Abstract
Introduction
Chronic kidney disease (CKD) is common in patients with type 2 diabetes (T2DM) and makes them particularly susceptible to safety/tolerability issues related to many classes of oral antihyperglycemic agents (OAHA). Dipeptidyl peptidase-4 inhibitors (DPP-4is) like sitagliptin are generally well tolerated in patients with T2DM and renal disease and therefore may be preferentially used in patients with CKD. To assess the extent of this preference, the characteristics of sitagliptin users with T2DM and CKD were compared with those of other (non-DPP-4i) OAHA users with T2DM and CKD.
Methods
Patients with T2DM and CKD with claims between 2006 and 2012 were identified from a United States insurance claims database. Patients starting sitagliptin or another OAHA as mono, dual, or triple therapy were compared. Demographic and clinical characteristics within 5 years before starting or escalating to new therapies were assessed.
Results
Compared to patients with CKD starting other OAHAs, patients with CKD starting sitagliptin as mono or dual therapy were older, had more physician visits, were more likely to have a history of heart failure and to use loop diuretics. In triple therapy patients, the differences between groups were not as pronounced, but the overall prevalences of comorbidities was higher.
Conclusion
Similar to prior observations in a general T2DM population, patients with T2DM and CKD prescribed sitagliptin tend to be older and have more comorbidities than those prescribed other classes of OAHA. If not recognized and analyzed appropriately, this channeling could lead to biased treatment effect estimates in comparative analyses that include users of sitagliptin.
Funding
Merck & Co., Inc., Kenilworth, NJ, USA.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-015-0133-z) contains supplementary material, which is available to authorized users.
Keywords: Channeling bias, Chronic kidney disease, Observational study, Oral antihyperglycemic agents, Treatment outcomes, Type 2 diabetes
Introduction
Chronic kidney disease (CKD) is a common condition in patients with type 2 diabetes (T2DM). An estimated 20–35% of patients with T2DM have moderate to severe renal impairment [1, 2]. However, many antihyperglycemic medications are contraindicated or need to be used with caution in patients with CKD, complicating T2DM treatment choices and management [3]. Patients with T2DM and CKD are particularly susceptible to safety and tolerability issues related to many classes of oral antihyperglycemic agents (OAHA). Dipeptidyl peptidase-4 inhibitors (DPP-4i) such as sitagliptin are well tolerated in a broad range of T2DM patient types, including those with renal disease, and may therefore be preferentially used in patients with CKD. Prior studies have demonstrated the preferential use of sitagliptin in several populations [4–7]. In general, patients initiating treatment with sitagliptin were older and had more complications of diabetes and comorbidities than patients initiating other antihyperglycemic therapies [4–7]. If not recognized and appropriately considered in the analysis, this preferential selection of patients with specific demographic and disease characteristics for treatment with sitagliptin (channeling bias) could lead to inaccurate treatment effect estimates in comparative analyses that include sitagliptin [8]. The objective of this study was to describe the baseline characteristics of patients with T2DM and CKD initiating treatment with sitagliptin or non-DPP-4i OAHAs to ascertain whether channeling exists in this patient population.
Methods
The Truven Health MarketScan® Databases (MarketScan, Truven Health Analytics, Ann Arbor, MI, USA) contain medical claims records for more than 150 million unique patients dating from 1996. The records are derived from outpatient and inpatient insurance claims for employees of over 100 employers participating in more than 12 health plans, and their beneficiaries in the United States. Records consist of commercial claims and healthcare encounters, including information on demographics, health plan membership, International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes, and Current Procedure Terminology (CPT) codes. The records of retirees with supplemental insurance are included in the database thus providing data on the elderly with continuity of care across those <65 and ≥65 years of age.
Patients ≥25 years of age with T2DM and CKD, with claims in the United States (US) between January 2006 and June 2012, were identified in MarketScan. Of these patients, those initiating sitagliptin or a non-DPP-4i OAHA were categorized by complexity of antihyperglycemic treatment.
Patients were identified as having T2DM if MarketScan records for the patient indicated at least one inpatient or outpatient diagnosis of diabetes and at least one prescription for OAHA medication.
Patients with CKD were identified by ICD-9-CM diagnostic codes (585, 585.3, 585.4, 585.5, 585.6, 585.9, 403, 403.0, 403.00, 403.01, 403.1, 403.10, 403.11, 403.9, 403.90, 403.91, 250.4, 404, 404.0, 404.00, 404.01, 404.02, 404.03, 404.1, 404.10, 404.11, 404.12, 404.13, 404.9, 404.90, 404.91, 404.92, 404.93, 582, 582.0, 582.1, 582.2, 582.4, 582.8, 582.81, 582.89, 582.9).
Antihyperglycemic treatment was defined as: (1) initiating monotherapy (≥1 new outpatient prescription record on or after the T2DM diagnosis); (2) escalating to dual combination therapy (≥1 new prescription for a 2nd class ≥90 days after the 1st class, with prescription for 1st class overlapping the index date of 2nd class); (3) escalating to triple combination therapy (≥1 new prescription for a 3rd class ≥90 days after the 2nd class, with prescriptions for 1st and 2nd classes overlapping the index date of 3rd class).
Patients were required to have at least 1 year of continuous enrollment in the database prior to initiation/escalation of antihyperglycemic treatment. Patients were excluded from the analysis if they had a diagnosis of type 1 diabetes, ketoacidosis, malnutrition-associated diabetes, drug-induced diabetes or gestational diabetes without a subsequent T2DM diagnosis code. Patients with ICD-9-CM codes explicit for mild renal disease (stage 1 and 2) and patients on insulin or other injectable therapy were also excluded from the analysis.
Demographics, and clinical conditions and health care resource utilization recorded up to 5 years before therapy initiation were assessed as baseline characteristics. Over 70 clinical conditions and comorbidities may have been recorded in the database, including diabetes complications, cancers, and cardiovascular (CV), metabolic, gastrointestinal, hepatic, infectious, psychiatric, pulmonary, and neurological events. Types of health care resource utilization recorded in the database included physician and emergency department visits, hospitalizations, days hospitalized, and number of medications received.
Differences between sitagliptin and non-DPP-4i OAHA treatment groups were compared using absolute standardized differences (ASD) [9]. ASD is the difference of two means or proportions divided by the pooled estimate of the standard deviation. Unlike the traditional p value, ASD is a measure of difference that is not influenced by large sample sizes and has been demonstrated to be a better measure of covariate balance [10, 12]. An ASD of at least 10% was used to indicate a meaningful difference between treatment groups [12].
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Results
A total of 35,922 patients with T2DM and CKD were identified as meeting the inclusion criteria. Over 45% of patients (46.7%; n = 16,742) initiated sitagliptin (n = 1234) or a non-DPP-4i OAHA monotherapy (n = 15,508), 40.5% (n = 14,540) initiated an escalation to dual combination therapy (sitagliptin, n = 2683; OAHA, n = 11,857), and 12.9% (n = 4640) initiated an escalation to triple combination therapy (sitagliptin, n = 1385; OAHA, n = 3255). Roughly, 15% of patients with T2DM and CKD (14.8%; n = 5302) initiated treatment with sitagliptin. In comparison, in the patients excluded from this analysis due to a lack of recorded CKD, the percentage of patients initiating sitagliptin was 7.4%.
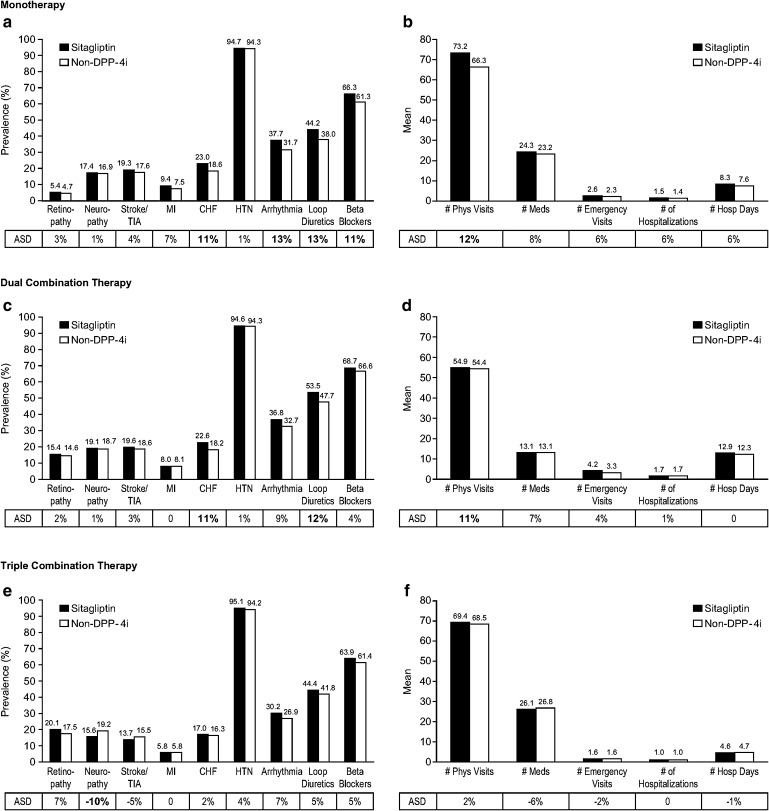
The greatest differences between treatment groups were observed in patients initiating monotherapy or an escalation to dual combination therapy. Compared to patients initiating monotherapy with non-DPP-4i OAHAs, patients initiating monotherapy with sitagliptin were older (mean [standard deviation (SD)]: sitagliptin 68.8 [12.5] years, non-DPP-4i 66.6 [12.8] years; ASD 17%), were more likely to have a history of heart failure (Fig. 1a; sitagliptin 23.0%, non-DPP-4i 18.6%; ASD 11%) or arrhythmia (Fig. 1a; sitagliptin 37.7%, non-DPP-4i 31.7%; ASD 13%), were more likely to use loop diuretics (Fig. 1a; sitagliptin 44.2%, non-DPP-4i 38.0%; ASD 13%) or beta-blockers (Fig. 1a; sitagliptin 66.3%, non-DPP-4i 61.3%; ASD 11%), and had more physician visits (Fig. 1b; mean [SD]: sitagliptin 73.2 [57.6] physician visits, non-DPP-4i 66.3 [55.4] physician visits; ASD 12%). The differences between treatment groups (non-DPP-4i OAHA users versus sitagliptin users) observed in patients initiating an escalation to dual therapy were similar to those observed in patients initiating monotherapy, with the exception that the between-group age difference was not as great (mean [SD]: sitagliptin 71.1 [11.1] years, non-DPP-4i 70.0 [11.0] years; ASD 10%) and the differences for history of arrhythmia and use of beta-blockers were not meaningful (Fig. 1c, d).
Fig. 1.
Baseline characteristics of patients with type 2 diabetes and chronic renal disease up to 5 years before initiating treatment with sitagliptin or non-DPP-4i oral antihyperglycemic agent as monotherapy or as part of dual or triple therapy. a, c, e Clinical conditions and comorbidities. b, d, f Health care resource utilization. ASD of ≥10% indicates a meaningful difference between treatment groups. For any between-group difference of ASD of at least 10%, the ASD value is in bold type. ASD absolute standardized difference, CHF congestive heart failure, DPP-4i dipeptidyl peptidase-4 inhibitor, Hosp hospital, HTN hypertension, Meds medications, MI myocardial infarction, Phys physician, TIA transient ischemic attack
In patients initiating an escalation to triple combination therapy, the differences between treatment groups (non-DPP-4i OAHA users versus sitagliptin users) were not as pronounced as those seen in patients initiating monotherapy or escalation to dual therapy, including the between-group age difference (mean [SD]: sitagliptin 68.9 [10.9] years, non-DPP-4i 68.4 [10.5] years; ASD 5%; Fig. 1e, f).
Discussion
In this study of patients with T2DM from an employee-based insurance database, sitagliptin was initiated in a higher percentage of patients with T2DM and CKD (14.8%) compared to patients with T2DM but no record of CKD (7.4%). Unlike many other OAHAs, sitagliptin is approved for patients with any stage of renal disease [11]. In light of this and its favorable renal safety profile [12–15], the higher use of sitagliptin in patients with CKD observed in the current analysis is not surprising.
In general, patients with T2DM and CKD who initiated treatment with sitagliptin tended to be older and were more likely to have a pre-treatment history of heart failure, arrhythmia, or use of loop diuretics or beta-blockers than patients initiating other classes of OAHA. In this context, it is worth noting the results of a large, recently completed clinical trial examining the effects of adding sitagliptin to usual care in patients with T2DM and CV disease [16]. In the overall study population, no difference in CV event rates compared with placebo was observed (hazard ratio [HR] for the primary composite CV outcome was 0.98; 95% confidence interval (CI): 0.88, 1.09; p < 0.001 for noninferiority) [16]. Additionally, in patient subgroups evaluated by renal function, no difference in CV risk was noted for patients with CKD [estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2; HR = 0.92; 95% CI: 0.78, 1.10) or those without CKD (eGFR ≥60 mL/min/1.73 m2; HR = 1.00; 95% CI: 0.89, 1.13) [16].
The most pronounced differences in baseline characteristics between the treatment groups were observed between patients initiating monotherapy. As treatment complexity increased, the differences in baseline characteristics between treatment groups persisted but were attenuated, presumably due to diminishing treatment options with increasing treatment complexity. These observations of channeling in patients receiving treatment with sitagliptin are similar to those previously reported in a general T2DM population [4–7].
While the MarketScan database includes insurance claims data on a large, diverse population from the US, these results may not be generalizable to the overall US population or to ex-US populations. In addition, the primary uses of these data are for administrative purposes, not research. Consequently, the database has missing or limited data on a number of important disease characteristics and comorbidities. Importantly for this study, patients with end-stage renal disease are likely underrepresented since these patients are Medicare eligible. Chronic renal disease was defined solely through ICD-9-CM codes as laboratory data are not available in our dataset.
Conclusions
This study further documents the presence of channeling in patients initiating treatment with sitagliptin. In this study, patients with CKD initiating treatment with sitagliptin were generally older and were more likely to have a pre-treatment history of heart failure, arrhythmia, or use of loop diuretics or beta-blockers than patients initiating other classes of oral therapies. If not recognized and analyzed appropriately, this channeling could lead to biased treatment effect estimates in comparative analyses, including those involving users of sitagliptin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
This analysis was funded by Merck & Co., Inc., Kenilworth, NJ, USA. Article processing charges for this publication were funded by Merck & Co., Inc. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published. The authors acknowledge Edward A. O’Neill, Ph.D., Alan Meehan, Ph.D., and Kristen Lewis, all of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, for editorial and submission assistance. Some of the data reported in this manuscript were previously presented as a poster at the 2014 American Diabetes Association Conference in San Francisco, CA, USA (Brodovicz K, Chen Y, Liu Z, Ritchey ME, Liao J, Engel SS. Characterization of sitagliptin use in patients with type 2 diabetes and chronic renal disease by cross-sectional analysis of a medical insurance claims database).
Disclosures
K. G. Brodovicz was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, at the time this analysis was carried out and may own stock and/or hold stock options in the company. Y. Chen is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ and may own stock and/or hold stock options in the company. Z. Liu is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ and may own stock and/or hold stock options in the company. M. E. Ritchey was an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, at the time this analysis was carried out and may own stock and/or hold stock options in the company. J. Liao is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ and may own stock and/or hold stock options in the company. S. S. Engel is an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ and may own stock and/or hold stock options in the company.
Compliance with ethics guidelines
This article does not contain any new studies with human or animal subjects performed by any of the authors.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
References
- 1.de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305:2532–2539. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koro CE, Lee BH, Bowlin SJ. Antidiabetic medication use and prevalence of chronic kidney disease among patients with type 2 diabetes mellitus in the United States. Clin Ther. 2009;31:2608–2617. doi: 10.1016/j.clinthera.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 3.Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brodovicz KG, Kou TD, Engel SS, Alexander CM, O’Neill EA, Girman CJ. Characteristics of older adult patients initiating sitagliptin and other oral antihyperglycemic agents in a large US medicare claims database (abstract) Diabetes. 2012;61(S1):A350. [Google Scholar]
- 5.Brodovicz KG, Kou TD, Alexander CM, O’Neill EA, Senderak M, Engel SS, Girman CJ. Recent trends in the characteristics of patients prescribed sitagliptin and other oral antihyperglycaemic agents in a large US claims database. Int J Clin Pract. 2013;67:449–454. doi: 10.1111/ijcp.12090. [DOI] [PubMed] [Google Scholar]
- 6.Cai B, Katz L, Alexander CM, Williams-Herman D, Girman CJ. Characteristics of patients prescribed sitagliptin and other oral antihyperglycaemic agents in a large US claims database. Int J Clin Pract. 2010;64:1601–1608. doi: 10.1111/j.1742-1241.2010.02516.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Q, Rajagopalan S, Mavros P, Engel SS, Davies MJ, Yin D, Radican L. Baseline characteristic differences between patients prescribed sitagliptin vs. other oral antihyperglycemic agents: analysis of a US electronic medical record database. Curr Med Res Opin. 2010;26:1697–1703. doi: 10.1185/03007995.2010.489029. [DOI] [PubMed] [Google Scholar]
- 8.Brookhart MA, Sturmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care. 2010;48:S114–S120. doi: 10.1097/MLR.0b013e3181dbebe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ali MS, Groenwold RH, Pestman WR, Belitser SV, Roes KC, Hoes AW, de Boer A, Klungel OH. Propensity score balance measures in pharmacoepidemiology: a simulation study. Pharmacoepidemiol Drug Saf. 2014;23:802–811. doi: 10.1002/pds.3574. [DOI] [PubMed] [Google Scholar]
- 11.US prescribing information for JANUVIA® (sitagliptin) Tablets, February, 2014. https://www.merckconnect.com/januvia/overview.html.14. Accessed Oct 22, 2014.
- 12.Engel SS, Round E, Golm GT, Kaufman KD, Goldstein BJ. Safety and tolerability of sitagliptin in type 2 diabetes: pooled analysis of 25 clinical studies. Diabetes Ther. 2013;4:119–145. doi: 10.1007/s13300-013-0024-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arjona Ferreira JC, Marre M, Barzilai N, Guo H, Golm GT, Sisk CM, Kaufman KD, Goldstein BJ. Efficacy and safety of sitagliptin versus glipizide in patients with type 2 diabetes and moderate-to-severe chronic renal insufficiency. Diabetes Care. 2013;36:1067–1073. doi: 10.2337/dc12-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arjona Ferreira JC, Golm GT, Goldstein BJ. Primary objective of study of sitagliptin in patients with ESRD on dialysis. Am J Kidney Dis. 2013;62:642. doi: 10.1053/j.ajkd.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 15.Arjona Ferreira JC, Corry D, Mogensen CE, Sloan L, Xu L, Golm GT, Gonzalez EJ, Davies MJ, Kaufman KD, Goldstein BJ. Efficacy and safety of sitagliptin in patients with type 2 diabetes and ESRD receiving dialysis: a 54-week randomized trial. Am J Kidney Dis. 2013;61:579–587. doi: 10.1053/j.ajkd.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 16.Green JB, Bethel MA, Armstrong PW, Buse JB, Engel SS, Garg J, Josse R, Kaufman KD, Koglin J, Korn S, Lachin JM, McGuire DK, Pencina MJ, Standl E, Stein PP, Suryawanshi S, Van de Werf W, Peterson ED, Holman RR. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N.Engl J Med. 2015;373:232–242. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.