Imagine looking out over the patrons in a restaurant as one of them looks up from their plate of food and makes eye contact with you. The change in the visual image on your retina is miniscule, yet the knowledge gained is great. A tiny change in the movements of another person’s eyes lets you know instantly that they are looking at you, know that you are also looking at them, and feel a particular emotion or have a particular intention. In this issue of Brain, Wolf et al. (2014) describe three patients with focal lesions of the ventromedial prefrontal cortex (vmPFC) who show strikingly atypical patterns of fixation onto faces: they do not look at a person’s eyes, with implications for social decision-making.
The eye region of faces is salient not only because it tells us where somebody else is directing their attention, but also because it informs us of their emotional state (the muscles around the eyes contribute substantially to certain emotional facial expressions, most particularly fear). Deficits in our ability to process this information may underlie aspects of paranoid schizophrenia (feeling as though somebody is watching you all the time) as well as autism spectrum disorders (where diminished eye contact is associated with social disengagement; Fig. 1). The ability to use our perception of another person’s gaze to guide our own attention emerges during specific stages of early development, and some aspects of this ability may be unique to humans (although dogs are also quite good at figuring out social attention signals). To understand these different facets of social attention, it is a high priority to elucidate the neural substrates involved.
Figure 1.
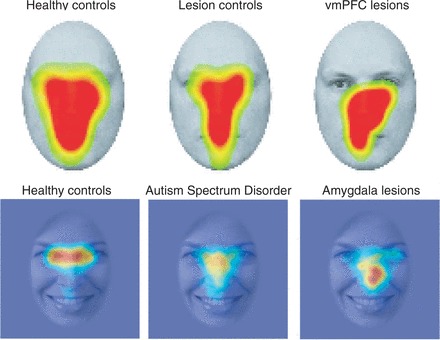
Failing to look at the eyes. Shown in each image are the regions of a face at which different groups of subjects look, as measured using eye-tracking. The hottest colours (red regions) denote those regions of the face where people look the most. Whereas this corresponds to the eye region of the face in healthy controls (far left), it is abnormal in certain clinical populations, including individuals with lesions of the vmPFC (top right) or amygdala (bottom right) and individuals with autism spectrum disorder (bottom centre) Top row: from Wolf et al. 2014. Bottom row: data from Michael Spezio, Daniel Kennedy, Ralph Adolphs. All images represent spatially smoothed data averaged across multiple fixations, multiple stimuli and multiple subjects within the indicated group.
The data obtained by Wolf et al. from the three patients with focal vmPFC lesions are intriguing for two reasons: first, they pose a challenge with regards to their incorporation into leading accounts of the functions of this brain region in decision-making; and second, they link the vmPFC to another brain structure, the amygdala, damage to which seems to produce very similar impairments.
The vmPFC has long been implicated in social behaviour, emotion and decision-making (Damasio, 1994), and is currently under intense investigation in relation to reward learning. Although there is some debate regarding whether vmPFC itself encodes the value of rewards (Jones et al., 2012), everyone seems to agree that it plays a key role in a particular type of decision-making that requires us to think about the consequences of our actions. This is variously reflected in ‘goal-directed’ or ‘model-based’ forms of reward learning, or an ability to imagine what will happen in one’s autobiographical future. All of these abilities activate the vmPFC in neuroimaging studies, and all are compromised by lesions to it; all are generally thought to be effortful and to diminish (and become more habitual) with practice.
Wolf et al. provide data that seem to argue for a different level of processing: the vmPFC may also be important for the early allocation of attention, required to select stimuli for further processing. This idea is consistent with evidence that top–down signals from the prefrontal cortex may guide visual imagery and visual search. Remarkably, strikingly similar findings are available for one other brain structure: the amygdala. Like the vmPFC, the amygdala is involved in social behaviour, emotion and decision-making (Murray, 2007). The amygdala was also recently shown to play an attentional role that influences how we fixate eyes within faces (Adolphs et al., 2005; Fig. 1).
How could impaired visual attention relate to impaired social behaviour and decision-making? If you do not pay proper attention to a face, you will not recognize its emotions, hence you will show impaired social behaviour, and you will have difficulty making social decisions. It may be that attention and decision-making simply rely on slightly different regions, or subpopulations of neurons, within the vmPFC. But it may also be that they reflect a more unitary function, just implemented at different points in time. Perhaps initial attentional selection determines whereabouts on the face we look first, and information about the value and significance of the facial features sampled then, in turn, influences where we look next. Exactly how this plays out in time, and how it depends on relative roles of the vmPFC and the amygdala, is a complex question that only electrophysiological methods can resolve (for an example in the case of reward learning, see Morrison et al., 2011). Similarly, considerably more work would be needed to circumscribe the deficit: is it specific to faces? To social stimuli? To complex visual stimuli? Additional experiments that would be informative in this regard are ones that measure fixations onto a larger range of visual stimuli, and under a range of task demands (e.g. spontaneous viewing, perceptual judgment or directed visual search).
There are plenty of caveats for how best to interpret the study by Wolf et al., caveats for which the authors themselves provide an admirably clear discussion in their paper. The lesions of course are not specific to a single Brodmann area, nor even to grey matter; the sample size is small; and no doubt there have been some plastic changes since the onset of the lesion (an issue of particular interest given the work on developmental-onset lesions of the vmPFC, e.g. Taber-Thomas et al., 2014). It is often unclear how best to establish the statistical reliability of findings from such small samples. Sometimes, they can be treated as multiple case studies and compared against a small group of suitably matched comparison subjects (often involving statistical tests that correct for small samples, such as those that John Crawford has long championed). Alternatively, they could be treated as a (very) small group of n = 3 (the approach chosen by Wolf et al.). There is also something to be said for refraining from assigning any sort of statistical significance to the findings altogether, and simply reporting the (typically large) effect in detail (Cumming, 2014), a quantitative foundation on which similar future studies can then build.
Perhaps in good part for reasons of statistical reliability, instructions for aspiring authors in Brain make a point of noting that case studies are discouraged, yet the present study offers what essentially amounts to three case studies. As someone who has authored his share of case studies, it will come as no surprise that I am rather fond of this category, for several reasons. The primary reason is simple: if you are going to publish a case study, your story had better be interesting and your effect size large. That is the case also with the data of Wolf et al.: not only does each of the three vmPFC patients show a strongly atypical eye-tracking pattern to faces, but, especially for fearful faces, the atypicality is remarkably consistent across the three patients. Weaker and less reliable effects would require larger samples to detect them. A second reason for my fondness for case studies is that they generally are about deficits (e.g. due to lesions, as in the present paper), and hence generally admit of a stronger causal link between brain and psychology than do the much more numerous neuroimaging studies of healthy individuals. It is worth noting that the title of the paper by Wolf et al., strictly speaking, does not follow from their findings: impaired visual attention following vmPFC lesions does not imply that the vmPFC therefore normally mediates this process. Showing that the vmPFC mediates the process would be much more difficult and would require experimentally replaying the pattern of activity normally seen in a healthy vmPFC to see if this engaged visual attention (something now becoming possible with optogenetic tools in rodents, but currently impossible in humans). Of course, neither lesions nor optogenetics would show that the process is ‘in’ the vmPFC, or that the vmPFC is sufficient for social attention, since the vmPFC is connected with the rest of the brain.
Which brings us to the final consideration of how best to interpret the findings of Wolf et al. We know that lesions in either vmPFC or in the amygdala produce similar deficits in social attention to the eyes in faces. Might lesions in one of these structures produce abnormal blood oxygenation level-dependent (BOLD) functional MRI signals in the other? We once carried out an experiment showing abnormal signals in the prefrontal cortex in patients who had bilateral amygdala lesions (Hampton et al., 2007), but the converse experiment remains to be done. Even more interesting are those cases or trials in which, despite their lesion, patients exhibit relatively normal performance—an occasional event that should not be dismissed as ‘variability’ or ‘noise’ as it constitutes valuable data in its own right. Lesions to the amygdala, for instance, can sometimes leave a relatively spared ability to recognize emotional facial expressions, but in these cases, there is an explanation that can be revealed with functional MRI: other regions of the brain seem to compensate (Becker et al., 2012). The extension to a whole-brain field of view (one of the undisputed virtues of functional MRI) will also provide a stronger link to psychiatric disorders in which vmPFC and/or amygdala are thought to be dysfunctional. Wolf et al. have thus provided a study that also suggests the experiment they should run next: put these three patients into a scanner and find out how their lesions influence processing in the rest of the brain.
References
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Becker B, Mihov Y, Scheele D, Kendrick K, Feinstein JS, Matusch A, et al. Fear processing and social networking in the absence of a functional amygdala. Biol Psychiatry. 2012;72:70–7. doi: 10.1016/j.biopsych.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Cumming G. The new statistics: why and how. Psychol Sci. 2014;25:7–29. doi: 10.1177/0956797613504966. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' Error: Emotion, Reason, and the Human Brain. New York: Grosset/Putnam; 1994. [Google Scholar]
- Hampton A, Adolphs R, Tyszka JM, O'Doherty J. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55:545–55. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Jones JL, Esber GR, McDannald MA, Gruber AJ, Hernandez A, Mirenzi A, et al. Orbitofrontal cortex supports behavior and learning using inferred but not cached values. Science. 2012;338:953–6. doi: 10.1126/science.1227489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, Saez A, Lau B, Salzman CD. Different time courses for learning-related changes in amygdala and orbitofrontal cortex. Neuron. 2011;71:1127–40. doi: 10.1016/j.neuron.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA. The amygdala, reward and emotion. Trends Cogn Sci. 2007;11:489–97. doi: 10.1016/j.tics.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Taber-Thomas B, Asp E, Koenigs M, Sutterer M, Anderson S, Tranel D. Arrested development: early prefrontal lesions impair the maturation of moral judgment. Brain. 2014;137:1254–61. doi: 10.1093/brain/awt377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf R, Philippi C, Motzkin J, Baskaya M, Koenigs M. Ventromedial prefrontal cortex mediates visual attention during facial emotion recognition. Brain. 2014 doi: 10.1093/brain/awu063. Advance Access published on March 31, 2014, doi:10.1093/brain/awu063. [DOI] [PMC free article] [PubMed] [Google Scholar]


