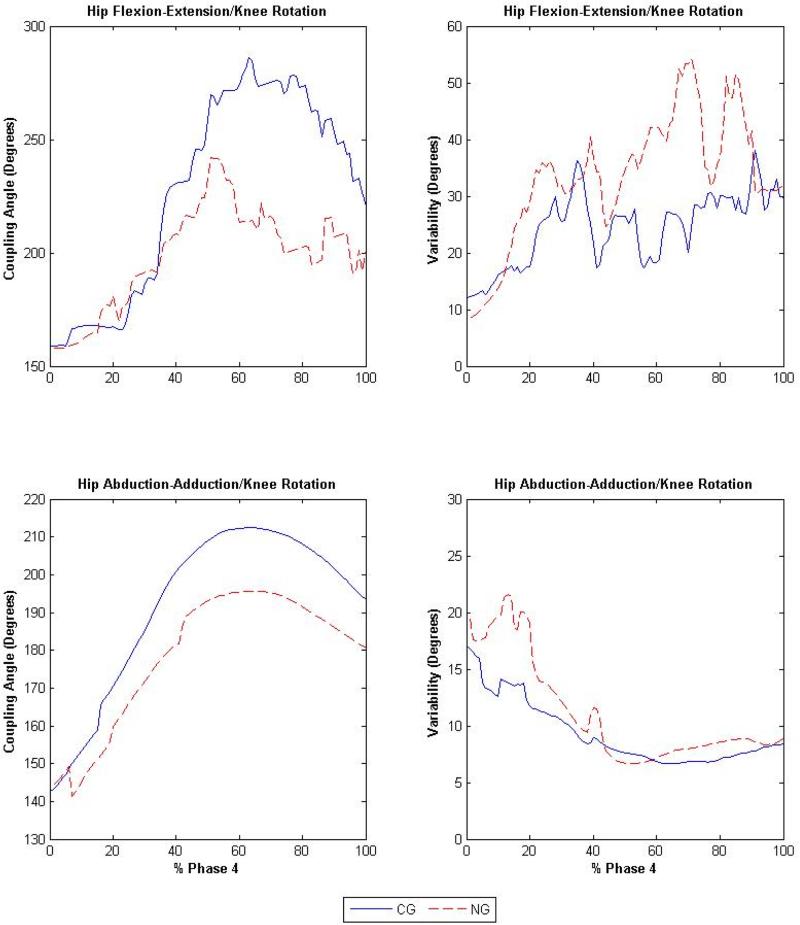
Significant differences were observed for hip and knee joint couplings in phases 1 and 4 between participants with and without acetabular cartilage lesions (Table 2). During phase 1, the LG demonstrated a decrease of 3.5° (F1,91 = 4.09, p = 0.04), 11.5° (F1,91 = 4.75, p = 0.03) and 11.5° (F1,91 = 6.92, p = 0.01) in variability, compared to the NG (Fig. 2), in the hip flexion-extension/knee rotation, hip abduction-adduction/knee rotation and hip rotation/knee rotation joint couplings, respectively. During phase 4, variability in the LG increased, compared to the NG (Fig. 3), by 9.9° (F1,91 = 7.41, p < 0.01) and 1.75° (F1,91 = 4.14, p = 0.04) in the hip flexion-extension/knee rotation and hip abduction-adduction/knee rotation joint couplings, respectively. The angle-angle plots of the hip flexion-extension/knee rotation, hip abduction-adduction/knee rotation, and hip rotation/knee rotation joint couplings can be found in figure 4.
Table 2.
Hip and knee joint coordination variability in degrees, mean(standard deviation), for the non-acetabulum (NG) and acetabular cartilage lesions (LG) groups. Statistically significant differences (p < 0.05) between joint couplings are indicated by an *.
| Joint Coupling | Phase 1 | Phase 4 | ||||
|---|---|---|---|---|---|---|
| NG | LG | P-Value | NG | LG | P-Value | |
| Hip flexion-extension/Knee flexion-extension | 6.05(3.19) | 6.69(6.34) | 0.46 | 2.19(4.09) | 2.57(3.61) | 0.32 |
| Hip flexion-extension/Knee abduction-adduction | 14.8(9.26) | 16.7(11.5) | 0.45 | 30.8(28.0) | 24.5(25.2) | 0.36 |
| Hip flexion-extension/Knee rotation | 15.5(10.4)* | 12.0(7.30)* | 0.04 | 24.4(16.2)* | 34.3(24.4)* | <0.01 |
| Hip abduction-adduction/Knee flexion-extension | 11.2(8.63) | 9.99(6.05) | 0.51 | 1.94(5.26) | 1.93(3.50) | 0.56 |
| Hip abduction-adduction/Knee abduction-adduction | 40.8(26.8) | 40.2(26.5) | 0.99 | 6.65(3.83) | 7.44(7.28) | 0.57 |
| Hip abduction-adduction/Knee rotation | 38.7(28.3)* | 27.2(21.9)* | 0.03 | 9.35(5.87)* | 11.1(7.82)* | 0.04 |
| Hip rotation/Knee flexion-extension | 11.1(5.58) | 11.9(7.05) | 0.84 | 2.59(4.69) | 3.25(4.89) | 0.21 |
| Hip rotation/Knee abduction-adduction | 33.2(22.7) | 27.8(15.0) | 0.42 | 10.5(8.38) | 11.2(9.49) | 0.95 |
| Hip rotation/Knee rotation | 37.2(26.8)* | 25.7(17.2)* | 0.01 | 11.0(6.35) | 11.2(7.82) | 0.69 |
Fig. 2.
Coupling angles (degrees) of the hip flexion-extension/knee rotation (top left), hip abduction-adduction/knee rotation (middle left) and hip rotation/knee rotation (bottom left) and the variability (degrees) of the hip flexion-extension/knee rotation (top right), hip abduction-adduction/knee rotation (middle right) and hip rotation/knee rotation (bottom right) joint couplings during phase 1, where the solid and dashed lines represent the non-acetabular lesion group (NG) and the acetabular lesion group (LG), respectively.
Fig. 3.
Coupling angles (degrees) of the hip flexion-extension/knee rotation (top left) and hip abduction-adduction/knee rotation (bottom left) and the variability (degrees) of the hip flexion-extension/knee rotation (top right) and hip abduction-adduction/knee rotation (bottom right) joint couplings during phase 4, where the solid and dashed lines represent the non-acetabular lesion group (NG) and the acetabular lesion group (LG), respectively.
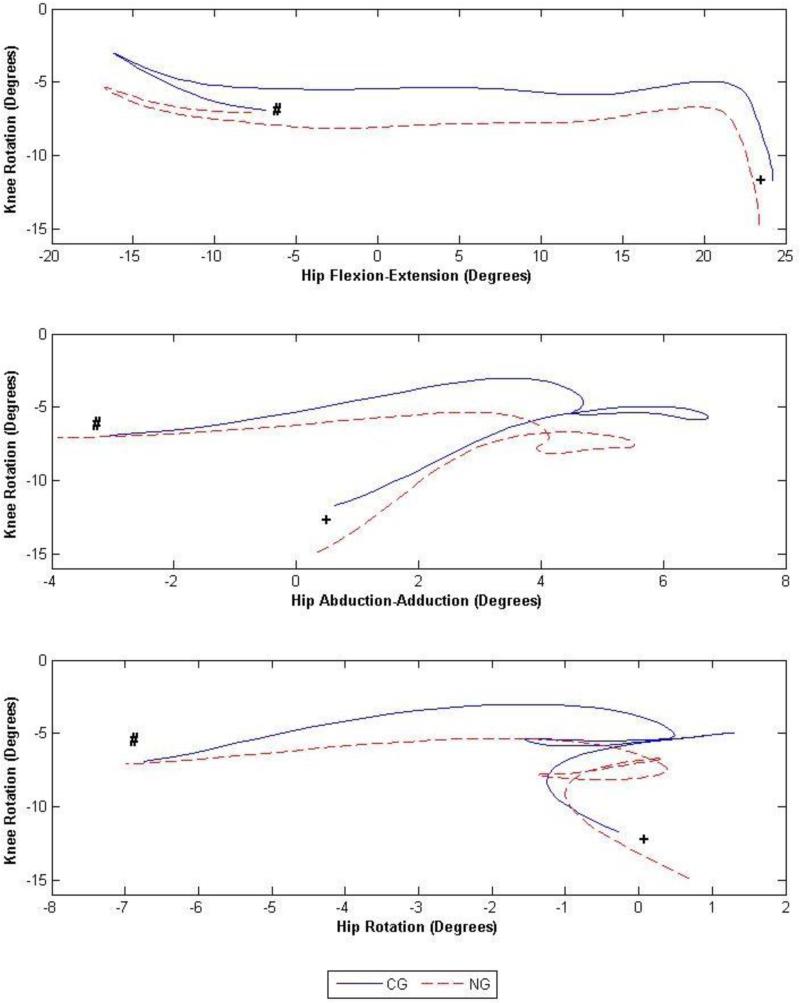
Fig. 4.
Angle-angle plots (degrees) of the hip flexion-extension/knee rotation (top left), hip abduction-adduction/knee rotation (top right) and hip rotation/knee rotation (bottom) joint couplings during the entire stance phase, where the solid and dashed lines represent the non-acetabular lesion group (NG) and the acetabular lesion group (LG), respectively. Initial contact and toe-off are indicated with a + and a #, respectively.
The minimum, maximum and range of motion of each degree of freedom during phases 1 and 4 are displayed in table 3. During phase 1, significant differences in minimum knee rotation (F1,91 = 5.41, p = 0.02), range of motion in the knee sagittal (F1,91 = 5.40, p = 0.02) and transverse planes (F1,91 = 4.85, p = 0.03) exist between the NG and LG. No differences exist in lower extremity joint kinematics during phase 4.
Table 3.
Hip and knee joint kinematics minimum (Min.), maximum (Max.) and range of motion (ROM) in degrees, mean(standard deviation), for the non-acetabulum (NG) and acetabular cartilage lesion (LG) groups. Statistically significant differences (p < 0.05) in the kinematic measures are indicated by an *.
| Phase 1 | Phase 4 | |||||
|---|---|---|---|---|---|---|
| Min. | Max. | ROM | Min. | Max. | ROM | |
| Hip flexion(+)/extension(−) | ||||||
| NG | 18.1(6.85) | 24.6(6.49) | 6.44(2.65) | −16.3(7.27) | −6.83(7.07) | 9.49(2.16) |
| LG | 16.8(7.45) | 23.7(7.81) | 6.90(2.72) | −16.9(8.10) | −7.71(7.70) | 9.23(2.34) |
| p-value | 0.26 | 0.52 | 0.19 | 0.76 | 0.48 | 0.19 |
| Hip abduction(−)/adduction(+) | ||||||
| NG | 0.62(2.89) | 6.39(3.29) | 5.78(1.69) | −3.04(2.79) | −4.34(2.21) | 7.39(2.10) |
| LG | 0.27(2.46) | 5.27(3.24) | 5.00(1.97) | −3.90(2.69) | −3.94(2.75) | 7.84(2.42) |
| p-value | 0.77 | 0.22 | 0.08 | 0.15 | 0.49 | 0.31 |
| Hip internal(+)/external(−) rotation | ||||||
| NG | −2.57(4.22) | 2.74(4.53) | 5.32(2.48) | −6.84(5.03) | −0.14(4.96) | 6.70(2.83) |
| LG | −2.73(5.56) | 3.18(5.24) | 5.91(3.68) | −7.08(5.93) | −0.25(4.89) | 6.82(3.29) |
| p-value | 0.46 | 0.26 | 0.52 | 0.94 | 0.96 | 0.84 |
| Knee flexion(−)/extension(+) | ||||||
| NG | −3.34(3.86) | −18.3(4.56) | 14.9(3.77)* | −8.17(4.39) | −44.9(5.32) | 36.8(4.38) |
| LG | −2.69(4.45) | −19.1(5.43) | 16.4(3.20)* | −7.13(4.62) | −45.1(4.38) | 38.0(4.57) |
| p-value | 0.53 | 0.22 | 0.02 | 0.40 | 0.87 | 0.50 |
| Knee abduction(−)/adduction(+) | ||||||
| NG | −1.74(3.06) | 0.82(3.31) | 2.56(1.39) | −3.41(3.54) | −0.12(3.33) | 3.29(2.16) |
| LG | −0.94(3.34) | 2.03(3.20) | 2.97(1.48) | −2.72(3.69) | 1.14(3.97) | 3.87(2.54) |
| p-value | 0.31 | 0.11 | 0.17 | 0.45 | 0.18 | 0.38 |
| Knee internal(+)/external(−) rotation | ||||||
| NG | −12.0(6.39)* | −4.04(4.93) | 7.97(3.97)* | −7.92(5.65) | −2.21(6.11) | 5.71(2.79) |
| LG | −15.5(5.63)* | −5.54(5.08) | 9.99(4.07)* | −8.72(5.67) | −4.01(5.66) | 4.70(2.43) |
| p-value | 0.02 | 0.29 | 0.03 | 0.96 | 0.48 | 0.15 |
4. Discussion
In this study, participants with acetabular cartilage defects, determined using a semi-quantitative MRI-based quantification method (Lee et al., 2014), were found to exhibit altered hip and knee joint coordination patterns during the loading response and terminal stance/pre-swing phases of the gait cycle. During loading response (phase 1), those with acetabular lesions demonstrated decreased joint coordination variability, compared to those without acetabular lesions and may suggest that those with acetabular lesions are limited in their movement strategies during this phase of the gait cycle. In terminal stance/pre-swing (phase 4), those with acetabular lesions demonstrated increased joint coordination variability compared to those without acetabular lesions, which may suggest increased movement strategies in order to compensate for the increased disability that those with acetabular lesions possess.
We observed that during early stance phase walking (phase 1), participants with acetabular cartilage lesions had decreased variability in the hip flexion-extension/knee rotation, hip abduction-adduction/knee rotation and hip rotation/knee rotation joint couplings. Previous studies have shown that participants with hip OA demonstrated altered kinematic patterns in the hip sagittal (Eitzen et al., 2012; Hurwitz et al., 1998; Hurwitz et al., 1997; Watelain et al., 2001; Zeni et al., 2014), frontal and transverse planes (Eitzen et al., 2012; Watelain et al., 2001). The loads experienced by the lower extremities are greatest during heel strike when the lower limb is near full extension (Schipplein and Andriacchi, 1991) and may cause those with acetabular cartilage lesions to modify their hip and knee joint coordination patterns during this phase of gait, in an attempt to provide increased shock absorption. Those with hip OA demonstrated altered gait patterns compared to a healthy population and these altered gait patterns were found to be related to pain (Hurwitz et al., 1997). The LG experience increased pain compared to the NG and the increased levels of pain may be associated with the altered hip and knee joint coordination patterns during phase 1, similar to those with hip osteoarthritis (Hurwitz et al., 1997). In addition, there may be a rotational misalignment of the knee joint near heel strike indicating a change in the normal load bearing contact region of the knee joint (Andriacchi and Mündermann, 2006). This rotational misalignment at the knee may cause altered movement patterns in the hip frontal and transverse planes. The differences in knee rotation kinematics between the NG and LG groups, during this phase, may support the notion that there is a rotational misalignment of the knee joint present during phase 1. Whether or not this misalignment in the knee joint is a result of the acetabular cartilage lesions, increased levels of pain or a diminished ability to properly react to the high loads at heel strike should be further evaluated. The differences in knee transverse plane kinematics between the NG and LG, may help explain the differences in the joint coordination patterns present during early stance phase of walking.
Similar to a previous study (Kumar et al., 2013), participants with acetabular lesions in the current study demonstrated worse pain and disability compared to those participants without acetabular cartilage lesions. The reduced coordination variability in the acetabular lesion group during the loading response phase may indicate a pain avoidance mechanism. Hence, it may be possible that the participants with acetabular cartilage lesions exhibit reduced coordination variability during phase 1, which may indicate less flexibility of the lower extremity (Newel and Corcos, 1993), as well as a decreased ability to compensate for the load placed on the hip joint and therefore, cause repetitive stress exposure to the hip joint and may lead to an increased risk of further injury and pain. In addition, reduced coordination variability in the hip abduction-adduction/knee rotation and hip rotation/knee rotation couplings may also be related to altered joint excursions (Eitzen et al., 2012; Hurwitz et al., 1998; Hurwitz et al., 1997; Zeni et al., 2014). The LG demonstrated an increased range of motion in the knee transverse plane which may be related to a possible rotational misalignment of the knee joint. The reduced coordination variability in participants with acetabular lesions may suggest reduced movement strategies during the loading response phase yet this should be systematically investigated (i.e. electromyography, etc.).
In late stance (phase 4), the LG demonstrated increased variability in the hip flexion-extension/knee rotation and hip abduction-adduction/knee rotation joint couplings, compared to the NG. During this phase, the hip joint undergoes flexion in order to prepare for the swing phase of gait. Earlier studies demonstrated that during the last 50% of the stance phase, hip and knee excursions were significantly reduced in people with hip OA (Eitzen et al., 2012) and those with hip OA demonstrated larger amounts of hip flexion compared to healthy controls at toe-off (Watelain et al., 2001). In addition, those with hip OA demonstrated reduced variability in hip sagittal plane angles throughout the entire stance phase of gait (Kiss, 2010) and may suggest that hip sagittal plane angles in the LG, compared to the NG, may also be altered during phase 4. Previous reports have suggested that hip extension range of motion is significantly associated with self-reported disability in those with hip OA (Steultjens et al., 2000). In the current study, the LG participants reported significantly increased disability compared to the NG yet no differences in kinematics exist during phase 4. Unlike those with hip OA, who demonstrated differences in hip abduction at toe-off compared to a control group (Watelain et al., 2001), a difference in hip frontal plane kinematics between the LG and NG group does not exist during phase 4, yet the LG group demonstrated significantly higher variability in the hip abduction-adduction/knee rotation joint coupling. The lack of planar kinematic differences in phase 4 may suggest that the altered hip and knee joint couplings may be indicative of the increased disability and pain in those with acetabular cartilage lesions compared to those without acetabular cartilage lesions. Increased coordination variability in the hip and knee joint couplings in participants with acetabular lesions compared to those without acetabular cartilage lesions may be evidence of dynamic instability or ineffective motor control strategies during this phase. Those with acetabular cartilage lesions may be using a larger amount of movement strategies during phase 4, in order to overcome their self-reported disability, yet the controlling mechanisms (i.e. muscle activations) that may cause these increased movement strategies were not quantified in the current study. The lack of kinematic differences between the NG and LG, during phase 4, may help support the notion that vector coding is a more sensitive measure to detect altered motion patterns compared to planar kinematic analyses (Armour Smith et al., 2014).
Limitations of this study exist in the methods used to classify the control and symptomatic groups. In this study, patients with acetabular cartilage lesions were compared to those without acetabular cartilage lesions. We did not account for other types of hip pathology such as femoral cartilage lesions, labrum tears, etc. Therefore, it is possible that the non-acetabular lesion group includes participants with other hip pathologies and the effects of these pathologies on joint coordination variability should be explored in the future. In addition, future studies should use participants with a KL grade of less than two in order to create a control group and to assess the effects of acetabular lesions on joint coordination variability, compared to this control group. Although many of the participants in this study presented with largely unilateral hip OA, some of the participants possess bilateral hip OA (~11% in the NG and 35% in the LG) and therefore, caution should be taken when interpreting the results of this study. The effects of bilateral hip OA were not explored in this study yet it is an important factor when analyzing gait, as bilateral hip OA can affect gait patterns. In this study, approximately 36% of the acetabular lesion group demonstrated bilateral hip OA yet the effects of bilateral hip OA on joint coordination variability during gait are unknown. Future studies should explore the effects of bilateral hip OA on joint coordination variability during gait. Another limitation exists in the experimental methods used to perform the gait task required of this study. In this study, all participants walked at a fixed pace yet those with hip OA, when asked to walk at a self-selected pace, demonstrated significant differences in speed when compared to healthy controls (Eitzen et al., 2012; Foucher et al., 2012; Watelain et al., 2001). Also, walking speed had an effect on various gait parameters in those with hip OA when compared to healthy controls (Kiss, 2010). The use of a fixed walking speed reduces the concern of differences in speed between groups yet may contribute to the altered joint coordination patterns presented in this study.
In conclusion, an MRI-based semi-quantitative scoring method (Lee et al., 2014) was used to distinguish those with and without acetabular cartilage lesions, in order to understand the effects of this pathology on hip and knee joint coordination variability during gait. Participants with acetabular cartilage defects, exhibited altered hip and knee joint coordination patterns during the loading response and terminal stance/pre-swing phases of the gait cycle. Those with acetabular lesions demonstrated reduced joint coordination variability during the loading response phase of gait and may indicate a pain avoidance mechanism or an inability to properly react to high loads during this phase of gait. On the other hand, during terminal stance/pre-swing (phase 4), the increased joint coordination variability may be indicative of increased movement strategies used to compensate for increased disability in those with acetabular lesions. In addition, these differences in movement variability may indicate underlying deficits in motor control patterns that could be related to future progression of hip cartilage degeneration. The analysis performed in the current study provided knowledge on the effects of acetabular cartilage lesions on knee joint kinematics, as knee rotation was a component in all of the significant joint couplings within early and late stance phase. Hence, vector coding analyses could be a useful technique to investigate gait mechanics at both the hip and knee joints in people with hip joint pathology. The information provided from vector coding may help clinicians develop better treatments and rehabilitation protocols for those with hip joint pathology. In addition, future longitudinal studies should be performed to assess if these differences in coordination variability are associated with progression of hip OA.
Supplementary Material
Highlights.
This study reports on joint coordination in those with acetabular cartilage lesions
Vector coding was used to analyze hip and knee joint coordination variability
People with acetabular cartilage lesions exhibit altered joint coordination variability
Vector coding may be a useful method in analyzing hip joint pathologies during gait
Acknowledgements
This study was supported by NIH-NIAMS P50 AR060752. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None of the authors have any financial or personal conflicts of interest that could potentially bias the work and conclusions presented in this manuscript.
References
- Andriacchi TP, Mündermann A. The role of ambulatory mechanics in the initiation and progression of knee osteoarthritis. Curr. Opin. Rheumatol. 2006;18:514–518. doi: 10.1097/01.bor.0000240365.16842.4e. [DOI] [PubMed] [Google Scholar]
- Armour Smith J, Popovich JM, Jr., Kulig K. The influence of hip strength on lower-limb, pelvis, and trunk kinematics and coordination patterns during walking and hopping healthy women. J. Orthop. Sports Phys. Ther. 2014;44:525–531. doi: 10.2519/jospt.2014.5028. [DOI] [PubMed] [Google Scholar]
- Barrett R, Vonk Noordegraaf M, Morrison S. Gender differences in the variability of lower extremity kinematics during treadmill locomotion. J. Motor Behav. 2008;40:62–70. doi: 10.3200/JMBR.40.1.62-70. [DOI] [PubMed] [Google Scholar]
- Batschelet E. Circular statistics in biology. Academic Press; London: 1981. [Google Scholar]
- Chang R, Van Emmerik R, Hamill J. Quantifying rearfoot–forefoot coordination in human walking. J. Biomech. 2008;41:3101–3105. doi: 10.1016/j.jbiomech.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Eitzen I, Fernandes L, Nordsletten L, Risberg MA. Sagittal plane gait characteristics in hip osteoarthritis patients with mild to moderate symptoms compated to healthy controls: A cross-sectional study. BMC Musculoskelet. Disord. 2012;13:258–270. doi: 10.1186/1471-2474-13-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher KC, Schlink BR, Shakoor N, Wimmer MA. Sagittal plane hip motion reversals during walking are associated with disease severity and poorer function in subjects with hip osteoarthritis. J. Biomech. 2012;45:1360–1365. doi: 10.1016/j.jbiomech.2012.03.008. [DOI] [PubMed] [Google Scholar]
- Hamill J, Haddad JM, McDermott WJ. Issues in quantifying variability from a dynamical systems perspective. J. Appl. Biomech. 2000;16:407–418. [Google Scholar]
- Heiderscheit BC, Hamill J, Van Emmerik EA. Variability of stride characterisitics and joint coordination among individuals with unilateral patellofemoral pain. J. Appl. Biomech. 2002;18:110–121. [Google Scholar]
- Hurwitz DE, Foucher KC, Sumner DR, Andriacchi TP, Rosenberg AG, Galante JO. Hip motion and moments during gait relate directly to proximal femoral bone mineral density in patients with hip osteoarthritis. J. Biomech. 1998;31:919–925. doi: 10.1016/s0021-9290(98)00097-9. [DOI] [PubMed] [Google Scholar]
- Hurwitz DE, Hulet CH, Andriacchi TP, Rosenberg AG, Galante JO. Gait compensations in patients with osteoarthritis of the hip and their relationship to pain and passive hip motion. J. Orthop. Res. 1997;15:629–635. doi: 10.1002/jor.1100150421. [DOI] [PubMed] [Google Scholar]
- Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Annals of the Rheumatic Diseases. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss RM. Effect of walking speed and severity of hip osteoarthritis on gait variability. J. Electromyogr. Kinesiol. 2010;20:1044–1051. doi: 10.1016/j.jelekin.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Kumar D, Dillon A, Nardo L, Link TM, Majumdar S, Souza RB. Differences in the association of hip cartilage lesions and cam-type femoroacetabular impingement with movement patterns: A preliminary study. PM&R. 2014;6:681–689. doi: 10.1016/j.pmrj.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Wyatt C, Chiba K, Lee S, Nardo L, Link TM, Majumdar S, Souza RB. Anatomic correlates of reduced hip extension during walking in individuals with mild-moderate radiographic hip osteoarthritis. J Orthop Res. 2015;33:527–534. doi: 10.1002/jor.22781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar D, Wyatt CR, Lee S, Nardo L, Link TM, Majumdar S, Souza RB. Association of cartilage defects, and other mri findings with pain and function in individuals with mild–moderate radiographic hip osteoarthritis and controls. Osteoarthr. Cartil. 2013;21:1685–1692. doi: 10.1016/j.joca.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Nardo L, Kumar D, Wyatt CR, Souza RB, Lynch J, McCulloch CE, Majumdar S, Lane NE, Link TM. Scoring hip osteoarthritis with mri (SHOMRI): A whole joint osteoarthritis evaluation system. J. Magn. Reson. Im. 2014 doi: 10.1002/jmri.24722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RH, Chang R, Baird JL, Van Emmerik REA, Hamill J. Variability in kinematic coupling assessed by vector coding and continuous relative phase. J. Biomech. 2010;43:2554–2560. doi: 10.1016/j.jbiomech.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Morgenroth DC, Medverd JR, Seyedali M, Czerniecki JM. The relationship between knee joint loading rate during walking and degenerative changes on magnetic resonance imaging. Clin. Biomech. 2014;29:664–670. doi: 10.1016/j.clinbiomech.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullineaux DR, Bartlett RM, Bennett S. Research design and statistics in biomechanics and motor control. J Sport Sci. 2001;19:739–760. doi: 10.1080/026404101317015410. [DOI] [PubMed] [Google Scholar]
- Newel K, Corcos D. Issues in variability and motor control. Human Kinetics Publishers; Champaign: 1993. [Google Scholar]
- Nilsdotter A, Lohmander L, Klassbo M, Roos E. Hip disability and osteoarthritis outcome score (HOOS) - validity and responsiveness in total hip replacement. BMC Musculoskelet. Disord. 2003;4 doi: 10.1186/1471-2474-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry J, Burnfield JM. Gait analysis: Normal and pathological function. 2nd ed. SLACK Incorporated; Thorofare, New Jersey: 2010. [Google Scholar]
- Schipplein OD, Andriacchi TP. Interaction between active and passive knee stabilizers during level walking. J. Orthop. Res. 1991;9:113–119. doi: 10.1002/jor.1100090114. [DOI] [PubMed] [Google Scholar]
- Sparrow WA, Donovan E, van Emmerik R, Barry EB. Using relative motion plots to measure changes in intra-limb and inter-limb coordination. J. Motor Behav. 1987;19:115–129. doi: 10.1080/00222895.1987.10735403. [DOI] [PubMed] [Google Scholar]
- Spoor CW, Veldpaus FE. Rigid body motion calculated from spatial co-ordinates of markers. J. Biomech. 1980;13:391–393. doi: 10.1016/0021-9290(80)90020-2. [DOI] [PubMed] [Google Scholar]
- Steultjens MPM, Dekker J, van Baar ME, Oostendorp RAB, Bijlsma JWJ. Range of joint motion and disability in patients with osteoarthritis of the knee or hip. Rheumatology. 2000;39:955–961. doi: 10.1093/rheumatology/39.9.955. [DOI] [PubMed] [Google Scholar]
- Watelain E, Dujardin F, Babier F, Dubois D, Allard P. Pelvic and lower limb compensatory actions of subjects in an early stage of hip osteoarthritis. Arch. Phys. Med. Rehabil. 2001;82:1705–1711. doi: 10.1053/apmr.2001.26812. [DOI] [PubMed] [Google Scholar]
- Zeni J, Pozzi F, Abujaber S, Miller L. Relationship between physical impairments and movement patterns during gait in patients with end-stage hip osteoarthritis. J. Orthop. Res. 2014;33:382–389. doi: 10.1002/jor.22772. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.