Abstract
Autism spectrum disorder (ASD) is a group of heritable disorders with complex and unclear etiology. Classic ASD symptoms include social interaction and communication deficits as well as restricted, repetitive behaviors. In addition, ASD is often comorbid with intellectual disability. Fragile X syndrome (FXS) is the leading genetic cause of ASD, and is the most commonly inherited form of intellectual disability. Several mouse models of ASD and FXS exist, however the intellectual disability observed in ASD patients is not well modeled in mice. Using the Fmr1 knockout mouse, and the eIF4E transgenic mouse, two previously characterized mouse models of fragile X syndrome and ASD respectively, we generated the eIF4E/Fmr1 double mutant mouse. Our study shows that the eIF4E/Fmr1 double mutant mice display classic ASD behaviors, as well as cognitive dysfunction. Importantly, the learning impairments displayed by the double mutant mice spanned multiple cognitive tasks. Moreover, the eIF4E/Fmr1 double mutant mice display increased levels of basal protein synthesis. The results of our study suggest that the eIF4E/Fmr1 double mutant mouse may be a reliable model to study cognitive dysfunction in the context of ASD.
Keywords: Autism spectrum disorders, protein synthesis, eIF4E, Fragile X syndrome, cognition, genetic models, rodent behavior
Introduction
Autism spectrum disorder (ASD) is a heterogeneous group of heritable disorders that are defined by the presence of two behavioral characteristics: persistent deficits in social communication and social interaction, and restricted, repetitive patterns of behavior, interests, or activities (Association, 2013). These core symptoms often are accompanied by a number of other characteristics including intellectual disability (ID), motor impairments, anxiety disorders, among others (Association, 2013). The rate of ID in patients with autism has been reported to be as high as 70% (Fombonne, 2003), although more recent studies indicate a more conservative estimate of approximately 55% (Charman et al., 2011). The variability of symptoms that are observed in ASD is wide and can span from mild to severely debilitating. ASD affects approximately 1 in 68 children in the United States alone, with a higher prevalence occurring in boys compared to girls (Centers for Disease Control and Prevention (CDC), 2014). There are two classifications of ASD: syndromic and idiopathic (non-syndromic). Idiopathic ASD is used to describe cases where ASD is the primary diagnosis and syndromic ASD consists of cases where ASD is a secondary diagnosis, which can be linked to common genetic variants such as Rett syndrome or fragile X syndrome (Carter and Scherer, 2013). As such, ASD is considered a polygenic disorder, which can arise from multiple different genetic variants, in addition to unidentified environmental factors (Carter and Scherer, 2013; Klei et al., 2012).
Fragile X syndrome (FXS) is a developmental disorder and one of the most common inherited forms of ID, characterized by moderate to severe ID and mild facial deformities. A recent meta-analysis reports FXS prevalence to be approximately 1 in 7000 males, and 1 in 11,000 females (Hunter et al., 2014). FXS is one of the aforementioned genetic variants that can lead to syndromic ASD (Carter and Scherer, 2013). In fact, FXS is the leading genetic cause of ASD (Coffee et al., 2009) and 60-74% of patients with FXS have at least one behavioral feature that can be classified as an ASD (McCary and Roberts, 2012). Shared symptoms between FXS and ASD include behavioral and cognitive deficits as well as poor eye contact, repetitive behaviors, and social deficits (Gabis et al., 2011). Children with FXS and ASD are diagnosed developmentally as the lowest functioning group in both adaptive and developmental measures (Kau et al., 2004; Rogers et al., 2001). Moreover, in FXS patients, autism measures scale with increasing age; similarly, there is a 10-fold increase in morbidity with time (Hatton et al., 2006; Sabaratnam et al., 2003). Because FXS is the leading cause of syndromic ASD, studying these two disorders in the context of one another in a mouse model is a promising way to study how FXS and ASD comorbidities may develop.
The Fmr1 knockout (KO) mouse is a widely used and well-established mouse model of FXS. These mice exhibit symptoms reminiscent of FXS through the deletion of the Fmr1 gene, which encodes for the fragile x mental retardation protein (FMRP). Behavioral and physical characteristics of these mice include stereotypic and repetitive behavior, macroorchidism, and some cognitive impairments (Bhattacharya et al., 2012). These mice also exhibit increased basal levels of protein synthesis (Bhattacharya et al., 2012). One limitation in studying the Fmr1 KO mouse is that strain differences can strongly influence phenotypes exhibited by these mice, leading to disparate outcomes (Dobkin et al., 2000; Paradee et al., 1999; Spencer et al., 2011). Other studies indicate that in general, background strain differences can influence behavioral outcomes, particularly measures of ASD-like behaviors. (Moy et al., 2008; 2007). In accordance with the overlap of symptoms observed between patients with FXS and ASD, mouse models of ASD share some behavioral traits with Fmr1 KO mice. For example, the eIF4E transgenic (Tg) mouse is a recently characterized mouse model of ASD that also exhibit exaggerated levels of translation via the overexpression of the eukaryotic initiation factor 4E (eIF4E) protein (Santini et al., 2013). Behaviorally, these mice exhibit repetitive and stereotypic behavior, impaired social interactions, and cognitive inflexibility. Similar to the Fmr1 KO mice (Huber et al., 2002), these mice also exhibit enhanced hippocampal metabotropic glutamate receptor-long term depression (mGluR-LTD) as well as enhanced striatal mGluR-LTD (Santini et al., 2013).
Despite the growing number of mouse models of ASD and FXS, there are surprisingly few mouse models that recapitulate the intellectual disability observed by patients with autism. Rodent studies that do report impaired cognitive function only show deficits across some behavioral tasks. For instance, one study showed that the Fmr1 KO mice have an impairment in a cross-shaped water maze task, whereas both contextual and cued fear memory were intact (Dobkin et al., 2000). Another study showed that strain differences can strongly influence the performance of the Fmr1 KO mice in the Morris water maze (Paradee et al., 1999). Our current understanding of the heritability of ASD suggests that ASD can arise from the mutation of several different genes, often in combination (Santini and Klann, 2014). Therefore, a mouse model that employs a genetic strategy impinging on multiple gene mutations may be a viable way to study how ASD and FXS occur co-morbidly. In addition, this type of approach could lead to a mouse model of ASD that encompasses a wider range of ASD-associated symptoms, including cognitive dysfunction.
In the current study, we utilized two previously characterized mouse models of FXS and ASD, Fmr1 KO mice and eIF4E Tg mice, respectively, to generate eIF4E/Fmr1 double mutant mice. We found that these mice exhibit stereotypic and repetitive behaviors, impaired social interactions, as well as impaired learning and memory across multiple cognitive tasks. Consistent with previous reports, these genetic alterations also lead to an overall increase in protein synthesis. Our results suggest that the eIF4E/Fmr1 double mutant mouse is a mouse model of ASD that reliably displays cognitive dysfunction in addition to core ASD-like behaviors.
Materials and Methods
Ethics Statement
All procedures were performed in accordance with protocols approved by the New York University Animal Welfare Committee and followed the NIH Guidelines for the use of animals in research. All mice were housed in the New York University animal facility and were compliant with the NIH Guide for Care and Use of Laboratory Animals.
Housing/Mice
The following four genotypes were used in this study: wildtype (WT), eIF4E Tg, Fmr1 KO, and eIF4E/Fmr1 double mutant (n = 10-12/genotype). Fmr1 KO mice were bred and maintained on a C57/BL6 (Jackson Labs) background, as were the eIF4E Tg mice, both of which have been described previously (Hou et al., 2006; Santini et al., 2013). Fmr1 KO mice were backcrossed routinely with C57BL/6 mice every three generations. eIF4E Tg/Fmr1 double mutant mice were initially generated by crossing heterozygous female mice carrying the Fmr1 deletion with heterozygous male mice carrying the eIF4E mutation. Subsequently, all animals used for experimentation were derived from the crossing of female XFmr1+XFmr1−/eIF4E+/− with males either XFmr1+Y/eIF4E+/− or XFmr1−Y/eIF4E+/−. WT, Fmr1 KO, eIF4E Tg and eIF4E/Fmr1 double mutant mice were obtained with the predicted Mendelian ratios. Male mice, approximately three months of age were used in this study.
Mice were housed with their littermates in groups of 2-3 animals per cage and kept on a 12 h regular light/dark cycle, with food and water provided ad libitum.
Behavior
A battery of tests was used to assess ASD-like behaviors and memory function (Silverman et al., 2010). The behavioral tests were conducted in order of increasing aversiveness: open field, elevated plus maze, marble-burying, social interaction, novel object recognition, Morris water maze, and fear conditioning. For all experiments, mice were acclimated to the testing room 30 min before behavioral training and all behavior apparatuses were cleaned between each trial with a 30% ethanol solution. The experimenters were blind to genotype of the mice while performing and scoring all behavioral tasks. Each experimental test was performed at approximately the same time of day for each animal. All measures are expressed as a mean ± SEM.
Open Field
The open field test was used to measure locomotor activity and anxiety-like behavior, and was performed as described previously (Santini et al., 2013). Mice were placed into the center of a clear Plexiglas (40 × 40 × 30 cm) open field arena and allowed to explore for 15 min. Illumination was provided by overhead lights (~800 lux) inside the arena. The amount of time each mouse spent in the center and periphery of the OF, and total distance traveled was measured. Experiments were performed in the presence of a 55 dB white noise. Data were collected at 2 min intervals controlled by a Digiscan optical animal activity system (RXYZCM, AccuScan Instruments, OH, USA).
Elevated Plus Maze
The plus maze consisted of two contralateral open arms (30 cm × 5 cm) and two contralateral enclosed arms (30 × 5 × 15 cm) elevated off the ground (40 cm), and was performed as described previously (Banko et al., 2007). Each mouse was placed in the center of the maze facing an open arm and allowed to explore the maze for a total of 5 min. The frequency of open and closed arm entries, as well as duration in all arms was recorded using Ethovision XT video tracking software (Noldus).
Marble Burying
The marble-burying task was used to assess repetitive behaviors and was performed as described previously (Santini et al., 2013). Twenty small black marbles were arranged in a 5 × 4 matrix in a clean plexiglass cage (27.5 × 18.5 × 22 cm) containing white house bedding approximately 3 inches deep. Mice were introduced into this test arena and allowed 30 min to explore under dim lighting. At the end of this time, the mouse was removed and the number of buried marbles was counted. A threshold of at least 75% coverage was used to arbitrate a marble as buried.
Social Interaction
Social behavior was measured using a 3-chambered social arena as described previously (Moy et al., 2007; Santini et al., 2013). Mice received a 5 min habituation session to the arena in the presence of two wire cages, one in each side of the chamber. A 5 min social preference test followed in which the mice were allowed to explore the three-chambered arena containing a caged mouse (stranger mouse) on one side, and a wire cage containing an inanimate object. The placement of social and non-social targets was counterbalanced between animals. The measured parameters of social interaction were: time spent in each chamber and interaction time with each target, calculated by Ethovision XT video tracking software (Noldus).
Novel Object Recognition
The novel object recognition task was performed as described previously (Hoeffer et al., 2008). Different shaped and colored lego blocks (approximately 1 × 1 × 2 in) were used for the familiar and novel objects. On days one and two, mice were habituated to the arena and two familiar objects for 10 min. The following day, the mice were exposed to the arena with the same two identical objects and the interaction time with either object was recorded during a 10 min period. On day four, a novel object replaced one of the familiar objects on the non-preferred side which was measured from day 3 and the mice were given 5 min to explore the familiar and novel objects. Interaction parameters were defined as contact with the object (distance < 2 cm). The preference index was calculated using the following equation:
Fear Conditioning
Contextual and cued fear conditioning was used to measure associative fear memory and was performed as described previously (Huynh et al., 2014). Mice received 3 d of habituation to the training context, consisting of a white house light and metal conducting grid floor. On the training day, mice were acclimated to the training context for 4 min, and then given two conditioned stimulus (30 s, 80 dB tone) – unconditioned stimulus (0.5 mA footshock) pair presentations, which were separated by a 2 min inter-trial interval (Huynh et al., 2014). To measure contextual fear memory, the mice were placed back into the original training context, and the amount of time spent freezing was measured over a 3 min period. Freezing behavior is represented as a percentage of time spent freezing while in the training context.
Cued fear conditioning was measured by placing the mouse into a novel context, which consisted of a red house light, a white plexiglass floor and vanilla scented bedding. Following a 4 min exploration time in the novel context, the mice received 3 tone presentations separated by a 2 min inter-trial interval. Freezing behavior was calculated as an average percentage of time the mouse spent freezing during the tone presentations.
Morris Water Maze
The hidden platform version of the Morris water maze was used to study spatial memory and was performed as described previously (Santini et al., 2013). The maze consisted of a circular pool (52 in diameter) filled with water, and colored with white paint to increase the water’s opacity.
The pool was separated into four virtual quadrants. There were both proximal as well as distant visual cues in place. Namely, there were visual cues placed both on the pool and around the room to mark the position of the different quadrants and a hidden platform was placed in one quadrant. The diameter of the platform was 10.5 cm, which was 1 cm beneath the surface of the water. The training phase consisted of three trials (60 s/trial) each day for six consecutive days, wherein each mouse received a maximum duration of 60 s to find the platform. On the first day only, the mice were gently taken to the platform position if they were unable to find the platform at the end of the 60 s trial. On each day, the swim start positions were in three of the quadrants such that on the next day, the quadrants that were used included the unused quadrant from the previous day. The latency to find the platform was recorded during the training phase.
A 60 s probe trial was administered at the beginning of training day four and on day seven. In the probe trial, the platform was removed from the pool, and the mice were given 60 s to explore. During the probe trial, the number of platform crossings was measured.
A visible platform test was conducted and swim speed was recorded to ensure that none of the mice were impaired in their visual acuity or swimming ability. A small flag was placed on the platform to indicate the platform position, and the latency to find the visible platform was measured. Swim speed was recorded using Ethovision XT video tracking software (Noldus).
Biochemistry
A protein synthesis assay was performed as described previously using SUnSET (Santini et al., 2013). Hippocampal slices were prepared from each genotype and incubated at 37° in artificial cerebral spinal fluid (aCSF) for 2 hr. At the end of the 2 hr incubation, puromycin (10 μg/mL in vehicle, a subthreshold concentration for total synthesis blockade) was added to the aCSF and the slices were further incubated for 45 min. During this incubation time, newly synthesized proteins were end-labeled with puromycin. Puromycin was removed from the incubation media with three successive washes of oxygenated aCSF and slices were flash-frozen on dry ice. Puromycin-treated samples were identified on blots using the mouse monoclonal antibody 12D10 (1:5,000 from a 5 mg ml−1 stock). Because only a small fraction of the brain proteins were labeled, signal from blots was identified using ECL-Advance (GEHealthcare).
Statistical Analysis
Group data are presented as mean ± SEM. A one-way ANOVA followed by Dunnett’s post hoc tests was used to analyze the behavioral and biochemical data and a repeated measures ANOVA followed by Dunnett’s post hoc tests was used to analyze the marble burying, and Morris water maze data. A repeated measures ANOVA followed by Bonferroni post hoc tests was used to analyze novel object recognition data. We also conducted a 2-way ANOVA using the gene mutations as separate factors to analyze the MWM data. A Student’s t-test was used to compare time spent with the stranger mouse compared to the inanimate object for the social interaction test.
Results
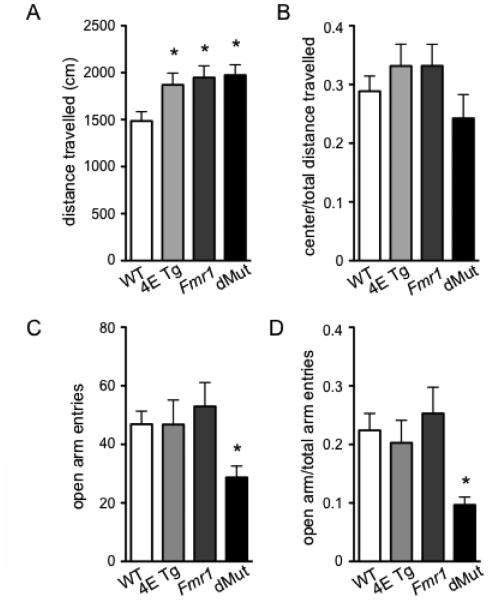
To study locomotor activity and anxiety-like behavior, we performed the open field (OF) and elevated plus maze (EPM) tasks on the eIF4E/Fmr1 double mutant mice and their wild-type (WT), eIF4E Tg and Fmr1 KO littermates. In the OF task, we found that the eIF4E Tg, Fmr1 KO and eIF4E/Fmr1 double mutant mice displayed hyperactivity compared to WT mice (Fig. 1A). Compared to the WT mice, all mutant mice traveled a higher cumulative distance over the 15 min OF task (one-way ANOVA, F (3,44) = 4.131, p = 0.0115, followed by Dunnett’s post hoc tests). There was no significant difference in the ratio of distance traveled in the center arena to total distance traveled in the OF (Fig. 1B). In the EPM task, the eIF4E/Fmr1 double mutant mice displayed anxiety-like behavior, which was not observed in any of the other three genotypes (Fig. 1C, D). The eIF4E/Fmr1 KO double mutant mice made fewer open arm entries than WT, eIF4E Tg, and Fmr1 KO mice, (one-way ANOVA, F (3,42) = 2.850, p = 0.049, followed by Dunnett’s post hoc test) and had a lower ratio of open arm entries to total arm entries (one-way ANOVA, F (3, 42) = 3.162, p = 0.0343, followed by Dunnett’s post hoc tests). The anxiety-like behavior was not a result of an overall decrease in locomotor activity by the eIF4E/Fmr1 double mutant mice, as they displayed hyperactivity in the OF, as mentioned above (Fig. 1A). These results indicate that the eIF4E/Fmr1 double mutant mice display hyperactivity, as well as anxiety-like behavior.
Figure 1. eIF4E/Fmr1 double mutant mice display hyperactivity and anxiety-like behavior.
(A) Locomotor activity measured in the Open Field (OF) test (*p < 0.05). (B) ratio of center distance to total distance traveled in the OF test. (C) Number of entries in the open arm (*p < 1.5) and (D) ratio of open arm/total arm entries in the Elevated Plus Maze (EPM) task (*p < 0.05). Data are represented as the mean ± SEM (n = 10-14). WT, 4E Tg, Fmr1 and dMut denote wild-type, eIF4E transgenic, Fmr1 knock-out and eIF4E/Fmr1 double mutant mice, respectively.
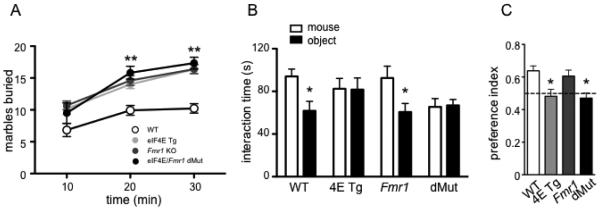
To study classic ASD-like behaviors in the eIF4E/Fmr1 double mutant mice, we used the marble burying task and 3-chambered social interaction task to measure repetitive/stereotypic behavior and social behavior, respectively. We observed that the eIF4E Tg, Fmr1 KO and eIF4E/Fmr1 double mutant mice all displayed stereotypic behavior in the marble burying task (Fig. 2A). Over the 30-min marble burying task, the eIF4E Tg, Fmr1 KO and eIF4E/Fmr1 double mutant mice buried more marbles than WT mice (one-way ANOVA, F (3, 36) = 17.95, p < 0.0001) and at a faster rate (repeated measure ANOVA, F (2, 36) = 59.92, p < 0.0001). For the social interaction task, both the eIF4E Tg and eIF4E/Fmr1 double mutant mice displayed deficits in social interaction (Fig. 2B, C). The WT and Fmr1 KO mice both preferred the stranger mouse to the inanimate object, (Student’s t-test, t (13) = 2.433, p = 0.0316 and t (11) = 2.816, p = 0.0183, respectively.) whereas the eIF4E Tg and eIF4E/Fmr1 KO mice did not (Fig. 2B). These results also were reflected in the preference index, which was calculated using the time spent with the stranger mouse divided by the total time spent with either the mouse or the object. Using the preference index, neither the eIF4E Tg nor eIF4E/Fmr1 double mutant mice preferred the stranger mouse to the object (Fig. 2C) (one-way ANOVA, F(3, 40) = 3.998, p = 0.0140, followed by Dunnett’s post hoc tests). These results indicate that the eIF4E/Fmr1 double mutant mice display classic ASD-like behaviors: repetitive and stereotypic behaviors as well as deficits in social interactions.
Figure 2. eIF4E/Fmr1 double mutant mice show repetitive behaviors and impairment in social interaction.
(A) Cumulative data of the number of marbles buried in 10-min intervals (**p < 0.01). (B) Time spent interacting with either the stranger mouse or inanimate object in the three-chambered arena test (*p < 0.05) and (C) preference index calculated as time of interaction with the stranger mouse over the total interaction time with either the mouse or the object (*p < 0.05). Data are represented as the mean ± SEM (n = 8-12). WT, 4E Tg, Fmr1 and dMut denote wild-type, eIF4E transgenic, Fmr1 knock-out and eIF4E/Fmr1 double mutant mice, respectively (B, C).
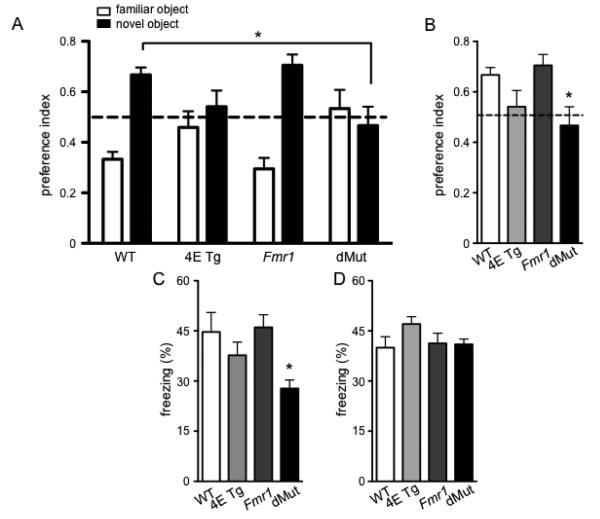
To examine learning and memory in the eIF4E/Fmr1 double mutant mice, we used the novel object recognition (NOR), fear conditioning, and Morris water maze (MWM) tasks. In the NOR task, a preference index was calculated using the amount of time the mouse spent interacting with the novel object divided by the total time spent with either the novel object or familiar object (Fig. 3A, B). A repeated measures ANOVA revealed an interaction between preference for the novel object and genotype (Fig. 3A) (F (3, 43) = 3.842, p < 0.05). Specifically, there was a preference for the novel object over the familiar object for the WT, eIF4E Tg, and Fmr1 KO mice (one-way ANOVA, F (1, 43) = 11.51, p < 0.01). However, bonferroni post hoc tests indicated that compared to WT mice, the eIF4E/Fmr1 double mutant mice did not exhibit this preference (Fig. 3B) (Bonferroni post hoc, p < 0.05). The eIF4E/Fmr1 double mutant mice also displayed impaired contextual fear memory, but not cued fear memory (Fig. 3C, D). Compared to WT, eIF4E Tg, and Fmr1 KO mice, the eIF4E/Fmr1 double mutant mice spent less time freezing in a context in which they received 2 tone-shock pairs (Fig. 3C) (one-way ANOVA, F (3, 37) = 3.276, p = 0.0316, followed by Dunnett’s post hoc tests.) In contrast, in the cued fear memory test, all mice displayed similar levels of freezing during the tone presentation (Fig. 3D) (one-way ANOVA, n.s.). The results obtained from the NOR and fear conditioning tasks demonstrate that the eIF4E/Fmr1 double mutant mice display impaired associative memory in both a neutral and fearful context.
Figure 3. eIF4E/Fmr1 double mutant mice display impaired associative memory.
(A) Preference index for the Novel Object Recognition task (NOR) calculated as time of interaction with either the familiar or the novel object over the total time spent with the novel and familiar objects (*p < 0.05). (B) Preference index for the NOR depicting the preference index for the novel object only. (*p < 0.05). (C) Percentage of time spent in freezing behavior in the context associated with the shocks (contextual fear memory, (*p < 0.05) and (D) in a novel context during the presentation of the tone associated with the shocks (cued fear memory). Data are represented as mean ± SEM (n = 10-14). WT, 4E Tg, Fmr1 and dMut denote wild-type, eIF4E transgenic, Fmr1 knock-out and eIF4E/Fmr1 double mutant mice, respectively.
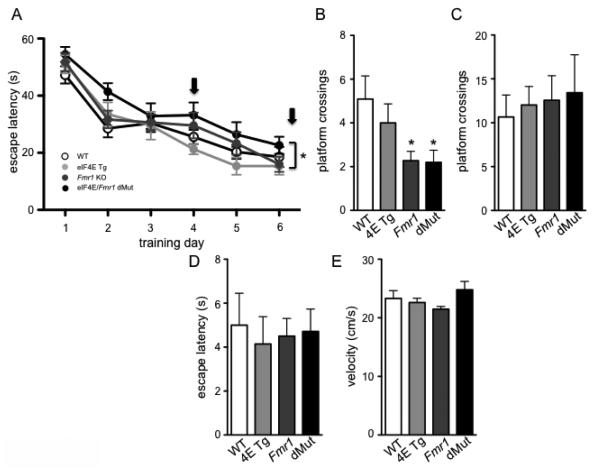
The performance of the eIF4E/Fmr1 double mutant mice in the MWM task revealed mixed results depending on which day the mice were probed for their spatial memory. Overall, in the training phase of the MWM, there was a main effect of time (repeated measure ANOVA, F (5, 44) = 53.06, p < 0.0001) and genotype (one-way ANOVA, F (3, 44) = 3.710, p = 0.0183) but no interaction between time and genotype (Fig. 4A). On day 4 of the probe trial, the Fmr1 KO and eIF4E/Fmr1 double mutant mice both made fewer platform crossings than the WT and eIF4E Tg mice (Fig. 4B)(one-way ANOVA, F (3, 40) = 3.158, p = 0.0350, followed by Dunnett’s post hoc tests). On the day 7 probe trial, all of the mice made similar numbers of platform crossings (Fig. 4C)(one-way ANOVA, n.s.), indicating that the Fmr1 KO and eIF4E/Fmr1 double mutant mice were no longer impaired in their memory for the platform position. We also performed a 2-way ANOVA to determine whether there was a differential contribution of either eIF4E overexpression or Fmr1 deletion on MWM performance across genotypes. We found that there was a main effect of the Fmr1 deletion during the training phase of the MWM (F (1,44) = 6.929, p = 0.012) as well as a main effect of the Fmr1 deletion on the day 4 probe trial (F (1, 43) = 8.490, p = 0.006). This analysis indicates that the deletion of Fmr1 in both the Fmr1 KO mice and eIF4E/Fmr1 double mutant mice may be responsible for the poor performance in the MWM compared to WT and eIF4E Tg mice. These results are consistent with previous reports that eIF4E overexpression does not affect performance in the MWM (Santini et al., 2013). The results obtained in the MWM were not due to either differences in swim speed or visual impairments as all mice performed similarly in the visual platform test (Fig. 4D) (one-way ANOVA, n.s.) and exhibited similar swim speeds (Fig. 4E)(one-way ANOVA, n.s.). These results indicate that the eIF4E/Fmr1 double mutant mice display mildly impaired hippocampal spatial learning and memory in the MWM.
Figure 4. eIF4E/Fmr1 double mutant mice show impaired spatial memory after short but not prolonged training protocols.
(A) Latency to find the hidden platform during the training phase (3 trials/day for 6 days) of the Morris Water Maze (MWM). Arrows denote the days on which the probe test was performed (*p < 0.05) (A). (B) Number of platform crossings during the probe test (platform removed) performed on day 4 (*p < 0.05) and day 7 (C). (D) Latency to find the platform during the visible phase of the test (platform flagged). (E) Swim speed recorded on day 7, during the probe test. Data are represented as the mean ± SEM (n = 10-14). WT, 4E Tg, Fmr1 and dMut denote wild-type, eIF4E transgenic, Fmr1 knock-out and eIF4E/Fmr1 double mutant mice, respectively.
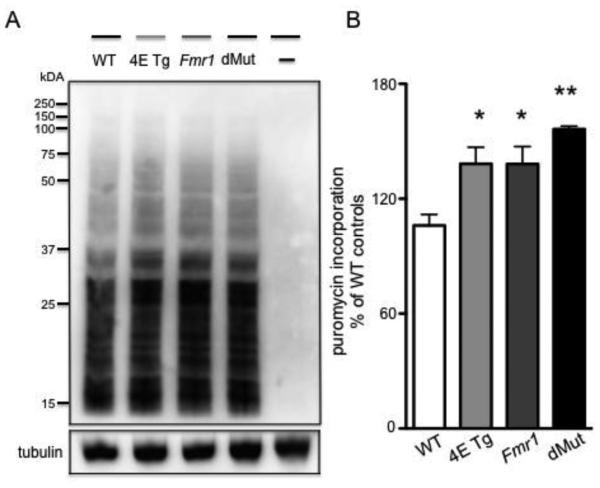
Finally, we performed the SunSET assay on hippocampal slices to measure basal protein synthesis in the four genotypes. Previous studies indicate that the eIF4E transgenic mice and Fmr1 KO mice both display exaggerated levels of protein synthesis (Bhattacharya et al., 2012; Santini et al., 2013). Our results indicate that the eIF4E Tg, Fmr1 KO and eIF4E/Fmr1 double mutant mice all display higher levels of protein synthesis compared to WT mice (Fig. 5A, B) (one-way ANOVA, F (3, 8) = 8.988, p = 0.0061). Moreover, Dunnett’s post hoc tests revealed that the eIF4E/Fmr1 double mutant mice exhibit a more statistically significant difference in protein synthesis than WT mice (p < 0.01) than either the eIF4E TG or Fmr1 KO mouse do in comparison to WT mice (p < 0.05). Although there was a trend towards increased protein synthesis in the eIF4E/Fmr1 double mutant compared to the single mutant mice, these results did not reach statistical significance. Overall, these results show that the eIF4E/Fmr1 double mutant mice display increased levels of protein synthesis.
Figure 5. eIF4E/Fmr1 double mutant mice display enhanced hippocampal protein synthesis.
Labeling of newly synthesized proteins performed with the SUnSET (see Materials and Methods) in hippocampal slices. (A) Representative blot (B) and summary of puromycin incorporation expressed as percentage of WT control and represented as means ± SEM (*p < 0.05; **p < 0.01, n= 4). WT, 4E Tg, Fmr1 and dMut denote hippocampal samples treated with puromycin obtained from wild-type, eIF4E transgenic, Fmr1 knock-out and eIF4E/Fmr1 double mutant mice, respectively. – denotes wild-type hippocampal slices untreated controls.
In summary, our results show that the eIF4E/Fmr1 double mutant mice display stereotypic/repetitive behavior, deficits in social interactions, cognitive impairments, and enhanced protein synthesis.
Discussion
Our study indicates that eIF4E/Fmr1 double mutant mice exhibit cognitive dysfunction in addition to ASD-like behaviors. Similar to the eIF4E Tg and Fmr1 KO mice, the eIF4E/Fmr1 double mutant mice displayed stereotypic behaviors, as well as deficits in social interactions. In contrast to the single mutant and WT mice, the eIF4E/Fmr1 double mutant mice also displayed cognitive deficits in a number of behavioral tasks. In the training phase of the Morris water maze (MWM), the double mutant mice took consistently longer than the WT mice to find the position of the hidden platform. Moreover, on probe tests on day 4, the double mutant mice displayed fewer platform crossings than WT mice. Interestingly, this effect was no longer apparent on the probe test on day 7, indicating that the double mutant mice are able to locate the position of the platform at similar rates as their WT littermates after several days of training. For the novel object recognition, the double mutant mice did not show a preference for the novel object whereas the WT, eIF4E Tg, and Fmr1 KO mice all preferred the novel object over the familiar object. Finally, the eIF4E/Fmr1 double mutant mice also showed impaired contextual fear memory compared to their WT and single mutant littermates; cued fear memory was unimpaired. The comparison of behavioral differences we observe in the eIF4E Tg, Fmr1 KO and eIF4E/Fmr1 double mutant mice is illustrated in Table 1.We also conducted SunSET assays to measure basal levels of protein synthesis in hippocampal slices and found that the double mutant mice have higher levels of protein synthesis compared to WT mice. Overall, our data show that the double mutant mice display ASD-like behaviors, impairments in several cognitive tasks, and exhibit increased levels of protein synthesis.
Table 1.
Summary of behavioral and biochemical phenotypes for eIF4E Tg, Fmr1 KO and eIF4E/Fmr1 double mutant mice in comparison to wild-type littermates.
| Phenotype | Tests | elF4E Tg Mice | Fmr1 KO Mice | elFAE/Fmrl
Double Mutant Mice |
|---|---|---|---|---|
|
| ||||
| anxiety-like behavior |
elevated plus maze |
− | − | + |
|
| ||||
| ASD-like behavior |
repetitive behavior (marble-burying, locomotor activity) |
+ | + | + |
| social behavior (social preference) |
+ | − | + | |
|
| ||||
| learning and memory |
novel object recognition |
− | − | + |
| contextual fear conditioning |
− | − | + | |
| Morris water maze |
− | +(partial) | +(partial) | |
|
| ||||
| exaggerated protein synthesis |
SUnSET | + | + | + |
The eIF4E/Fmr1 double mutant mouse is a reliable model of ASD because it displays the phenotypes of the eIF4E Tg and Fmr1 KO single mutant mice, along with cognitive dysfunction, which is not always observed in other mouse models of ASD (Peça et al., 2011; Santini et al., 2013). In this study, both the eIF4E Tg and Fmr1 KO mice exhibited stereotypic and repetitive behaviors as previously reported, but only the Fmr1 KO mice have been shown to display learning impairments, albeit there are conflicting reports (Bhattacharya et al., 2012; Dobkin et al., 2000; Santini et al., 2013). One study reported a learning deficit in a water maze task in Fmr1 KO mice when the mice were generated under a FVB/129 background, but not when the mice were generated under a pure C57BL/6 background (Dobkin et al., 2000). The Fmr1 KO mice in our study were generated on a pure C57BL/6 background, and although they did not exhibit an impairment in the NOR as previously reported from our lab (Bhattacharya et al., 2012), they did show an impairment on the day 4 probe test in the MWM. Notably, the Fmr1 KO mice that were studied previously were generated on a mixed C57BL/6 and SveJ/129 background, which may underlie the reason for the behavioral differences observed in comparison to the current study. Several other independent studies are in support of the finding that strain differences in the Fmr1 KO mice can lead to disparate behavioral outcomes (Paradee et al., 1999; Spencer et al., 2011). Moreover, differences in genetic background have also been shown to affect behavior across a multitude of tasks including the elevated plus maze, MWM, and T-maze tasks (Moy et al., 2007).
Our results indicate that although the eIF4E/Fmr1 double mutant mice were impaired in the early phases of the MWM test (Fig. 4A), the position of the hidden platform could be learned by day seven of training (Fig. 4C). This is an important observation because it suggests that the memory impairment in the eIF4E/Fmr1 double mutant mice can be overcome with multiple days of training. In clinical populations, there is evidence that ASD patients rely heavily on declarative memory in order to compensate for other memory and social deficits (Ullman and Pullman, 2015). Moreover, reports in patients with ASD suggest that declarative memory is largely intact in high and mid-level functioning ASD patients (Boucher et al., 2012). On the other hand, high functioning ASD patients, although having only moderate ID, often have comorbidities with at least two anxiety disorders (Salazar et al., 2015; Ung et al., 2013). These findings are consistent with the fact that autism presents as a spectrum and that there is a broad range of clinical symptoms observed in human patients.
FXS is the leading genetic cause of ASD, with 5% of ASD cases being attributed to FXS. Although it is not currently known how FXS can lead to autism, there are several working hypotheses. One current hypothesis is that the reduced FMRP can lead to atypical brain development in regions that are associated with autism (McCary and Roberts, 2012). Another hypothesis posits that there is an additive effect of FMRP relating to autism in conjunction with a secondary medical condition that may increase the risk for autism (García-Nonell et al., 2008). Our findings indicate that the eIF4E/Fmr1 double mutant mice retain phenotypic characteristics of the eIF4E Tg and Fmr1 KO mice, with an added component of cognitive dysfunction. Another observation from the current study is that the eIF4E/Fmr1 double mutant mice exhibited similar levels of stereotypic/repetitive behaviors as the eIF4E Tg and Fmr1 KO mice, although this may be because the single mutant mice demonstrate ceiling effects in the behavioral tasks that were used. ASD is a complex disorder that is often comorbid with other disorders, including intellectual disability. The comorbidity of ASD in FXS patients is estimated at between 30-50%, with an even higher percentage of FXS patients (60-74%) exhibiting at least one behavior that classifies as an ASD (McCary and Roberts, 2012). In addition, the behavioral profile of patients with FXS and ASD can be different than in individuals with either disorder alone. One study indicated that children with both ASD and FXS exhibit less of a social behavior deficit than children with FXS alone (Kau et al., 2004). However, other behaviors in patients with FXS and ASD are exacerbated. For instance, the same study showed that children with both FXS and ASD showed more impairments in overall cognition and adaptive behaviors compared to children with FXS only (Kau et al., 2004). These observations are in support of the need to study comorbidities in ASD and FXS.
To study ASD in mice, several genetic mouse models have been used which impact proteins that impinge on translational control, whereas other mouse models exist in which genes encoding for synaptic proteins are mutated (Santini and Klann, 2014). For example, mice with a Shank3 deletion, which is a component of postsynaptic densities, exhibit extreme grooming behavior, anxiety-like behavior and are also impaired in social interactions (Peça et al., 2011). Shank3 is a protein that is strongly expressed in the striatum, indicating both a critical role of this synaptic protein in modulating stereotypic behavior and of a role of the striatum in repetitive behavior. It is interesting to note that repetitive and stereotypic behaviors are among the most reproducible of the ASD-like phenotypes across various mouse models (Peça et al., 2011; Santini et al., 2013; Santini and Klann, 2014). These behaviors are largely dependent on the striatum, whereas many cognitive behaviors, particularly the ones used in this study, rely on the hippocampus. Indeed, although the Shank3 mice show exaggerated grooming behavior and deficits in social behavior, these mice do not exhibit any impairments in the MWM (Peça et al., 2011). It will be of interest to study the contribution of the hippocampus and striatum in generating ASD-like behaviors, and specifically, how exaggerated translation may differentially impact these brain regions.
Our current study employs a genetic mouse model that alters levels of eIF4E and FMRP, which are proteins that are critically involved with the regulation of protein synthesis. eIF4E is a translation factor that is activated when its repressor protein, 4E-BP is phosphorylated by the mammalian target of rapamycin complex 1 (mTORC1), allowing eIF4E to associate with eIF4G, thereby triggering cap-dependent translation initiation (Gingras et al., 2001). FMRP on the other hand, is thought to be involved with translation elongation via ribosomal stalling (Darnell and Klann, 2013); however, there also is evidence of a role for FMRP in translation initiation by interacting with eIF4E via CYFIP1 (Napoli et al., 2008). Consistent with previous findings, our results indicate that either genetically increasing levels of eIF4E or loss of the Fmr1 gene leads to enhanced protein synthesis (Bhattacharya et al., 2012; Santini et al., 2013). Although the eIF4E/Fmr1 double mutant mice did not exhibit statistically higher rates of protein synthesis compared to either single mutant, Dunnett’s post hoc tests revealed that the rate of protein synthesis in double mutants (p < 0.01) compared to WT mice is different than either single mutant (p < 0.05) compared to WT (Fig. 5B). This suggests that although the effect of altering both levels of eIF4E and FMRP on translation is not additive, protein synthesis rates are nonetheless altered compared to either the eIF4E Tg or Fmr1 KO mice. Indeed, independent research indicates that eIF4E and FMRP regulate the translation of specific subsets of messenger RNAs (mRNAs); however it is unknown how these mRNAs may be regulated in the instance where levels of both eIF4E and FMRP are altered (Darnell et al., 2011; Thoreen et al., 2012). Because eIF4E and FMRP have been found to work in concert in the context of translation regulation (Napoli et al., 2008), it is likely that there is overlap between the mRNAs that are regulated by eIF4E and FMRP, which may explain why our results do not indicate a statistically higher rate of protein synthesis in the double mutant mice.
Herein, we have shown that eIF4E/Fmr1 double mutant mice display cognitive dysfunction in addition to classic ASD-like behaviors. By genetically increasing levels of eIF4E and deleting FMRP, two proteins that are critically involved with translation regulation, we have produced a mouse model of ASD that displays learning and memory impairments. This novel mouse model should aid in ongoing attempts to study the role of dysregulated protein synthesis in generating ASD-like behaviors in mice. Moreover, it may provide valuable insight on why some, but not all, ASD patients display cognitive impairments.
Highlights.
The eIF4E Tg/Fmr1 KO double mutant mouse is a novel mouse model of ASD
eIF4E/Fmr1 double mutant mice exhibit ASD-like behaviors
eIF4E/Fmr1 double mutant mice exhibit impaired memory in multiple behavioral tasks
eIF4E/Fmr1 double mutant mice exhibit enhanced protein synthesis
The eIF4E/Fmr1 double mutant mouse models multiple clinical features of ASD
Acknowledgements
T.N.H., E.S. and E.K. designed the research. T.N.H., M.S., K.S.F., E.S. performed the behavioral experiments. T.N.H, S.Y.K. and E.S. performed the biochemical experiments. T.N.H., E.S. and E.K. analyzed the data. T.N.H., E.S. and E.K. wrote the manuscript, which was edited by all of the authors. This work was funded by grants from the National Institutes of Health grants (NS034007, NS047384, and HD082013) and the Simons Foundation (274864) to E.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Association AP. The Diagnostic and Statistical Manual of Mental Disorders. Fifth. 2013. bookpointUS. [Google Scholar]
- Banko JL, Merhav M, Stern E, Sonenberg N, Rosenblum K, Klann E. Behavioral alterations in mice lacking the translation repressor 4E-BP2. Neurobiology of Learning and Memory. 2007;87:248–256. doi: 10.1016/j.nlm.2006.08.012. doi:10.1016/j.nlm.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Kaphzan H, Alvarez-Dieppa AC, Murphy JP, Pierre P, Klann E. Genetic Removal of p70 S6 Kinase 1 Corrects Molecular, Synaptic, and Behavioral Phenotypes in Fragile X Syndrome Mice. Neuron. 2012;76:325–337. doi: 10.1016/j.neuron.2012.07.022. doi:10.1016/j.neuron.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher J, Mayes A, Bigham S. Memory in autistic spectrum disorder. Psychol Bull. 2012;138:458–496. doi: 10.1037/a0026869. doi:10.1037/a0026869. [DOI] [PubMed] [Google Scholar]
- Carter MT, Scherer SW. Autism spectrum disorder in the genetics clinic: a review. Clin. Genet. 2013;83:399–407. doi: 10.1111/cge.12101. doi:10.1111/cge.12101. [DOI] [PubMed] [Google Scholar]
- Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, Baird G. IQ in children with autism spectrum disorders: data from the Special Needs and Autism Project (SNAP) Psychol Med. 2011;41:619–627. doi: 10.1017/S0033291710000991. doi:10.1017/S0033291710000991. [DOI] [PubMed] [Google Scholar]
- Coffee B, Keith K, Albizua I, Malone T, Mowrey J, Sherman SL, Warren ST. Incidence of fragile X syndrome by newborn screening for methylated FMR1 DNA. Am. J. Hum. Genet. 2009;85:503–514. doi: 10.1016/j.ajhg.2009.09.007. doi:10.1016/j.ajhg.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Klann E. The translation of translational control by FMRP: therapeutic targets for FXS. Nature Publishing Group. 2013 doi: 10.1038/nn.3379. doi:10.1038/nn.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KYS, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell. 2011;146:247–261. doi: 10.1016/j.cell.2011.06.013. doi:10.1016/j.cell.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators, Centers for Disease Control and Prevention (CDC) Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63:1–21. [PubMed] [Google Scholar]
- Dobkin C, Rabe A, Dumas R, Idrissi A. El, Haubenstock H, Brown WT. Fmr1 knockout mouse has a distinctive strain-specific learning impairment. NSC. 2000;100:423–429. doi: 10.1016/s0306-4522(00)00292-x. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: an update. J Autism Dev Disord. 2003;33:365–382. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- Gabis LV, Baruch YK, Jokel A, Raz R. Psychiatric and Autistic Comorbidity in Fragile X Syndrome Across Ages. Journal of Child Neurology. 2011;26:940–948. doi: 10.1177/0883073810395937. doi:10.1177/0883073810395937. [DOI] [PubMed] [Google Scholar]
- García-Nonell C, Ratera ER, Harris S, Hessl D, Ono MY, Tartaglia N, Marvin E, Tassone F, Hagerman RJ. Secondary medical diagnosis in fragile X syndrome with and without autism spectrum disorder. Am. J. Med. Genet. 2008;A 146A:1911–1916. doi: 10.1002/ajmg.a.32290. doi:10.1002/ajmg.a.32290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. doi:10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- Hatton DD, Sideris J, Skinner M, Mankowski J, Bailey DB, Roberts J, Mirrett P. Autistic behavior in children with fragile X syndrome: Prevalence, stability, and the impact of FMRP. Am. J. Med. Genet. 2006;A 140A:1804–1813. doi: 10.1002/ajmg.a.31286. doi:10.1002/ajmg.a.31286. [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E. Removal of FKBP12 Enhances mTOR-Raptor Interactions, LTP, Memory, and Perseverative/Repetitive Behavior. Neuron. 2008;60:832–845. doi: 10.1016/j.neuron.2008.09.037. doi:10.1016/j.neuron.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic Translational and Proteasomal Regulation of Fragile X Mental Retardation Protein Controls mGluR-Dependent Long-Term Depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. doi:10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Huber KM, Gallagher SM, Warren ST, Bear MF. Altered synaptic plasticity in a mouse model of fragile X mental retardation. Proc. Natl. Acad. Sci. U.S.A. 2002;99:7746–7750. doi: 10.1073/pnas.122205699. doi:10.1073/pnas.122205699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, Leal J. Epidemiology of fragile X syndrome: a systematic review and meta-analysis. Am. J. Med. Genet. 2014;A 164A:1648–1658. doi: 10.1002/ajmg.a.36511. doi:10.1002/ajmg.a.36511. [DOI] [PubMed] [Google Scholar]
- Huynh TN, Santini E, Klann E. Requirement of Mammalian target of rapamycin complex 1 downstream effectors in cued fear memory reconsolidation and its persistence. Journal of Neuroscience. 2014;34:9034–9039. doi: 10.1523/JNEUROSCI.0878-14.2014. doi:10.1523/JNEUROSCI.0878-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau ASM, Tierney E, Bukelis I, Stump MH, Kates WR, Trescher WH, Kaufmann WE. Social behavior profile in young males with fragile X syndrome: characteristics and specificity. Am. J. Med. Genet. 2004;A 126A:9–17. doi: 10.1002/ajmg.a.20218. doi:10.1002/ajmg.a.20218. [DOI] [PubMed] [Google Scholar]
- Klei L, Sanders SJ, Murtha MT, Hus V, Lowe JK, Willsey AJ, Moreno-De-Luca D, Yu TW, Fombonne E, Geschwind D, Grice DE, Ledbetter DH, Lord C, Mane SM, Martin CL, Martin DM, Morrow EM, Walsh CA, Melhem NM, Chaste P, Sutcliffe JS, State MW, Cook EH, Roeder K, Devlin B. Common genetic variants, acting additively, are a major source of risk for autism. Mol Autism. 2012;3:9. doi: 10.1186/2040-2392-3-9. doi:10.1186/2040-2392-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCary LM, Roberts JE. Early identification of autism in fragile X syndrome: a review. J Intellect Disabil Res. 2012;57:803–814. doi: 10.1111/j.1365-2788.2012.01609.x. doi:10.1111/j.1365-2788.2012.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Nonneman RJ, Segall SK, Andrade GM, Crawley JN, Magnuson TR. Social approach and repetitive behavior in eleven inbred mouse strains. Behav. Brain Res. 2008;191:118–129. doi: 10.1016/j.bbr.2008.03.015. doi:10.1016/j.bbr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Young NB, Perez A, Holloway LP, Barbaro RP, Barbaro JR, Wilson LM, Threadgill DW, Lauder JM, Magnuson TR, Crawley JN. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav. Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. doi:10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, Witke W, Costa-Mattioli M, Sonenberg N, Achsel T, Bagni C. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. doi:10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Paradee W, Melikian HE, Rasmussen DL, Kenneson A, Conn PJ, Warren ST. Fragile X mouse: strain effects of knockout phenotype and evidence suggesting deficient amygdala function. NSC. 1999;94:185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- Peça J, Feliciano C, Ting JT, Wang W, Wells MF, Venkatraman TN, Lascola CD, Fu Z, Feng G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature. 2011;472:437–442. doi: 10.1038/nature09965. doi:10.1038/nature09965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, Wehner DE, Hagerman R. The behavioral phenotype in fragile X: symptoms of autism in very young children with fragile X syndrome, idiopathic autism, and other developmental disorders. J Dev Behav Pediatr. 2001;22:409–417. doi: 10.1097/00004703-200112000-00008. [DOI] [PubMed] [Google Scholar]
- Sabaratnam M, Murthy NV, Wijeratne A, Buckingham A, Payne S. Autistic-like behaviour profile and psychiatric morbidity in Fragile X Syndrome. European Child & Adolescent Psychiatry. 2003;12:172–177. doi: 10.1007/s00787-003-0333-3. doi:10.1007/s00787-003-0333-3. [DOI] [PubMed] [Google Scholar]
- Salazar F, Baird G, Chandler S, Tseng E, O’sullivan T, Howlin P, Pickles A, Simonoff E. Co-occurring Psychiatric Disorders in Preschool and Elementary School-Aged Children with Autism Spectrum Disorder. J Autism Dev Disord. 2015:1–12. doi: 10.1007/s10803-015-2361-5. doi:10.1007/s10803-015-2361-5. [DOI] [PubMed] [Google Scholar]
- Santini E, Huynh TN, MacAskill AF, Carter AG, Pierre P, Ruggero D, Kaphzan H, Klann E. Exaggerated translation causes synaptic and behavioural aberrations associated with autism. Nature. 2013;493:411–415. doi: 10.1038/nature11782. doi:10.1038/nature11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Klann E. Reciprocal signaling between translational control pathways and synaptic proteins in autism spectrum disorders. Science Signaling. 2014;7 doi: 10.1126/scisignal.2005832. re10. doi:10.1126/scisignal.2005832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. doi:10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer CM, Alekseyenko O, Hamilton SM, Thomas AM, Serysheva E, Yuva-Paylor LA, Paylor R. Modifying behavioral phenotypes in Fmr1KO mice: genetic background differences reveal autistic-like responses. Autism Res. 2011;4:40–56. doi: 10.1002/aur.168. doi:10.1002/aur.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoreen CC, Chantranupong L, Keys HR, Wang T, Gray NS, Sabatini DM. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature. 2012;485:109–113. doi: 10.1038/nature11083. doi:10.1038/nature11083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullman MT, Pullman MY. A compensatory role for declarative memory in neurodevelopmental disorders. Neuroscience & Biobehavioral Reviews. 2015;51:205–222. doi: 10.1016/j.neubiorev.2015.01.008. doi:10.1016/j.neubiorev.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ung D, Wood JJ, Ehrenreich-May J, Arnold EB, Fujii C, Renno P, Murphy TK, Lewin AB, Mutch PJ, Storch EA. Clinical characteristics of high-functioning youth with autism spectrum disorder and anxiety. Neuropsychiatry. 2013;3:147–157. doi: 10.2217/npy.13.9. doi:10.2217/npy.13.9. [DOI] [PMC free article] [PubMed] [Google Scholar]