Abstract
Purpose
Using CJLAT, a chimeric herpes simplex virus (HSV-1) that produces a high incidence of herpes stromal keratitis (HSK) in latently infected rabbits, we characterized, by in vivo confocal microscopy (CM), the cellular events that precede development of HSK.
Methods
Thirty days post infection, in vivo CM was performed daily for 10 days and then weekly for up to 80 days post infection.
Results
We detected three types of subclinical corneal lesions prior to clinically apparent HSK: i)Small epithelial erosions; ii)Regenerating epithelium overlying small, cell infiltrates within the basal epithelial cell layer; and iii)Dendritic-like cells within the basal epithelial layer overlying stromal foci containing infiltrating cells. Sequential in vivo CM observations suggested that subclinical foci resolved over time, but were larger and more abundant with CJLAT than wild type HSV-1 McKrae. Active HSK was observed only with CJLAT and was initially associated with a large epithelial lesion overlying stromal immune cell infiltrates.
Conclusions
These results suggest that replication in the cornea of reactivated virus from trigeminal ganglia produces epithelial lesions which recruit immune cell infiltrates into the basal epithelial layer and anterior stroma. The virus is usually cleared rapidly eliminating viral antigens (Ags) prior to the arrival of the immune cells, which disperse. However, if the virus is not cleared rapidly, or if an additional reactivation results in an additional round of virus at the same site before the immune cells disperse, then the immune cells are stimulated and may induce an immunopathological response leading to the development of HSK.
Keywords: Herpes simplex virus, herpes stromal keratitis, rabbit, confocal microscopy
INTRODUCTION
In developed countries, recurrent HSV-1 induced eye disease, called herpetic stromal keratitis (HSK) or more properly recurrent-HSK, is a major cause of infectious corneal blindness 1, with ~500,000 afflicted individuals in the US alone 2, 3. Primary ocular HSV-1 infection is typically very mild or asymptomatic. By its conclusion, virus has ascended through axons and established lifelong latent infection in neurons of the trigeminal ganglia (TG). As a result of poorly understood cellular and molecular mechanisms, the virus periodically reactivates and returns via axons to the cornea where it replicates, is shed in tears, and can produce HSK.
Most episodes of HSK begin as superficial epithelial keratitis (HEK) that progresses to stromal disease 4–6. Although long term antiviral therapy can reduce HSK by ~40% 7 (presumably by decreasing viral replication after reactivation), antiviral treatment has no significant impact on preventing progression from HEK to HSK 7, 8. This suggests that reactivated virus is involved in recurrent HEK, but additional virus replication is not needed to progress to stromal disease. In contrast, immunosuppressants such as corticosteroids and cyclosporine A, which are not suitable for long term therapeutic use, are useful during recurrent HSK 9. This suggests that progression from recurrent HEK to HSK involves a pathologic inflammatory response. For these and other reasons, it is generally thought that HSK is an immunopathologic disorder in response to virus that returns to the cornea following reactivation from latency in the TG. Although the specific underlying immunopathologic mechanism(s) and the specific viral antigens (Ags) remain to be determined, the viral Ags appear to trigger an already highly primed local immune response, producing exacerbated immunopathological and inflammatory responses with accompanying tissue damage and stromal scarring 10. HSV-1 specific cellular immune responses are implicated because immunosuppressed individuals have decreased HSK despite often having increased viral reactivation (i.e., shedding of virus) 11. It has also been proposed that HSK is triggered by small amounts of viral Ags remaining in the cornea after clearance of the primary infection, or that the cornea may be an alternate site of latent or persistent viral infection 12, 13.
Since long term oral acyclovir only reduces ocular HSV-1 recurrences by ~40% 7 and no prophylactic or therapeutic clinical vaccines against ocular herpes are currently available, there is an urgent need for a better understanding of the cellular and molecular mechanisms leading to HSK for the eventual development of efficacious interventions. To address this problem, we have developed a unique ocular rabbit model in which clinically relevant, recurrent HSK occurs in approximately 80% of the eyes. CJLAT is an HSV-1 (strain McKrae) chimera in which the first 1.5 kb of the 8.3 kb HSV-1 latency associated transcript (LAT) gene was replaced by the latency related (LR) gene of bovine herpes virus 1 14, 15. We have previously reported that acute eye disease following CJLAT ocular infection of rabbits is similar to that produced by wild type McKrae 16, in that clinical corneal disease resolves completely by day 21 post infection and upon clinical exam the eyes are unremarkable. However, in CJLAT infected eyes starting between about day 33 to 80 post infection, up to 80% of the corneas develop clinical HSK 16, while less than 2% of corneas from rabbits latently infected with wild type HSV-1 McKrae (or other HSV-1 strains) develop any overt recurrent disease. Although the virological, immunological, and molecular mechanisms that lead to the high level of HSK produced by CJLAT have not yet been determined, the rabbit-CJLAT model has provided us with the first clinically relevant small animal model with which to systematically study the development of recurrent HSK. We report here on our initial in vivo confocal microscopic studies of HSK in the rabbit-CJLAT model.
MATERIALS AND METHODS
Viruses and tissue culture cells
All viruses were triple plaque purified and passaged only 2 or 3 times in rabbit skin (RS) cells prior to use. CJLAT has been previously described 15, 16. RS cells were grown in Eagles minimal essential media (MEM) supplemented with 5% fetal calf serum (FCS).
Rabbits
Eight to ten week old New Zealand White (NZW) female rabbits were used. Rabbits were treated in accordance with ARVO (Association for Research in Vision and Ophthalmology), AALAC (American Association for Laboratory Animal Care), and National Institutes of Health guidelines for the care and use of animals in research. Rabbits were bilaterally infected without scarification or anesthesia by placing, as eye drops, 2×105 pfu of virus into the conjunctival cul-de-sac, closing the eye, and rubbing the lid gently against the eye for 30 seconds as we previously described 15, 17. Both eyes were used since in our experience there is no significant correlation between the left and right eyes of a rabbit for virus shedding or ocular disease17. Thus the eyes can be treated as independent variables.
Cohorts of latently infected rabbits
Five rabbits were infected with wild type HSV-1 McKrae (WT) without corneal scarification and 18 were identically infected with CJLAT. One rabbit was maintained as a control. Seven CJLAT infected rabbits, 3 WT infected rabbits, and the uninfected rabbit survived to day 31 post infection (p.i.). At this time, eyes were clinically evaluated by a hand held slit lamp to assess acute disease, and only eyes that appeared clear (11 of 14 CJLAT infected eyes; 6 of 6 WT infected eyes; and both uninfected eyes) were subsequently studied. At day 31 pi, 2 CJLAT infected rabbits with clinically normal corneas were removed from this study for use in a different study (not shown). Six of the 11 eyes (3 eyes were previously excluded) in the 7 CJLAT infected rabbits that were initially clear developed HSK at 39, 59, 60 and 79 days p.i. None of the 6 eyes from WT infected rabbits developed HSK. The experiment was terminated at day 80 p.i. and the remaining animals euthanized.
In Vivo Confocal Microscopy
Starting at day 31, clinically normal eyes from CJLAT, wild type or control latently infected rabbits were evaluated daily to day 40 and then weekly to day 80 by in vivo confocal microscopy for the development of recurrent HSK using methods previously described 18. Briefly, rabbits were anesthetized with intramuscular ketamine HCl (50 mg/kg body weight, Phoenix Pharmaceutical Inc., St Joseph, MO) and xylazine (5 mg/kg body weight, Phoenix Pharmaceutical Inc., St Joseph, MO) and topical 0.5% proparacaine HCl (Bausch & Lomb Inc., Tampa, FL). Rabbit eyes were then scanned using a Tandem Scanning Confocal Microscope (TSCM, Tandem Scanning Corporation, Reston, VA) with a 24x surface contact objective (NA = 0.6 and WD = 1.5 mm) and 2.5% hydromethylcellulose coupling solution (Gonak, Akorn Inc., Buffalo Grove, IL). The axial focal plane position of the objective was controlled by an Oriel 18011 encoder Mike Controller (Oriel Corp., Stratford, CT). Images were captured using a Dage MTI VE-1000 camera (Dage MTI, Michigan City, IN) and digitized using a digitizing board (Data Translatations, Marlboro, MA) controlled by specially designed software 19. For each examination, images were collected of the corneal epithelium, basal lamina, and stroma from the central, nasal, temporal and inferior corneal regions as well as selected areas showing epithelial damage. Within each region a through focus series of images (a 3D data set) extending from the corneal epithelium to the corneal endothelium was also collected and projections along the XZ and YZ plane were generated.
RESULTS
Corneas from uninfected rabbits showed light scattering from superficial corneal epithelium, stromal keratocyte nuclei and stromal nerves by in vivo CM
In vivo confocal microscopy of healthy, uninfected rabbit corneas (Fig. 1) was consistent with our previous studies 20, 21 showing light scattering from only the superficial corneal epithelium (Fig. 1A), stromal keratocyte nuclei (Fig. 1B; arrows) and stromal nerves (Fig. 1B; arrowhead). Major light scattering structures in the normal cornea are better identified in XZ projections through the 3D data set (Fig. 1C) in which the corneal surface epithelium (Epi), basement membrane (BM) and endothelium (Endo) are detected.
Figure 1.
In vivo confocal microscopic images of the rabbit cornea showing, (A) the normal superficial epithelial cell surface layer, (B) the corneal stroma containing keratocyte cell nuclei (arrows) and a stromal nerve (arrowhead) and (C) an XZ, cross-sectional view of the 3D data set showing the epithelium (Epi), epithelial basement membrane (BM), and the corneal endothelium (Endo).
Small numbers of sub-clinical foci in corneas of rabbits latently infected with wild type HSV-1
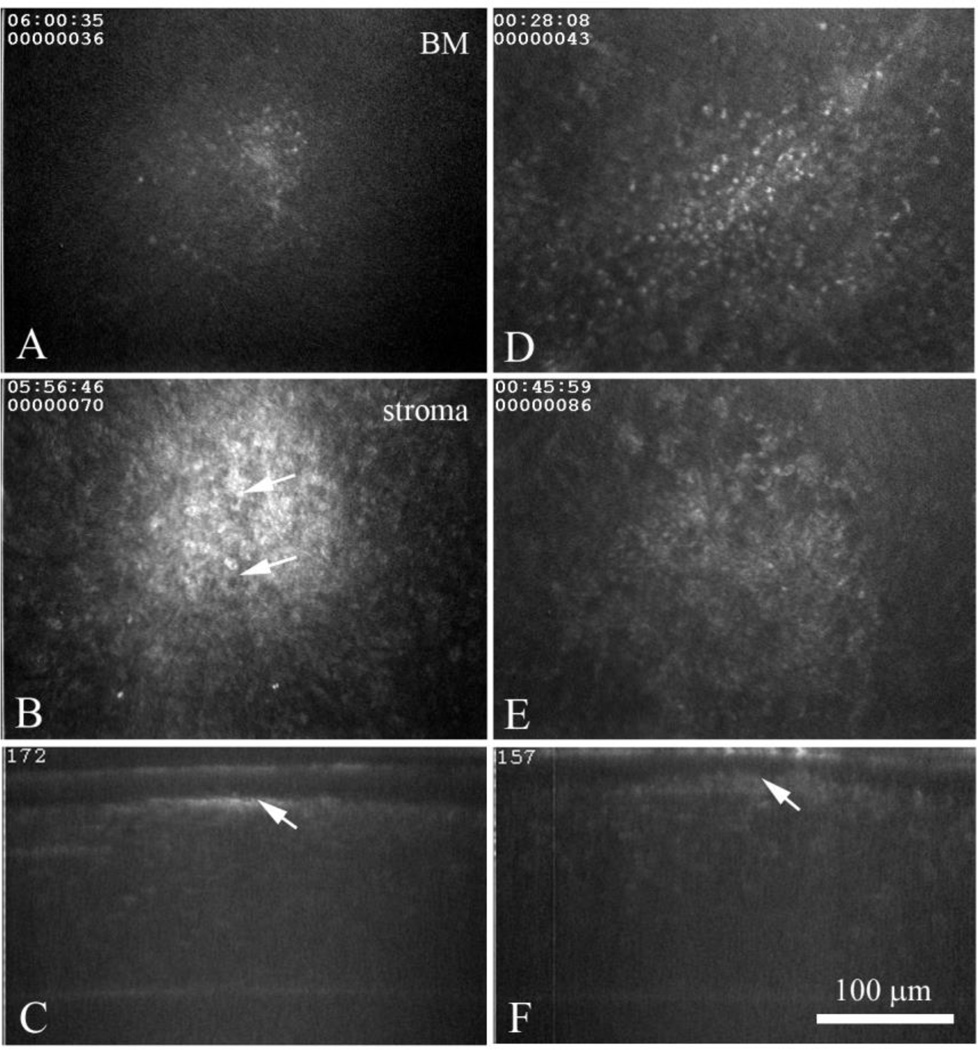
In contrast to uninfected corneas, a few corneas from the wild type (WT) latently infected group of rabbits showed small numbers of isolated regions containing brightly reflecting small cells at the level of the basement membrane (Fig. 2A) which overlaid regions in the stroma that contained granular deposits (Fig. 2B). These granular deposits appeared to contain cellular infiltrates (arrows). We designated these regions (i.e., basement membrane + stroma) sub-clinical foci, since these corneas were clear by slit lamp examination without detectable acute disease. XZ projections showed that these sub-clinical foci are small, measuring approximately 100 – 150 µm in diameter, and are limited to the basement membrane/anterior stroma region (Fig. 2C, arrow). These isolated sub-clinical foci were identified at all time points evaluated (Fig. 2D-F) and showed differing degrees of cell infiltration both at the basement membrane (Fig. 2D) and within the stroma (Fig. 2E), but in the stroma these foci always appeared limited to the anterior stroma (Fig. 2F, arrow). Sub-clinical foci could not be identified in every wild type infected cornea at every time point due both to the difficulty in locating these small lesions and to their rapid resolution/disappearance over time.
Figure 2.
In vivo confocal microscopic images from a wild type, latently infected rabbit eye at 35 Days (A-C) and 60 Days (D-F) post infection. Images through the basement membrane region (A and D) detected brightly reflecting, small cell infiltrates of variable size that overlaid granular deposits (B and E) that appeared to be composed of immune cell infiltrates (B, arrows). In the XZ projections (C and F) note the small region of light scattering (arrow) located at the basement membrane anterior stroma.
Numerous sub-clinical foci revealed by in vivo confocal microscopy of corneas from rabbits latently infected with CJLAT
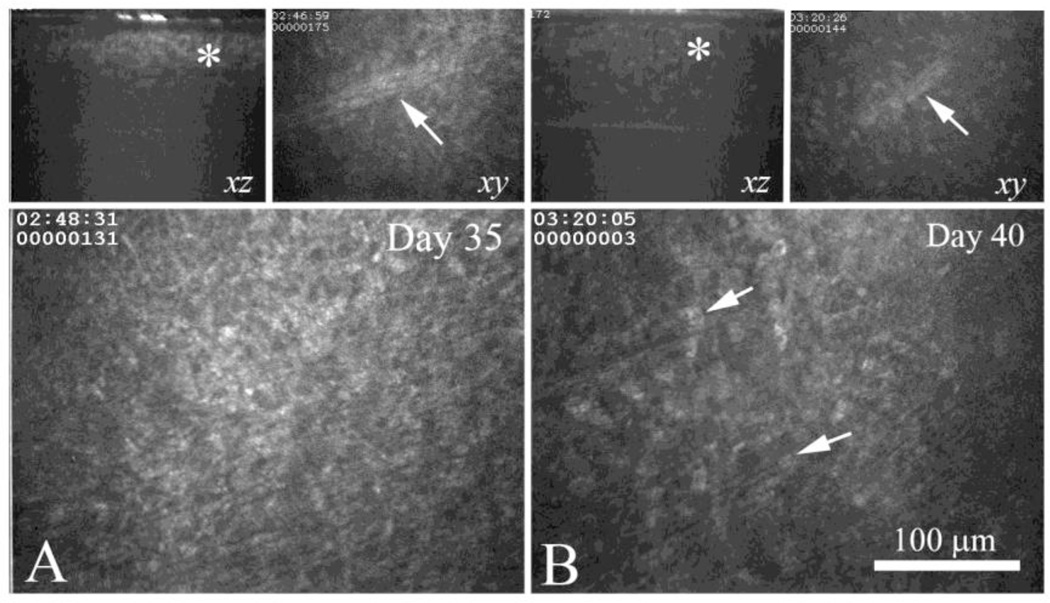
In CJLAT-rabbit corneas sub-clinical foci were much more abundant. They were easily detected by in vivo confocal microscopy in every CJLAT infected eye at all times examined after day 31 p.i. They appeared similar to the sub-clinical foci in WT infected corneas (Fig. 3A) except that they extended deeper within the corneal stroma (Fig. 3A, upper left panel, asterisk). These larger, subclinical foci in the CJLAT infected rabbit corneas appeared to contain cellular infiltrates similar to those identified in WT infected corneas (Fig. 3A, lower panel). Using a mid-stromal nerve identified in the 3D data set at Day 35 (3A, upper right panel, arrow), the same region was identified at Day 40 (Fig. 3B, upper right panel, arrow). At this time, the stromal foci appeared less localized and scattered considerably less light (Fig. 3B, left upper panel, asterisk). They also appeared to contain fewer infiltrating cells (Fig. 3B, lower panel arrows) suggesting resolution of the foci over time.
Figure 3.
In vivo confocal microscopy of the same eye from a CJLAT latently infected rabbit at Day 35 (A) and Day 40 (B). Upper left panels in A and B shows XZ projection through the same sub-clinical foci (asterisk), upper right panel shows the a nerve (arrow) that was located immediately below the sub-clinical foci in the same region. Lower panel shows the anterior stroma in the region of the sub-clinical foci, immediately below the basement membrane. Note that the sub-clinical foci appear less infiltrated (arrows) at Day 40 compared to Day 35 suggesting resolution of the lesion.
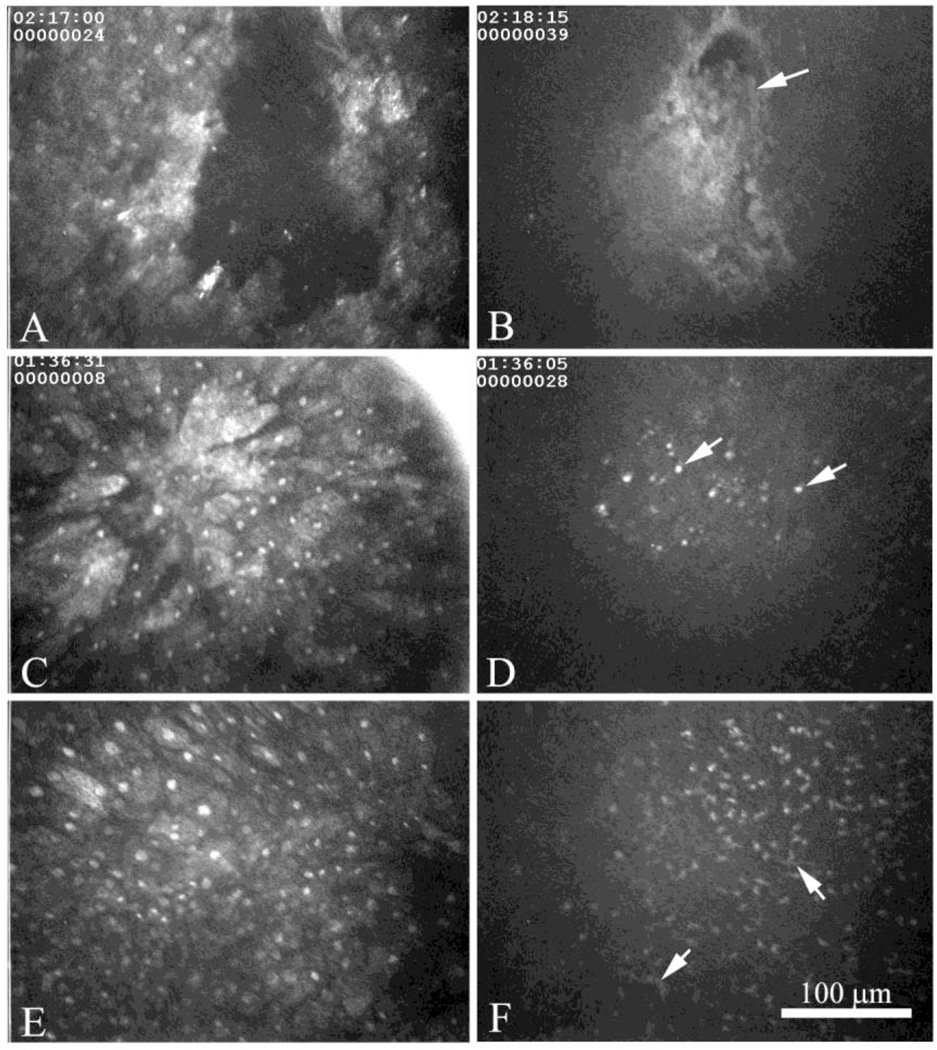
No epithelial damage was noted in normal corneas or any of the corneas from rabbits latently infected with WT HSV-1. In contrast, epithelial damage was fairly common in corneas of the CJLAT latently infected rabbits and ranged from small epithelial defects (Fig. 4A), to elongated surface epithelial cells suggesting migration (Fig. 4C), to disorganized surface epithelial cells (Fig. 4E). Epithelial defects or epithelial lesions appeared to extend deep within the epithelial sheet, showing necrotic basal epithelial cells adjacent to the basement membrane (Fig. 4B, arrow). Interestingly, regions of epithelial migration, suggesting epithelial lesion healing, were associated with infiltrates of small round cells at the level of the basement membrane (Fig. 4D, arrow). Regions of disorganized surface epithelial cells (late epithelial lesion healing) were also associated with small cell infiltrates within the basement membrane region intermingled with cells that appeared to have dendritic processes (Fig. 4F, arrows). Taken together these data suggest that reactivated CJLAT virus from the TG produced self-limiting regions of damage that healed by epithelial migration and re-stratification, and that epithelial lesions recruited small migrating cells that appear to be the start of a sub-clinical focus.
Figure 4.
In vivo confocal microscopic images of the surface epithelium (A, C, and E) and basement membrane (B, D, and F) showing epithelial erosion (A and B), migrating epithelium (C and D) and disorganized surface epithelium (E and F). At the level of the basement membrane epithelial erosions appear to contain necrotic basal epithelial cells (B, arrow), while regions of regenerating epithelium (C and E), were associated with varying degrees of small cell infiltrates (D and F, arrows).
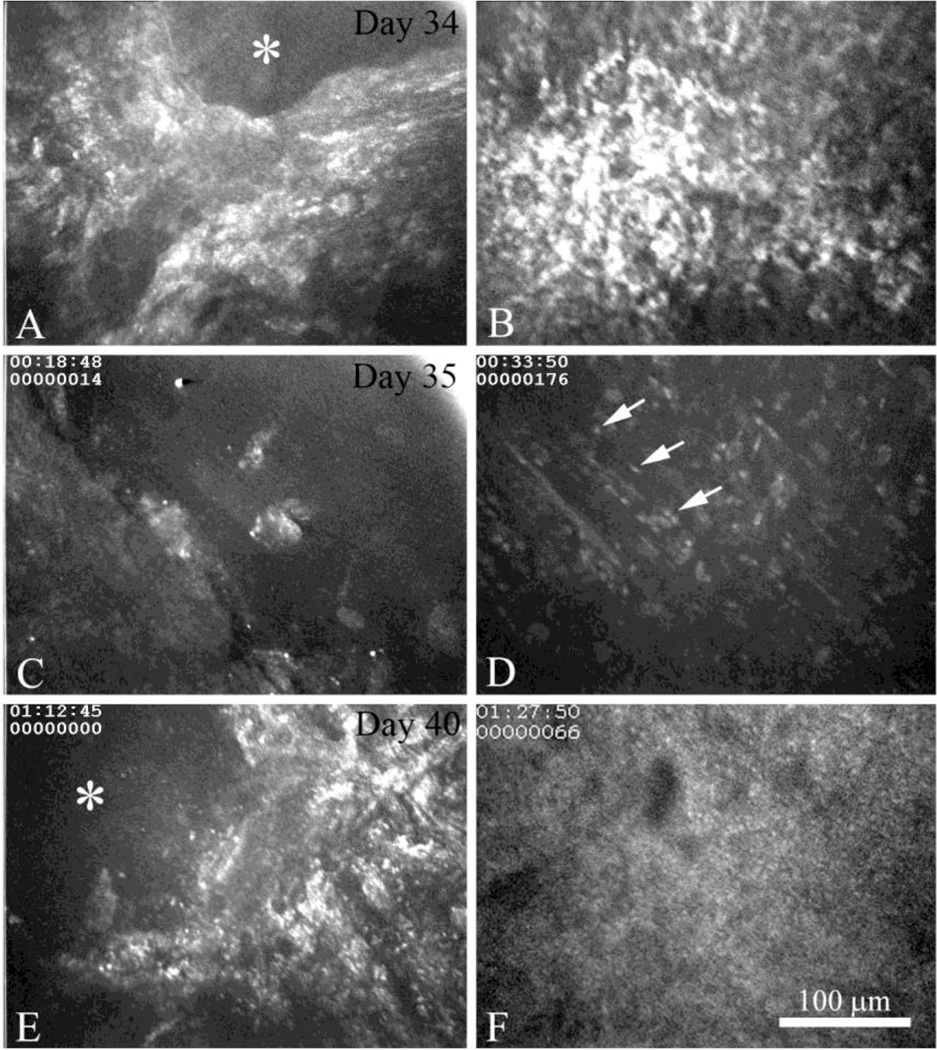
In all 6 of the corneas from the CJLAT rabbit group that went on to develop active recurrent HSK, clinically recognizable HSK was preceded by the development of an epithelial lesion (Fig. 5A, asterisk, day 34) with an underlying sub-clinical focus (Fig. 5B) similar to the sub-clinical foci noted pre-HSK above (see Fig 3). Interestingly, these overlapping epithelial lesions and sub-clinical foci persisted and could be detected the next day as regions of bare basement membrane (Fig. 5C, day 35) and the beginning of acute inflammatory cell infiltration into the underlying stroma (Fig. 5D, arrows) respectively. Over the next 5 days, there was a persistent epithelial lesion with bare basement membrane (Fig. 5E, asterisk, day 40) with continued acute inflammatory cell infiltration and massive polymorphonuclear cell (PMN) infiltration of the stroma that completely obscured normal stromal details (Fig. 5F). Such lesions persisted over the next days and weeks, later developing corneal neovascularization and stromal scarring (not shown).
Figure 5.
In vivo confocal microscopic images of active recurrent HSK in the same eye beginning at Day 34 (A and B) and extending to Day 35 (C and D) and Day 40 (E and F). Images are taken at the level of the corneal surface (A, C, and E) and anterior corneal stroma (B, D, and F) in the same 3D data set. The first initiation of recurrent HSK at Day 35 was associated with the appearance of an epithelial lesions and exposure of the basement membrane (A, asterisk) that immediately overlaid a sub-clinical foci (B). Progression of recurrent HSK at Day 36 was associated with persistent epithelial erosion (C) and the infiltration of the anterior stroma with acute inflammatory cells (D, arrows). Four days later at Day 40, persistent epithelial erosion (E, asterisk) was associated with massive polymorphonuclear cell infiltration (F).
DISCUSSION
Because of the lack of a reliable animal model, there is a paucity of information regarding the early events in the cornea that lead to recurrent HSK. Human studies on the development of recurrent HSK are generally limited to ex vivo studies of HSK corneal buttons obtained during corneal replacement surgery or in vivo studies of full blown or resolved HSK in individuals with a history of repeated recurrent HSK. In either situation, it is difficult to extrapolate from the full blown or resolved disease state back to the important early steps of the disease process. In addition, even in studies of individuals with repeated episodes of recurrent HSK, the early stages of the first recurrent episode cannot be studied.
In the mouse model, severe corneal disease can occur following acute HSV-1 infection and this has been studied as a model for recurrent HSK (reviewed in 22), even though it is not a recurrent infection and the gross features of the corneal disease do not closely mimic clinical HSK. Spontaneous recurrent HSK does occasionally occur in the rabbit ocular model of HSV-1 latency and reactivation and the clinical corneal disease is very similar to that seen in humans 16. However, as in humans, only a very small fraction of rabbit eyes develop recurrent HSK (<2%), making the model refractory to detailed analytical study 16. Our model of recurrent HSK in rabbits latently infected with the McKrae chimera, CJLAT 15, 16, has allowed us for the first time to investigate early stages of recurrent HSK by in vivo confocal microscopy.
Acute HSV-1 infection in rabbits (and humans) initially induces epithelial keratitis that is self-limiting and usually resolves within 7–10 days. It involves viral replication, recruitment of acute inflammatory cells and macrophages which scavenge infected cells, and release of cytokines attracting circulating lymphocytes. While it is generally believed that the presence of lymphoid cells after acute inflection is a transient phenomenon, recent clinical in vivo confocal microscopy studies have identified clusters of dendritic-like cells in the basal corneal epithelium that overlie regions of “granular fibrosis” in approximately 60% of cases with prior recurrent HSK 23. The authors speculated that the dendritic-like cells/structures/particles are clusters of Langerhan cells (LC) or dendritic, antigen presenting cells (APCs) recruited to the cornea in response to viral antigens. While LC are not specific to viral infection and have been seen occasionally in normal peripheral cornea and following keratitis of various pathogenic mechanisms, the authors suggest that identification of LC by in vivo confocal microscopy may be a valuable tool for the diagnosis of HSK 23. Interestingly, our in vivo confocal microscopy data evaluating the progression of recurrent HSV-1 corneal disease in eyes of rabbits latently infected with CJLAT shows cellular and structural changes in the cornea that appear identical to those observed by in vivo confocal microscopy of human cases of resolved HSK 23.
Thus, in the studies presented here, corneas of rabbits latently infected with CJLAT contained numerous small, sub-clinical foci containing dendritic-like cells within the basal epithelium and stromal cell infiltrates or “granular fibrosis” immediately underneath. These sub-clinical foci and our in vivo confocal microscopy images of pre-HSK CJLAT cornea are consistent with the description and in vivo confocal microscopy images reported in over half of human corneas with prior recurrent HSK 23.
We also performed some ex vivo confocal microscopy studies on cornea from these studies. Unfortunately, although several different mAbs can be used to detect a variety of rabbit immune cells by FACS, the only mAb we found useful for detection of rabbit immune cells by ex vivo confocal microscopy was a pan-leukocyte CD45 antibody. Since CD45 is a marker for bone marrow derived immune cells we were able to confirm that the infiltrating cells seen in the cornea by in vivo CM were in fact immune cells (not shown), but the type of immune cells present could only be extrapolated based on relative size and morphology. Based on the in vivo CM studies and the knowledge that the infiltrating cells seen were CD45 positive, it appears that sub-clinical foci develop following transient focal epithelial injury (epithelial lesion; perhaps due to return of reactivated virus to the corneal epithelium), which in turn recruits epithelial dendritic cells/Langerhans cells (DCs/LCs) and stromal immune cell infiltrates. Sub-clinical foci also appeared to resolve over time. In eyes with no clinical disease, active epithelial lesions and active sub-clinical foci were never seen to overlap. In contrast and importantly, in all 6 CJLAT eyes that developed HSK, the recurrent HSK appeared to develop around sub-clinical foci that had an overlying epithelial lesion. These combined foci (epithelial lesion + sub-clinical foci) appeared to persist for days, later involving infiltration of what appeared to be acute inflammatory cells and developing into HSK. Thus, there appears to be an important relationship between sub-clinical foci, and particularly a sub-clinical foci combined with an epithelial lesion (epithelial lesion + sub-clinical foci), and HSK. Interestingly, sub-clinical foci in the CJLAT infected corneas were larger and much more frequent than those seen in eyes of rabbits infected with wild type McKrae. If these sub-clinical foci are important for the ensuing development of HSK, the increased size and number in CJLAT compared to wild type infected eyes is consistent with the much greater frequency of HSK development in rabbits latently infected with CJLAT compared to wild type McKrae. Unfortunately, because of the relatively small number of eyes in the above studies, a rigorous statistical analysis to quantify the frequency at which sub-clinical foci resulted in HSK was not possible.
We propose a model of the relationship between reactivated virus from the trigeminal ganglia returning to the cornea, epithelial lesions, sub-clinical foci, and the development of HSK. Primary ocular infection, usually clinically unnoticed, results in priming of T-cells in draining lymph nodes (DLNs), lifelong viral latency in trigeminal ganglia (TG), clearing of corneal disease, and a normal appearing cornea. Spontaneously reactivated HSV-1 from the TG returns to the cornea, infects and kills epithelial cells producing small transient punctate epithelial lesions lasting less than 24 hours. Antigens (Ags) produced by viral replication are taken up by APCs (LCs/DCs and macrophages) cause signaling to the cornea which recruits immune cells to the site in 2–4 days, by which time the epithelial lesion has resolved. In the absence of target viral Ags, the infiltrating immune cells remain as sub-clinical foci composed of DCs/LCs in the basal epithelium and leukocytes in the underlying anterior stroma. These sub-clinical foci are detectable by in vivo confocal microscopy but not by clinical exam. In the absence of new viral Ag, the immune cells slowly leave (~3–7 days) and the sub-clinical foci are resolved. This cycle can occur repeatedly at the same and/or different locations in the cornea, resulting in new sub-clinical foci comprised of increasingly more specific and more stimulated functional leukocytes. In the rare event that a new epithelial lesion forms over the sub-clinical foci before it has had time to resolve or the original epithelial lesion persists until the sub-clinical foci fully forms, a fast and robust immunopathologic response is induced in the sub-clinical foci due to viral Ags, APCs stimulation and cross-priming of local memory T-cells. Since the memory T-cells are highly responsive due to numerous previous rounds of sub-clinical foci, even small amounts of remaining viral Ags are likely sufficient to cause multiple rounds of immunopathological responses resulting in persistent and more extensive epithelial lesions similar to human epithelial keratitis (HEK) and the development of early HSK, immunopathologic damage to both the stroma and epithelium, and ongoing HSK.
In summary, these studies provide new insights into the cellular mechanisms that lead to the development of HSV-1 induced immunopathological recurrent HSK, and will be useful for helping to direct the development of effective anti-viral immunotherapeutic and antiviral drug strategies.
ACKNOWLEDGEMENTS
This work was supported by Public Health Service grants EY018171, EY013191, 1R56AI098985, 1R56AI093133, EY07348, EY016663, EY14900, EY019896, and EY024618, Agriculture and Food Research Initiative Competitive Grants Program (USDA National Institute of Food and Agriculture) (13-01041), The Discovery Center for Eye Research, and a Research to Prevent Blindness Challenge grant.
Footnotes
CONFLICT OF INTEREST
All of the authors declare that there is no conflict of interest.
LITERATURE CITED
- 1.Smith RE, McDonald HR, Nesburn AB, et al. Penetrating keratoplasty: changing indications 1947 to 1978. Arch Ophthalmol. 1980;98:1226–1229. doi: 10.1001/archopht.1980.01020040078009. [DOI] [PubMed] [Google Scholar]
- 2.Liesegang TJ, Melton LJ, 3rd, Daly PJ, et al. Epidemiology of ocular herpes simplex. Incidence in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1155–1159. doi: 10.1001/archopht.1989.01070020221029. [DOI] [PubMed] [Google Scholar]
- 3.Whitley RJ, Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 4.Koelle DM, Reymond SN, Chen H, et al. Tegument-specific, virus-reactive CD4 T cells localize to the cornea in herpes simplex virus interstitial keratitis in humans. J Virol. 2000;74:10930–10938. doi: 10.1128/jvi.74.23.10930-10938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liesegang TJ. Epidemiology of ocular herpes simplex. Natural history in Rochester, Minn, 1950 through 1982. Arch Ophthalmol. 1989;107:1160–1165. doi: 10.1001/archopht.1989.01070020226030. [DOI] [PubMed] [Google Scholar]
- 6.Liesegang TJ. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea. 1999;18:127–143. doi: 10.1097/00003226-199903000-00001. [DOI] [PubMed] [Google Scholar]
- 7.HEDS. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. Herpetic Eye Disease Study Group. N Engl J Med. 1998;339:300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 8.HEDS. A controlled trial of oral acyclovir for the prevention of stromal keratitis or iritis in patients with herpes simplex virus epithelial keratitis. The Epithelial Keratitis Trial. The Herpetic Eye Disease Study Group. Arch Ophthalmol. 1997;115:703–712. doi: 10.1001/archopht.1997.01100150705001. [DOI] [PubMed] [Google Scholar]
- 9.Heiligenhaus A, Steuhl KP. Treatment of HSV-1 stromal keratitis with topical cyclosporin A: a pilot study. Graefes Arch Clin Exp Ophthalmol. 1999;237:435–438. doi: 10.1007/s004170050257. [DOI] [PubMed] [Google Scholar]
- 10.Streilein JW, Dana MR, Ksander BR. Immunity causing blindness: five different paths to herpes stromal keratitis. Immunol Today. 1997;18:443–449. doi: 10.1016/s0167-5699(97)01114-6. [DOI] [PubMed] [Google Scholar]
- 11.Kuo T, Wang C, Badakhshan T, et al. The challenges and opportunities for the development of a T-cell epitope-based herpes simplex vaccine. Vaccine. 2014;32:6733–6745. doi: 10.1016/j.vaccine.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cantin E, Chen J, Willey DE, et al. Persistence of herpes simplex virus DNA in rabbit corneal cells. Invest Ophthalmol Vis Sci. 1992;33:2470–2475. [PubMed] [Google Scholar]
- 13.Polcicova K, Biswas PS, Banerjee K, et al. Herpes keratitis in the absence of anterograde transport of virus from sensory ganglia to the cornea. Proc Natl Acad Sci U S A. 2005;102:11462–11467. doi: 10.1073/pnas.0503230102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mott KR, Osorio N, Jin L, et al. The bovine herpesvirus-1 LR ORF2 is critical for this gene's ability to restore the high wild-type reactivation phenotype to a herpes simplex virus-1 LAT null mutant. J Gen Virol. 2003;84:2975–2985. doi: 10.1099/vir.0.19421-0. [DOI] [PubMed] [Google Scholar]
- 15.Perng GC, Maguen B, Jin L, et al. A gene capable of blocking apoptosis can substitute for the herpes simplex virus type 1 latency-associated transcript gene and restore wild-type reactivation levels. J Virol. 2002;76:1224–1235. doi: 10.1128/JVI.76.3.1224-1235.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barsam CA, Brick DJ, Jones C, et al. A viral model for corneal scarring and neovascularization following ocular infection of rabbits with a herpes simplex virus type 1 (HSV-1) mutant. Cornea. 2005;24:460–466. doi: 10.1097/01.ico.0000138833.34865.39. [DOI] [PubMed] [Google Scholar]
- 17.Perng GC, Dunkel EC, Geary PA, et al. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol. 1994;68:8045–8055. doi: 10.1128/jvi.68.12.8045-8055.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jester JV, Petroll WM, Cavanagh HD. Measurement of tissue thickness using confocal microscopy. Methods Enzymol. 1999;307:230–245. doi: 10.1016/s0076-6879(99)07016-0. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Jester JV, Cavanagh HD, et al. On-line 3-dimensional confocal imaging in vivo. Invest Ophthalmol Vis Sci. 2000;41:2945–2953. [PubMed] [Google Scholar]
- 20.Petroll WM, Boettcher K, Barry P, et al. Quantitative assessment of anteroposterior keratocyte density in the normal rabbit cornea. Cornea. 1995;14:3–9. [PubMed] [Google Scholar]
- 21.Maurer JK, Parker RD, Petroll WM, et al. Quantitative measurement of acute corneal injury in rabbits with surfactants of different type and irritancy. Toxicol Appl Pharmacol. 1999;158:61–70. doi: 10.1006/taap.1999.8686. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta G, BenMohamed L. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine. 2011;29:5824–5836. doi: 10.1016/j.vaccine.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenberg ME, Tervo TM, Muller LJ, et al. In vivo confocal microscopy after herpes keratitis. Cornea. 2002;21:265–269. doi: 10.1097/00003226-200204000-00006. [DOI] [PubMed] [Google Scholar]