Abstract
Background
Although melanoma can elicit robust tumor antigen-specific immune responses, advanced melanoma is associated with immune tolerance. We have previously described several mechanisms of melanoma-induced immunosuppression, including the skewing of the immune response towards a Th2 cytokine profile and the induction of regulatory T cells. Since dendritic cells (DCs) are potentially important players that can direct other cells of the immune system towards a cytotoxic, humoral, or regulatory phenotype, we hypothesized that melanoma-produced factors directly affect the maturation and function of DCs, influencing the nature and magnitude of the resulting immune response.
Materials and Methods
To test this hypothesis, immature myeloid-derived DCs (mdDCs) were derived with cytokines from CD14+ peripheral blood mononuclear cells (PBMCs) and exposed to 20% melanoma-conditioned media (MCM). After 2 d, the expression of maturation markers and the function of these mdDCs, measured by cytokine production, the amount of endocytosis, expression of the inhibitory molecule indoleamine 2,3-dioxygenase (IDO), and the ability to stimulate T cells were determined.
Results
We found that incubation with MCM did not inhibit the expression of maturation markers or IDO, the production of cytokines, the amount of antigen uptake, or the ability to induce T cell proliferation in mixed-lymphocyte reactions by mdDC.
Conclusions
These results suggest that the immunosuppressive effects of melanoma-produced factors are independent of directly measurable changes in mdDC function or maturation in vitro.
Keywords: melanoma, immunosuppression, dendritic cells, indoleamine 2, 3-dioxygenase (IDO)
INTRODUCTION
Dendritic cells (DCs) circulate throughout the blood and lymphoid tissues and are recruited to peripheral sites such as the gut and skin after exposure to foreign antigens [1, 2]. Immature DCs are extremely efficient at antigen capture, and after exposure to pathogens, they become effective antigen-presenting cells that migrate to draining lymphoid tissues and direct adaptive immune responses [2]. Depending on the pathogen and the nature of the inflammatory environment, DCs can induce immunogenic Th1, Th2, or Th17 profiles or they can promote the development of tolerogenic regulatory T cells (Tregs [3]). DCs also influence the activity of cytotoxic T cells, B cells, and natural killer (NK) cells [2]. Thus, given their unique function in antigen presentation and immune programming, DCs also play a critical role in the immune response to cancer.
DCs are a critical component of the antitumor immune response induced by melanoma [4]. A variety of immunotherapies, including DC adjuvants and DC-based tumor vaccines have been developed to stimulate tumor-specific immune responses [5]. However, most cancer immunotherapies ultimately fail and advanced melanoma is characterized by overall immunosuppression. These failures suggest the development of immunoregulatory mechanisms, which may include the modulation of DC function [6]. Thus, although dendritic cells (DCs) are among the first leukocytes to infiltrate a tumor, they can evoke antitumor immune responses or mediate the development of immune tolerance [7].
The functional defects of tumor-infiltrating DCs impair responses of other immune cells, allowing tumor cells to escape immune cell-mediated destruction. Most tumor-infiltrating DCs are immature and have reduced expression of maturation markers after activation compared with circulating DCs [8]. They also have functional defects, including impaired cytokine production, reduced antigen presentation, and reduced migration [8, 9]. In melanoma studies, DCs that infiltrate primary tumors are predominantly immature and DCs that infiltrate melanoma metastases induce immune tolerance [10, 11]. However, evidence for direct effects of melanoma cells or melanoma-produced factors on DC function is lacking.
We and others have shown that the number of regulatory T cells and suppressive DCs are increased in melanoma patients and are associated with poor survival [12, 13]. Furthermore, using an in vitro model of melanoma, we have shown that soluble factors produced by melanoma tumors cells induce an increased frequency of Tregs and skew the immune response towards a Th2 profile [14, 15]. One possible mechanism of this Treg induction is indirect; melanoma-produced factors may influence DC maturation or function, leading to tolerogenic changes that promote Treg development [16]. Therefore, we investigated the effects of exposure to melanoma-conditioned media (MCM) on the maturation, cytokine secretion, endocytic ability, and tolerogenicity of in vitro-derived DCs. We hypothesized that soluble factors produced by melanoma tumor cells induce immunosuppressive defects in the maturation or function of DCs that may contribute to immune evasion. However, we found that incubation with MCM does not alter the state of myeloid-derived DCs (mdDCs) in vitro, indicating that melanoma-produced soluble factors may not directly alter the function of mdDCs.
MATERIALS AND METHODS
Media
Control media consisted of Roswell Park Memorial Institute (RPMI) 1640 media supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM L-glutamine ± 10% pooled human serum (HS; Gemini Bio-Products, West Sacramento, CA). MCM was generated by bringing a metastatic melanoma cell line (A375; American Type Culture Collection, ATCC, Manassas, VA) to 80%–90% confluence in control media with 10% HS, then changing the media to fresh control media without HS, and collecting the serum-free conditioned media 24 h later. This media was then centrifuged at 350 × g, passed through a 0.22 μm filter, and stored at −70°C until use. As previously reported, the conditioned media was diluted to 20% in control media without HS to ensure the presence of adequate levels of growth factors and micronutrients [17, 18].
Dendritic Cell Derivation
Peripheral blood was collected from healthy volunteers without a history of melanoma and the peripheral blood mononuclear cell (PBMC) fraction was isolated using a Ficoll-Paque density gradient (Amersham Biosciences AB, Uppsala, Sweden). Cells were washed with Dulbecco’s phosphate-buffered saline (DPBS) three times prior to their use or frozen in 10% DMSO solution in control media with 10% HS at −70°C until use. DCs were derived from monocytes as previously described [1, 2, 19–21]. In Brief, CD14+ cells were isolated from the PBMCs by magnetic immunosorting according to the manufacturer’s instructions (MACS cell separation; Miltenyi Biotec, Auburn, CA). The PBMCs were incubated with anti-CD14 microbeads for 15 min at 0°C, washed in PBS with 0.5% bovine serum albumin (BSA) and 2 mM EDTA, and exposed to a magnetic field according the manufacturer’s instructions. The CD14+ cells were washed again in PBS with 0.5% BSA and 2mMEDTA, and some were frozen in 10% DMSO solution in control media without HS at −70°C until use for phenotypic assays. The remainder of the CD14+ cells were in plated in 1 mL control media without HS on a flat-bottomed, 24-well plate (Corning, Corning, NY) with 1000 U/mL human recombinant IL-4 (Cell Sciences, Canton, MA) and 1000 U/mL human recombinant GM-CSF for 5 d (US Biological, Swampscott, MA) at 37°C in 5% CO2. MCM or additional serum-free control media was added on d 6 at 20% concentration by volume for an additional 48 h ± 10 μg/mL lipopolysaccharide (LPS; Sigma, St. Louis, MO) for the final 24 h. mdDCs were then harvested after 7 d total in culture for phenotypic or functional assays.
mdDC Maturation Assays
Immature or LPS-matured mdDCs or uncultured, sorted CD14+ cells were stained with antibodies specific for human CD14 (FITC-conjugated; Miltenyi Biotec, Auburn, CA), human HLA-DR (PerCP-conjugated; BD Biosciences, San Jose, CA), human CD83 (APC-conjugated; BD Biosciences), human CD86 (PE-conjugated; Invitrogen, Carlsbad, CA) molecules, and with human Fc receptor (FcR) blocking reagent (Miltenyi Biotec) for 15 min at room temperature. The cells were then fixed with fixation/permeabilization solution according to the manufacturer’s instructions (eBioscience, San Diego, CA) and stored in 1% paraformaldehyde (PFA) in PBS solution in the dark at 4°C until analysis. The cells were analyzed by flow cytometry on a FACSCaliber (Becton Dickinson, Mountain View, CA). Flow cytometry data was analyzed using the FlowJo software program (Tree Star, Ashland, OR) with gating based on forward versus side scatter for live mdDCs or monocytes (among the mdDCs or uncultured, sorted CD14+ cells, respectively) and the mean fluorescence intensity (MFI) of CD14, HLA-DR, CD83, and CD86 was calculated from this gate. Negatively or singly-stained cells were used as compensation controls as well as the appropriate isotype controls for nonspecific binding (Beckman Coulter, Fullerton, CA and Invitrogen).
Cytokine Analysis
Cell supernatants were collected from the maturation experiments after exposure to LPS by aspirating the media prior to cell collection, centrifugation at 300 × g, and isolation and storage of the supernatant at −70°C until analysis. IL-10, IL-12(p70), TNF-α, IL-6, and IFN-γ levels were analyzed by cytometric bead array according to the manufacturer’s instructions (Bio-Rad, Hercules, CA).
Dextran Endocytosis Assay
Five hundred μg/mL 40 kDa dextran (FITC-conjugated; Invitrogen) was added to mdDCs for 2 h at 37°C after exposure to serum-free control media or MCM ± LPS according to the previously described derivation assay. The cells were then harvested, washed twice with DPBS, and fixed and stored in 1% PFA in PBS at 4°C in the dark until analysis. The cells were analyzed by flow cytometry to determine the MFI of dextran-FITC after gating on live mdDCs by forward versus side scatter.
Mixed Leukocyte Reaction Assay
MdDCs matured in LPS and control media or MCM for 24 h were harvested and enumerated. Allogeneic CD4+ T cells were separated from PBMCs using magnetic immunosorting according to the manufacturer’s instructions (MACS cell separation; Miltenyi Biotec) and stained with 2 μM carboxy-fluorescein diacetate succinimidyl ester (CFSE) for 15 min at 37°C (Invitrogen); 1 × 105 allogeneic CFSE-stained CD4+ T cells were cultured alone with 1 μg/mL soluble CD3 and 1 μg/mL soluble CD28 antibodies (BD Biosciences) or cocultured with various numbers of mdDCs for 5 d on 96-well plates (Corning). The cells were then harvested and stained with antibodies specific for human CD4 molecules (TC-conjugated; Invitrogen). The percentage of divided CD4+ lymphocytes was determined by flow cytometry, gating on the CFSE diluted population.
Western Blot Analysis
Total cell lysates were extracted from mdDCs that were exposed to control media or MCM ± LPS. Lysates were solubilized in trisbuffered saline Tween (TBST) and boiled at 100°C for 5 min before SDS-polyacrylamide gel electrophoresis (PAGE). Equal amounts of total protein were loaded per lane as calculated by the bicinchoninic acid assay (BCA; Pierce, Rockford, IL). PAGE-separated proteins were then transferred to a nitrocellulose membrane (Bio-Rad, Hercules, CA) and blocked with 5% nonfat dry milk in phosphate-buffered saline Tween (PBST) overnight at 4°C. Immunostaining was performed with 1 μg/mL of a rabbit primary antibody specific for human indoleamine 2,3-dioxygense (IDO) molecules (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h at room temperature and with 1 μg/mL of a goat secondary antibody conjugated to horseradish peroxidase. The blots were developed with the appropriate detection kit according to the manufacturer’s instructions (Abcam, Inc., Cambridge, MA). Membranes were also probed for heavy-chain clathrin (HC clathrin; BD Biosciences, San Jose, CA) as an additional loading control.
RESULTS
Melanoma-Conditioned Media Does Not Alter Dendritic Cell Maturation
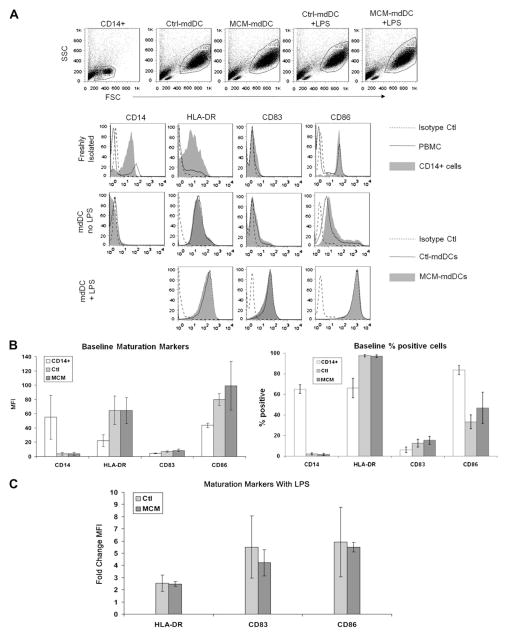
To determine the effect of MCM on the maturation of mdDCs, expression of maturation markers was evaluated before and after incubation with the TLR4 ligand, LPS. The mdDCs were harvested and the amount of CD14, HLA-DR, CD83, and CD86 expression was assessed by flow cytometry. The gating strategies of freshly-isolated PBMC, CD14+ cells, and mdDCs, and the histograms used to evaluate expression of maturation markers are depicted in the representative flow plots in Figure 1A. Consistent with in vitro derivation of mdDCs, CD14 expression was decreased compared with freshly sorted CD14+ cells (Fig. 1B). Prior to maturation with LPS, the staining intensities of HLA-DR, CD83, and CD86 antibodies were not different between control mdDCs (ctl-mdDCs) and MCM-exposed mdDCs (MCM-mdDCs); with a mean fluorescence intensity (MFI) of 65 ± 20 versus 65 ± 18 for HLA-DR, 6.9 ± 1.1 versus 8.5 ± 1.7 for CD83, and 80 ± 8 versus 99 ± 34 for CD86 for ctl-mdDCs versus MCM-mdDCs, respectively, P >0.05 (Fig. 1B). After exposure to LPS, all three maturation markers were upregulated in both groups. The fold change in the level of expression of maturation markers after LPS exposure was similar between ctl-mdDCs and MCM-mdDCs (2.5 ± 0.7 versus 2.5 ± 0.2 for HLA-DR, 5.5 ± 2.6 versus 4.2 ± 1.1 for CD83, and 5.9 ± 2.9 versus 5.5 ± 0.4 for CD86 for ctl-mdDCs versus MCM-mdDCs, respectively, P > 0.05; Fig. 1C). In addition, the percentage of cells that expressed these markers was the same between the ctl-mdDCs and the MCM-mdDCs (Fig. 1B). Thus, exposure to MCM did not alter the surface marker expression of mdDCs.
FIG. 1.
MCM does not alter expression of maturation markers on mdDC. (A) Representative flow plots depict freshly isolated PBMC, sorted uncultured CD14+ cells or mdDCs in control media (Ctl-mdDC) or MCM (MCM-mdDC) ± LPS overlaid with isotype control samples. Cells are gated as depicted and the MFI of CD14, HLA-DR, CD83, and CD86 was determined. (B) Cumulated data from three independent experiments depicting the baseline MFI of maturation markers on mdDCs (left) and the percentage of positive cells (right) in control media or MCM. Expressed as mean ± SEM. (C) The fold change in the level of expression of maturation markers after LPS exposure is expressed for ctl-mdDCs versus MCM-mdDCs.
Melanoma-Conditioned Media Does Not Alter mdDC Cytokine Expression
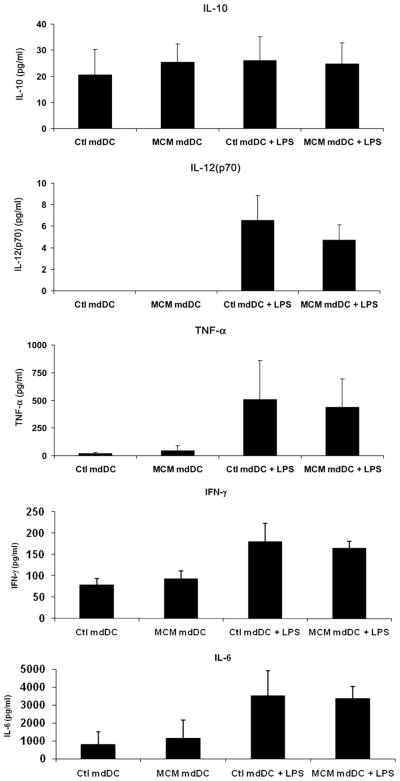
To determine the amount and nature of the cytokines secreted by melanoma-exposed mdDCs, the levels of IL-10, IL-12(p70), and TNF-α were measured in the cell supernatants from the previous maturation experiments using a cytokine bead array. Prior to incubation with LPS, the levels of TNF-α were low and not different between groups (21 ± 11 versus 45 ± 45 pg/mL for ctl-mdDCs versus MCM-mdDCs, respectively, P > 0.05) and levels of secreted IL-12(p70) were undetectable in both groups (Fig. 2). IL-10, IL-6, and IFN-γ levels were also not different between groups (20 ± 10 versus 25 ± 7 pg/mL IL-10, 806 ± 700 versus 1153 ± 1017 pg/mL IL-6, and 78 ± 15 versus 93 ± 18 pg/mL IFN- γ for ctl-mdDCs versus MCM-mdDCs, respectively). After LPS maturation, the levels of TNF-α, IL-12(p70), IL-6, and IFN- γ increased in all groups but were not different between ctl-mdDCs and MCM-mdDCs (509 ± 352 versus 438 ± 256 pg/mL TNF-α, 6.5 ± 2.3 versus 4.7 ± 1.4 pg/mL IL-12(p70), 3527 ± 1385 versus 3365 ± 676 pg/mL IL-6, and 180 ± 43 versus 438 ± 255 pg/mL IFN-γ for matured ctl-mdDCs versus MCM-mdDCs, respectively, P > 0.05). Levels of IL-10 did not increase with LPS exposure and remained similar between the two groups (26±9 versus 25±8 pg/mL for matured ctl-mdDCs versus MCM-mdDCs, respectively, P > 0.05). Taken together, these results indicate that exposure of mdDCs to MCM did not alter cytokine secretion either before or after maturation with LPS.
FIG. 2.
MCM does not alter mdDC cytokine secretion. Secretion of IL-10, IL-12(p70), TNF-α, IFN-γ, and IL-6 is shown from mdDCs exposed to control media or MCM ± LPS. Cumulated data from three independent experiments are depicted and expressed as the mean ± SEM.
Melanoma-Conditioned Media Does Not Inhibit the Endocytic Function of mdDCs
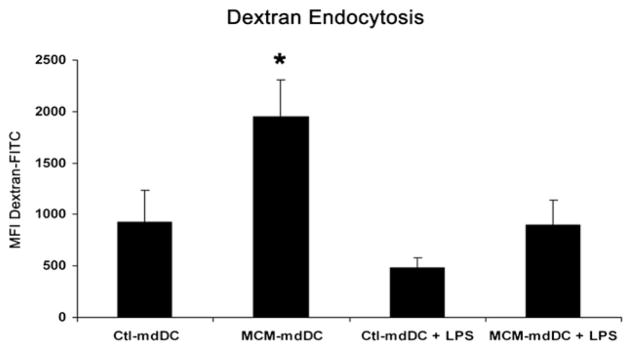
To further characterize the effects of MCM exposure on the functional capabilities of mdDCs, endocytosis was evaluated by uptake of fluorescently-conjugated dextran. Immature mdDCs exposed to MCM displayed greater endocytic function than immature mdDCs exposed to control media (1946 ± 365 versus 927 ± 309 MFI FITC-dextran for MCM-mdDCs versus ctl-mdDCs, respectively, P < 0.05; Fig. 3). As expected, LPS maturation decreased the endocytic capacity of mdDCs in both groups and the MFIs were not different between groups (896 ± 244 versus 481 ± 96 MFI for MCM-mdDCs versus ctl-mdDCs, respectively; Fig. 3). Thus, MCM exposure did not impair, but actually enhanced the endocytic function of immature mdDCs.
FIG. 3.
MCM does not inhibit the endocytic function of mdDCs. The endocytic function of mdDCs exposed to control media or MCM ± LPS expressed as the MFI of endocytosed fluorescently-conjugated dextran. Cumulated data from five independent experiments are depicted and are expressed as the mean ± SEM (*P < 0.05, Ctl-mdDC versus MCM-mdDC).
Melanoma-Conditioned Media Does Not Inhibit mdDC-Mediated T Cell Stimulation
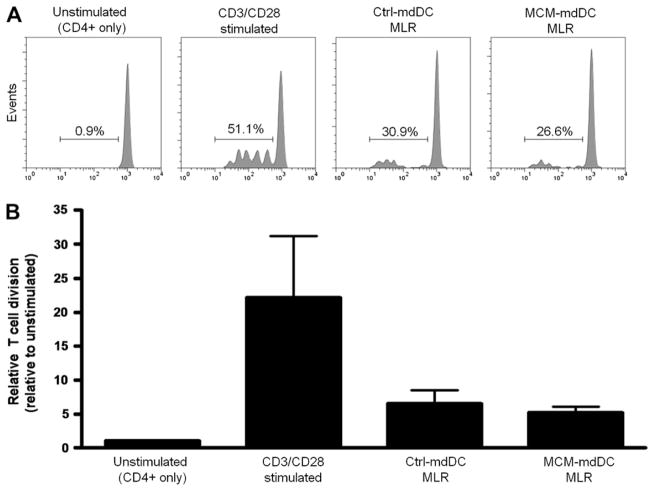
Another important function of DCs is the ability to activate T cells. Therefore, the capacity of MCM-exposed mdDCs to induce allogeneic CD4+ T cell proliferation was assessed in a mixed leukocyte reaction (MLR). mdDCs were derived as described and co-cultured with purified allogeneic CD4+ T cells for 5 d. Proliferation of the CD4+ T cells was determined by CFSE dilution, relative to the percentage of divided cells in the unstimulated samples. The relative proliferation induced by the MCM-exposed and control mediaexposed mdDCs was similar (5.2 ± 0.9 versus 6.6 ± 2.0 for MCM-mdDCs versus ctl-mdDCs, respectively, P > 0.05; Fig. 4). Thus, melanoma-exposed mdDCs activated allogeneic responder CD4+ T cells and induced proliferation to a similar extent as mdDCs exposed to control media.
FIG. 4.
MCM does not alter mdDC-mediated T cell activation. (A) Representative flow plots depict the proliferation of responder allogeneic CD4+ T cells cultured alone, with mdDCs exposed to control media or MCM, or stimulated with antibodies specific for CD3 and CD28 molecules. (B) The average relative cell division from three independent experiments was combined, error bars represent the SEM. Relative T cell proliferation was determined by dividing the percentage of CD4+ CFSElow cells in each sample by the percentage of divided cells in the unstimulated samples.
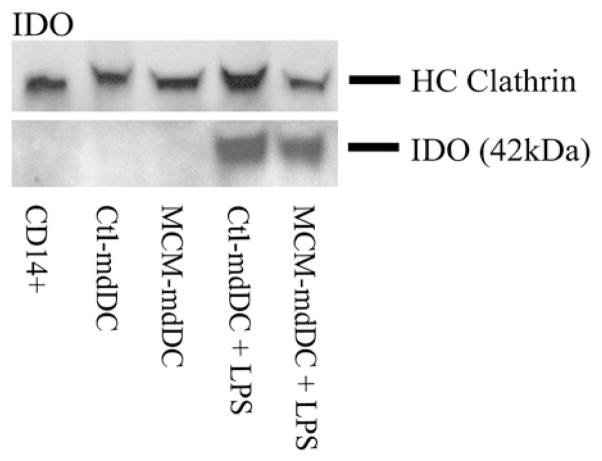
Melanoma-Conditioned Media Does Not Alter IDO Expression
Since MCM exposure did not impair the maturation, cytokine secretion, endocytosis, or T cell activation of mdDCs, we next sought to determine if MCM exposure rendered DCs tolerogenic by a mechanism independent of these functions. The expression of IDO, an enzyme that catabolizes tryptophan and is known to inhibit T cell function, was evaluated by Western blot in mdDCs exposed to control media or MCM. Immature ctl-mdDCs and MCM-mdDCs had undetectable levels of intracellular IDO. However, after LPS maturation, IDO expression increased in both ctl-mdDCs and MCM-mdDCs to a similar extent (Fig. 5). Thus, MCM exposure does not alter the expression of IDO, a marker of tolerogenicity, in mdDCs.
FIG. 5.
MCM does not alter IDO expression. Western blot shown depicts IDO expression for freshly sorted, uncultured CD14+ cells or mdDCs cultured in control media or MCM ± LPS.
DISCUSSION
In prior experiments, we identified the immunosuppressive effects of MCM, which skews cells towards a Th2 response and induces Treg cells [12, 15]. To elucidate the mechanism of this immunosuppression, we sought to determine if exposure to melanoma directly alters the phenotype or function of monocyte-derived DCs. Circulating DCs from peripheral blood are rare, representing less than 1% of the PBMC fraction [11] and, thus, are not easily obtained for adequate in vitro study. Therefore, using a standard DC derivation protocol, mdDCs were exposed to MCM for 48 h and analyzed for maturation, cytokine secretion, and function. MCM exposure did not alter levels of HLA-DR, CD83, and CD86 on immature mdDCs. After exposure to LPS, the surface expression of these markers increased and was similar to mdDCs exposed to control media. In addition, secretion of the cytokines IL-10, IL-12, and TNF-α was not altered by MCM exposure, nor was the expression of the immunomodulatory enzyme, IDO. However, we observed an increase in the uptake of dextran after exposure to MCM. One could speculate that this observation suggests that MCM contributes to a state of relative immaturity in mdDCs that was not measured by the expression of maturation markers or function. Overall, it appears that exposure to this otherwise immunosuppressive melanoma-conditioned media does not directly alter DC phenotype or function in vitro.
Enk et al. first described the effects of melanoma on DCs in patients. Comparing DCs from progressing and responding melanoma metastases, they found that tumor-infiltrating DCs from progressing metastases had reduced CD86 expression, demonstrated a reduced ability to activate T cells in MLRs, and had an increased ability to induce T cell anergy [11]. In contrast, other investigators found that mdDCs exposed to melanoma-secreted factors had increased expression of the co-stimulatory molecule CD86 [16]. It is unclear why the results of the current study do not corroborate these earlier findings; however, there are a number of important differences in the approaches of each study. Perhaps treating mdDCs with tumor-conditioned media does not accurately represent the conditions encountered by DCs infiltrating the tumor, where DCs are in direct contact with tumor cells, surrounding stromal cells, and other infiltrating lymphocytes, possibly including regulatory T cells [22, 23]. Thus, perhaps the function or maturation of mdDCs would be altered by exposure to MCM if they were also exposed to these other immunosuppressive signals. In addition, the cytokines necessary to derive mdDCs may overwhelm our ability to detect differences in these experiments. Alternatively, since mdDCs express increased levels of co-stimulatory molecules and function differently after stimulation [24–26], mdDCs may not be as susceptible to immunosuppression as freshly-isolated DCs. Thus, perhaps different experimental conditions, including different ex vivo protocols, are responsible for diverse results in melanoma DC studies. Nevertheless, we did not find any evidence for direct effects of melanoma-secreted factors on DC maturation or function.
Despite concerns about the physiologic relevance of mdDCs, DCs derived and matured in culture are routinely used for human DC research and as a therapeutic tool in melanoma vaccine studies [27]. Since melanoma cells are often used as a source of tumor antigens prior to vaccination, the current finding that melanoma exposure does not directly alter mdDC phenotype or function provides promise for future investigations of mdDCs in therapeutic strategies. Furthermore, these results indicate that the immune tolerance often seen after vaccination with mdDCs may occur independently of the exposure of these cells to melanoma in vitro.
Several limitations of this study exist. We investigated the effects of MCM from one well-described metastatic melanoma cell line that had demonstrated immunosuppressive effects by inducing T regulatory cells and a Th2 cytokine profile from PBMCs. Therefore, it is possible that conditioned media from other melanoma cell lines or from directly explanted tumors would have detectable effects on mdDCs. In addition, due to the complexity and plasticity of DCs, it is possible that treatment with MCM altered a function that was not examined in the current study or not easily assayed with modern techniques. Finally, in vitro conditions that more accurately mimic the tumor microenvironment, for example, direct incubation with melanoma cells, may alter the phenotype or function of mdDCs. However, MCM-exposure during the derivation process and incubation with higher concentrations of MCM (up to 50% by volume) did not alter the phenotype or function of mdDCs (data not shown). In addition, MCM-treated mdDCs stimulated with other maturation signals, such as TNF-α, had a similar phenotype as mdDCs matured with LPS (data not shown).
In conclusion, we found that melanoma-secreted factors do not directly alter mdDC phenotype or function in vitro following LPS stimulation. These findings suggest that soluble factors mediating melanoma-induced immunosuppression on Tregs and Th2 cells in vitro may be independent of direct modifications of mdDCs.
References
- 1.Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142. doi: 10.1084/jem.137.5.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 3.Cools N, Ponsaerts P, Van Tendeloo VF, et al. Balancing between immunity and tolerance: An interplay between dendritic cells, regulatory T cells, and effector T cells. J Leukoc Biol. 2007;82:1365. doi: 10.1189/jlb.0307166. [DOI] [PubMed] [Google Scholar]
- 4.Ferrantini M, Capone I, Belardelli F. Dendritic cells and cytokines in immune rejection of cancer. Cytokine Growth Factor Rev. 2008;19:93. doi: 10.1016/j.cytogfr.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Peng JC, Thomas R, Dredge K. Dendritic cell immunotherapy for melanoma. Rev Recent Clin Trials. 2006;1:87. doi: 10.2174/157488706776876517. [DOI] [PubMed] [Google Scholar]
- 6.Anichini A, Vegetti C, Mortarini R. The paradox of T-cell-mediated antitumor immunity in spite of poor clinical outcome in human melanoma. Cancer Immunol Immunother. 2004;53:855. doi: 10.1007/s00262-004-0526-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Talmadge JE, Donkor M, Scholar E. Inflammatory cell infiltration of tumors: Jekyll or Hyde. Cancer Metastasis Rev. 2007;26:373. doi: 10.1007/s10555-007-9072-0. [DOI] [PubMed] [Google Scholar]
- 8.Gottfried E, Kreutz M, Mackensen A. Tumor-induced modulation of dendritic cell function. Cytokine Growth Factor Rev. 2008;19:65. doi: 10.1016/j.cytogfr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 9.Perrot I, Blanchard D, Freymond N, et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J Immunol. 2007;178:2763. doi: 10.4049/jimmunol.178.5.2763. [DOI] [PubMed] [Google Scholar]
- 10.Vermi W, Bonecchi R, Facchetti F, et al. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J Pathol. 2003;200:255. doi: 10.1002/path.1344. [DOI] [PubMed] [Google Scholar]
- 11.Enk AH, Jonuleit H, Saloga J, et al. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int J Cancer. 1997;73:309. doi: 10.1002/(sici)1097-0215(19971104)73:3<309::aid-ijc1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Baumgartner J, Wilson C, Palmer B, et al. Melanoma induces immunosuppression by up-regulating FOXP3(+) regulatory T cells. J Surg Res. 2007;141:72. doi: 10.1016/j.jss.2007.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCarter MD, Baumgartner J, Escobar GA, et al. Immunosuppressive dendritic and regulatory T Cells are upregulated in melanoma patients. Ann Surg Oncol. 2007;10:2854. doi: 10.1245/s10434-007-9488-3. [DOI] [PubMed] [Google Scholar]
- 14.Baumgartner JM, Gonzalez R, Lewis KD, et al. Increased survival from stage IV melanoma associated with fewer regulatory T Cells. J Surg Res. 2009;154:13. doi: 10.1016/j.jss.2008.04.043. [DOI] [PubMed] [Google Scholar]
- 15.McCarter M, Clarke J, Richter D, et al. Melanoma skews dendritic cells to facilitate a T helper 2 profile. Surgery. 2005;138:321. doi: 10.1016/j.surg.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Gray CP, Arosio P, Hersey P. Heavy chain ferritin activates regulatory T cells by induction of changes in dendritic cells. Blood. 2002;99:3326. doi: 10.1182/blood.v99.9.3326. [DOI] [PubMed] [Google Scholar]
- 17.Jeras M, Bergant M, Repnik U. In vitro preparation and functional assessment of human monocyte-derived dendritic cells-potential antigen-specific modulators of in vivo immune responses. Transpl Immunol. 2005;14:231. doi: 10.1016/j.trim.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs B, Wuttke M, Papewalis C, et al. Dendritic cell subtypes and in vitro generation of dendritic cells. Horm Metab Res. 2008;40:99. doi: 10.1055/s-2007-1022561. [DOI] [PubMed] [Google Scholar]
- 19.Ganguly D, Paul K, Bagchi J, et al. Granulocyte-macrophage colony-stimulating factor drives monocytes to CD14low CD83+ DCSIGN-interleukin-10-producing myeloid cells with differential effects on T-cell subsets. Immunology. 2007;121:499. doi: 10.1111/j.1365-2567.2007.02596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tedder TF, Jansen PJ. Isolation and generation of human dendritic cells. Curr Protoc Immunol. 2001;7:32.1. doi: 10.1002/0471142735.im0732s23. [DOI] [PubMed] [Google Scholar]
- 21.Dillon S, Agrawal S, Banerjee K, et al. Yeast zymosan, a stimulus for TLR2 and dectin-1, induces regulatory antigen-presenting cells and immunological tolerance. J Clin Invest. 2006;116:916. doi: 10.1172/JCI27203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rissoan MC, Soumelis V, Kadowaki N, et al. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 23.Hubert P, Jacobs N, Caberg JH, et al. The cross-talk between dendritic and regulatory T. cells: Good or evil? J Leukoc Biol. 2007 doi: 10.1189/jlb.1106694. [DOI] [PubMed] [Google Scholar]
- 24.Wilson NS, El-Sukkari D, Belz GT, et al. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood. 2003;102:2187. doi: 10.1182/blood-2003-02-0513. [DOI] [PubMed] [Google Scholar]
- 25.Jefford M, Schnurr M, Toy T, et al. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: Differential regulation of function by specific classes of physiologic stimuli. Blood. 2003;102:1753. doi: 10.1182/blood-2002-12-3854. [DOI] [PubMed] [Google Scholar]
- 26.Osugi Y, Vuckovic S, Hart DN. Myeloid blood CD11c(+) dendritic cells and monocyte-derived dendritic cells differ in their ability to stimulate T lymphocytes. Blood. 2002;100:2858. doi: 10.1182/blood.V100.8.2858. [DOI] [PubMed] [Google Scholar]
- 27.Lesterhuis WJ, Aarntzen EH, De Vries IJ, et al. Dendritic cell vaccines in melanoma: From promise to proof? Crit Rev Oncol Hematol. 2008;66:118. doi: 10.1016/j.critrevonc.2007.12.007. [DOI] [PubMed] [Google Scholar]