Abstract
Background
Microcystins (MC), representing >100 congeners being produced by cyanobacteria, are a hazard for aquatic species. As MC congeners vary in their toxicity, the congener composition of a bloom primarily dictates the severity of adverse effects and appears primarily to be governed by toxicokinetics, i.e. whether transport of MCs occurs via organic anion transporting polypeptides (Oatps). Differences in observed MC toxicity in various fish species suggest differential expression of Oatp subtypes leading to varying tissue distribution of the very same MC congener within different species.
Objectives
Functional characterization and analysis of the tissue distribution of Oatp subtypes in zebrafish (Danio rerio) as a surrogate model for cyprinid fish.
Methods
Zebrafish Oatps (zfOatps) were cloned and the organ distribution was determined at the mRNA level. zfOatps were transiently expressed in HEK293 cells for functional characterization using the Oatp substrates estrone-3-sulfate, taurocholate and methotrexate and specific MC-congeners (MC-LR, -RR, -LF and -LW).
Results
Novel zfOatp isoforms were isolated. Among these isoforms, the organ specific expression of zfOatp1d1 and of members of the zfOatp1f subfamily was identified. At the functional level, zfOatp1d1, zfOatp1f2, zfOatp1f3 and zfOatp1f4 transported at least one of the Oatp substrates, and zfOatp1d1, zfOatp1f2 and zfOatp1f4 were shown to transport MC congeners. MC-LF and MC-LW were generally transported faster than MC-LR and MC-RR.
Conclusions
The subtype specific expression of zfOatp1d1 and of members of the zfOatp1f subfamily as well as differences in the transport of MC congeners could explain the MC congener dependent differences in toxicity in cyprinids.
Keywords: microcystin, Oatp, zebrafish, toxin transport
INTRODUCTION
Mass occurrences of cyanobacteria present a threat to livestock worldwide, since various toxins are released upon breakdown of the bloom. The composition of the cyanobacteria species and the produced toxins play an important role in the severity of toxicity (Dietrich et al. 2008; Sivonen and Jones 1999). Due to mass-occurrences in nearly all water bodies, cyanobacterial blooms have been associated with massive kills of fish and other aquatic organisms. Beyond the acute intoxication, subchronic or chronic exposure to cyanobacterial toxins e.g. microcystins (MCs) may lead to the loss of the species with the highest susceptibility to cyanotoxins and thus to a shift in the species distribution of a given aquatic ecosystem (Ernst et al. 2009). MCs are toxic cyclic heptapeptides of which >100 different MC congeners have been described (Puddick et al. 2014). Intoxications by MCs largely depend on the individual MC congener's toxicodynamics and toxicokinetics, whereby the latter depends on the physicochemical properties of the MC congener in question. The toxicodynamic portion of the toxicity is primarily governed by the capacity of MCs to inhibit Ser/Thr protein phosphatases (ser/thrPP), consequently resulting in inhibition of crucial cell signal-transduction processes, protein trafficking, and cytoskeletal homeostasis (Campos and Vasconcelos 2010). It is noteworthy that the more lipophilic MCs, namely MC-LW and MC-LF and the more hydrophilic MC-LR and MC-RR inhibit ser/thrPP activities at approximately equimolar concentrations and thus physicochemical properties do not explain the large differences observed for the apical toxicity (Fischer et al. 2010; Höger et al. 2007). Consequently, the key differences in toxicity most likely stem from differences in toxicokinetics. Indeed, MCs were demonstrated to be transported across cell membranes via organic anion transporting polypeptides (humans and rodents: OATPs; fish: Oatps according to HUGO and ZFIN guidelines) (Fischer et al. 2005; Lu et al. 2008; Meier-Abt et al. 2007; Steiner et al. 2014). To date, more than 300 OATPs/Oatps have been described and different OATP/Oatp subtypes are found within a single species (Hagenbuch and Stieger 2013). Computational analysis and experimental evidence suggest a membrane topology with 12 transmembrane domains (TMD) for the rat rOATP1A1 (Wang et al. 2008), a feature also common to fish Oatps and other mammalian OATPs. Although of great importance for the hepatic transport of bile salts, steroids and steroid conjugates, thyroid hormones, prostaglandins and oligo-peptides as well as for the clearance of xenobiotics, OATPs are present in almost every organ or tissue. Depending on the subtype, OATPs are expressed ubiquitously or in specific organs (Hagenbuch and Stieger 2013).
Transport of MCs was demonstrated for hOATP1A2, which is mainly expressed in the brain, and for the liver specific hOATP1B1 and hOATP1B3 in humans (Fischer et al. 2010; Fischer et al. 2005; Monks et al. 2007) as well as for rOATP1B2 in rats and mOATP1B2 in mice (Fischer et al. 2005; Lu et al. 2008). Likewise in fish, MC transport is mediated by liver specific Oatps as shown for Oatp1d1 in little skate and rtOatp1d1 in rainbow trout (Meier-Abt et al. 2007; Steiner et al. 2014). However, MC transport by OATPs/Oatps is largely MC congener dependent, as demonstrated for hOATP1B1 and hOATP1B3 expressed in HEK293 or HeLa cells with MC-LR, MC-RR, MC-LW, and MC–LF (Fischer et al. 2010; Monks et al. 2007). Indeed, while some OATPs lacked transport of MC-RR, transport was demonstrated for MC-LF, MC-LW and to a comparably minor extent for MC-LR (Fischer et al. 2010). The latter may be key for the systemic distribution of MC congeners within a given organism (fish or mammalian) and consequently for the development of species specific MC mediated organ toxicity. Thus, the differences in susceptibility toward MC intoxications observed in fish may be explained by differential expression of Oatp subtypes and by Oatp subtype-selective transport of specific MC congeners. To test the latter hypothesis, zebrafish (Danio rerio) Oatp subtypes were cloned and expressed in HEK293 cells to allow characterization of Oatp substrate and MC congener dependent transport.
METHODS
Experimental fish, reagents and materials
Zebrafish (Danio rerio) were obtained from the animal research facility of the University of Konstanz. [3H] taurocholic acid (TCA) (209.8 Giga Becquerel (GBq)/mmol), [3H] estrone sulfate ammonium salt (E3S) (2009.1 GBq/mmol) and [3H] methotrexate disodium salt (MTX) (1824.1 GBq/mmol) were purchased from American Radiolabeled Chemicals Inc. and Hartmann Analytic GmbH (Braunschweig, Deutschland). MC congeners (-LR, -LF, -LW and -RR) were purchased from Enzo Life Science, Inc. (New York, USA). Reverse-transcription and PCR reagents were purchased from New England Biolabs (Ipswich, UK) unless indicated otherwise. Cell culture material was from PAA Laboratories (Cölbe, Germany). All other chemicals and antibodies, unless stated otherwise, were from Sigma-Aldrich (Taufkirchen, Germany).
RNA preparation and cDNA Synthesis
RNA was isolated from muscle, intestine, liver, kidney, brain and gills (pooled from 15 zebrafish, indiscriminant of gender) and cDNA was prepared as described previously in detail (Steiner et al. 2014).
Cloning of Oatp sequences in eukaryotic expression plasmids
To generate zfOatp expression plasmids, the full-length open reading frames (ORFs) of the Oatp encoding slco genes were cloned into the vector ptracer™CMV/Bsd (Invitrogen, Carlsbad, CA, USA). Based on the annotated zfOatp sequences (Popovic et al. 2010), specific primers including flanking restriction sites were designed to PCR amplify the ORFs of the different zfOatps (Table S1). cDNA from zebrafish (wild type strain) was used as template and PCR was carried out using Phusion High Fidelity DNA-Polymerase (NEB) with proof-reading activity to exclude that sequence variations occurred due to methodical reasons. PCR was performed as follows: Initial DNA denaturing for 30 sec at 98 °C, 35 cycles of denaturing for 10 sec at 98 °C, primer annealing for 30 sec at 52 °C and elongation for 2.5 min at 72 °C followed by a final extension of DNA-fragments for 5 min at 72 °C. Before ligation of the vector backbone ptracer™CMV/Bsd with the ORF, both were digested with the same restriction enzymes. Transformation of chemical competent E. coli was performed and ligation success was validated by E. coli colony PCR screening and sequencing.
Phylogenetic analysis and topology prediction
The resulting cDNAs were sequenced on both strands and aligned with their respective annotated sequences retrieved from the NCBI database (http://www.ncbi.nlm.nih.gov/). Global alignments of the two sequences were performed with the ClustalW algorithm. Membrane topology was predicted by comparing 3 different programs and associated models: TMHMM (http://www.cbs.dtu.dk/services/TMHMM-2.0/), TMpred (http://embnet.vitalit.ch/software/TMPRED_form.html) and TOPCONS (http://topcons.cbr.su.se/). The accession numbers of the zfOatp subtype sequences cloned in this study are listed in Table S2.
Cell culture and transient transfection
Human embryonic kidney cells (HEK293) were cultured as described by Steiner et al. (2014). For expression of zfOatps, 60-80 % confluent HEK293 cells were transiently transfected either with the empty vector as control (ev-HEK293) or with one of the vector constructs containing the respective zfOatp ORF using FuGENE transfection reagent (Promega, Madison, WI, USA). The presence of a green fluorescence protein (GFP) ORF on ptracer™CMV/Bsd allowed verification of transfection success.
Uptake experiments
HEK293 cells were seeded in poly-D-lysine coated 24 well plates and transfected as described above. 48 h post-transfection HEK293 cells were incubated with Oatp substrates for 5 min as previously described (Steiner et al. 2014). Uptake was normalized to total protein concentration. Experiments were repeated three times (true replicates) in triplicates each (technical replicates). An Oatp was considered to transport a substrate when the uptake into cells expressing the Oatp was significantly higher than the uptake into control cells transfected with the empty vector. Significance was determined with the Mann-Whitney test (GraphPad Prism Software Inc.) with p ≤ 0.05 (one-tailed) from three independent experiments (n=3).
In addition, uptake of [3H] E3S was also performed for up to 30 min with zfOatp1f1 and Oatp1f2-1 to rule out that [3H] E3S transport could not have been seen due to high affinity/low capacity transport properties of zfOatp1f1 and Oatp1f2-1. However, no [3H] E3S transport was observed for these two Oatps (Figure S1).
Microcystin transport detected via immunoblot
All MC uptake experiments were conducted 48 h post-transfection of HEK293 cells with ptracer™CMV/Bsd-Oatp. Time- and concentration dependent uptake of MC-congeners was determined via immunoblotting. MC congeners were dissolved in methanol (MeOH ≤ 0.2 %) and 0.2% MeOH served as solvent control. Empty vector transfected HEK293 (ev-HEK293) served as negative control. For immunoblotting, proteins were isolated from cells with cold buffer containing 10 mM Tris-base, pH 7.5, 140 mM NaCl, 5 mM EDTA, 0.1 % (v/v) Triton X-100, pH 7.5 and 1 % protease inhibitor followed by centrifugation at 10,000 × g. Supernatant protein concentration was measured using a BCA assay kit (see above). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed with 15 μg protein/lane on 10 % gels at 200 V according to Laemmli (1970). Subsequent to electrophoresis, proteins were transferred onto a nitrocellulose membrane (Whatman, Dassel, Germany) at 300 mA for 90 min as described elsewhere (Towbin et al. 1992). Membranes were blocked in Tris-buffered Saline with 1 % Tween 20 containing 1 % bovine serum albumin. To detect MC bound to phosphatases, nitrocellulose membranes were incubated with the MC-specific primary monoclonal anti-Adda- antibody (clone AD4G2, 1:10) (Zeck et al. 2001) over night at 4 °C. GAPDH or actin served as immunoblot as well as protein loading control. Quantification was carried out using greyscale analysis (Quantity One version 4.6.9).
All cloned zfOatps were screened for their MC transport capability via incubation of the zfOatp-HEK293 cells with 50 nM of a single MC congener for 24 h. The time-dependent uptake of individual MC congeners was tested via incubation of the zfOatp-HEK293 cells for 1 h, 6 h and 24 h (and 48 h in the case of zfOatp1f2) with 50 nM MC (data not shown). Based on the latter results, optimized time-windows for individual zfOatp and MC congener combinations were selected that allowed time dependent detection of MC congener transport. For improved kinetic understanding zfOatp1d1-HEK293 cells were exposed to MC-LR for 3 h and to MC-LF for 30 min at MC concentrations ranging between 10 nM and 1000 nM. Quantification of MC transport was achieved via immunoblotting and densitometric greyscale analysis. The Michaelis-Menten equation was used to obtain Vmax and Km values. Data were fitted using non-linear regression analysis with GraphPad Prism.
Quantitative real-time PCR
To determine the tissue specific expression of zfOatp1d1 and zfOatp1f1-4, cDNA was synthesized as described above from adult zebrafish tissues and used as a template for semi-quantitative real time PCR. A 1:5 dilution of the cDNA was used. 18S-rRNA served as normalization control due to its equal expression in all tissues (data not shown). Gene specific primers were designed for Oatp1d1 (forward 5′-CACAATCCTCCTGCCAGCAAA-3′, reverse 5′-CCCTATGAAACCACTGACTTGT-3′) and Oatp1f (forward 5′-GGTATAGGAACACTGCTAATGG-3′, reverse 5′-CAGAGGGATAATACTGGCTTC-3′), whereby primer pairs for zfOatp1f1-4 were 100 % identical for all individual sequences. SensiMix™ SYBR® & Fluorescein Kit (Bioline, London, UK) was used according to the manufacturer's instructions. A melting curve analysis of the products was performed at the end of the PCR reaction to confirm the detection of a single PCR product. Standard curves for primers used were carried out in n=2-4 independent replicates, whereby each replicate consisted of technical triplicates. The resulting efficiency of amplification was 104±4 % for 18s-rRNA (n=4), 98±1 % for zfOatp1d1 (n=3) and 103±0 % for zfOatp1f (n=2) (data not shown). Data were analyzed according to the Δct method.
RESULTS
Sequence analysis
In order to characterize transport properties of several zfOatps, annotated or predicted zfOatp sequences found in the refseq database of NCBI (http://www.ncbi.nlm.nih.gov/refseq/) were used as templates for the amplification of the respective open reading frames, and zfOatp1c1, zfOatp1d1, zfOatp1f1, zfOatp1f2, zfOatp1f3, zfOatp1f4, zfOatp2b1 and zfOatp4a1 were cloned into the ptracer™CMV/Bsd plasmid. zfOatp3a1, zfOatp2a1 and zfOatp5a1 could not be cloned for unknown reasons. The integrity of the cloned zfOatp sequences was validated by sequencing and alignment to the respective annotated amino acid sequences (Table 1). The zfOatp1c1 sequence differed in 5 amino acids within the first 630 amino acids. Furthermore, the cDNA suggested the presence of a splice variant in that the last exon was spliced in a way that resulted in 5 additional nucleotides thus encoding a protein of 691 instead of 689 amino acids with a completely different C-terminal end. Thus, zfOatp1c1 was excluded from further analysis. zfOatp1d1 contained an insertion of 76 additional amino acids compared to the reference sequence. Sequence variations were observed in all zfOatps with the exception of zfOatp2b1 (Table 1), suggesting the presence of single nucleotide polymorphisms. For zfOatp1f2, zfOatp1f4 and zfOatp4a1 two expression variants were found (zfOatp1f2-1 and zfOatp1f2-2, zfOatp1f4-1 and zfOatp1f4-2 respective zfOatp4a1-1 and zfOatp4a1-2), which differed from each other as well as from the annotated sequence by several amino acids. zfOatp1f2-1 contained a deletion of 88 amino acids after position 361 compared to the annotated zfOatp1f2, most likely representing a splice variant with a missing exon.
Table 1.
Variations of cloned amino acid sequences compared to annotated sequences
| Protein name | length | Variations compared to annotated sequence at aa level | ||
|---|---|---|---|---|
| Number of deviating aa | aa stretches/gaps | Other | ||
| Oatp1c1 | 691 | 5 | - | Frame difference at aa 632 |
| Oatp1d1 | 689 | 2 | insertion (76 aa) after aa 377 | - |
| Oatp1f1 | 561 | 14 | - | - |
| Oatp1f2-1 | 662 | 4 | - | - |
| Oatp1f2-2 | 574 | - | Deletion (88 aa) after aa 362 | - |
| Oatp1f3 | 588 | 12 | - | - |
| Oatp1f4-1 | 644 | 9 | - | - |
| Oatp1f4-2 | 644 | 13 | - | - |
| Oatp2b1 | 677 | - | - | - |
| Oatp4a1-1 | 742 | 4 | - | - |
| Oatp4a1-2 | 742 | 3 | - | - |
For detailed amino acid replacement information and accession numbers of the annotated reference sequences see Supplemental Figures 2 to 8.
The membrane topology of the cloned zfOatps was predicted using 3 different algorithms (see Materials and Methods). Depending on the algorithm used, the number of predicted TMD varied. zfOatp1f1, zfOatp1f2-2 and zfOatp1f3, which contained shorter amino acid sequences, were also predicted to have only 8-10 TMDs rather than the 12 TMDs typically found in mammalian OATPs (Wang et al. 2008). Based on multiple alignments, both zfOatp1f1 and zfOatp1f3 were missing the first TMD while zfOatp1f2-2 lacked at least TMD 8 (Table S3).
Substrate specificity
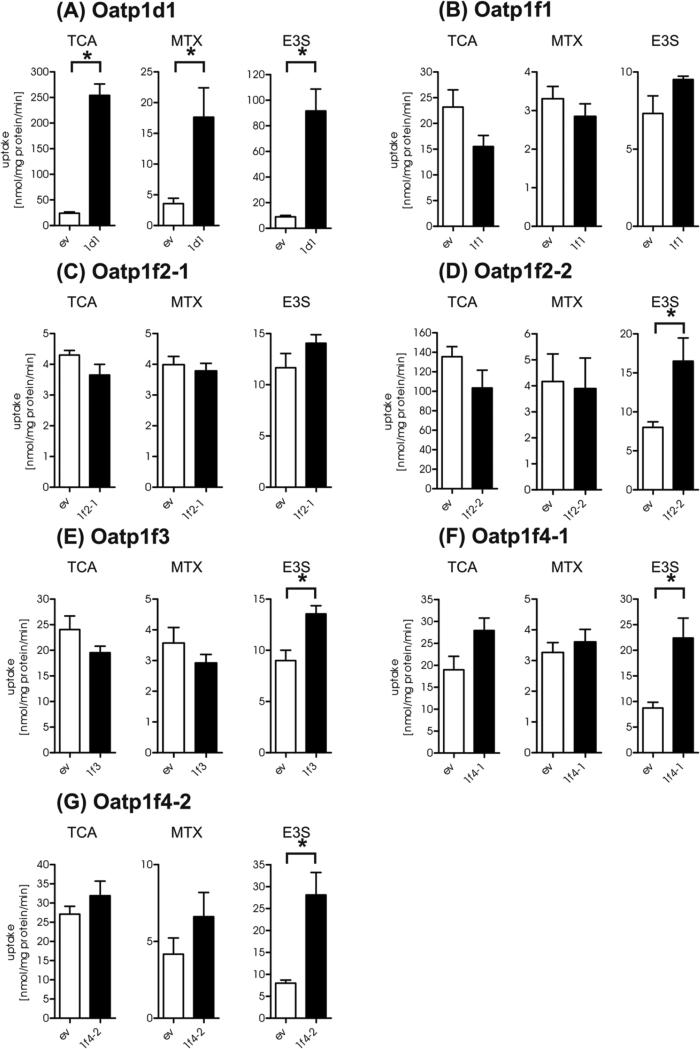
An initial functional screening of the cloned zfOatps was carried out with the known OATP/Oatp substrates taurocholate (TCA), estrone-3-sulfate (E3S) and methotrexate (MTX) (Figure 1). zfOatp1d1 transported all three substrates. E3S was also transported by zfOatp1f2-2, zfOatp1f3, zfOatp1f4-1 and zfOatp1f4-2. However, with the exception of zfOatp1d1, none of the other zfOatps transported TCA or MTX. Furthermore, zfOatp1f1, zfOatp1f2-1, zfOatp2b1 and zfOatp4a1 did not transport any of the three substrates. Thus, zfOatp1d1 seemed to represent a multi-specific Oatp while the other transporting zfOatps appeared to be more selective.
Fig. 1.
Transport of Oatp substrates. 5 min uptake of 30 nM [3H]TCA, 6.0 nM [3H]MTX and 6.1 nM [3H]E3S in HEK293 cells expressing different zfOatps (black bar, (A)-(G)) was assessed two days after transfection and compared to the uptake into empty vector transfected HEK293 cells (white bar). Significant transport is marked with a star. Columns represent means ± SEM from three independent experiments run in three technical replicates. Statistics: Mann-Whitney test (GraphPad Prism Software Inc.) and p ≤ 0.05 (one-tailed).
Microcystin congener dependent uptake by zfOatp
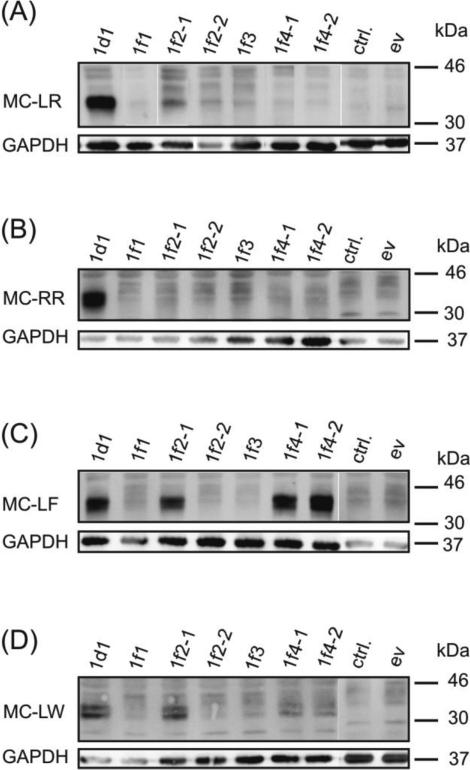
Due to the fact that zfOatp2b1 and zfOatp4a1 did not show transport of OATP/Oatp substrates, these zfOatps were excluded from further testing with MCs. Although the same holds true for zfOatp1f1 and zfOatp1f2-1, these zfOatps were included in the initial MC uptake studies as they are part of the zfOatp1 family for which transport of MCs was expected (Feurstein et al. 2010; Fischer et al. 2010; Fischer et al. 2005; Komatsu et al. 2007; Lu et al. 2008; Meier-Abt et al. 2007; Monks et al. 2007; Steiner et al. 2014). All cloned members of the zfOatp1 family were tested for the uptake of different MC congeners. Therefore, HEK293 cells transiently expressing zfOatp1 subtypes were exposed to 50 nM of MC-LR, MC-LW, MC-RR or MC-LF for 24 h followed by the detection of covalently bound MC-congeners in western blots with the anti-Adda-antibody (see Materials and Methods). As already observed for the OATP/Oatp model substrates, zfOatp1d1 also transported all tested MC-congeners (Figure 2). Although zfOatp1f2-2 and zfOatp1f3 were able to transport E3S, they did not transport any of the four MC-congeners tested. Similar absence of MC transport was observed for zfOatp1f1. In contrast zfOatp1f2-1 that did not transport TCA, MTX and E3S, indeed did take up MC-LR, MC-LF and MC-LW. In addition, zfOatp1f4 (variants 1 and 2), which transported E3S, only transported the MC-congener MC-LF.
Fig. 2.
zfOatp1d1 and zfOatp1f-subtype mediated MC transport. Western blot of MC in zfOatp transfected cells, empty vector (ev) and non-transfected cells (ctrl.), treated for 24 h with 50 nM MC-LR (A), MC-RR (B), MC-LF (C) and MC-LW (D).
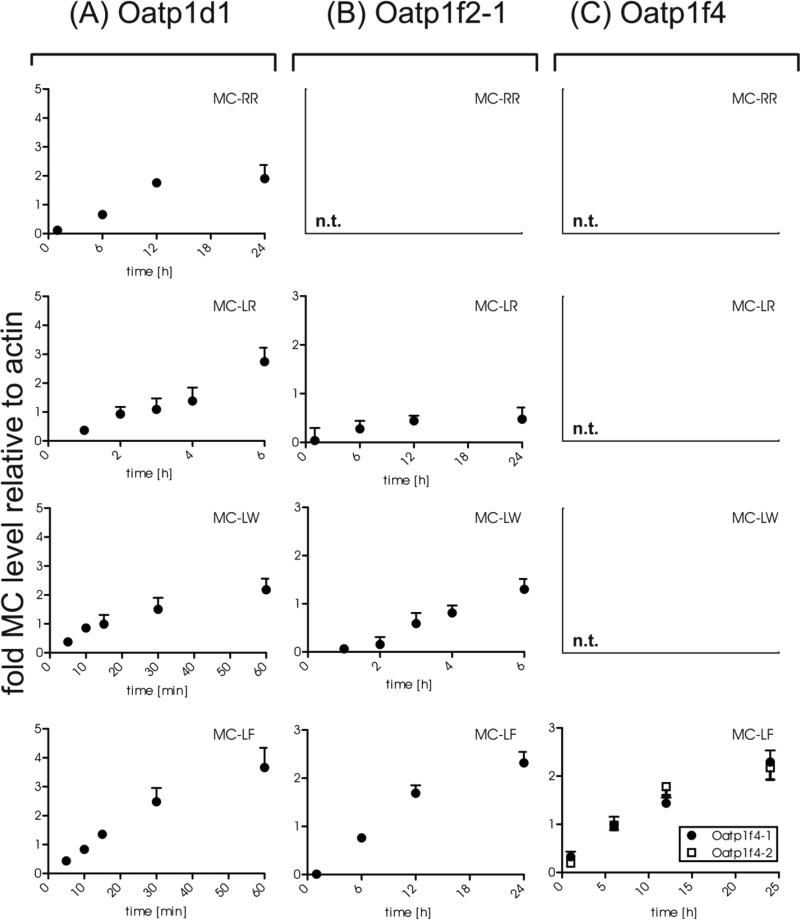
Based on the above results, time and MC-congener dependent transport studies were conducted with zfOatp1d1, zfOatp1f2-1 and zfOatp1f4 (variants 1 and 2). MC-RR exposure of zfOatp1d1 expressing HEK293 cells resulted in a nearly linear uptake plateauing after 12 h, while a more rapid uptake, linear up to 6 h, was observed for MC-LR (Figure 3). In contrast, transport of MC-LF and MC-LW occurred within 1 h. A similar MC congener specific uptake was observed in HEK293 cells expressing zfOatp1f2-1. Thereby MC-LR was taken up much slower than MC-LW and MC-LF. However, transport of MC-LF and MC-LW by zfOatp1f2-1 was slower than that observed for zfOatp1d1. Comparable transport of MC-LF to that observed for zfOatp1f2-1 was found for the two zfOatp1f4 variants.
Fig. 3.
Quantification of time dependent MC uptake by zfOatps via densitometry analysis. Data are means + SEM from three independent experiments.
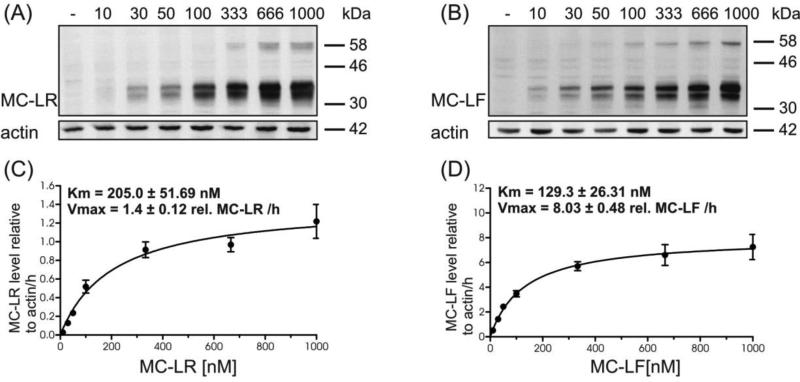
In view of the slower transport velocities for the more hydrophilic MC-LR and MC-RR when compared to the more lipophilic MC-LF and MC-LW, a more in-depth characterization of the transport kinetics was considered crucial. For this, MC-congener concentration dependent transport studies were carried out with zfOatp1d1 and MC congeners MC-LR and MC-LF, whereby the time range for linear uptake for each of the MC congeners was chosen. MC-LR and MC–LF uptake by zfOatp1d1 expressing HEK293 cells was saturable with increasing substrate concentrations ranging between 10 and 1000 nM (Figure 4). The relative Vmax values calculated based on MC densitometry suggested a 6-fold higher uptake velocity for MC-LF than for MC-LR. The data also suggest a 2-fold higher affinity of zfOatp1d1 for MC-LF compared to MC-LR. It should be noted however, that these kinetic parameters can merely be used for a comparison within this study and do not lend themselves for a comparison with kinetic values obtained from experiments using radiolabelled MCs in various transient or stable OATP/Oatp expression systems.
Fig. 4.
Concentration dependent uptake of MC-LR and MC-LF (10 nM-1000 nM) mediated by zfOatp1d1 using immunoblotting with anti-ADDA antibody. HEK293-zfOatp1d1 cells were exposed to increasing concentrations of MC-LR for 3 h (A) and MC-LF for 30 min (B), β-actin served as loading control. Quantification of transported MC-LR (C) and MC-LF (D) was carried out using anti-ADDA Westernblot and densitometric analysis. Vmax and Km were obtained by fitting the data from the greyscale analysis to the Michelis-Menten equation. Data are means ± SEM from three independent experiments.
Organ distribution of zfOatp1d1 and members of the zfOatp1f subfamily
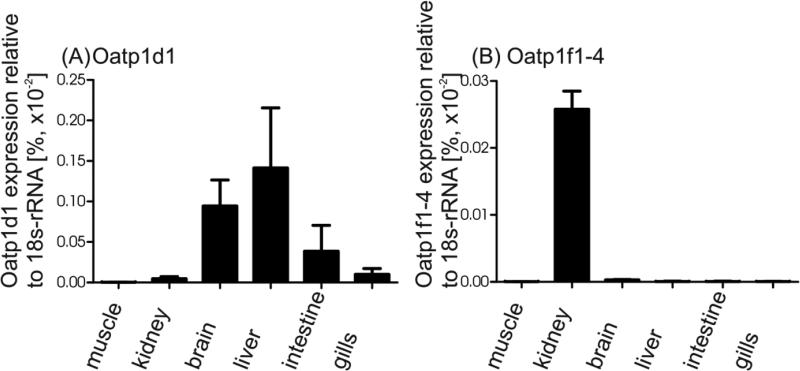
The organ distribution of zfOatps was determined by semi-quantitative real time PCR and demonstrated that zfOatp1d1 had a comparably high expression in brain and liver, followed by a moderate expression in the intestine, a low expression in the kidney and the gills and a marginal expression in muscle tissue (Figure 5). The zfOatp1f subfamily was exclusively expressed in the kidney.
Fig. 5.
RNA expression of (A) zfOatp1d1 and (B) zfOatp1f using semi-quantitative real time PCR. Expression was normalized to the reference gene 18s-rRNA. Data are mean ± SEM of 3 biological replicates containing the combined organs of 15 fish each.
DISCUSSION
Differences in MC apical toxicity were reported for coregonids and cyprinids, either after intraperitoneal injection or gavage of specific MC congeners or gavage of bloom material containing various MC congeners (Ernst et al. 2006; Ernst et al. 2007; Fischer and Dietrich 2000; Kotak et al. 1996; Tencalla and Dietrich 1997). As the toxicodynamic properties of different MC congeners do not differ dramatically, i.e. a maximum of a factor 2 for PP1 was reported (Fischer et al. 2010; Höger et al. 2007), it was assumed that the primary factor responsible for the overt differences in apical toxicity observed between fish species is toxicokinetics. It was already suggested from earlier studies in mammalian cells, expression systems and whole animal studies that the overall kinetics depends on the MC congener to be transported, the capability of a given OATP/Oatp subtype of transporting a specific MC congener and finally on the OATP subtype expressed in a given organ/tissue. The finding that the zebrafish zfOatp1d1, zfOatp1f2-1 and both cloned expression variants of Oatp1f4 are capable of MC-congener transport, whereby the hydrophobic congeners MC-LF and MC-LW were transported faster in all zfOatp subtypes tested (Figure 4), corroborated the MC congener dependent transport already observed for mammalian OATPs. Moreover, when comparing zfOatp subtypes, differences in transport velocities for a given MC congener were observed (Figure 3), as previously suggested for the human transporter hOATP1B1 and hOATP1B3 (Fischer et al. 2010). Finally, the cloned zfOatps demonstrated variant organ expression (Figure 5), suggesting that zfOatp1d1 is expressed in multiple organs, while zfOatp1f1-4, similar to other representatives of the zfOatp1f1 subfamily, is primarily expressed in the kidney. The expression pattern of zfOatp1d1 observed in this study is similar to the one reported earlier by Popovic et al. (2013). In the latter study zfOatp1d1 showed the highest expression in liver and brain with about 30 to 50-fold lower expression in skeletal muscle, gills and intestine. The study presented here confirmed the highest expression in liver and brain and 15 to 30-fold lower expression in skeletal muscle, gills and kidneys. However, the intestinal expression was higher than previously reported. Overall, Oatp1d1 expression in zebrafish differed from the expression pattern of the orthologous Oatp1d1 in rainbow trout and little skate (Cai et al. 2002; Steiner et al. 2014), where this Oatp appears to be exclusively expressed in the liver. Obviously the expression of Oatp subtypes, e.g. zfOatp1d1, and their inherent affinity and capacity of transporting MC congeners can play a major role within the context of toxicological impact of cyanobacterial blooms. While some organs like the liver and kidney are capable of regeneration upon MC induced cytotoxicity as long as MC exposure was insufficiently high to produce mortality, other organs, e.g. the brain, cannot. Consequently, subchronic and chronic exposure of zebrafish to cyanobacterial blooms may result in continued accumulation of MC congeners in the brain, brain pathology and thus possibly behavioral changes in the individual fish, e.g. swimming activity (Cazenave et al. 2008; Ernst et al. 2007; Tencalla and Dietrich 1997). In a similar scenario, rainbow trout or little skate subchronically and chronically exposed to the same cyanobacterial bloom may primarily experience liver and kidney damage followed by regeneration possibly without having any tangible effect on the behavior or overall survival of the individual or the local population. Moreover, in a cyanobacterial bloom where primarily MC-LR is present at low concentrations, uptake of MC-LR via zfOatp1d1 in the gastrointestinal tract would evolve slowly due to the lower affinity and capacity of zfOatp1d1. Assuming a bloom would primarily contain the more lipophilic MC-LF, rapid uptake from the gastrointestinal tract would ensue due to the higher affinity and capacity of zfOatp1d1 for MC-LF despite the lower presence of zfOatp1d1 in the gastrointestinal tract. Systemic distribution of MC-LF, if not completely removed via the first-pass effect in the liver, could result in neurotoxicity and renal toxicity due to the fact that zfOatp1d1, zfOatp1f2-1 and zfOatp1f4 are expressed in kidney and are well capable of MC-LF transport. Indeed, the latter scenario is supported by the findings of Fischer and Dietrich (2000) in carp exposed to oral concentrations of MC-LR, where time-dependent pathological changes were observed in carp hepatopancreas and kidney and detectable MC-LR was observed in brain and muscle tissue at the end of the exposure.
Remarkably, zfOatps cloned in this study demonstrated significant differences in their amino acid sequence when compared to the annotated isoforms. Whether these differences stem from alternate splicing or represent single nucleotide polymorphisms (SNPs) is difficult to ascertain with the present dataset. Indeed, confirmation at the genomic level would be required. However, the genetic variations observed in this study might be due to the fact that sequences of wild-derived zebrafish have been compared with annotated sequences from inbred strains. Indeed, intra-strain and inter-strain variations are commonly found in zebrafish (Guryev et al. 2006) suggesting that these could most likely also occur amongst the Oatp sequences analyzed. In human hOATP1B1, hOATP1B3 and hOATP2B1 more than 100 SNPs were reported (Nies et al. 2013), whereby some of the SNPs may lead to alterations in OATP expression, localization and/or function (Kalliokoski and Niemi 2009). Thus, despite that SNPs have not been described in zfOatps to date, the different transport properties of the two zfOatp1f2 expression variants regarding their transport of MC as well as common OATP substrates may be explained by their amino acid difference (Table 1) and thus could support the presence of SNPs. Furthermore, the other differences observed in the zfOatp sequences e.g. the additional/missing amino acid stretches in zfOatp1d1/zfOatp1f2-2, most likely result from alternative splicing. A comparison of zfOatp1d1 with the annotated sequence as well as with the genomic sequence (Acc.#: NC_007115.6) revealed exon number 9 encoding 65 amino acids to be missing in the annotated reference sequence at position 375. Furthermore, exon number 10 contained 11 additional amino acids. This results in a shorter protein, predicted to have only 613 and not 689 amino acids as found for zfOatp1d1 in this study. Despite the fact that this led to one TMD less in the annotated reference sequence, the functionality of this splice variant did not seem to be impaired, as a recent characterization of this Oatp also showed multi-specific transport (Popovic et al. 2013). Hence, the missing TMD in the annotated zfOatp1d1 does not appear to be decisive for the transport function of the protein. In contrast, a shorter splice variant of rat OATP1B2 (Kakyo et al. 1999) missing one exon was demonstrated to have a narrower substrate spectrum than the full-length rOATP1B2 (Cattori et al. 2000; Choudhuri et al. 2000), thereby suggesting that the localization of the missing exon and resulting missing TMD can be crucial for the transport function. Notably, both Oatp1f-members containing only 9-10 TMD (zfOatp1f1 and zfOatp1f3) did not exhibit any transport capabilities for the tested substrates, suggesting that a minimum of 11 TMD might be required for a functional zfOatp or that the missing TMD results in aberrant expression of the protein.
CONCLUSIONS
In conclusion the data presented confirm that not only zfOatp1d1 but also zfOatp1f2-1 and both cloned expression variants of zfOatp1f4 are capable of transporting MC-congeners in zebrafish. The organ-specific expression of zfOatp subtypes and their inherent MC congener transporting capabilities are key to the apical toxicity observed. Largely depending on the MC congener and concentration present in a given environmental cyanobacterial bloom scenario, apical toxicity could range from overt fish mortality and moribund fish to fish with altered behavioral patterns. The identification of Oatps as the key parameter for interpreting potential impacts of MCs in fish thus allows for better understanding of the impact of toxic cyanobacterial blooms on fish populations and the diversity of aquatic ecosystems.
Supplementary Material
ACKNOWLEDGEMENTS
The financial support from the DFG Center Grant, International Max Planck Research School for Organismal Biology (IMPRS), Marie Curie International Research Staff Exchange Scheme Fellowship (PIRSES-GA-2011-295223), the National Center for Research Resources (RR021940), and the National Institute of General Medical Sciences (GM077336 and GM103549) of the National Institutes of Health are acknowledged.
LIST OF ABBREVIATIONS
- OATP
organic anion transporting polypeptide
- MC
Microcystin
- MC-LR
Microcystin-LR
- MC-RR
Microcystin-RR
- MC-LW
Microcystin-LW
- MC-LF
Microcystin-LF
- ser/thrPP
Ser/Thr protein phosphatases
- PP1
protein phosphatase 1
- Oatp
Organic anion transporting polypeptide
- zf
zebrafish
- h
human
- m
mouse
- ORF
open reading frame
- TCA
taurocholic acid
- E3S
estrone sulfate ammonium salt
- MTX
methotrexate disodium salt
- TMD
transmembrane domain
- HEK
human embryonic kidney
- HeLa
Henrietta Lacks
- GFP
green fluorescent protein
- slco
solute carrier organic anion transporter family
REFERENCES
- Cai S-Y, Wang W, Soroka CJ, Ballatori N, Boyer JL. An evolutionarily ancient Oatp: insights into conserved functional domains of these proteins. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2002;282(4):G702–G710. doi: 10.1152/ajpgi.00458.2001. [DOI] [PubMed] [Google Scholar]
- Campos A, Vasconcelos V. Molecular mechanisms of microcystin toxicity in animal cells. International journal of molecular sciences. 2010;11(1):268–287. doi: 10.3390/ijms11010268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattori V, Hagenbuch B, Hagenbuch N, et al. Identification of organic anion transporting polypeptide 4 (Oatp4) as a major full-length isoform of the liver-specific transporter-1 (rlst-1) in rat liver. FEBS letters. 2000;474(2):242–245. doi: 10.1016/s0014-5793(00)01596-9. [DOI] [PubMed] [Google Scholar]
- Cazenave J, Nores M, Miceli M, Díaz M, Wunderlin D, Bistoni M. Changes in the swimming activity and the glutathione S-transferase activity of Jenynsia multidentata fed with microcystin-RR. Water research. 2008;42(4-5):1299–1307. doi: 10.1016/j.watres.2007.09.025. [DOI] [PubMed] [Google Scholar]
- Choudhuri S, Ogura K, Klaassen CD. Cloning of the full-length coding sequence of rat liver-specific organic anion transporter-1 (rlst-1) and a splice variant and partial characterization of the rat lst-1 gene. Biochemical and biophysical research communications. 2000;274(1):79–86. doi: 10.1006/bbrc.2000.3105. [DOI] [PubMed] [Google Scholar]
- Dietrich D, Fischer A, Michel C, Hoeger S. Toxin mixture in cyanobacterial blooms-a critical comparison of reality with current procedures employed in human health risk assessment. Advances in experimental medicine and biology. 2008;619:885. doi: 10.1007/978-0-387-75865-7_39. [DOI] [PubMed] [Google Scholar]
- Ernst B, Hoeger S, O'Brien E, Dietrich D. Oral toxicity of the microcystin-containing cyanobacterium Planktothrix rubescens in European whitefish (Coregonus lavaretus). Aquatic toxicology. 2006;79(1):31. doi: 10.1016/j.aquatox.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Ernst B, Hoeger SJ, O'Brien E, Dietrich DR. Physiological stress and pathology in European whitefish (Coregonus lavaretus) induced by subchronic exposure to environmentally relevant densities of Planktothrix rubescens. Aquatic Toxicology. 2007;82:15–26. doi: 10.1016/j.aquatox.2007.01.007. [DOI] [PubMed] [Google Scholar]
- Ernst B, Hoeger SJ, O'Brien E, Dietrich DR. Abundance and toxicity of Planktothrix rubescens in the pre-alpine Lake Ammersee, Germany. Harmful Algae. 2009;8(2):329–342. [Google Scholar]
- Feurstein D, Kleinteich J, Heussner AH, Stemmer K, Dietrich DR. Investigation of Microcystin Congener-Dependent Uptake into Primary Murine Neurons Environmental Health Perspectives. 2010;118(10):1370–1375. doi: 10.1289/ehp.0901289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer A, Hoeger S, Stemmer K, et al. The role of organic anion transporting polypeptides (OATPs/SLCOs) in the toxicity of different microcystin congeners in vitro: A comparison of primary human hepatocytes and OATP-transfected HEK293 cells. Toxicology and Applied Pharmacology. 2010;245(1):9–20. doi: 10.1016/j.taap.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Fischer W, Dietrich D. Pathological and Biochemical Characterization of Microcystin-Induced Hepatopancreas and Kidney Damage in Carp (Cyprinus carpio). Toxicology and Applied Pharmacology. 2000;164(1):73–81. doi: 10.1006/taap.1999.8861. [DOI] [PubMed] [Google Scholar]
- Fischer WJ, Altheimer S, Cattori V, Meier PJ, Dietrich DR, Hagenbuch B. Organic anion transporting polypeptides expressed in liver and brain mediate uptake of microcystin. Toxicology and applied pharmacology. 2005;203(3):257–263. doi: 10.1016/j.taap.2004.08.012. [DOI] [PubMed] [Google Scholar]
- Guryev V, Koudijs E, Berezikov E, Johnson S L, Plasterk H, van Eeden FJM, Cuppen E. Genetic variation in the zebrafish. Genome Research. 2006;16(4):491–497. doi: 10.1101/gr.4791006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenbuch B, Stieger B. The SLCO (former SLC21) superfamily of transporters. Molecular Aspects of Medicine. 2013;34(2-3):396–412. doi: 10.1016/j.mam.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höger SJ, Schmid D, Blom JF, Ernst B, Dietrich DR. Analytical and functional characterization of microcystins [Asp3] MC-RR and [Asp3, Dhb7] MC-RR: consequences for risk assessment? Environmental science & technology. 2007;41(7):2609–2616. doi: 10.1021/es062681p. [DOI] [PubMed] [Google Scholar]
- Kakyo M, Sakagami H, Nishio T, et al. Immunohistochemical distribution and functional characterization of an organic anion transporting polypeptide 2 (oatp2). FEBS letters. 1999;445(2):343–346. doi: 10.1016/s0014-5793(99)00152-0. [DOI] [PubMed] [Google Scholar]
- Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. British journal of pharmacology. 2009;158(3):693–705. doi: 10.1111/j.1476-5381.2009.00430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Furukawa T, Ikeda R, et al. Involvement of mitogen-activated protein kinase signaling pathways in microcystin-LR–induced apoptosis after its selective uptake mediated by OATP1B1 and OATP1B3. Toxicological sciences. 2007;97(2):407–416. doi: 10.1093/toxsci/kfm054. [DOI] [PubMed] [Google Scholar]
- Kotak B, Semalulu S, Fritz D, Prepas E, Hrudey S, Coppock R. Hepatic and renal pathology of intraperitoneally administered microcystin-LR in rainbow trout (Oncorhynchus mykiss). Toxicon: official journal of the International Society on Toxinology. 1996;34(5):517–525. doi: 10.1016/0041-0101(96)00009-8. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lu H, Choudhuri S, Ogura K, et al. Characterization of organic anion transporting polypeptide 1b2-null mice: essential role in hepatic uptake/toxicity of phalloidin and microcystin-LR. Toxicological sciences. 2008;103(1):35–45. doi: 10.1093/toxsci/kfn038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Abt F, Hammann-Hänni A, Stieger B, Ballatori N, Boyer J. The organic anion transport polypeptide 1d1 (Oatp1d1) mediates hepatocellular uptake of phalloidin and microcystin into skate liver. Toxicology and applied pharmacology. 2007;218(3):274–279. doi: 10.1016/j.taap.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Monks NR, Liu S, Xu Y, Yu H, Bendelow AS, Moscow JA. Potent cytotoxicity of the phosphatase inhibitor microcystin LR and microcystin analogues in OATP1B1-and OATP1B3-expressing HeLa cells. Molecular cancer therapeutics. 2007;6(2):587–598. doi: 10.1158/1535-7163.MCT-06-0500. [DOI] [PubMed] [Google Scholar]
- Nies AT, Niemi M, Burk O, et al. Genetics is a major determinant of expression of the human hepatic uptake transporter OATP1B1, but not of OATP1B3 and OATP2B1. 2013. [DOI] [PMC free article] [PubMed]
- Popovic M, Zaja R, Fent K, Smital T. Molecular Characterization of Zebrafish Oatp1d1 (Slco1d1), a Novel Organic Anion-transporting Polypeptide. Journal of Biological Chemistry. 2013;288(47):33894–33911. doi: 10.1074/jbc.M113.518506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic M, Žaja R, Smital T. Organic anion transporting polypeptides (OATP) in zebrafish (Danio rerio): phylogenetic analysis and tissue distribution. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2010;155(3):327–335. doi: 10.1016/j.cbpa.2009.11.011. [DOI] [PubMed] [Google Scholar]
- Puddick J, Prinsep MR, Wood SA, Kaufononga SA, Cary SC, Hamilton DP. High Levels of Structural Diversity Observed in Microcystins from Microcystis CAWBG11 and Characterization of Six New Microcystin Congeners. Marine drugs. 2014;12(11):5372–5395. doi: 10.3390/md12115372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivonen K, Jones G. Cyanobacterial toxins. In: Chorus I, Bartram J, editors. Toxic cyanobacteria in water: A guide to their public health consequences, monitoring and management. Spon Press; 1999. pp. 26–68. [Google Scholar]
- Steiner K, Hagenbuch B, Dietrich DR. Molecular cloning and functional characterization of a rainbow trout liver Oatp. Toxicology and applied pharmacology. 2014;280(3):534–542. doi: 10.1016/j.taap.2014.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tencalla F, Dietrich D. Biochemical characterization of microcystin toxicity in rainbow trout (Oncorhynchus mykiss). Toxicon: official journal of the International Society on Toxinology. 1997;35(4):583. doi: 10.1016/s0041-0101(96)00153-5. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biotechnology. 1992;24:145–9. [PubMed] [Google Scholar]
- Wang P, Hata S, Xiao Y, Murray JW, Wolkoff AW. Topological assessment of oatp1a1: a 12-transmembrane domain integral membrane protein with three N-linked carbohydrate chains. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2008;294(4):G1052–G1059. doi: 10.1152/ajpgi.00584.2007. [DOI] [PubMed] [Google Scholar]
- Zeck A, Weller MG, Bursill D, Niessner R. Generic microcystin immunoassay based on monoclonal antibodies against Adda. Analyst. 2001;126(11):2002–2007. doi: 10.1039/b105064h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.