Abstract
BACKGROUND
The external ear represents a site with high ultraviolet exposure and thin skin overlying cartilage. The aim of this study was to determine if ear melanomas have different characteristics than cutaneous melanomas in other anatomic sites.
METHODS
The evaluation of patients treated at a tertiary care center.
RESULTS
Sixty patients were treated for ear melanoma (87% male, mean age = 56.7, mean thickness = 1.65 mm). Seven of thirty-two patients (22%) who underwent sentinel lymph node biopsy had positive nodes. Twenty (33%) patients had recurrence including 6 patients with negative sentinel lymph nodes (SLNs) and 5 patients with positive SLNs. Three of 10 patients (30%) treated with Mohs surgery had local recurrence.
CONCLUSIONS
The overall local and systemic recurrences are similar to those previously reported. There is a higher recurrence rate than expected in patients with a negative SLN and a high local recurrence rate after Mohs surgery. Our data suggest that SLN evaluation may be less accurate in ear melanomas and that Mohs surgery may be associated with a relatively high local recurrence rate.
Keywords: Melanoma, Ear, Sentinel lymph node, Recurrence
The American Cancer Society estimated that 68,130 new melanomas will be diagnosed in the United States alone during 2010.1 The increasing incidence and prevalence of melanoma are in stark contrast to the overall decrease in the incidence of other cancers such as prostate, breast, and colorectal. Despite the increase in new cases, the 5-year survival from melanoma has steadily increased from 82% to 93%, which is likely secondary to earlier detection.2 Head and neck melanoma accounts for approximately 20%3 of melanoma diagnoses, with external ear melanoma accounting for approximately 3% to 20%4,5 of that number. Overall, ear melanoma accounts for only 1% to 4%5 of all cutaneous melanoma diagnoses.
The external ear represents a site with high ultraviolet exposure, thin skin overlying cartilage, and multiple areas of lymphatic drainage. These features make melanoma of the ear unique and cause one to consider if the management of these lesions should be different as well. To confound the surgical management, patients desire a cosmetically pleasing result without sacrificing oncologic control.
Earlier reports had attributed a poor prognosis to ear melanoma and postulated that a more aggressive biologic process was at work, necessitating a more aggressive surgical approach.6–10 More recent data dispute this assumption and suggest that wider margins in head and neck melanoma do not change overall survival.5,8,11 As a result, current therapy generally adheres to that of cutaneous melanoma treatment in other locations (ie, wide local excision with 1- to 2-cm margins, sentinel lymph node biopsy [SLNB] for lesions >1 mm in depth or other adverse pathologic features, and completion lymph node dissection [CLND] for patients with positive SLNBs).
There are numerous publications on cutaneous melanoma occurring on the head and neck; however, there are only a handful of publications describing melanoma of the external ear, many from decades ago.3,5 Because of the potentially unique nature of ear melanoma and the relatively few scientific articles describing it, we sought to better define its behavior and treatment at our institution.
Methods
The University of Colorado Tumor Registry was queried for melanoma of the ear from 1994 to 2009. A retrospective chart review was conducted to verify the diagnosis and location. Only patients with malignant melanoma of the external ear were selected, and study variables included age, sex, location, Clark’s level, Breslow depth, type of surgical treatment, a history of previous skin cancer, lymph node (LN) evaluation, recurrence, and overall survival. Ulceration and the presence or absence of mitotic figures were included when available. The University of Colorado Institutional Review Board approved this study.
Surgical treatment was undertaken by a surgical oncologist; ear, nose, and throat specialist; or a dermatologist. Surgical definitions are as follows: wide local excision removes the skin and subcutaneous tissue; wedge resection removes the skin, subcutaneous tissue, and cartilage; and partial amputation removes up to one fourth on the ear. Mohs surgery is the removal of a biopsy specimen around the entire defect, and all surgical margins are subsequently examined by horizontal histologic frozen sections.
A discussion regarding SLNB was undertaken in all qualified patients and was performed using technectium-99 and/or isosulfan blue dye (Covidien, Mansfield, MA, USA). Patients selected for SLNB underwent preoperative lymphoscintigraphy with technetium-99–labeled colloid tracer at the primary site or just posterior to the lobule. Finally, isosulfan blue was injected in the operating room immediately before the procedure. Intraoperatively, a handheld gamma counter was used to localize sentinel lymph nodes (SLNs); all blue nodes or those with at least 10% of the counts of the highest (hottest) SLN were removed.
Pathologic examination of SLNs was performed using a standard melanoma algorithm and included serial sectioning and S-100 and HMB-45 immunohistochemical stains. No frozen sectioning was used. If an SLN was found to have melanoma metastasis on evaluation of the fixed tissue, completion LN dissection was undertaken at a separate appointment.
Summary statistics were used for demographic variables. The mean and standard deviation were reported for continuous variables, whereas the number and frequency were reported for categoric variables. Two group t tests were used to test the mean difference between 2 groups. The chi-square or Fisher exact test was used to test the difference in proportion between groups. The Kaplan-Meier product-limit estimate was used to generate survival distribution. Variables including age at diagnosis, primary lesion thickness, the number of positive LNs during SLN, and the number of positive nodes during complete neck dissection were used in the multivariate analysis model.
Results
Sixty patients with external ear melanoma met criteria for inclusion in this study. The mean age at diagnosis was 57 years (16 to 97 years), and patients had a median follow-up of 38 months. In accordance with previous studies, the incidence of ear melanoma at our institution is 3.8% of all cutaneous melanoma diagnoses. There is a significant male predilection with disease (87%, 52/60) but a relatively even anatomic distribution of right sided versus left sided (48% vs 53%). Previous skin cancer was noted in over one fourth of patients, with basal cell carcinoma accounting for 50% of these lesions.
The majority of the lesions were classified as superficial-spreading melanoma (33%) although lentigo and nodular melanoma were both observed in significant quantities (20% and 18%, respectively). The minority (5%) were desmoplastic lesions. The mean Breslow depth was 1.65 mm with a median of 1.22 mm and a Clark level of IV. One third of patients experienced recurrence at a mean of 29 months from diagnosis. Recurrent lesions were characterized as local only (9/20), metastatic (7/20), or both (4/20) (Table 1).
Table 1.
Patient characteristics and features of external ear melanoma
| Sex (%) | |
| Male | 87 (52/60) |
| Female | 13 (8/60) |
| Age (y) | |
| Mean | 57 |
| Median | 56 |
| Range | 16–97 |
| Location (%) | |
| Left ear | 52 (31/60) |
| Right ear | 48 (29/60) |
| Previous skin cancer (%) | 27 (16/60) |
| BCC | 13 (8/60)* |
| SCC | 7 (4/60) |
| Melanoma | 7 (4/60) |
| Pathology (%) | |
| Superficial spreading | 33 (20/60) |
| Lentigo | 20 (12/60) |
| Nodular | 18 (11/60) |
| Demoplastic | 5 (3/60) |
| Malig blue nevus | 2 (1/60) |
| Spindle | 2 (1/60) |
| Not defined | 23 (14/60) |
| Clark level (%) | |
| I | 3 (2/60) |
| II | 13 (8/60) |
| III | 15 (9/60) |
| IV | 68 (41/60) |
| Breslow level (mm) | |
| Mean | 1.65 |
| Median (range) | 1.22 (in situ–5) |
| Overall follow-up (mo) | |
| Mean | 40.4 |
| Median (range) | 37.5 (1–129) |
| Time to recurrence (mo) | |
| Mean (range) | 29 |
| Median | 16 |
| Range | 5–92 |
| Recurrence (%) | 33.3 (20/60) |
| None | 66.6 (40/60) |
| Local | 15 (9/60) |
| Metastatic | 12 (7/60) |
| Both | 7 (4/60) |
BCC = basal cell carcinoma; SCC = squamous cell carcinoma.
One patient had both melanoma and basal cell skin cancer before the diagnosis and treatment of the external ear melanoma.
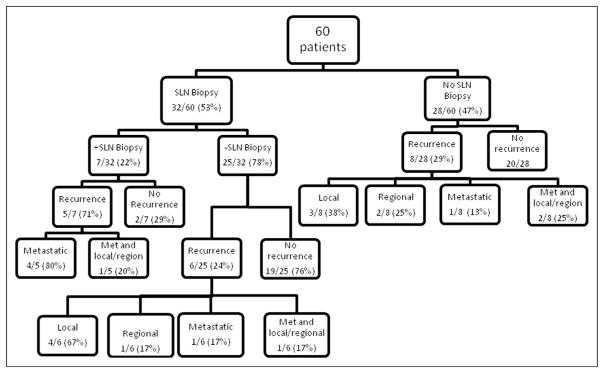
The surgical treatment of patients is as follows: 82% underwent wide local excision, 17% had Mohs surgery, and 1 patient had partial amputation. Over half of the patients underwent SLNB (52.3%), and CLND was undertaken in all positive biopsies (7 patients) and in 1 patient at the time of initial surgery at the surgeon’s discretion. Three of these 8 patients (37%) were found to have additional positive nodes after complete neck dissection (Table 2). A comparison of outcomes between patients who had SLN biopsies versus none is shown in Fig. 1.
Table 2.
Surgical management
| Primary management (%) | |
| Wide local excision | 82 (49/60) |
| Mohs | 17 (10/60) |
| Partial amputation | 1 (1/60) |
| Regional management (%) | |
| SLNB | 53 (32/60) |
| Positive SLN | 22 (7/32) |
| Neck dissection | 13 (8/60) |
| Positive nodes | 37 (3/8) |
SLNB = sentinel lymph node biopsy; SLN = sentinel lymph node.
Figure 1.
Patient treatment and recurrence patterns.
Twenty patients experienced recurrence defined as local, metastatic, or both. Patients with a positive LN biopsy were 1.3 times more likely to have a recurrence event. Although patients with recurrence were younger (53.8 vs 58.2 years old) and had thicker primary lesions (1.96 vs 1.50 mm), these 2 variables did not reach statistical significance. Patients who had a positive LN after completion neck dissection were also not significantly associated with recurrence (Table 3). Of the 10 patients who underwent Mohs surgery in this population, 3 developed local recurrence only.
Table 3.
Comparison of patients with and without recurrence
| No recurrence (40/60) | Recurrence (20/60) | P value | |
|---|---|---|---|
| Sex (%) | .1 | ||
| Male | 93 (37/40) | 75 (15/20) | |
| Female | 7 (3/40) | 25 (5/20) | |
| Age (y) | .3 | ||
| Mean (SD) | 58 (15.4) | 53.8 (15.3) | |
| Median (range) | 57.5 (16–88) | 53 (27–91) | |
| Previous skin cancer (%) | 32.5 (13/40) | 15 (3/20) | .15 |
| BCC | 20 (8/40) | 5 (1/20) | |
| SCC | 7.5 (3/40) | 5 (1/20) | |
| Melanoma | 5 (2/40) | 10 (2/20)* | |
| Side (%) | .86 | ||
| Right ear | 48 (19/40) | 50 (10/20) | |
| Left ear | 52 (21/40) | 50 (10/20) | |
| Pathology (%) | .63 | ||
| Superficial spreading | 32 (13/40) | 35 (7/20) | |
| Lentigo | 15 (6/40) | 30 (6/20)† | |
| Nodular | 20 (8/40) | 10 (3/20) | |
| Demoplastic | 2 (2/40) | 0 | |
| Malig blue nevus | 0 | 5 (1/20) | |
| Spindle | 2.5 (1/40) | 5 (1/20) | |
| Not defined | 25 (10/40) | 20 (4/20) | |
| Clark level (%) | .4 | ||
| I | 5 (2/40) | 0 | |
| II | 12.5 (5/40) | 15 (3/20) | |
| III | 10 (4/40) | 25 (5/20) | |
| IV | 72.5 (29/40) | 60 (12/20) | |
| Breslow thickness (mm) | .14 | ||
| Mean (SD) | 1.50 (.99) | 1.96 (1.41) | |
| Median (range) | 1.1 (in situ–5) | 1.64 (.29–5) | |
| Surgical treatment (%) | |||
| SLNB | 52.5 (21/40) | 55 (11/20) | .86 |
| Positive SLN | 9.5 (2/21) | 45 (5/11) | .03 |
| Neck dissection | 7.5 (3/40)‡ | 25 (5/20) | .1 |
| Positive nodes | 0 | 60 (3/5) |
BCC = basal cell carcinoma; SCC = squamous cell carcinoma; SD = standard deviation; SLNB = sentinel lymph node biopsy.
One patient had previously been diagnosed with a melanoma and BCC.
One patient had lentiginous and superficial spreading lesions, another patient had both a lentiginous and nodular lesion.
One patient had a neck dissection at the time of initial operation instead of a SLN biopsy at the discretion of the surgeon.
A comparison of patients with positive SLNB versus those with negative SLNB is shown in Table 4. Twenty percent of patients who underwent SLNB had positive nodes, and all underwent CLND. Overall, patients with positive SLNs were older (62.5 vs 57.4 years), had higher rates of previous skin cancer (43% vs 24%), and had thicker lesions (2.7 vs 2.1 mm). Overall recurrence was more common in patients with positive SLNs (72% [5/7] vs 24% [6/25]). These patients recurred sooner (ie, at a median of 12.9 months after diagnosis vs 19 months) and were more likely to have distant metastases (ie, 60% vs 33%). Of the recurrences in the SLN− group, 4 were local.
Table 4.
Comparison of positive and negative SLNB patients
| Negative SLNB | Positive SLNB | P value | |
|---|---|---|---|
| Sex (%) | 1.00 | ||
| Male | 88 (22/25) | 85.7 (6/7) | |
| Female | 12 (3/25) | 14.3 (1/7) | |
| Age (y) | |||
| Mean (SD) | 57 (12.9) | 61.5 (14) | .49 |
| Median (range) | 57 (36–91) | 65 (49–78) | |
| Previous skin cancer (%) | 24 (6/25) | 43 (3/7) | .37 |
| BCC | 12 (3/25) | 29 (2/7) | |
| SCC | 4 (1/25) | 14 (1/7) | |
| Melanoma | 8 (2/25) | 0 (0/7) | |
| Side (%) | |||
| Right | 44 (11/25) | 57.1 (4/7) | .68 |
| Left | 56 (14/25) | 42.9 (3/7) | |
| Pathology (%) | .84 | ||
| Superficial spreading | 28 (7/25)* | 14.3 (1/7) | |
| Lentigo | 20 (5/25)* | 14.3 (1/7) | |
| Nodular | 24 (6/25)* | 14.3 (1/7) | |
| Desmoplastic | 8 (2/25) | 14.3 (1/7) | |
| Spindle | 4 (1/25) | 0 (0/7) | |
| Not defined | 24 (6/25) | 42.9 (3/7) | |
| Clark level (%) | 1.00 | ||
| I | 0 (0/25) | 0 (0/7) | |
| II | 0 (0/25) | 0 (0/7) | |
| III | 12 (3/25) | 0 (0/7) | |
| IV | 88 (22/25) | 100 (7/7) | |
| Breslow thickness (mm) | .37 | ||
| Mean (SD) | 2.18 (1.26) | 2.65 (1.15) | |
| Median (range) | 2.1 (.6–5) | 2.15 (1.5–4.4) | |
| Number of nodes removed | .04 | ||
| Mean (SD) | 4.88† (5.88) | 2.14 (1.35) | |
| Median (range) | 3 (1–28) | 2 (1–5) | |
| Postoperative recurrence (%) | 24 (6/25) | 71 (5/7) | .03 |
| Local/regional | 66 (4/6) | 0 (0/7) | |
| Metastatic | 17 (1/6) | 57 (4/7) | |
| Both | 17 (1/6) | 14 (1/7) | |
| Time to recurrence (mo) | .04 | ||
| Mean | 26.4 | 13.4 | |
| Median (range) | 19 (14–43) | 12 (7–23) |
BCC = basal cell carcinoma; SCC = squamous cell carcinoma; SD = standard deviation; SLNB = sentinel lymph node biopsy.
One patient had lentiginous and superficial spreading lesions, and 1 patient had lentiginous and nodular lesions.
One patient had multiple SLNBs.
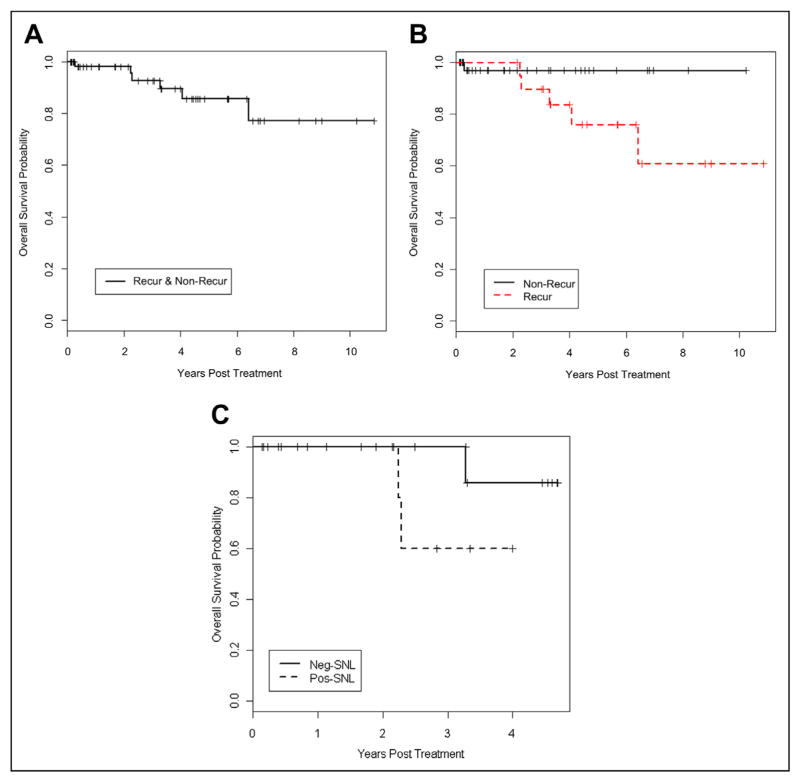
The overall survival was 85% (95% confidence interval [CI], 74% to 99%) at 5 years and 77% (95% CI, 60% to 99%) at 10 years. Comparing patients with recurrence versus nonrecurrence, the 5-year survival probability is 97% (95% CI, 91% to 100%) for patients without recurrence and 75% (95% CI, 58% to 100%) for patients with recurrence. When comparing SLN+ and SLN− patients, survival probability is 60% (95% CI, 29% to 100%) at 3 years for SLN+ patients and 92% (95% CI, 79% to 100%) at 5 years for SLN− patients (Fig. 2A–C).
Figure 2.
(A) Overall survival. (B) Survival for patients with and without recurrence. (C) Survival for patients with +/− SLN biopsies.
Comments
External ear melanoma is a cancer characterized by an inordinate amount of sun exposure in comparison to the rest of the body and an unusual anatomy with nearby underlying cartilage. Additionally, it is a rare lesion that accounts for just 1% to 4%6 of all cutaneous melanomas. These features and a 10-year survival rate of 70%3 have led to increased interest regarding the management of external ear melanoma. External ear melanoma had been considered to be of a more aggressive nature in the 1970s and 1980s; however, recent reports have shown evidence to the contrary. Thus, current treatment has realigned with that of melanoma in other locations, allowing for both an oncologic resection and an appropriate cosmetic result with unchanged recurrence.
The histologic types of melanoma observed in our patient population were similar to previous results (33% superficial spreading [45% to 46%], 22% lentigo [19.6% to 26%], and 18% nodular [16% to 22%]). The majority of our patients underwent wide local excision for disease control; however, compared with previous studies, the majority of our patients also underwent SLNBs (53%), reflecting a referral population. Our overall recurrence rate of 33% was also similar to previous reported rates, which range from 23% to 58%.10,11 Our local recurrence rate was 15% (9/60) and accounted for almost half (9/20) of all recurrences. The SLN status of patients was significant for prognosis, with patients with positive SLNs developing recurrences sooner and more frequently. In addition to SLN status, increased thickness of the primary lesion, previous skin cancer, and age were factors that trended toward significance for recurrence.
Although Mohs surgery has been reported to be effective for head and neck melanoma,12 other previous studies have indicated that having a minimal excision margin for ear melanoma, given its particular anatomy, had a negative effect on recurrence.6,10 Our patient population who underwent Mohs surgery is still too small to make any definitive conclusions (10/60 patients); however, caution should be exercised in using Mohs for ear melanoma because the 30% recurrence rate is significantly greater than generally reported local recurrence rates. Nonetheless, all recurrences in the Mohs group remained local and had no evidence of further local or regional disease.
The difficulty of identifying the exact draining nodal basin remains great in lesions of the head and neck. Common nodal basins include both pre- and postauricular sites, anterior or posterior cervical chains, and the parotid gland. The large variety of draining nodal basins highlights an appropriate use for SLNBs.13 However, 45% (11/20 patients) of our patients who did recur did not have an SLNB, whereas the recurrence rate for patients with a negative SLNB was 24%. Although a larger proportion of our patients did have an SLNB, the recurrence rate in SLN− patients was generally higher than reported for all SLN–patients with melanoma arising from other body regions. Reports from larger cohorts of head and neck melanoma have reported a high false-negative rate in SLNBs; for ear melanoma alone, previous studies report recurrence from 0% to 22% in SLN− patients. These rates along with the higher reported overall recurrence for head and neck melanoma could reflect a tendency to accept closer margins for head and neck melanoma to preserve cosmesis as well as lower accuracy for SLNB because of the considerable variability in the drainage patterns from head and neck melanomas. This shows the need for continued close observation for all patients with head and neck melanoma regardless of SLN status.14
There are several limitations inherent to this type of retrospective review. Pathological and surgical margins were individualized at the surgeon’s discretion. As a tertiary care center, our patient population may have more advanced lesions than would be observed at an initial encounter, reflecting a possible referral bias. Many patients may have continued their care with their local physicians; therefore, the long-term history of these lesions is unknown. In addition, there were a limited number of patients in each category, specifically SLN+, making overall survival difficult to compare with other groups. The limited numbers of patients may also account for the lack of statistical significance for patients with positive LNs after neck dissection and recurrence.
As with other melanomas of the head and neck, tumor thickness remains the most consistent prognostic factor for recurrence with ear melanoma patients. Treatment standards for all cutaneous melanoma are applicable to ear melanoma. Although narrower margins and Mohs resection are gaining acceptance in select cases, treatment should continue to follow standard melanoma treatment guidelines with an emphasis on improving local control of the primary disease and close surveillance even after SLNB.
Footnotes
The authors declare no conflict of interest.
Presented at the 63rd Annual Meeting of The Southwestern Surgical Congress, April 4–8, 2011, Ko Olina, HI.
References
- 1. [Accessed May 15, 2011];American Cancer Society Facts and Figures. 2005 Available at: www.cancer.org.
- 2.Jemal A, Siegel R, Xu J, et al. Cancer statistics 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 3.Larson DL, Larson JD. Head and neck melanoma. Clin Plast Surg. 2010;37:73–7. doi: 10.1016/j.cps.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Pino Rivero V, González Palomino A, Pantoja Hernández CG, et al. Malignant melanoma of the pinna. Importance of its diagnosis, treatment and prognostic factors. An Otorrinolaringol Ibero Am. 2006;33:545–50. [PubMed] [Google Scholar]
- 5.Jahn V, Breuninger H, Garbe C, et al. Melanoma of the ear: prognostic factors and surgical strategies. Br J Dermatol. 2006;154:310–8. doi: 10.1111/j.1365-2133.2005.07065.x. [DOI] [PubMed] [Google Scholar]
- 6.Byers RM, Smith JL, Russell N, et al. Malignant melanoma of the external ear: review of 102 cases. Am J Surg. 1980;140:518–21. doi: 10.1016/0002-9610(80)90203-2. [DOI] [PubMed] [Google Scholar]
- 7.Wanebo HJ, Cooper PH, Young DV, et al. Prognostic factors in head and neck melanoma: effect of lesion location. Cancer. 1988;62:831–7. doi: 10.1002/1097-0142(19880815)62:4<831::aid-cncr2820620432>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Pockaj BA, Jaroszewski DE, DiCaudo DJ, et al. Changing surgical therapy for melanoma of the external ear. Ann Surg Oncol. 2003;10:689–96. doi: 10.1245/aso.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 9.van Akkooi AC, Voit CA, Verhoef C, et al. New developments in sentinel node staging in melanoma: controversies and alternatives. Curr Opin Oncol. 2010;22:169–77. doi: 10.1097/CCO.0b013e328337aa78. [DOI] [PubMed] [Google Scholar]
- 10.Hudson DA, Krige JE, Strover RM, et al. Melaignant melanoma of the external ear. Br J Plast Surg. 1990;43:608–11. doi: 10.1016/0007-1226(90)90129-n. [DOI] [PubMed] [Google Scholar]
- 11.Narayan D, Ariyan S. Surgical considerations in the management of malignant melanoma of the ear. Plast Reconstr Surg. 2001;107:20–4. doi: 10.1097/00006534-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Bricca GM, Brodland DG, Ren D, et al. Cutaneous head and neck melanoma treated with Mohs micrographic surgery. J Am Acad Dermatol. 2005;52:92–100. doi: 10.1016/j.jaad.2004.08.038. [DOI] [PubMed] [Google Scholar]
- 13.Patel SF, Coit DG, Shaha AR, et al. SLN biopsy for cuteanous head and neck melanoma. Arch Otolaryngol Head Neck Surg. 2002;128:285–91. doi: 10.1001/archotol.128.3.285. [DOI] [PubMed] [Google Scholar]
- 14.Salthman BE, Ganly I, Patel SG, et al. Prgonostic implication of sentinel lymph node biopsy in cutaneous head and neck melanoma. Head Neck. 2010;32:1686–92. doi: 10.1002/hed.21390. [DOI] [PubMed] [Google Scholar]