Abstract
Introduction
A1chieve® (ClinicalTrials.gov identifier NCT00869908) was a 24-week observational study evaluating certain insulin analogs and not insulin analogs in general in 66,726 people with type 2 diabetes (T2D) in routine clinical care in 28 non-Western countries. This study demonstrated that insulin analogs improved self-management and metabolic control in patients with T2D. We investigated the effectiveness and clinical characteristics of patients with T2D showing better response to basal insulin (BI) (detemir), using data from the A1chieve study performed in Korea.
Methods
Subjects were classified into two groups according to the achievement of target glycated hemoglobin (A1c) level of <7.5%. Multivariate logistic regression analysis was performed to determine the variables independently associated with the achievement of target A1c level.
Results
Baseline A1c, postprandial glucose (PPG), difference between PPG and fasting plasma glucose, and duration of diabetes were independently associated with better response to BI after adjusting for other risk factors. Compared to patients with BI use at evening, those who took BI in the morning demonstrated a larger reduction in A1c level.
Conclusion
Once-daily BI therapy appears to be effective in Korean subjects with type 2 diabetes who had a shorter duration of diabetes and a smaller postprandial glucose excursion.
Funding
Novo Nordisk Pharma Korea and Novo Nordisk International Operations.
Electronic supplementary material
The online version of this article (doi:10.1007/s13300-015-0140-0) contains supplementary material, which is available to authorized users.
Keywords: A1chieve® study, Basal insulin responder, Detemir, Korea
Introduction
The A1chieve® study (ClinicalTrials.gov identifier NCT00869908) enrolled 66,726 patients with type 2 diabetes (T2D) and was a 24-week, prospective, multinational (28 countries), open-labeled, observational study that evaluated the clinical safety and effectiveness of insulin analogs in routine clinical care in non-Western countries [1]. This study showed that the treatment with insulin analogs provides a valuable option for broad improvements in self-management and metabolic control, regardless of the types of insulin used [2]. In the A1chieve study conducted in Korea, therapy with modern insulin analogs significantly reduced the levels of glycated hemoglobin (A1c), fasting plasma glucose (FPG), and postprandial plasma glucose (PPG) (−1.6 ± 2.2%, −2.5 ± 4.7 mmol/L, and −4.0 ± 6.4 mmol/L, respectively) at 24 weeks. In addition, no major hypoglycemic episodes were observed, and the rate of minor hypoglycemic episodes was marginally decreased [3]. However, many questions remain regarding the optimal approach for initiating insulin analogs, including type(s) of insulin (basal, rapid-acting, biphasic, or a combination of basal and rapid-acting), continuation or discontinuation of oral anti-diabetic drugs with insulin analogs in insulin-naïve subjects, as well as insulin regimen (basal, rapid-acting, biphasic, or a combination of basal and rapid-acting). Although previous studies have evaluated the characteristics of Korean T2D patients showing better response to biphasic insulin analog (BIA) therapy [4–6], it is still uncertain regarding the characteristics of patients who show better response to basal insulin (BI) analog therapy. In addition, the latest sub-analysis from the A1chieve study conducted in Korea included 3074 patients who were analyzed in three groups regarding A1c <7.5%/7.5–9%/>9%. No significant A1c reduction was observed with any insulin regimens in Korean patients with relatively well-controlled T2D (A1c <7.5%), a basal–bolus regimen may be adequate for Korean patients with poorly controlled T2D (A1c ≥9.0%) [7]. Therefore, using data from the A1chieve study in Korea, we investigated the effectiveness of the BI analog, insulin detemir (Levemir®) in Korean patients with T2D. In particular, we provide the characteristics of patients showing better response to insulin detemir in Korean patients with type 2 diabetes.
Methods
Patients and Study Design
A total of 104 sites from South Korea were involved in this study. The study population and design were described in a previous report [3]. Briefly, the inclusion criteria were Korean patients with T2D who planned to use or who had started the study products (biphasic insulin aspart 30 [BIA30], insulin detemir, or insulin aspart) within 4 weeks of inclusion in this study. Patients were excluded for the following reasons: hypersensitivity to the study products, pregnancy, breastfeeding, or intention of becoming pregnant within the next 6 months. We selected subjects who were treated with insulin detemir once daily. The data were collected at baseline, at an interim visit approximately 12 weeks after the baseline visit, and at a final visit approximately 24 weeks after the baseline visit. The primary endpoint was serious adverse drug reactions including major hypoglycemic events. The secondary study endpoints were effectiveness (changes in FPG, PPG after breakfast, A1c and lipid profile) and safety (changes in number of hypoglycemic events and nocturnal hypoglycemic events, number of adverse drug reactions). According to usual practices, the physicians were free to determine all decisions on subsequent treatment, as well as the discontinuation of insulin. The study was performed in accordance with the Declaration of Helsinki 1964, as revised in 2013, and the guidelines for Good Pharmacoepidemiology Practices. The protocol was reviewed and approved by independent institutional review boards at 104 study sites (Representative site: Yonsei University College of Medicine Institutional Review Board number: 4-2009-0359), and all participants provided written informed consent before any trial-related activity.
Analysis Design
The A1chieve study in Korea reported a decrease in A1c from 9.4 ± 1.9% at baseline to 8.0 ± 1.4% at 24 weeks in insulin detemir-treated subjects [3] and no significant A1c reduction with any insulin regimens (BI, bolus regimen and basal–bolus regimen and biphasic regimen) in Korean patients with relatively sub-optimally controlled (A1c <7.5%) T2D [7]. Another previous observational study, similar result showed that reduction in A1c was 7.6% of final A1c from 8.9% of baseline A1c. Higher baseline A1c leads to more decline of A1c. However, their final A1c levels were not less than 7%, but around 7.5% [8]. Based on these findings, we hypothesized that achieving A1c levels less than 7.5% might be optimal in real clinical practice and classified the study subjects into two groups according to the response to insulin detemir. Group I had an A1c <7.5%, and group II had an A1c ≥7.5%. We investigated the variables predictive of achieving the glycemic control target with BI analogs in T2D patients. We also compared the effectiveness and safety of insulin detemir according to the time of injection. We excluded subjects who did not have records about insulin administration time.
Statistical Procedures
Data were expressed as mean ± standard deviation or as proportion. The comparison of effectiveness of the endpoints based on A1c level was performed using analysis of variance (ANOVA) with repeated measures. The mean improvement from baseline A1c and corresponding 95% confidence interval (CI) was calculated and compared between treatment groups using the t test. The association between the effect of the treatment group and the degree of hyperglycemia was represented by n (%) at different levels of A1c at the end of the trial. To determine the variables predictive of achieving the glycemic control target with BI analogs in T2D patients, multiple logistic regression analysis was performed with clinically relevant variables and established parameters that significantly differed between responders and non-responders in our study. Multivariate regression analysis was used to estimate multiple correlations between achievement of an A1c less than 7.5% and clinical and laboratory risk factors. A variance inflation factor >10 suggests an erroneous model; all such items were omitted from the models. All data were analyzed by Novo Nordisk using SAS (Version 9.1.3, SAS Institute Inc. North Carolina, USA). p values <0.05 were considered statistically significant.
Results
Study Population
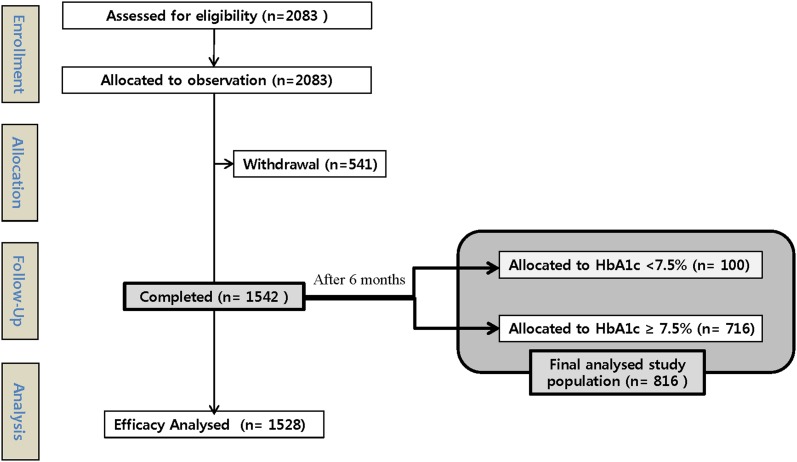
The study population and analysis design were described in previous reports [3, 7]. Briefly, of the 4058 patients who were administered study insulin at least once in the full analysis set (FAS), 3074 had A1c level measured at baseline and at the final visit; and 2952 (72.7% of the FAS) who received one of the four insulin analog regimens were eligible for analysis. In clinical practice, more Korean physicians chose insulin detemir over BIA30 as a BI (2083 vs. 1434, respectively). Of the 2083 BI-enrolled subjects, 1542 completed the 6-month study. As per the analysis data sets, 14 subjects were not part of the efficacy analysis, and thus there was a difference in the number of subjects between the completed and efficacy analysis sets. Of the 1528 subjects on insulin detemir who were eligible for efficacy analysis, 816 had data sufficient for sub-analysis of the efficacy and safety in T2D according to injection time and responsiveness (Fig. 1).
Fig. 1.
Flowchart illustrating the number of patients included in the A1chieve® sub-study in Korea. HbA1c glycated hemoglobin
There were no restrictions on entry into the study, with relationship to baseline A1c levels. Participants were free to withdraw at will at any time. Therefore, it could not be identified why individuals were registered and what made physicians prescribe insulin regimen. Possible causes include impaired cardiac, liver, and/or renal function; oral ingestion discomfort such as dysphagia; need for a stable glycemic control; active demand for new treatment; requirements due to adverse effects before treatment; and willing to participate in research and insurance policies—reimbursement guideline, etc.
In this study, the insulin therapies were prescribed by a physician in the course of clinical practice, were commercially available and were funded according to local practice in routine care.
Baseline Clinical and Laboratory Characteristics at Study Entry According to Target A1c
Patients were allocated to group I (A1c <7.5%, n = 100; 55 men and 45 women) and group II (A1c ≥7.5%, n = 716; 378 men and 338 women) based on responsiveness to insulin detemir. The male-to-female ratio in the two groups was similar (p = 0.6787). Table 1 shows the baseline characteristics of the study patients according to the achievement of a target A1c of 7.5%. For groups I and II, the mean age, the duration of diabetes and average body mass index (BMI) were similar. Compared to patients who were non-responsive to once-daily insulin detemir (group II, A1c ≥7.5%), responsive subjects had significantly lower baseline glucose parameter markers such as FPG, PPG, and serum A1c level. The patients in group II showed a tendency for greater difference in glucose level (Δ glucose = PPG − FPG) than those in group I. Total cholesterol and triglycerides were significantly higher in group II than in group I (p = 0.0024 and 0.0386, respectively). In addition, non-HDL cholesterol (mmol/L) was also statistically different between the two groups (3.1 in group I vs. 3.6 in group II, p = 0.0132). The patients in group II were more significantly dependent on sulfonylurea (SU)-treatment (SU alone or SU plus metformin). Systolic blood pressure and other plasma lipids were not significantly different between the two groups.
Table 1.
Baseline characteristic of the subjects treated with insulin detemir according to achievement of an A1c 7.5%
| Groups according to A1c at final follow-up | p value | ||
|---|---|---|---|
| <7.5% (N = 100) | ≥7.5% (N = 716) | ||
| Age (years) | 58.2 ± 12.7 | 57.6 ± 12.4 | 0.6747 |
| Male (N, %) | 55 (55.0%) | 378 (52.8%) | 0.6787 |
| Diabetes duration (years) | 10.4 ± 7.6 | 10.0 ± 7.2 | 0.5888 |
| Body weight (kg) | 64.4 ± 10.9 | 63.8 ± 10.9 | 0.57 |
| BMI (kg/m2) | 24.5 ± 3.5 | 24.3 ± 3.3 | 0.565 |
| Systolic BP (mmHg) | 127.0 ± 16.4 | 127.1 ± 15.5 | 0.9613 |
| Creatinine (mmol/L) | 88.5 ± 31.7 | 85.3 ± 27.8 | 0.4152 |
| Plasma glucose level (mmol/L) | |||
| FPG | 7.7 ± 2.7 | 11.4 ± 4.3 | <0.0001 |
| PPG | 11.6 ± 3.7 | 16.3 ± 5.6 | <0.0001 |
| PPG minus FPG | 3.4 ± 2.8 | 5.0 ± 4.8 | 0.0515 |
| Baseline A1c (%) | 6.9 ± 0.5 | 9.8 ± 1.8 | <0.0001 |
| Lipid (mmol/L) | |||
| T. Chol. | 4.2 ± 1.1 | 4.8 ± 1.3 | 0.0024 |
| TG | 1.5 ± 0.8 | 1.9 ± 1.3 | 0.0386 |
| HDL | 1.1 ± 0.4 | 1.2 ± 0.3 | 0.1011 |
| LDL | 2.6 ± 1.3 | 2.8 ± 1.0 | 0.2889 |
| Concomitant OAD [baseline (%)/at final follow-up (%)] | |||
| S alone | (10.0/7.0) | (6.70/6.84) | <0.000/<0.000 |
| M alone | (11.0/17.0) | (9.36/26.26) | <0.000/<0.000 |
| T alone | (1.0/1.0) | (0.28/0.84) | 0.5637/0.0588 |
| S + M | (22.0/20.0) | (28.07/27.37) | <0.000/<0.000 |
| S + T | (0.0/0.0) | (0.84/0.0) | ND |
| M + T | (0.0/0.0) | (2.23/1.12) | ND |
| Agent other than the above OADs or their combinations | (37.0/37.0) | (36.31/27.51) | <0.000/<0.000 |
A1c glycated hemoglobin, BMI body mass index, BP blood pressure, FPG fasting plasma glucose, HDL HDL cholesterol, LDL LDL cholesterol, M metformin, ND not determined, OAD oral anti-diabetic drugs, PPG postprandial glucose, S sulfonylurea, T. Chol total cholesterol, TG triglyceride, T thiazolidinedione
The baseline characteristics of age, diabetes duration, body weight, BMI, systolic BP, FPG, PPG, PPG minus FPG, A1C, total cholesterol, triglyceride, HDL cholesterol, and LDL cholesterol were analyzed using the t test at a 5% level of significance. Other parameters such as proportion of male patients (%) and concomitant OAD use (baseline/at last follow-up) were analyzed using Chi-square test. The p values were presented for testing statistical significance. Agent other than the above OADs or their combinations included glinide, α-glucosidase inhibitor, DPP4-inhibitor.
Efficacy and Safety of 6 Months of Insulin Detemir Treatment According to Injection Time
To compare whether the effectiveness and safety of BI depends on injection time, the subjects were classified into two groups depending on whether BI was injected in the morning or in the evening. Of the 816 subjects, 91.9% (n = 750) were injected with insulin detemir in the morning, whereas 66 patients had evening injections (Table 2). The reductions in A1c were significantly larger in subjects who were injected with insulin detemir in the morning than those who were injected with insulin detemir in the evening. No major hypoglycemic episodes were reported, but 267 patients in the original A1chieve study in Korea reported 559 minor episodes, including 451 diurnal and 108 nocturnal episodes [3]. In this sub-analysis of BI, subjects who were injected in the morning received a larger amount of insulin dose than those who were injected in the evening (0.38 vs. 0.34 U/kg, respectively, p = 0.049) and frequently experienced minor (5.35% vs. 5.20%, respectively, p < 0.001) and nocturnal hypoglycemic episodes (1% vs. 0%, respectively, p = 0.004). Body weight gain was not different between the two groups.
Table 2.
Efficacy and safety comparison after 6 months of treatment with insulin detemir according to injection time
| Breakfast (n = 750) | Dinner (n = 66) | p value | |
|---|---|---|---|
| Δ Body weight (kg) | 0.39 ± 2.8 | 0.10 ± 2.1 | NS |
| Plasma Glucose Level (mmol/L) | |||
| Δ FPG | −3.2 ± 4.1 | −2.9 ± 4.2 | NS |
| Δ PPG | −4.2 ± 6.3 | −3.8 ± 5.6 | NS |
| Δ PPG minus FPG | −0.6 ± 5.6 | 0.3 ± 4.6 | NS |
| Δ A1c (%) | −1.6 ± 2.0 | −0.9 ± 1.8 | 0.01 |
| Insulin dose (U/kg) | 0.38 ± 0.17 | 0.34 ± 0.15 | 0.049 |
| In A1c ≤6.5% | 0.30 ± 0.15 | 0.22 ± 0.12 | NS |
| In A1c <7.0% | 0.34 ± 0.15 | 0.32 ± 0.12 | NS |
| In A1c <7.5% | 0.35 ± 0.16 | 0.32 ± 0.11 | NS |
| Hypoglycemia (N, %) | |||
| Minor | 59 (5.35%) | 3 (5.20%) | <0.0001 |
| Major | 0 (0.00%) | 0 (0.00%) | ND |
| Nocturnal | 11 (1.00%) | 1 (0.00%) | 0.004 |
A1c glycated hemoglobin, FPG fasting plasma glucose, ND not detected NS not significant, PPG postprandial glucose
For multiple logistic regression analysis, achievement of an A1c less than 7.5% was used as a dependent variable. Age, gender, BMI, duration of diabetes, total cholesterol, and glycemic indices including PPG and Δ glucose were independent variables based on the results of Table 1 and the conventional risk variables in Table 2. In this analysis, we used two statistical models with different independent variables. We adopted PPG and Δ glucose as the glycemic index in models 1 and 2, respectively. Age was independently correlated with more responsive glycemic control in both models (age, p = 0.01 and 0.02 in models 1 and 2, respectively). The A1c, PPG, and Δ glucose were also independently correlated with less responsive glycemic control after multivariate adjustment for other risk factors in models 1 and 2 (A1c, p < 0.01 in both models; PPG, p < 0.01, model 1; Δ glucose, p < 0.01, model 2). The duration of diabetes was significantly correlated with less responsive glycemic control in model 2 (p = 0.01 in model 2) but only showed a trend in model 1 (p = 0.03 but the 95% CI included 1.0) (Table 3).
Table 3.
Multivariable logistic regression models for factors associated with more responsive glycemic control on basal insulin
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Age | 1.04 (1.01–1.07) | 0.01 | 1.04 (1.01–1.07) | 0.02 |
| Sex | 0.51 (0.25–1.05) | 0.07 | 0.73 (0.35–1.49) | 0.38 |
| BMI | 0.99 (0.90–1.09) | 0.82 | 0.98 (0.89–1.08) | 0.71 |
| Duration of diabetes | 0.95 (0.90–1.00) | 0.03 | 0.93 (0.89–0.99) | 0.01 |
| Total cholesterol | 1.01 (0.70–1.45) | 0.96 | 1.13 (0.78–1.64) | 0.51 |
| A1C | 0.76 (0.63–0.92) | <0.01 | 0.72 (0.59–0.88) | <0.01 |
| PPG | 0.70 (0.62–0.80) | <0.01 | ||
| PPG-FPG (Δ glucose) | 0.84 (0.74–0.95) | <0.01 | ||
A1c glycated hemoglobin, BMI Body mass index, FPG fasting plasma glucose, ND not determined OR odd ratio, PPG postprandial glucose
The comparison of efficacy and safety parameters of changes in FPG, PPG, PPG-FPG, A1C, and body weight from baseline to after 6 months of treatment with insulin detemir according to injection time (Breakfast, n = 1102; Dinner, n = 97) were analyzed using the t test at a 5% level of significance (Table 2). The proportion of hypoglycemia events was analyzed using Chi-square test. The p values are presented for testing statistical significance. The p values were presented as ‘NS’ for p > 0.05.
The factors associated with more responsive glycemic control on basal insulin such as PPG and Δ glucose were analyzed using multiple logistic regression analysis based on an A1c threshold of 7.5% as the dependent variable and age, gender, BMI, duration of diabetes, total cholesterol, and glycemic indices including PPG and Δ glucose as independent variables (Table 3). The odds ratio and corresponding 95% CI as well as the p values are presented for testing statistical significance.
Discussion
Despite the greater glycemic effectiveness of twice-daily injection of BIA or basal–bolus regimens compared to once-a-day BI [4, 9, 10], patients are often reluctant to start with twice-daily injections of BIA or basal–bolus regimens, especially those who are insulin naïve [11, 12]. In addition to the obstacles in initiating basal insulin therapy, many questions remain regarding the optimal target A1c and the approach for initiating insulin analog therapy. Basal insulin therapy works to control fasting glycemia, and it is expected that other drugs will suffice apropos postprandial glycemia. In many patients, however, basal insulin is unable to achieve adequate glycemic control. This might be associated with basal insulin inadequacy. All basal insulins are not alike. Each basal insulin and basal analogue has a unique structure and characteristics including duration of action, glycemic variability and risk of hypoglycemia. These differences may bring that one basal insulin substitute for another, in case adequate control is not achieved with a particular preparation [13]. Studies investigating the predictive characteristics of patients who respond well to BI analog (detemir) therapy have been especially lacking in Korean patients with T2D.
This sub-analysis of A1chieve study in Korea revealed that once-daily basal insulin given in the morning is preferable in Korean patients with T2D. There was a better response in patients who had older age, shorter duration of diabetes, well-controlled glycemic status (i.e., patients with lower A1c, lower PPG and lower Δ glucose) and were not taking sulfonylurea.
A recent paper, confirming the predictive factors of insulin analog user in A1chieve study, reported that a higher baseline A1c resulted in a comparatively lower final A1c. This study was the result of analysis of patients from 28 various countries [14]. Whereas in our analysis of Korean patients, the patients with lower A1c showed better response, which could be attributed to racial differences in insulin response. The biggest difference is considered from the origin of study analysis methods. In this study, the responders were defined as having final A1c lower than 7.5%, but on the other hand, the previous study had adopted delta A1c as a dependent value.
In the previous study, with every increase of 1.0% in baseline A1c, the final A1c decreased by 0.7–0.8% units. The A1c decreased from 9.5 ± 1.7% (at baseline) to about 2% final A1c in insulin-naïve subjects. In the present study, in group II (A1c ≥7.5%), baseline A1c was 9.8 ± 1.8%, which is similar to the existing research. This study is not for the degree of A1c reduction, but for an analysis about what conditions could target A1c less than 7.5%. These are the reasons why the results are different from the previous study. A number of studies have shown that a higher baseline A1c group has a large delta A1c changes, which is similar to the findings in this study. However, in the case of basal insulin treatment, people with higher baseline A1c might have difficulty to reach the target A1c less than 7.5% in spite of greater decrease during 6 months.
The appropriate choice of an insulin regime for initiation of therapy has always been difficult. There were several simple objective ways to select the basal insulin; High FPG levels should prompt a basal insulin prescription, postprandial glucose excursion (mg%) <40, low prandial:fasting index <0.4, high ratio of fasting plasma glucose/A1c ≥20 (mg%/%). The diurnal glucose profile is useful in selecting the form of insulin therapy in type 2 diabetes [15]. These previous findings are consistent with the result of this study that once-daily detemir therapy was more effective in patients who had well-controlled glycemic status (lower A1c, smaller prandial glucose excursion, lower postprandial glucose). Reduction in HbA1c levels was found across both older and younger age groups without an increased risk of hypoglycemia or weight gain [8]. Biphasic and prandial insulin regimens may lead to better glycemic control than basal insulin therapy [16]. This could make inferences that the patients who had preserved beta cell function in shorter duration of diabetes, and not taking sulfonylurea would be able to have better response to the basal insulin regimen.
Most Korean physicians preferred morning injections of insulin detemir over evening injections. Once-daily injections in the morning showed significant A1c-lowering effects but increased overall hypoglycemic episodes. These desirable glycemic effects and undesirable hypoglycemic episodes might be due to increased insulin doses in the morning injections. Although the doses of insulin in the morning injections were significantly higher, this did not affect the increase in the body weight. Previous studies showed consistent with the findings in our study which was once daily insulin detemir is not associated with weight gain [17]. A majority of the patients were injected with detemir in the morning in real clinical practice. While an evening injection is recommended by the available guidelines [18–20], results have shown that morning and evening administration of detemir were associated with reductions in A1c [21]. The morning administration group had a larger insulin dose, but they had fewer hypoglycemic events and only 500 g more weight gain compared to the evening administration group [21]. The Korean Diabetes Association does not clearly indicate the injection time of BI treatment. The primary physicians preferred morning injection of BI analog might be more comfortable for the patients and effective in glycemic control. This might be an explanation for morning preference in BI therapy. Observational studies include a wide range of study designs, a defining feature of which is that any intervention studied is determined by clinical practice and not by the protocol. Data from large, prospective observational studies provide information about the safety and efficacy of medicines in daily clinical use. However, observational trials have inherent limitations in terms of their susceptibility to bias, restricting their ability to define causality. However, their strengths include that they reflect daily clinical practice more closely than randomized controlled trials, both in terms of the heterogeneous patient populations that are included, and the medical interventions that they receive [22]. Similarly, our study has several disadvantages of an observational study. First, we did not adjust for a number of confounding variables faced in actual clinical practice. Second, the comparison between patients injecting detemir in the evening with those injecting in the morning is weakened by a small number of patients in the former group. The A1chieve study, by nature of its design, has a number of limitations that must be considered when interpreting the data [2]. Third, in terms of the subjects’ baseline characteristics, we could not figure out detailed withdrawal reasons, previous drug use including statin, and there were no statistical results on the difference between the subjects who withdrew from the study and the subjects who completed the study.
Conclusion
This analysis was a sub-analysis of the A1chieve study in Korea which demonstrated the efficacy and safety of once-daily BI therapy administered in the morning. Once-daily BI therapy might be preferable in patients with T2D with non-SU-treated diabetes of a shorter duration with smaller postprandial glucose excursion.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Sponsorship and article processing charges for this study were funded by Novo Nordisk Pharma Korea and Novo Nordisk International Operations. Medical writing assistance for this study was provided by Nomita S Saxena, Medical writer, Novo Nordisk. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval for the version to be published. Y.-C. Hwang and S. O. Song created the tables and figures, interpreted the data, and wrote the manuscript. B.-W. Lee designed the study, analyzed and interpreted the data, and reviewed/edited the manuscript. K.-J. Ahn, B. S. Cha, and Y. D. Song contributed to the discussion and reviewed the manuscript. Novo Nordisk Pharma Korea and Novo Nordisk International Operations collected the data.
Disclosures
S. O. Song, Y.-C Hwang, K.-J Ahn, B. S. Cha, Y. D. Song, D. W. Lee and B.-W Lee declare that they have no competing interest.
Compliance with ethics guidelines
The study was performed in accordance with the Declaration of Helsinki 1964, as revised in 2013, and the guidelines for Good Pharmacoepidemiology Practices. The protocol was reviewed and approved by independent institutional review boards at 104 study sites (Representative site: Yonsei University College of Medicine Institutional Review Board number: 4-2009-0359), and all participants provided written informed consent before any trial-related activity.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Sun Ok Song and You-Cheol Hwang contributed equally to this work.
References
- 1.Shah SN, Litwak L, Haddad J, Chakkarwar PN, Hajjaji I. The A 1 chieve study: a 60,000-person, global, prospective, observational study of basal, meal-time, and biphasic insulin analogs in daily clinical practice. Diabetes Res Clin Pract. 2010;88:S11–S16. doi: 10.1016/S0168-8227(10)70003-6. [DOI] [PubMed] [Google Scholar]
- 2.Home P, El Naggar N, Khamseh M, Gonzalez-Galvez G, Shen C, Chakkarwar P, et al. An observational non-interventional study of people with diabetes beginning or changed to insulin analogue therapy in non-Western countries: the A 1 chieve study. Diabetes Res Clin Pract. 2011;94(3):352–363. doi: 10.1016/j.diabres.2011.10.021. [DOI] [PubMed] [Google Scholar]
- 3.Yoo HJ, Park KY, Park KS, Ahn KJ, Min KW, Park JH, et al. Safety and efficacy of modern insulin analogues. Diabetes Metab J. 2013;37(3):181–189. doi: 10.4093/dmj.2013.37.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YH, Lee BW, Chun S, Cha B, Lee H. Predictive characteristics of patients achieving glycaemic control with insulin after sulfonylurea failure. Int J Clin Pract. 2011;65(10):1076–1084. doi: 10.1111/j.1742-1241.2011.02755.x. [DOI] [PubMed] [Google Scholar]
- 5.Jung CH, Park JY, Cho JH, Yoon KH, Yang HK, Lee YH, et al. The optimal morning:evening ratio in total dose of twice-daily biphasic insulin analogue in poorly controlled Type 2 diabetes: a 24-week multi-centre prospective, randomized controlled, open-labelled clinical study. Diabet Med. 2014;31(1):68–75. doi: 10.1111/dme.12322. [DOI] [PubMed] [Google Scholar]
- 6.Lee Y-H, Lee B-W, Kwon HJ, Kang ES, Cha BS, Lee HC. Higher morning to evening ratio in total dose of twice-daily biphasic insulin analog might be effective in achieving glucose control in patients with poorly controlled type 2 diabetes. Diabetes Technol Ther. 2012;14(6):508–514. doi: 10.1089/dia.2011.0208. [DOI] [PubMed] [Google Scholar]
- 7.Hwang YC, Kang JG, Ahn KJ, Cha BS, Ihm SH, Lee S, et al. The glycemic efficacies of insulin analogue regimens according to baseline glycemic status in Korean patients with type 2 diabetes: sub-analysis from the A(1)chieve((R)) study. Int J Clin Pract. 2014;68(11):1338–1344. doi: 10.1111/ijcp.12482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karnieli E, Baeres FM, Dzida G, Ji Q, Ligthelm R, Ross S, et al. Observational study of once-daily insulin detemir in people with Type 2 diabetes aged 75 years or older. Drugs Aging. 2013;30(3):167–175. doi: 10.1007/s40266-013-0054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raskin P, Allen E, Hollander P, Lewin A, Gabbay RA, Hu P, et al. Initiating insulin therapy in type 2 diabetes A comparison of biphasic and basal insulin analogs. Diabetes Care. 2005;28(2):260–265. doi: 10.2337/diacare.28.2.260. [DOI] [PubMed] [Google Scholar]
- 10.Choe EY, Lee YH, Lee BW, Kang ES, Cha BS, Lee HC. Glycemic effects of once-a-day rapid-acting insulin analogue addition on a basal insulin analogue in Korean subjects with poorly controlled type 2 diabetes mellitus. Diabetes Metab J. 2012;36(3):230–236. doi: 10.4093/dmj.2012.36.3.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nam S, Chesla C, Stotts NA, Kroon L, Janson SL. Factors associated with psychological insulin resistance in individuals with type 2 diabetes. Diabetes Care. 2010;33(8):1747–1749. doi: 10.2337/dc10-0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mollema E, Snoek F, Heine R, Van der Ploeg H. Phobia of self-injecting and self-testing in insulin-treated diabetes patients: opportunities for screening. Diabet Med. 2001;18(8):671–674. doi: 10.1046/j.1464-5491.2001.00547.x. [DOI] [PubMed] [Google Scholar]
- 13.Kalra S, Gupta Y. Basal insulin inadequacy versus failure—using appropriate terminology. Eur Endocrinol. 2015;11(2):79–80. doi: 10.17925/EE.2015.11.02.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Home PD, Shen C, Hasan MI, Latif ZA, Chen J-W, Gálvez GG. Predictive and explanatory factors of change in HbA1c in a 24-week observational study of 66,726 people with type 2 diabetes starting insulin analogs. Diabetes Care. 2014;37(5):1237–1245. doi: 10.2337/dc13-2413. [DOI] [PubMed] [Google Scholar]
- 15.Kalra S, Gupta Y. Insulin initiation: bringing objectivity to choice. J Diabetes Metab Disord. 2015;14(1):17. doi: 10.1186/s40200-015-0146-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lasserson D, Glasziou P, Perera R, Holman R, Farmer A. Optimal insulin regimens in type 2 diabetes mellitus: systematic review and meta-analyses. Diabetologia. 2009;52(10):1990–2000. doi: 10.1007/s00125-009-1468-7. [DOI] [PubMed] [Google Scholar]
- 17.Yale J-F, Damci T, Kaiser M, Karnieli E, Khunti K, Liebl A, et al. Initiation of once daily insulin detemir is not associated with weight gain in patients with type 2 diabetes mellitus: results from an observational study. Diabetol Metab Syndr. 2013;5(1):56. doi: 10.1186/1758-5996-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herbst KL, Hirsch IB. Insulin strategies for primary care providers. Clin Diabetes. 2002;20(1):11–17. doi: 10.2337/diaclin.20.1.11. [DOI] [Google Scholar]
- 19.Hirsch IB. Insulin analogues. N Engl J Med. 2005;352(2):174–183. doi: 10.1056/NEJMra040832. [DOI] [PubMed] [Google Scholar]
- 20.Devries J, Nattrass M, Pieber T. Refining basal insulin therapy: what have we learned in the age of analogues? Diabetes Metab Res Rev. 2007;23(6):441–454. doi: 10.1002/dmrr.762. [DOI] [PubMed] [Google Scholar]
- 21.Philis-Tsimikas A, Charpentier G, Clauson P, Ravn GM, Roberts VL, Thorsteinsson B. Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther. 2006;28(10):1569–1581. doi: 10.1016/j.clinthera.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 22.Yang W, Zilov A, Soewondo P, Bech OM, Sekkal F, Home PD. Observational studies: going beyond the boundaries of randomized controlled trials. Diabetes Res Clin Pract. 2010;88:S3–S9. doi: 10.1016/S0168-8227(10)70002-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.