Abstract
Background
Cardiovascular disease (CVD) can occur in individuals with low LDL-cholesterol (LDL-c). We investigated whether detailed measures of LDL subfractions and other lipoproteins can be used to assess CVD risk in a population with both low LDL-c and high C-reactive protein that was randomized to high-intensity statin or placebo.
Methods and Results
In 11,186 JUPITER participants, we tested whether lipids, apolipoproteins, and ion mobility (IM)-measured particle concentrations at baseline and after random allocation to rosuvastatin 20 mg/d or placebo were associated with first CVD events (n=307) or CVD/all-cause death (n=522). In placebo-allocated participants, baseline LDL-c was not associated with CVD (adjusted HR per SD, 1.03, 95% CI 0.88-1.21). In contrast, associations with CVD events were observed for baseline non-HDL-cholesterol (non-HDL-c: 1.18, 1.01-1.38), apolipoprotein B (apoB: 1.28, 1.11-1.48), and IM-measured non-HDL particles (non-HDL-p: 1.19, 1.05-1.35) and LDL particles (LDL-p: 1.21, 1.07-1.37). Association with CVD events was also observed for several LDL and VLDL subfractions, but not for IM-measured HDL subfractions. In statin-allocated participants, CVD events were associated with on-treatment LDL-c, non-HDL-c, and apoB; these were also associated with CVD/all-cause death, as were several LDL and VLDL subfractions albeit with a pattern of association that differed from the baseline risk.
Conclusions
In JUPITER, baseline LDL-c was not associated with CVD events, in contrast with significant associations for non-HDL-c and atherogenic particles: apoB and IM-measured non-HDL-p, LDL-p, and select subfractions of VLDL-p and LDL-p. During high-intensity statin therapy, on-treatment levels of LDL-c and atherogenic particles were associated with residual risk of CVD/all-cause death.
Keywords: inflammation, lipids, lipoproteins, prevention, statins
Low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c), and triglycerides are the standard blood lipid-related laboratory measurements used for cardiovascular disease (CVD) risk assessment and management, yet a significant burden of CVD risk is not revealed by these standard blood lipid measurements. Recent data raise concerns regarding the standardization of LDL-c, whether calculated from the Friedewald equation or measured directly, with significant variability and discrepant clinical results noted among various methods for LDL-c determination.1-3 Moreover, a sizeable proportion of CVD events occur in individuals who have LDL-c levels that are not traditionally considered to be at elevated CVD risk.4 It has been hypothesized that some of this increased risk is due to high particle concentrations (numbers) of LDL (LDL-p), other atherogenic lipoproteins (very-low-density lipoprotein [VLDL-p] and intermediate-density lipoprotein [IDL-p]), or their subfractions; and that risk due to these particles may not be reflected by levels of LDL-c, triglycerides, or estimated non-HDL-c.5 Thus, a more direct laboratory determination of lipoprotein particle number may reveal clinically relevant findings masked by the standard estimation of lipoprotein cholesterol concentration. Atherogenic lipoproteins (VLDL, IDL, LDL), regardless of their size, each contain one apolipoprotein (apo) B molecule per particle; hence, apoB level is a measure of total atherogenic lipoprotein concentration.5 On the other hand, HDL-c determination involves estimation based on either masking or removing apoB-containing particles to measure HDL-c.6 The estimation of HDL-c would also affect the estimated non-HDL-c.
The current study addresses the potential role of a novel laboratory method (ion mobility, IM) that directly determines lipoprotein number across the entire lipoprotein spectrum (VLDL, IDL, LDL, and HDL) independent of the particles’ cholesterol composition. Ion mobility determines particle number after separating lipoprotein particles by size using gas-phase electrophoresis and directly counting the size-separated particles. To date, IM lipoprotein subfractions and CVD events have been evaluated in the Malmö Diet and Cancer Study cohort of middle aged Europeans, finding that among individuals not classified into a statin benefit group, LDL-p determined by ion mobility was associated with incident coronary events after adjustment for standard lipids.7, 8
It is uncertain whether these more specific measures of particle concentration and size for LDL and other lipoproteins are related to CVD risk when LDL-c levels are low, although risk tracks better with particle concentration than cholesterol when these measures disagree. 9 Furthermore, it is unknown whether lipoprotein subfractions contribute to the residual risk of CVD during high-intensity statin therapy. This is important because variation in lipoprotein subfractions may influence CVD risk and may be selectively manipulated. The newly discovered function of the SORT1 gene exemplifies the biological and potential therapeutic relevance of selective regulatory pathways for lipoprotein subfractions- the SORT1 gene modulates levels of hepatic apoB secretion and uptake, preferentially altering plasma levels of small and very small LDL subfractions, and the risk of myocardial infarction.10, 11 Therefore, in the JUPITER trial cohort which is characterized by low LDL-c (<130 mg/dL) and triglycerides <500 mg/dL but elevated high-sensitivity C-reactive protein (hsCRP), we investigated whether IM-measured lipoproteins or their subfractions predict CVD events after allocation to placebo or high-intensity statin therapy.
Methods
Study population
The JUPITER design has been previously published (ClinicalTrial.gov, NCT00239681).12 Asymptomatic individuals (women ≥ 60 years, men ≥ 50 years) without prior history of CVD were randomized into the trial if they had LDL-c <130 mg/dL and hsCRP ≥2.0 mg/L. The JUPITER trial exclusion criteria included triglycerides ≥500 mg/dL, current use of hormone therapy, previous or current use of lipid-lowering therapy or immunosuppressant agents. The trial protocol stipulated a baseline and 12-month visit at which time points blood was drawn for standard assays at a central laboratory as described below. Remaining blood samples were sent to the Clinical Coordinating Center at the Brigham and Women's Hospital (Boston, MA) and stored in liquid nitrogen. Four to five years after trial completion, IM measurements were performed on 11,277 of the 13,658 individuals with a stored baseline sample for whom sufficient sample remained. For the present analysis, we additionally excluded individuals who were missing any baseline standard lipid or apolipoprotein measurements (n=91), resulting in a total sample size of 11,186. Of these, 9,430 had both baseline and 12-month IM measurements.
Laboratory measurements
Standard lipids, apolipoproteins, hsCRP, and glucose measurements were performed in a central laboratory on fasting blood samples as previously described (Supplemental Methods).13 Consistent with previous JUPITER analyses, on-treatment concentrations were defined as values obtained after one year of randomized treatment.11-14 IM lipoproteins were measured at Quest Diagnostics Nichols Institute (San Juan Capistrano, CA) (Supplemental Methods and Supplemental Table 1).
Outcomes
On March 30, 2008, the Independent Data and Safety Monitoring Board terminated the JUPITER trial early upon determination that the accumulated evidence from the trial and other sources constituted proof beyond a reasonable doubt that rosuvastatin was indicated for a specified group of participants (after 1.9 year median follow-up, maximal follow-up 5.0 years).12 The primary endpoint of the trial was a composite CVD endpoint, defined as myocardial infarction, stroke, hospitalization for unstable angina, arterial revascularization, or cardiovascular death. We also pre-specified examining the expanded secondary endpoint of CVD or all-cause death, as previously done.14 Reported endpoints were adjudicated by an independent endpoint committee blinded to randomized treatment.
Statistical Analyses
Statistical analyses were performed with STATA, version 10.1. Change from baseline to one year levels was depicted in boxplots and compared with the Wilcoxon signed rank test. The Wilcoxon rank-sum test was used to test whether change from baseline to one year levels differed according to treatment group allocation.
Associations with outcomes were performed according to the treatment to which participants were randomized. Exposure time was calculated as the time from randomization to occurrence of the primary endpoint event, date of death, last visit, withdrawal, loss to follow-up, or March 30, 2008, whichever came first. Cox proportional hazard models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs), with robust standard errors reported. Biomarkers were modeled as continuous variables with results reported per standard deviation [SD] of the baseline distribution and per tertiles, consistent with prior JUPITER analyses.14 Analyses were adjusted for age, sex, race, smoking status, family history of premature coronary disease, body-mass index, systolic blood pressure, fasting glucose, and the natural logarithm (ln) of hsCRP. Some analyses also adjusted for LDL-c, HDL-c, and ln triglycerides in order to determine if the subfractions were independently associated with CVD risk after accounting for their correlation with standard lipids. Each IM subfraction was assessed in a separate model unless otherwise noted. We fit additional models that evaluated the incremental prognostic value of the panel of IM subfractions by entering them as a set15, 16 added to a base model with the established risk factors including standard lipids. Next, a parsimonious set of subfractions was selected using backward elimination (retention threshold, p<0.05), forcing the established risk factors (including standard lipids) in the model. A multivariable p value for the full and parsimonious set was obtained from the likelihood-ratio test comparing the base model plus subfractions with the base model only. Model discrimination was examined using the c-index,17 a generalization of the area under the receiver operator characteristic curve that is applicable to survival data. The likelihood ratio χ2 statistic was used to evaluate for treatment by lipoprotein interaction. P-values were two-tailed.
Results
Baseline characteristics for individuals with IM measurements were similar to the overall JUPITER population12 except that the current study had more whites (Supplemental Table 2). Spearman correlation coefficients of baseline lipids and apolipoproteins with IM lipoproteins are shown in Supplemental Table 3.
Similar to the main trial findings, 12 rosuvastatin 20 mg/day decreased LDL-c by 48.5%, non-HDL-c by 42.6%, apoB by 39.1%, and triglycerides by 16.3%, all p<0.0001 (Table 1). Profiles of IM-measured apoB-containing lipoproteins in JUPITER participants at baseline and after one year of rosuvastatin therapy are shown in Figure 1.In the rosuvastatin arm, there were smaller relative reductions of non-HDL-p (29.5%) and LDL-p (27.8%) compared with non-HDL-c or apoB. The larger LDL subfractions were lowered more by rosuvastatin (28 to 37%) than the smaller ones (<8%), resulting in a shift of the predominant LDL peak to a smaller particle diameter (Table 1 and Supplemental Figure 1). For HDL measures, rosuvastatin resulted in small increases in HDL-c (6.4%) and apoA-I (1.7%), but small decreases in IM HDL-p and its subfractions.
Table 1.
Median, 25th, and 75th percentile values of lipid and lipoprotein measures among the placebo and rosuvastatin arms.
| Baseline | Year 1* | Change† | % Change‡ | ||
|---|---|---|---|---|---|
| Lipids and apolipoproteins, mg/dL | |||||
| LDL cholesterol | Placebo | 109 (96, 119) | 110 (94,125) | 3 (−8, 15) | 2.8 (−7.5, 14.8) |
| Rosuvastatin | 108 (94,119) | 54 (44, 71) | −50 (−64, −30) | −48.5 (−57.9, −32.0) | |
| Non-HDL cholesterol | Placebo | 134 (119, 146) | 136 (118, 154) | 3 (−9, 16) | 2.4 (−6.6, 12.5) |
| Rosuvastatin | 134 (118, 147) | 75 (63, 95) | −55 (−70, −33) | −42.6 (−51.1, −27.5) | |
| Apolipoprotein B | Placebo | 109 (95, 121) | 105 (91, 118) | −3 (−14, 6) | −3.1 (−12.2, 6.4) |
| Rosuvastatin | 109 (95, 122) | 66 (56, 80) | −41 (−54, −26) | −39.1 (−47.5, −26.2) | |
| Triglycerides | Placebo | 114 (83, 163) | 116 (85, 162) | 1 (−23, 24) | 1.2 (−18.4, 24.6) |
| Rosuvastatin | 116 (84, 164) | 97 (72, 133) | −17 (−47, 4) | −16.3 (−33.3, 4.6) | |
| HDL cholesterol | Placebo | 50 (41,61) | 51 (42,62) | 1 (−3, 5) | 2.1 (−6.5, 11.4) |
| Rosuvastatin | 50 (41,61) | 53 (44,65) | 3 (−2, 8) | 6.4 (−2.8, 17.1) | |
| Apolipoprotein A-I | Placebo | 164 (146, 185) | 163 (144, 184) | −1 (−14, 12) | −0.5 (−8.4, 7.6) |
| Rosuvastatin | 165 (146, 186) | 167 (147, 191) | 3 (−12, 17) | 1.7 (−6.9, 10.5) | |
| Ion mobility | |||||
| Non-HDL particles, nmol/L | Placebo | 2292 (1852, 2775) | 2210 (1764, 2700) | −62 (−347, 213) | −3.0 (−14.7, 10.4) |
| Rosuvastatin | 2298 (1844, 2781) | 1625 (1291, 2026) | −647 (−1015, −265) | −29.5 (−41.0, −13.1) | |
| VLDL particles, nmol/L | |||||
| Total | Placebo | 155 (119, 194) | 143 (109, 182) | −9 (−35, 13) | −6.5 (−21.3, 10.1) |
| Rosuvastatin | 156 (121, 195) | 96 (72, 126) | −54 (−86, −25) | −37.7 (−51.4,−19.4) | |
| Large | Placebo | 14.5 (10.2, 19.6) | 13.8 (9.5, 18.6) | −0.7 (−3.9, 2.3) | −5.6 (−25.3, 18.7) |
| Rosuvastatin | 14.5 (10.2, 19.6) | 8.8 (5.8, 12.8) | −5.1 (−8.8, −1.8) | −39.3 (−55.3, −16.1) | |
| Medium | Placebo | 55.9 (42.1, 71.3) | 51.9 (38.7, 66.6) | −3.6 (−13.2, 5.8) | −7.0 (−22.9, 11.8) |
| Rosuvastatin | 56.3 (42.9, 71.1) | 34.1 (25.1, 46.0) | −19.8 (−31.9, −8.6) | −38.5 (−52.4, −19.3) | |
| Small | Placebo | 83.1 (64.8, 104.7) | 76.7 (59.1, 97.2) | −5.0 (−18.8, 6.7) | −6.6 (−21.4, 9.3) |
| Rosuvastatin | 84.0 (65.3, 104.9) | 52.0 (39.7, 67.9) | −29.1 (−46.1, −13.3) | −37.2 (−50.6, −18.8) | |
| IDL particles, nmol/L | |||||
| Total | Placebo | 428 (340, 528) | 409 (321, 511) | −16 (−74, 42) | −3.8 (−16.9, 10.8) |
| Rosuvastatin | 431 (343, 536) | 292 (226, 376) | −131 (−207, −54) | −32.0 (−44.6, −14.8) | |
| Large | Placebo | 222 (176, 270) | 206 (161, 256) | −12 (−44, 17) | −5.8 (−19.6, 8.8) |
| Rosuvastatin | 224 (179, 275) | 146 (113, 188) | −72 (−114, −33) | −34.6 (−46.3, −17.6) | |
| Small | Placebo | 196 (150, 254) | 192 (147, 252) | −2 (−34, 28) | −0.9 (−16.5, 16.5) |
| Rosuvastatin | 198 (152, 259) | 142 (109, 187) | −52 (−95, −16) | −28.5 (−42.6, −9.4) | |
| LDL particles, nmol/L | |||||
| Total | Placebo | 1689 (1349,2069) | 1641 (1286, 2028) | −35 (−253, 165) | −2.4 (−14.6, 11.4) |
| Rosuvastatin | 1689 (1341, 2085) | 1224 (970, 1541) | −447 (−734, −164) | −27.8 (−39.9, −11.4) | |
| I (largest) | Placebo | 328 (233, 452) | 325 (226, 445) | −4 (−61, 51) | −1.4 (−18.1, 17.9) |
| Rosuvastatin | 330 (233, 450) | 208 (153, 282) | −109 (−200, −40) | −36.8 (−51.2, −16.4) | |
| II a | Placebo | 273 (202, 358) | 268 (197, 353) | −3 (−49, 43) | −1.3 (−17.8, 17.6) |
| Rosuvastatin | 273 (202, 354) | 173 (128, 228) | −93 (−158, −36) | −37.1 (−51.1, −16.9) | |
| II b | Placebo | 303 (228, 397) | 297 (218, 388) | −4 (−55, 44) | −1.6 (−17.8, 16.9) |
| Rosuvastatin | 301 (228, 395) | 205 (156, 269) | −90 (−162, −26) | −31.8 (−46.9, −11.2) | |
| III a | Placebo | 250 (181, 362) | 241 (176, 351) | −6 (−50, 36) | −2.7 (−18.6, 16.4) |
| Rosuvastatin | 249 (179, 369) | 193 (147, 256) | −50 (−129, −4) | −22.5 (−40.7, −2.0) | |
| III b | Placebo | 93 (69, 135) | 90 (67, 130) | −3 (−20, 13) | −3.5 (−19.8, 15.8) |
| Rosuvastatin | 94 (69, 137) | 83 (63, 110) | −12 (−37, 6) | −13.3 (−33.0, 7.6) | |
| IV a | Placebo | 116 (89, 156) | 112 (84, 150) | −5 (−24, 13) | −4.6 (−19.3, 12.9) |
| Rosuvastatin | 117 (88, 158) | 110 (84, 142) | −8 (−32, 12) | −7.8 (−25.4, 11.8) | |
| IV b | Placebo | 104 (82, 135) | 99 (77, 129) | −6 (−21, 9) | −5.5 (−18.7, 9.7) |
| Rosuvastatin | 106 (81, 138) | 102 (78, 131) | −4 (−22, 13) | −4.4 (−19.2, 13.7) | |
| IV c (smallest) | Placebo | 99 (78, 127) | 92 (72, 120) | −6 (−19, 6) | −6.1 (−18.4, 7.2) |
| Rosuvastatin | 99 (78, 130) | 94 (73, 123) | −5 (−20, 8) | −5.7 (−18.5, 9.3) | |
| LDL peak diameter, Å | Placebo | 220.0 (215.8, 223.7) | 220.0 (215.8, 223.7) | 0 (−2.4, 1.9) | 0 (−1.1, 0.9) |
| Rosuvastatin | 220.1 (215.8, 223.7) | 218.2 (214.6, 221.2) | −1.2 (−4.3, 1.2) | −0.6 (−1.9, 0.6) | |
| HDL particles, μmol/L | |||||
| Total | Placebo | 41.4 (32.7, 53.3) | 38.9 (30.7, 49.2) | −2 (−8, 2) | −6.0 (−17.4, 6.0) |
| Rosuvastatin | 41.9 (32.7, 53.8) | 39.6 (31.2, 49.4) | −3 (−8, 3) | −6.6 (−18.9, 7.3) | |
| Large | Placebo | 10.8 (8.3, 14.3) | 10.0 (7.7, 13.1) | −0.8 (−2, 0.5) | −7.3 (−20.0, 5.3) |
| Rosuvastatin | 11.1 (8.4, 14.5) | 10.5 (8.1, 13.6) | −0.6 (−2, 1) | −5.5 (−19.1, 10.2) | |
| Small | Placebo | 30.4 (24.0, 39.1) | 28.7 (22.6, 36.3) | −2 (−6, 2) | −5.6 (−17.3, 7.0) |
| Rosuvastatin | 30.7 (23.9, 39.6) | 28.8 (22.7, 36.3) | −2 (−6, 2) | −6.9 (−19.2, 6.7) | |
Values obtained from individuals with both baseline and year 1 measurements (N=9,548).
P values from the Wilcoxon signed rank test comparing baseline and year 1 values were p<0.01 for all except for triglycerides and LDL peak diameter among the placebo group (p=0.21 and 0.34, respectively).
P values from the Wilcoxon rank-sum test comparing the change among the rosuvastatin group with the change among the placebo group were <0.01 for all except for LDL-IVc and total HDL particles ((p=0.11 and 0.37, respectively). All changes were in the direction of rosuvastatin > placebo except for LDL-IVb.
100*( Year 1 – baseline)/baseline
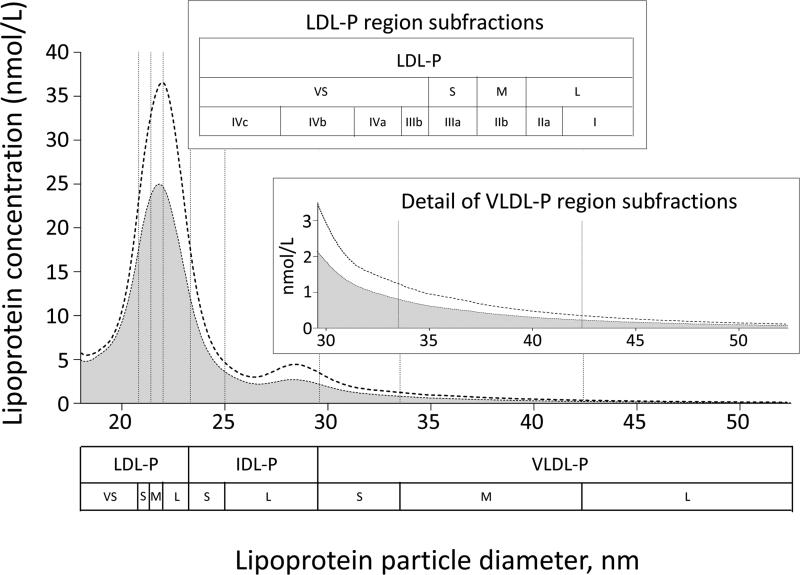
Figure 1.
Ion mobility apoB-containing subfraction concentrations at baseline (dashed line) and after 1 year of rosuvastatin (shaded area) based on a random subset of 4,000 baseline and 4,000 1-year rosuvastatin samples in JUPITER participants. Abbreviations: VS: very small, S: small, M: medium, L: large.
Baseline measures and incident CVD events
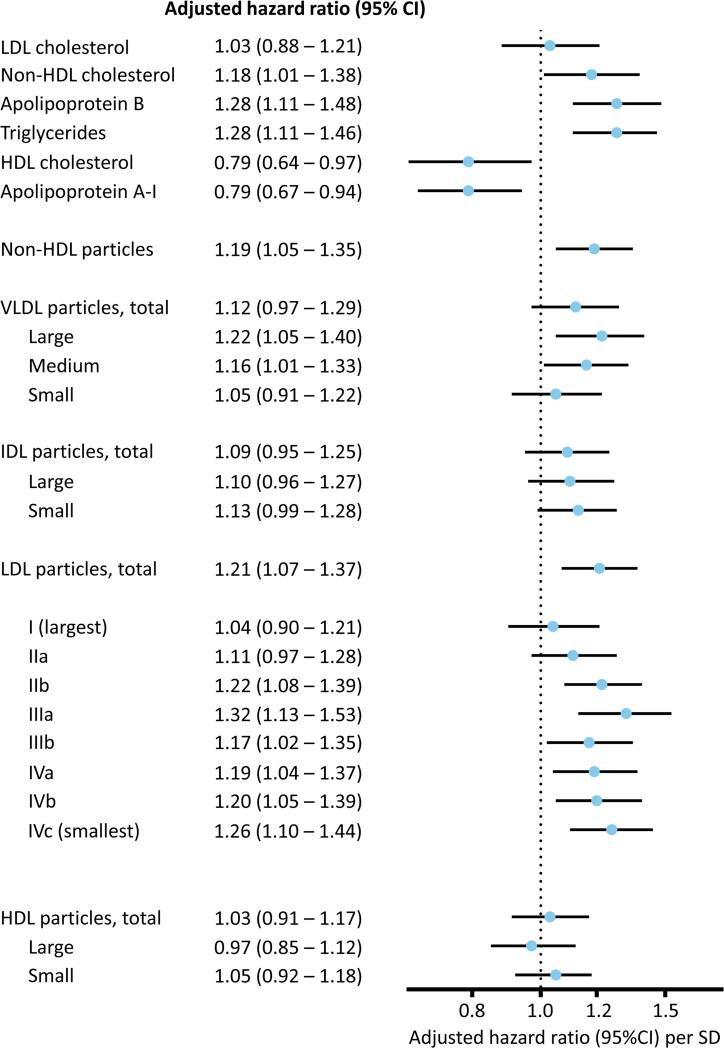
During a median follow-up of 1.9 years (maximum 5.0), the 11,186 participants (5,600 placebo/5,586 rosuvastatin) experienced 307 first primary CVD events (199 placebo/108 rosuvastatin) and 522 combined CVD and all-cause death events (322 placebo/200 rosuvastatin). In the placebo-allocated arm (Table 2 and Figure 2), baseline LDL-c was not associated with CVD (adjusted HR per SD, 1.03, 95% CI 0.88-1.21, p=0.71); in contrast, associations (HR, 95% CI, p value) were observed for baseline non-HDL-c (1.18, 1.01-1.38, p=0.036), apoB (1.28, 1.11-1.48, p=0.001), triglycerides (1.28, 1.11-1.46, p<0.001), and IM-measured non-HDL-p (1.19, 1.05-1.35, p=0.005) and LDL-p (1.21, 1.07-1.37, p=0.002). After additionally adjusting for standard lipids, associations were slightly attenuated for apoB (1.27, 1.03-1.56, p=0.024), non-HDL-p (1.15, 1.01-1.31, p=0.035), and LDL-p (1.16, 1.02-1.32, p=0.028).
Table 2.
Baseline lipid and lipoprotein measures in relation to incident CVD events among the placebo arm.
| CVD | CVD & all-cause death | ||||
|---|---|---|---|---|---|
| HR per SD higher* (95% CI) | P value | HR per SD higher* (95% CI) | P value | ||
| Lipids and apolipoproteins, mg/dL (SD) | |||||
| LDL cholesterol | 18.5 | 1.03 (0.88-1.21) | 0.71 | 0.92 (0.82-1.03) | 0.16 |
| Non-HDL cholesterol | 23.3 | 1.18 (1.01-1.38) | 0.036 | 1.01 (0.90-1.14) | 0.81 |
| Apolipoprotein B | 21.0 | 1.28 (1.11-1.48) | 0.001‡ | 1.07 (0.96-1.20) | 0.22 |
| Triglycerides | 72.0 | 1.28 (1.11-1.46) | <0.001‡ | 1.17 (1.05-1.30) | 0.005 |
| HDL cholesterol | 15.4 | 0.79 (0.64-0.97) | 0.028 | 0.79 (0.68-0.93) | 0.004‡ |
| Apolipoprotein A-I | 30.5 | 0.79 (0.67-0.94) | 0.009 | 0.77 (0.67-0.89) | <0.001‡ |
| Ion mobility, (SD) | |||||
| Non-HDL particles, nmol/L | 733 | 1.19 (1.05-1.35) | 0.005‡ | 1.06 (0.95-1.17) | 0.32 |
| VLDL particles, nmol/L | |||||
| Total | 59.7 | 1.12 (0.97-1.29) | 0.12 | 0.98 (0.87-1.10) | 0.69 |
| Large | 7.6 | 1.22 (1.05-1.40) | 0.004 | 1.11 (0.99-1.25) | 0.083 |
| Medium | 22.9 | 1.16 (1.01-1.33) | 0.036 | 1.02 (0.91-1.14) | 0.76 |
| Small | 32.0 | 1.05 (0.91-1.22) | 0.52 | 0.92 (0.81-1.03) | 0.15 |
| IDL particles, nmol/L | |||||
| Total | 156 | 1.09 (0.95-1.25) | 0.20 | 0.98 (0.88-1.10) | 0.74 |
| Large | 78 | 1.10 (0.96-1.27) | 0.17 | 0.96 (0.86-1.08) | 0.52 |
| Small | 93 | 1.13 (0.99-1.28) | 0.068‡ | 1.05 (0.94-1.17) | 0.40‡ |
| LDL particles, nmol/L | |||||
| Total | 571 | 1.21 (1.07-1.37) | 0.002‡ | 1.08 (0.97-1.20) | 0.15 |
| I (largest) | 162 | 1.04 (0.90-1.21) | 0.59‡ | 0.95 (0.84-1.07) | 0.42 |
| II a | 115 | 1.11 (0.97-1.28) | 0.14‡ | 0.98 (0.87-1.10) | 0.69 |
| II b | 129 | 1.22 (1.08-1.39) | 0.002‡ | 1.06 (0.95-1.18) | 0.30 |
| III a | 151 | 1.32 (1.13-1.53) | <0.001‡ | 1.15 (1.02-1.30) | 0.018 |
| III b | 78 | 1.17 (1.02-1.35) | 0.027 | 1.09 (0.97-1.21) | 0.15 |
| IV a | 84 | 1.19 (1.04-1.37) | 0.013 | 1.13 (1.01-1.26) | 0.026 |
| IV b | 54 | 1.20 (1.05-1.39) | 0.010 | 1.21 (1.08-1.35) | 0.001‡ |
| IV c (smallest) | 49 | 1.26 (1.10-1.44) | 0.001‡ | 1.36 (1.23-1.52) | <0.001‡ |
| LDL peak diameter, Å | 5.9 | 0.84 (0.73-0.96) | 0.012 | 0.87 (0.78-0.98) | 0.018 |
| HDL particles, μmol/L | |||||
| Total | 17.8 | 1.03 (0.91-1.17) | 0.67 | 0.99 (0.89-1.09) | 0.82 |
| Large | 4.9 | 0.97 (0.85-1.12) | 0.71 | 0.94 (0.84-1.05) | 0.26 |
| Small | 13.4 | 1.05 (0.92-1.18) | 0.48 | 1.01 (0.91-1.11) | 0.91 |
Per 1-SD increment in the lipid or lipoprotein variable, adjusted for age, sex, race, smoking, family history, BMI, systolic blood pressure, glucose, and ln hsCRP
Triglycerides, LDL IIIa through IVc, and LDL peak diameter were natural log transformed; values shown are the untransformed SDs.
All measures shown in this table were also evaluated in models that included LDL cholesterol, HDL cholesterol, ln triglycerides in addition to age, sex, race, smoking, family history, BMI, systolic blood pressure, glucose, and ln hsCRP, with variables that met statistical significance (P<0.05) indicated by ‡.
Figure 2.
Adjusted hazard ratios (per 1-SD higher) and 95% confidence intervals according to intention-to-treat analysis (placebo group) for the primary endpoint by baseline lipids, apolipoproteins, and IM-measured lipoproteins and subfractions, adjusted for age, sex, race, smoking, family history, BMI, systolic blood pressure, glucose, and ln hsCRP. Log-transformed variables were triglycerides and LDL III a – IV c subfractions.
Of the VLDL subfractions, the triglyceride-enriched large and medium subfractions were associated with CVD, similar to chemically-measured triglycerides. Within IDL subfractions, only the smaller subfraction showed a trend toward association, which was strengthened and became statistically significant (1.24, 1.09-1.40, p=0.001) after additionally adjusting for LDL-c, HDL-c, and triglycerides. None of the HDL subfractions were associated with CVD. Furthermore, LDL subfractions were associated with CVD, but associations differed according to the sizes of the LDL particles and adjustment for standard lipids (Table 2, Supplemental Table 4). Before such adjustment, associations were noted for all but the largest LDL (LDL-I and IIa) subfractions. However, as was also seen with the adjacent small IDL subfraction, after further adjustment for lipids (in particular triglycerides and HDL-c), subfractions LDL-I through IIIa (LDL Large [LDL-I and IIa], LDL Medium [LDL-IIb] and LDL Small [LDL-IIIa]) were significantly associated with CVD events (LDL-I: 1.19, 1.02-1.39, p=0.030; LDL-IIa: 1.16, 1.01-1.34, p=0.039; LDL-IIb: 1.18, 1.03-1.35, p=0.018; LDL-IIIa: 1.20, 1.01-1.42, p=0.040) as was the smallest LDL subfraction (LDL-IVc: 1.22, 1.06-1.40, p=0.005).
When examined in relation to the expanded secondary endpoint of CVD and all-cause death that occurred in the placebo group (No. events/N=322/5600; Table 2), the smaller LDL-p subfractions remained significantly associated with increased risk, in particular LDL-IVc (1.36, 1.23-1.52, p<0.001), which remained significant after additionally adjusting for standard lipids. Overall, generally similar results were obtained when the lipids and lipoproteins were examined as tertiles (Supplemental Table 5), with particularly high risk (2 to 2.4-fold) seen for the top versus bottom tertile of LDL-IVc.
Residual risk during high-intensity statin therapy
Among rosuvastatin-allocated individuals with complete on-treatment data, significant associations were noted for on-treatment LDL-c, non-HDL-c, and apoB with both residual risk of CVD events (No. events/N: 73/4,597; Table 3, Supplemental Tables 4 and 6, Supplementary Figure 2) and with residual risk of the expanded endpoint of CVD and all-cause death (No. events/N: 108/4,597). While none of the IM-measured lipoprotein fractions were significantly associated with residual risk of the primary endpoint in the subgroup of rosuvastatin-treated participants, effect estimates were consistent with the statistically significant associations seen with the expanded endpoint of CVD and all-cause death that included a greater number of events. In particular, increased residual risk was noted for non-HDL-p, VLDL-p (medium and small subfractions), IDL-p and its subfractions, and LDL-p (medium to large subfractions). Tests for treatment by lipoprotein interaction yielded significant differences for both the primary and expanded endpoints for small VLDL-p, IDL-p and its subfractions, and large LDL-p subfractions.
Table 3.
On-treatment lipid and lipoprotein measures in relation to residual risk of CVD among the rosuvastatin arm.
| CVD | CVD & all-cause death | |||
|---|---|---|---|---|
| HR per 1-SD higher* (95% CI) | P Value | HR per 1-SD higher* (95% CI) | P Value | |
| Lipids and apolipoproteins, mg/dL | ||||
| LDL cholesterol | 1.20 (1.05-1.37) | 0.006 | 1.20 (1.07-1.35) | 0.002 |
| Non-HDL cholesterol | 1.20 (1.03-1.40) | 0.017 | 1.23 (1.08-1.40) | 0.002 |
| Apolipoprotein B | 1.29 (1.08-1.55) | 0.005 | 1.32 (1.12-1.55) | 0.001 |
| Triglycerides | 1.05 (0.83-1.32) | 0.68 | 1.15 (0.95-1.40) | 0.14 |
| HDL cholesterol | 0.86 (0.67-1.10) | 0.23 | 0.80 (0.66-0.97) | 0.026 |
| Apolipoprotein A-I | 0.87 (0.66-1.15) | 0.34 | 0.77 (0.62-0.96) | 0.022 |
| Ion mobility, (SD) | ||||
| Non-HDL particles, nmol/L | 1.15 (0.87-1.51) | 0.34 | 1.30 (1.02-1.65) | 0.034 |
| VLDL particles, nmol/L | ||||
| Total | 1.23 (0.92-1.66) | 0.16 | 1.29 (1.02-1.64) | 0.033 |
| Large | 1.10 (0.92-1.32) | 0.29 | 1.14 (1.02-1.28) | 0.020 |
| Medium | 1.23 (0.92-1.63) | 0.16 | 1.30 (1.04-1.62) | 0.021 |
| Small | 1.25 (0.93-1.68) | 0.14 | 1.29 (1.01-1.65) | 0.042 |
| IDL particles, nmol/L | ||||
| Total | 1.20 (0.90-1.60) | 0.21 | 1.27 (1.00-1.61) | 0.047 |
| Large | 1.18 (0.88-1.58) | 0.28 | 1.30 (1.01-1.67) | 0.041 |
| Small | 1.21 (0.93-1.59) | 0.16 | 1.24 (0.99-1.54) | 0.060 |
| LDL particles, nmol/L | ||||
| Total | 1.12 (0.84-1.48) | 0.44 | 1.29 (1.01-1.64) | 0.040 |
| I (largest) | 1.22 (0.89-1.68) | 0.23 | 1.25 (0.97-1.61) | 0.080 |
| II a | 1.20 (0.88-1.63) | 0.25 | 1.35 (1.05-1.72) | 0.019 |
| II b | 1.12 (0.85-1.49) | 0.42 | 1.31 (1.03-1.66) | 0.029 |
| III a | 1.13 (0.86-1.48) | 0.37 | 1.25 (1.00-1.57) | 0.050 |
| III b | 0.91 (0.68-1.22) | 0.52 | 1.03 (0.80-1.33) | 0.81 |
| IV a | 0.94 (0.72-1.24) | 0.66 | 1.08 (0.86-1.37) | 0.50 |
| IV b | 0.95 (0.75-1.21) | 0.67 | 1.04 (0.85-1.29) | 0.68 |
| IV c (smallest) | 0.99 (0.79-1.26) | 0.97 | 1.07 (0.87-1.32) | 0.50 |
| LDL peak diameter, Å | 1.08 (0.83-1.42) | 0.55 | 1.06 (0.86-1.31) | 0.56 |
| HDL particles, μmol/L | ||||
| Total | 1.00 (0.80-1.26) | 0.99 | 1.04 (0.86-1.26) | 0.70 |
| Large | 0.90 (0.71-1.15) | 0.41 | 0.97 (0.80-1.18) | 0.76 |
| Small | 1.04 (0.84-1.29) | 0.71 | 1.06 (0.89-1.28) | 0.51 |
73 CVD events and 108 CVD/all-cause death events occurred among 4597 individuals with complete 12-month lipid/lipoprotein data.
Per 1-SD increment in the lipid or lipoprotein variable, adjusted for age, sex, race, smoking, family history, BMI, systolic blood pressure, glucose, and ln hsCRP.
Incremental prognostic value of the set of IM subfractions
For baseline risk associations with CVD, adding the full or parsimonious set of IM subfractions to a model with established risk factors (including standard lipids) improved model prediction (Supplementary Table 7): C statistics were 0.681 (established risk factors), 0.705 (plus full set of IM subfractions; p=0.0002 for likelihood ratio test, d.f.=15), and 0.703 (plus parsimonious set of IM subfractions; p<0.0001 for likelihood ratio test, d.f.=6), and similarly for CVD and all-cause death. Improvements in risk prediction were also noted for residual risk of CVD or CVD and all-cause death among rosuvastatin-allocated individuals with the parsimonious model of on-treatment subfractions.
Discussion
In the JUPITER trial population, recruited based on low LDL-c and elevated hsCRP, baseline LDL-c was not associated with incident CVD. In contrast, incident CVD was associated with a greater atherogenic particle burden, as estimated by non-HDL-c, measured by an immunoassay for apoB or by the IM method for non-HDL-p and LDL-p and select subfractions (primarily large and medium VLDL, and medium to very small LDL). During high-intensity statin therapy, on-treatment apoB, non-HDL-c, and LDL-c were associated with residual risk. However, the pattern of lipoprotein subfractions that was associated with residual risk differed from the baseline risk, with a shift towards more prominent residual risk associations for smaller VLDL and larger LDL subfractions. These results indicate that CVD risk can be increased despite low LDL-c as a result of a higher number of atherogenic particles within the VLDL-LDL particle spectrum. This study also suggests that on-treatment levels of atherogenic particles can contribute to residual risk during statin therapy, potentially indicating inadequate statin efficacy. Finally, risk prediction was improved by adding a set of IM subfractions to models with established risk factors including standard lipids, BMI, and hsCRP.
The present findings are consistent with the growing literature from multiple population-based studies of mostly statin-naive individuals in whom CVD risk tracked with discordantly elevated particle-based measures when LDL-c was low.8, 9, 18, 19 The present study, conducted in a multinational clinical trial, adds to the only other prospective analysis of IM lipoproteins in relation to CVD events, which also found risk to be associated with non-HDLc, IM non-HDL-p and LDL-p.7 Furthermore, in the present study, adjusting for LDL-c, HDL-c, and triglycerides did not impact the associations of apoB or IM non-HDL-p or LDL-p with CVD, indicating that the increased risk is attributable more to atherogenic particle concentrations than to the particles’ load of cholesterol or triglycerides.
Rosuvastatin therapy resulted in reductions across the spectrum of atherogenic apoB-containing particles, although to a lesser degree than was seen for LDL-c. The most pronounced reductions were seen in the larger atherogenic particles, with less of an effect on the smaller particles, resulting in a slight shift in the LDL-p distribution towards a smaller size. Prior studies that assessed the effects of statins on lipoprotein subfractions had fewer participants, used different laboratory methods and various statins: these studies had mixed results, with most studies finding no change in peak or average LDL size,20-22,23-25 while others found an increase26 or a slight decrease.27 The preferential reduction of larger, cholesterol-rich LDL particles in the present study is consistent however with previous findings for other statins28 and this effect likely contributed to the relatively greater reduction in LDL-c versus apoB and other measures of LDL particle concentration.
Higher levels of LDL-c, non-HDL-c, or apoB during statin therapy were associated with a higher residual risk of CVD, consistent with previous reports.14, 29 Notably, this risk was related to on-treatment levels of the LDL subfractions (LDL-I to IIIa) that were predominantly lowered by rosuvastatin. Residual risk was also associated with on-treatment levels of smaller VLDL and large IDL, which may represent remnants of triglyceride-rich lipoproteins that were also insufficiently reduced by rosuvastatin. Therefore, these lipoprotein fractions may be targeted by more aggressive lifestyle therapies or potentially with newer pharmacologic agents if they are proven to be efficacious in outcomes-driven clinical trials.
Interestingly, while levels of larger LDL subfractions were not related to increased CVD risk within the placebo-allocated arm in risk-factor adjusted models, a significant association with risk emerged after further adjustment for triglycerides and HDL-c, while the risk associated with the smaller LDL subfractions diminished after this adjustment (except for LDL-IVc). The attenuation of the association of the larger LDL subfractions with CVD when triglycerides and HDL-c were not taken into account suggests that triglycerides and HDL-c may negatively confound this association; conversely, the strengthening of the association of the smaller LDL subfractions with CVD when triglycerides and HDL-c were not taken into account suggests that triglycerides and HDL-c may positively confound this association.30 These observations are consistent with results from another statin clinical trial, where large predominant LDL peak size (measured by gel electrophoresis) was associated with increased recurrent CVD events, an association that was strengthened after adjusting for standard lipids in the placebo group and not observed in the statin group.31
Finally, unlike the Malmö study, we found that IM-measured HDL-p was not statistically significantly associated with CVD risk.7 This also contrasts with our prior finding in JUPITER that HDL-p as measured by NMR was inversely associated with CVD among both the placebo- and rosuvastatin-allocated arms.32 This could relate to differences in the lipoprotein isolation method for the IM method that were introduced since the Malmö study was performed and/or to differences between the IM and NMR methods. The modified lipoprotein isolation method used in the present study avoided ultracentrifugation, which may have resulted in measuring other proteins in the size range for HDL-p that otherwise would have been sedimented in the centrifugation process used to prepare samples for IM measurements in the Malmö study (see Supplemental Methods). Alternatively, it could be that the HDL particles detected by IM may be more protein-rich and less lipid loaded compared with the NMR HDL-p measurement. Moreover, in a population such as JUPITER that is enriched for individuals with chronic inflammation, some HDL particles may be dysfunctional, which may be more closely related to protein-rich HDL (potentially better measured by the IM method) than lipid-rich HDL (potentially better measured by the NMR method). Indeed, although it was not statistically significant, the direction of effect for the top versus bottom tertile of small HDL-p was positively associated with CVD in both treatment arms, as was seen in other studies with the small lipid-poor prebeta-1 HDL, possibly due to impaired cholesterol efflux or esterification.33 This finding merits further investigation in future studies.
Strengths and Limitations
Strengths of this study include the prospective analysis from the JUPITER trial of the effects of high-intensity statin therapy versus placebo on a wide variety of standard lipids, apolipoproteins, and the novel IM lipoprotein subfractions, measured both at baseline and on-treatment, and the assessment of associations with incident CVD events before and after random allocation to statin therapy versus placebo. The present study also has potential limitations. Median duration of follow-up in JUPITER was 1.9 years (maximum 5.0 years) due to early termination of the trial for benefit, and associations with events occurring over a longer term could not be assessed. The absolute number of CVD events was low and the results may not apply to other population groups. The results may not apply to a general population, since JUPITER excluded individuals with known CVD, diabetes, high triglycerides, or who did not meet entry criteria for LDL-c and hsCRP. Regression coefficients for some of the measures may depend on their study-specific variability, which is also influenced by the trial eligibility criteria. We performed multiple comparisons that increase the chance of a type I error. However, lipids and lipoproteins are correlated, and we interpreted the results emphasizing the magnitude of effects and the consistency with prior experimental and epidemiological studies. While the enhanced resolution of lipoprotein subfractions obtained by IM shed new light on the relationship of these particles to CVD events, the role of other unmeasured factors should not be excluded. Finally, we are unable to rule out possible association for some of the biomarkers with residual risk of the primary endpoint of CVD because of the relatively small number of primary CVD events in the rosuvastatin arm. Our results should be viewed as hypothesis-generating and will require further evaluation in other studies.
Conclusions
Despite the low levels of LDL-c among JUPITER participants, first CVD events were associated with higher baseline levels of atherogenic particles, as assessed by non-HDL-c, apoB, and IM non-HDL-p and LDL-p and select subfractions (primarily large and medium VLDL, and medium to very small LDL). During high-intensity statin therapy, residual risk was influenced by on-treatment levels of atherogenic particles and LDL-c. However, the pattern of lipoprotein subfractions that was associated with residual risk differed from that seen with baseline risk, with a shift towards more prominent residual risk associations for smaller VLDL and larger LDL subfractions, which may indicate inadequacy of the statin response and the potential for targeting these particles by additional therapies for further reducing residual CVD risk.
Supplementary Material
Acknowledgments
We thank James J. Devlin, PhD, and Dov Shiffman, PhD for their helpful comments on the manuscript, as well as Varin Tsai and Chiara Cabrera for their technical help, Julia Larsen, RN, James Morton, PharmD, William Patten, and Veronica Garcia, all at Quest Diagnostics, for their diligent assistance.
Funding Sources: Research reported in this publication was supported by NHLBI R01HL117861 to Dr Mora. JUPITER was financially supported by AstraZeneca, who collected trial data but had no role in the design or conduct of the current study, including data analysis or interpretation, drafting or editing this report, or in preparation, review or the decision to submit the manuscript for publication. Quest Diagnostics absorbed the cost of performing in a blinded manner the ion mobility measurements.
Footnotes
Clinical Trial Registration Information - ClinicalTrials.gov. Identifier: NCT00239681.
Disclosures: Dr. Mora has received research support from AstraZeneca, Atherotech Diagnostics, and NHLBI, served as a consultant to Quest Diagnostics, Lilly, Pfizer, and Cerenis Therapeutics. Drs Caulfield, Wohlgemuth, Chen, and Rowland are employees of Quest Diagnostics. Dr. Wohlgemuth also reports significant equity in Quest Diagnostics. Dr. Glynn has received research support from AstraZeneca, Novartis, and NIH. Dr. Superko has served as a consultant for Quest Diagnostics. Dr. Ridker has received research grant support from AstraZeneca, Novartis, Amgen, and NHLBI, and has served as a consultant to Genzyme, Jannsen, Aegerion, ISIS, Vascular Biogenics, Boston Heart, Pfizer, and Merck. Dr. Ridker is listed as a co-inventor on patents held by the Brigham and Women's Hospital related to the use of inflammatory biomarkers in CVD (licensed to AstraZeneca and Siemens). Dr. Krauss reports research funding from Merck, Roche, Sanofi-Regeneron, Quest Diagnostics, serving as a consultant for Merck, Roche, Genentech, and Celera/Quest Diagnostics, and receiving royalties from patents for ion mobility and gradient gel electrophoresis.
References
- 1.Martin SS, Blaha MJ, Elshazly MB, Toth PP, Kwiterovich PO, Blumenthal RS, Jones SR. Comparison of a novel method vs the Friedewald equation for estimating low-density lipoprotein cholesterol levels from the standard lipid profile. JAMA. 2013;310:2061–2068. doi: 10.1001/jama.2013.280532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oliveira MJ, van Deventer HE, Bachmann LM, Warnick GR, Nakajima K, Nakamura M, Sakurabayashi I, Kimberly MM, Shamburek RD, Korzun WJ, Myers GL, Miller WG, Remaley AT. Evaluation of four different equations for calculating LDL-c with eight different direct HDL-c assays. Clin Chim Acta. 2013;423:135–140. doi: 10.1016/j.cca.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal M, Spencer HJ, Faas FH. Method of LDL cholesterol measurement influences classification of LDL cholesterol treatment goals: Clinical research study. J Invest Med. 2010;58:945–949. doi: 10.231/JIM.0b013e3181fb7ca7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wald NJ, Hackshaw AK, Frost CD. When can a risk factor be used as a worthwhile screening test? BMJ. 1999;319:1562–1565. doi: 10.1136/bmj.319.7224.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barter PJ, Ballantyne CM, Carmena R, Castro Cabezas M, Chapman MJ, Couture P, de Graaf J, Durrington PN, Faergeman O, Frohlich J, Furberg CD, Gagne C, Haffner SM, Humphries SE, Jungner I, Krauss RM, Kwiterovich P, Marcovina S, Packard CJ, Pearson TA, Reddy KS, Rosenson R, Sarrafzadegan N, Sniderman AD, Stalenhoef AF, Stein E, Talmud PJ, Tonkin AM, Walldius G, Williams KM. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: Report of the thirty-person/ten-country panel. J Intern Med. 2006;259:247–258. doi: 10.1111/j.1365-2796.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 6.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 7.Musunuru K, Orho-Melander M, Caulfield MP, Li S, Salameh WA, Reitz RE, Berglund G, Hedblad B, Engstrom G, Williams PT, Kathiresan S, Melander O, Krauss RM. Ion mobility analysis of lipoprotein subfractions identifies three independent axes of cardiovascular risk. Arterioscler Thromb Vasc Biol. 2009;29:1975–1980. doi: 10.1161/ATVBAHA.109.190405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melander O, Shiffman D, Caulfield MP, Louie JZ, Rowland CM, Devlin JJ, Krauss RM. Low-density lipoprotein particle number is associated with cardiovascular events among those not classified into statin benefit groups. J Am Coll Cardiol. 2015;65:2571–2573. doi: 10.1016/j.jacc.2015.02.077. [DOI] [PubMed] [Google Scholar]
- 9.Sniderman AD, St-Pierre AC, Cantin B, Dagenais GR, Despres JP, Lamarche B. Concordance/discordance between plasma apolipoprotein B levels and the cholesterol indexes of atherosclerotic risk. Am J Cardiol. 2003;91:1173–1177. doi: 10.1016/s0002-9149(03)00262-5. [DOI] [PubMed] [Google Scholar]
- 10.Musunuru K, Strong A, Frank-Kamenetsky M, Lee NE, Ahfeldt T, Sachs KV, Li X, Li H, Kuperwasser N, Ruda VM, Pirruccello JP, Muchmore B, Prokunina-Olsson L, Hall JL, Schadt EE, Morales CR, Lund-Katz S, Phillips MC, Wong J, Cantley W, Racie T, Ejebe KG, Orho-Melander M, Melander O, Koteliansky V, Fitzgerald K, Krauss RM, Cowan CA, Kathiresan S, Rader DJ. From noncoding variant to phenotype via SORT1 at the 1p13 cholesterol locus. Nature. 2010;466:714–719. doi: 10.1038/nature09266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strong A, Ding Q, Edmondson AC, Millar JS, Sachs KV, Li X, Kumaravel A, Wang MY, Ai D, Guo L, Alexander ET, Nguyen D, Lund-Katz S, Phillips MC, Morales CR, Tall AR, Kathiresan S, Fisher EA, Musunuru K, Rader DJ. Hepatic sortilin regulates both apolipoprotein B secretion and LDL catabolism. J Clin Invest. 2012;122:2807–2816. doi: 10.1172/JCI63563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr., Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 13.Glynn RJ, MacFadyen JG, Ridker PM. Tracking of high-sensitivity c-reactive protein after an initially elevated concentration: The JUPITER study. Clin Chem. 2009;55:305–312. doi: 10.1373/clinchem.2008.120642. [DOI] [PubMed] [Google Scholar]
- 14.Mora S, Glynn RJ, Boekholdt SM, Nordestgaard BG, Kastelein JJ, Ridker PM. On-treatment non-high-density lipoprotein cholesterol, apolipoprotein B, triglycerides, and lipid ratios in relation to residual vascular risk after treatment with potent statin therapy: JUPITER (Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin). J Am Coll Cardiol. 2012;59:1521–1528. doi: 10.1016/j.jacc.2011.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, Jacques PF, Rifai N, Selhub J, Robins SJ, Benjamin EJ, D'Agostino RB, Vasan RS. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–2639. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 16.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlov J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 17.Harrell FE, Jr., Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Mora S, Buring JE, Ridker PM. Discordance of low-density lipoprotein (ldl) cholesterol with alternative LDL-related measures and future coronary events. Circulation. 2014;129:553–561. doi: 10.1161/CIRCULATIONAHA.113.005873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin SS, Michos ED. Are we moving towards concordance on the principle that lipid discordance matters? Circulation. 2014;129:539–541. doi: 10.1161/CIRCULATIONAHA.113.007207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frost RJ, Otto C, Geiss HC, Schwandt P, Parhofer KG. Effects of atorvastatin versus fenofibrate on lipoprotein profiles, low-density lipoprotein subfraction distribution, and hemorheologic parameters in type 2 diabetes mellitus with mixed hyperlipoproteinemia. Am J Cardiol. 2001;87:44–48. doi: 10.1016/s0002-9149(00)01270-4. [DOI] [PubMed] [Google Scholar]
- 21.Otvos JD, Shalaurova I, Freedman DS, Rosenson RS. Effects of pravastatin treatment on lipoprotein subclass profiles and particle size in the PLAC-I trial. Atherosclerosis. 2002;160:41–48. doi: 10.1016/s0021-9150(01)00544-5. [DOI] [PubMed] [Google Scholar]
- 22.Blake GJ, Albert MA, Rifai N, Ridker PM. Effect of pravastatin on LDL particle concentration as determined by NMR spectroscopy: A substudy of a randomized placebo controlled trial. Eur Heart J. 2003;24:1843–1847. doi: 10.1016/j.ehj.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Kappelle PJ, Dallinga-Thie GM, Dullaart RP. Diabetes Atorvastatin Lipid Intervention study g. Atorvastatin treatment lowers fasting remnant-like particle cholesterol and LDL subfraction cholesterol without affecting LDL size in type 2 diabetes mellitus: Relevance for non-HDL cholesterol and apolipoprotein B guideline targets. Biochim Biophys Acta. 2010;1801:89–94. doi: 10.1016/j.bbalip.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 24.Choi YJ, Roberts BK, Wang X, Geaney JC, Naim S, Wojnoonski K, Karpf DB, Krauss RM. Effects of the PPAR-delta agonist MBX-8025 on atherogenic dyslipidemia. Atherosclerosis. 2012;220:470–476. doi: 10.1016/j.atherosclerosis.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 25.Superko HR, Krauss RM, DiRicco C. Effect of fluvastatin on low-density lipoprotein peak particle diameter. Am J Cardiol. 1997;80:78–81. doi: 10.1016/s0002-9149(97)00288-9. [DOI] [PubMed] [Google Scholar]
- 26.Karalis DG, Ishisaka DY, Luo D, Ntanios F, Wun CC. Effects of increasing doses of atorvastatin on the atherogenic lipid subclasses commonly associated with hypertriglyceridemia. Am J Cardiol. 2007;100:445–449. doi: 10.1016/j.amjcard.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Soedamah-Muthu SS, Colhoun HM, Thomason MJ, Betteridge DJ, Durrington PN, Hitman GA, Fuller JH, Julier K, Mackness MI, Neil HA, Investigators C. The effect of atorvastatin on serum lipids, lipoproteins and NMR spectroscopy defined lipoprotein subclasses in type 2 diabetic patients with ischaemic heart disease. Atherosclerosis. 2003;167:243–255. doi: 10.1016/s0021-9150(02)00428-8. [DOI] [PubMed] [Google Scholar]
- 28.Krauss RM, Pinto CA, Liu Y, Johnson-Levonas AO, Dansky HM. Changes in LDL particle concentrations after treatment with the cholesteryl ester transfer protein inhibitor anacetrapib alone or in combination with atorvastatin. J Clin Lipidol. 2015;9:93–102. doi: 10.1016/j.jacl.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosa JC, Nestel PJ, Simes RJ, Durrington P, Hitman GA, Welch KM, DeMicco DA, Zwinderman AH, Clearfield MB, Downs JR, Tonkin AM, Colhoun HM, Gotto AM, Jr., Ridker PM, Kastelein JJ. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: A meta-analysis. JAMA. 2012;307:1302–1309. doi: 10.1001/jama.2012.366. [DOI] [PubMed] [Google Scholar]
- 30.Rothman KJ, Lash TL, Greenland S. Modern epidemiology. In: Rothman KJ, Lash TL, Greenland S, editors. Modern epidemiology. Lippincott, Williams & Wilkins; Philadelphia, PA: 2012. [Google Scholar]
- 31.Campos H, Moye LA, Glasser SP, Stampfer MJ, Sacks FM. Low-density lipoprotein size, pravastatin treatment, and coronary events. JAMA. 2001;286:1468–1474. doi: 10.1001/jama.286.12.1468. [DOI] [PubMed] [Google Scholar]
- 32.Mora S, Glynn RJ, Ridker PM. High-density lipoprotein cholesterol, size, particle number, and residual vascular risk after potent statin therapy. Circulation. 2013;128:1189–1197. doi: 10.1161/CIRCULATIONAHA.113.002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kane JP, Malloy MJ. Prebeta-1 HDL and coronary heart disease. Curr Opin Lipidol. 2012;23:367–371. doi: 10.1097/MOL.0b013e328353eef1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.