Abstract
Chemical mutagenicity is a major hazard that is important to workers' health. Despite the use of large amounts of allyl chloride, the available mutagenicity data for this chemical remains controversial. To clarify the mutagenicity of allyl chloride and because a micronucleus (MN) test had not yet been conducted, we screened for MN induction by using male ICR mice bone marrow cells. The test results indicated that this chemical is not mutagenic under the test conditions. In this paper, the regulatory test battery and several assay combinations used to determine the genotoxic potential of chemicals in the workplace have been described. Further application of these assays may prove useful in future development strategies of hazard evaluations of industrial chemicals. This study also should help to improve the testing of this chemical by commonly used mutagenicity testing methods and investigations on the underlying mechanisms and could be applicable for workers' health.
Keywords: allyl chloride, Globally Harmonized System of Classification and Labeling of Chemicals, micronucleus, mutagenicity, occupational disease
1. Introduction
Chemicals may have various hazardous effects on human health or the environment, and chemical hazard evaluations are important for workers' health and work environments. Depending on toxicity, classified substances and their mixtures may require restricted exposure in workplaces. There is an increased need for chemical hazard assessments because the number of workers that are exposed to chemicals has risen with the development of many industries, and it is necessary to determine what these substances are and how they are regulated [1].
One chemical, allyl chloride (CAS number 107-05-1), is used in many industries, which has led to concerns about possible threats to the health of workers. Only insufficient or controversial information is available concerning the potential related hazards of allyl chloride; therefore, an in vivo micronucleus (MN) assay was conducted to gain additional information concerning any such hazards. Furthermore, toxicological information [e.g. the Safety Data Sheet (SDS)] from this study could be applied for workers' rights in several industries.
Allyl chloride is used in the synthesis of allyl compounds [2]; as an intermediate for the manufacture of polymers, resins, plastics [3]; for varnishes and adhesives; and in the synthesis of pharmaceuticals and insecticides [4]. In the United States, this chemical is listed as a high production volume (HPV) chemical (65FR81686), which means that >1 million pounds was produced or imported into the United States in 1990 and/or in 1994 [5]. In workplaces where allyl chloride is produced or used, occupational exposure to allyl chloride may occur through inhalation and dermal contact [6].
Allyl chloride has already been tested for mutagenicity by the following short-term tests: Salmonella reversion test with strains TA1535 and TA100 (with and without activation), a forward and back mutation system in Streptomyces coelicolor, and two forward mutation systems in Aspergillus nidulans. Spot and plate incorporation assay techniques are also employed. Allyl chloride was active in Salmonella typhimurium and Salmonella coelicolor and negative in A. nidulans [7]. In our previous study [8], allyl chloride did not induce chromosomal aberrations in Chinese hamster lung (CHL/IU) cells; we therefore proceeded to perform an in vivo MN assay. This was necessary to improve the evaluation of the carcinogenic potential of this compound.
The purpose of the MN test is to screen for cytogenetic damage that results in the formation of micronuclei containing lagging chromosome fragments or whole chromosomes. Micronuclei were first used to quantify chromosomal damage and are now recognized as one of the most successful and reliable assays for genotoxic carcinogens [9].
In the in vivo MN test, mammalian bone marrow cells are treated with allyl chloride. Many toxicological studies other than the MN test have been conducted, although the available genotoxic data on allyl chloride remain controversial with and without mammalian metabolic activation (S9). Therefore, to secure quality assurance, further study was necessary that was based on good laboratory practice (GLP) guidelines.
Table 1 shows physicochemical and toxicological information regarding allyl chloride. Using the Globally Harmonized System of Classification and Labeling of Chemicals (GHS) classification, a “mutagen” is an agent that increases the occurrence of mutations in populations of cells and/or organisms. Substances and mixtures in this hazard class are assigned to one of two hazard categories. Category 1 has two subcategories (Table 2). Many studies have focused on the mutagenicity of allyl chloride (Table 3; however, with the exception of our previous study [8], no study has used GLP tests. Allyl chloride is nevertheless has category 2 germ cell mutagen notified classification and labeling, according to Classification, Labelling, and Packaging (CLP) criteria [10] on the evidence of these non-GLP dataset. The CLP regulation ensures that the hazards presented by chemicals are clearly communicated to workers and consumers in the European Union (EU) through the classification and labeling of chemicals. A public notice of the Ministry of Employment and Labor (MoEL) of Korea (Sejong, Korea) [11] has also classified it as a Category 2 germ cell mutagen following European Union Classification, Labelling, and Packaging classification (EU-CLP).
Table 1.
Physicochemical and toxicological information of allyl chloride∗
| Chemical name | Allyl chloride |
| CAS No. | 107-05-1 |
| Synonyms | 1-Chloro-2-propene 1-Propene, 3-chloro- 2-Propenyl chloride 3-Chloropropene Chlorallylene p-Aminopropiofenon 1-Chloropropylene |
| Molecular formula | C3H5Cl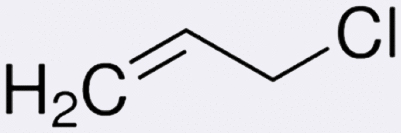
|
| Molecular weight | 76.5 |
| Melting point | −135°C |
| Forms | Colorless liquid |
| Partition coefficient | 2.1 |
| Boiling point | 45°C |
| Water solubility | 0.337 g/100 mL at 25°C |
| Stability & reactivity | Chemical stability: stable under recommended storage conditions Conditions to avoid: heat, flames, & sparks; temperature extremes; & direct sunlight Materials to avoid: oxidizing agents, boron trifluoride, sulfuric acid, nitric acid, & strong oxidizing agents |
| Toxicity | Target organs: liver, respiratory system Human LCLo inhalation, 3000 ppm Mouse LC50 inhalation, 11500 mg/m3/2H Mouse LD50 intraperitoneal, 155 mg/kg Mouse LD50 oral, 425 mg/kg Rabbit LCLo inhalation, 22500 mg/m3/2 h Rabbit LD50 skin, 2066 mg/kg Rat LC50 inhalation, 11 gm/m3/2H Rat LD50 oral, 460 mg/kg Guinea pig LC50 inhalation, 5800 mg/m3/2 h Allyl chloride is classified as Group 3 (i.e., not classifiable regarding its carcinogenicity to humans) in IARC, A3 in ACGIH, & Carc 2 in EU-CLP |
| GHS classification | Flammable liquids (Category 2) Acute toxicity, oral (Category 4) Acute toxicity, inhalation (Category 3) Skin irritation (Category 2) Eye irritation (Category 2) Carcinogenicity (Category 2) in MoEL of Korea & the IARC Group 3 Germ cell mutagenicity (Category 2) in the MoEL of Korea Specific target organ toxicity-single exposure (Category 3), respiratory system |
ACGIH, American Conference of Governmental Industrial Hygienists; CAS, Chemical Abstract Service; EU-CLP, European Union Classification, Labelling, and Packaging; GHS, Globally Harmonized System of Classification and Labeling of Chemicals; IARC, International Agency for Research on Cancer; LC50, median lethal concentration; LCLo, lowest lethal concentration; MoEL, Ministry of Employment and Labor.
The information is mostly obtained from searching ChemIDplus Advanced, U.S. National Library of Medicine (Rockville Pike, Bethesda, MD; http://chem.sis.nlm.nih.gov/chemidplus/rn/107-05-1), and Material Safety Data Sheet in KOSHANET (Ulsan, Korea; http://msds.kosha.or.kr/kcic/msdsdetail.do). The searches were conducted using keywords, the chemical name, and/or the CAS number.
Table 2.
Germ cell mutagenicity classification and standard assays in GHS classification
| Category 1. Known/presumed Known to produce heritable mutations in human germ cells | |
| Subcategory 1A Positive evidence from epidemiological studies Subcategory 1B Positive results in:
|
|
| Category 2. Suspected/possible | |
| |
GHS, Globally Harmonized System of Classification and Labeling of Chemicals.
Note. From: A Guide to The Globally Harmonized System of Classification and Labeling of Chemicals (GHS) [Internet]. Washington (DC):Occupational Safety and Health Administration (OSHA). 2003 [cited 2015 Jun 18]. Available from: https://www.osha.gov/dsg/hazcom/ghs.html#3.2. Copyright 2013, Occupational Safety and Health Administration (OSHA), Reprinted by permission.
Table 3.
Summary of studies focusing on in vitro and in vivo mutagenicity and carcinogenicity of allyl chloride∗
| Tests | Species | Protocol | Results | Refs |
|---|---|---|---|---|
| Genetic toxicity in vitro | ||||
| bacteria test (gene mutation) |
Salmonella typhimurium | Plate inc. assay | 2 tests negative, 1 positive in TA1535 with S9 | Dean et al [40] McCoy et al [41] |
| Spot test | 2 tests positive (in TA1535 with S9) (in TA1535 with & without S9) |
Neudecker & Henschler [42] | ||
| Liquid susp. assay | Positive in TA100 without S9 | Eder et al [43] | ||
| Escherichia coli | Spot test | Positive with & without S9 | ||
| Nonbacterial in vitro test (gene mutation) | Streptomyces coelicolor | Plate inc. assay | Positive for both forward & reverse mutation | Bignami et al [7] |
| Spot test | Positive for both forward & reverse mutation | |||
|
Aspergillus nidulans Saccharomyces cerevisiae |
Plate inc. assay Spot test Liquid suspension assay |
Negative Negative Positive both with & without S9 |
||
| Nonbacterial in vitro test (chromosomal aberration) |
A. nidulans Rat liver RL1 |
Other Other |
Increase in haploid segregants & diploid nondisjunctional sectors negative |
Crebelli et al [44] Dean et al [40] |
| Human HeLa S3 | H3-thymidine incorp. | Positive UDS | Schiffmann et al [45] | |
| DNA-modifying activity | E. coli | Other | Positive in pol A1 | McCoy et al [41] |
| Genetic toxicity in vivo | Rat/CD Rat/DC |
Micronucleus test Dominant lethal assay Sperm abnormality |
Negative Negative |
McGregor [46] |
| Mouse/B6C3F1 | SLRL test | Negative | ||
| Drosophila melanogaster | Negative | |||
SLRL, sex-linked recessive lethal; UDS, unscheduled DNA synthesis.
Information is mostly obtained by searching in United Nations Environment Programme (UNEP). OECD SIDS report: chloropropene (CAS no.: 107-05-1). Nairobi (Kenya): UNEP Publications; 1996.
Therefore, the MN assay accorded by GLP guidelines [12] was necessary to determine its mutagenicity exactly and propose it to a regulatory body such as the MoEL of Korea.
2. Materials and methods
2.1. Chemicals, animals, and experimental design
The test compound used in the in vivo MN test was allyl chloride (98.5%; Sigma-Aldrich, St. Louis, MO, USA; lot number MKBP7862V; cat. number 236306). Olive oil (Sigma-Aldrich; lot number BCBM3643V; cat. number O1514) was used as a solvent in accordance with the results of the solubility test. The positive control was mitomycin C (MMC; Sigma-Aldrich; lot number SLBF9516V,Cat. No. M4287).
The mouse (Mus musculus) bone marrow MN test was performed in accordance with the Organisation for Economic Co-operation and Development (OECD) Test TG 474 guidelines [12], and in accordance with Hayashi [13] and Heddle et al [14]. Groups of specific pathogen-free male ICR mice were treated with the test substance at three dosage levels. The highest dosage level was the estimated maximum tolerated dose or the standard limit dose for the MN test, whichever was lower. Concurrent negative group (i.e., olive oil) and positive control group (MMC, 0.5 mg/kg) were also treated. This study used 7-week old male ICR mice that were administered allyl chloride at 100 mg/kg, 200 mg/kg, and 400 mg/kg doses. At 24 hours post-treatment, six male animals that had been administered allyl chloride orally were used for each group. The Animal Ethics Committee of OSHRI, KOSHA approved the animal studies protocol (approval number IACUC-1403) to ensure appropriate care before the animals were obtained for research.
2.2. Bone marrow preparation and MN test
Twenty-four hours after the administration of allyl chloride the animals were euthanized and bone marrow cells were harvested from the mice femurs because of the cell cyle. Immature erythrocytes could be differentiated by using a variety of staining techniques that rely on the relatively high content of the cells' residual DNA. Mature erythrocytes with a low nucleic acid content appear pink to orange when stained with 5% Giemsa, whereas immature erythrocytes stain blue. Based on the cell cycle and maturation times of the erythrocytes, the bone marrow was harvested after 24 hours.
The bone marrow was flushed from the femurs and spread onto slides. The slides were air-dried, fixed, and stained with a fluorescent DNA-specific stain that easily illuminates any micronuclei that may be present. To evaluate the cytotoxicity, the preliminary tests were performed as a limit test to determine the maximum dosage. The inhibition of proliferation in the bone marrow cells was not observed in this test. To indicate chemically induced toxicity, the percentage of polychromatic erythrocytes (PCEs) among the 500 erythrocytes in the bone marrow was recorded for each dosage group.
At least 2,000 polychromatic erythrocytes (PCEs; e.g., reticulocytes, immature erythrocytes) were scored per animal with regard to the frequency of micronucleated cells in each of the six animals per dosage group. The presence of micronucleated PCEs was visually scored (at least 2,000 cells per mouse) by optical microscopy using a fluorescence microscope (Opti phot-2; Nikon, Tokyo, Japan) with a BA-2 filter. Cells were considered micronucleated if they neatly contained defined chromatin corpuscles with a diameter less than one-third the diameter of the cell nucleus, and if they stained an equal or lighter shade than the nucleus of the cell from which the micronucleated cell was developed. To reduce observer bias, only one reader was involved in scoring cells.
2.3. Evaluation and interpretation of the results
According to OECD TG 474 Test (i.e., Mammalian Erythrocyte Micronucleus Test) [12], the evaluation and interpretation of results were based on data that were presented as the mean number of micronucleated cells per 2,000 cells for each treatment group. The experimental and control MN frequency for each specimen within and between different mice strains were compared with a one-way analysis of variance test using SigmaStat version 3.11 software (Systat Software, Inc., San Jose, CA, USA). There is no requirement for verification of a clear positive or clear negative response.
2.4. Journal and book review
With particular emphasis on three topics—chemical mutagenicity, mutagenic tests associated with industrial chemicals, and the prevention of occupational diseases—we would like to discuss the prospects for developing a strategy for applying novel mutagenicity assays that are applicable for workers' health issues such as occupational cancer.
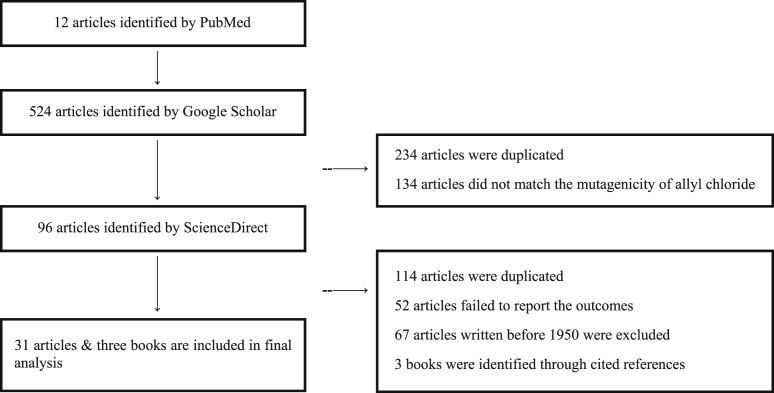
Searches were performed on the following sites: PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Google Scholar (http://scholar.google.com), and ScienceDirect (www.sciencedirect.com). The search strategy used a combination of the following Medical Subject Headings (MeSH; National Center for Biotechnology Information, Bethesda, MD) terms and keywords: “allyl chloride” and “mutagenicity test” or “workers” or “occupations.” The search results were further narrowed by reviewing titles and abstracts by two reviewers (the authors). Inclusion criteria were epidemiology, in vitro and in vivo mutagenicity and carcinogenicity studies. Additional missing case reports were identified by reviewing the references of review articles and bibliographies found on scholar.google.com. Disagreements in article and conference abstract identification were resolved by mutual discussion. Based on the literature review results, our search strategy identified 632 potential articles (Fig. 1). The reviewers agreed on 31 articles (i.e., references [13], [14], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]) and three books (i.e. [15], [34], [35]) that met the inclusion criteria for detailed analysis.
Fig. 1.
Flow diagram of article identification.
3. Results and discussion
There were no specific symptoms among the animals that were orally exposed to allyl chloride. The body weight of the animals exposed to this chemical ranged 35.74–40.20 g (Table 4). There were no environmental factors that may have affected the quality or integrity of the study results, which includes any significant behavioral changes (i.e., neurophysiological activity).
Table 4.
Animal body weight in micronucleus tests after oral exposure to allyl chloride
| Exposure method | Concentration | No. of animals | Average body weight (g; mean ± SD) |
|---|---|---|---|
| Orally exposed to allyl chloride for 24 h | Negative control (olive oil) | 6 | 37.97 ± 1.59 |
| 100 mg/kg b.w. | 6 | 37.98 ± 1.86 | |
| 200 mg/kg b.w. | 6 | 38.08 ± 1.53 | |
| 400 mg/kg b.w. | 6 | 37.89 ± 1.55 | |
| Positive control (MMC, 0.5 mg/kg b.w.) | 6 | 37.68 ± 1.86 |
b.w., body weight; MMC, mitomycin C.
Preliminary tests were performed to determine the maximum dosage. The proliferation of bone marrow cells was not inhibited in this test. The presence of micronucleated PCEs was visually scored by optical microscopy using a fluorescence microscope (Fig. 2).
Fig. 2.
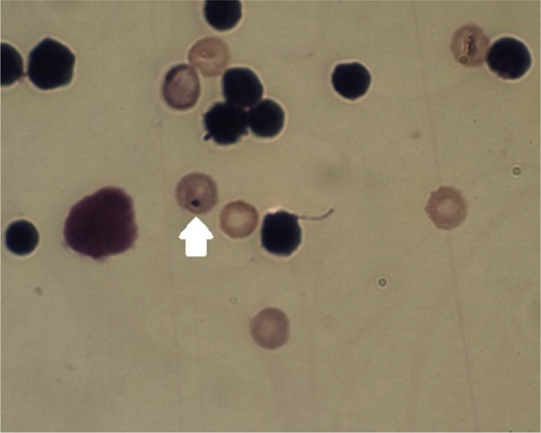
The presence of micronucleated polychromatic erythrocytes. Micronucleated mouse bone marrow cells with Giemsa staining under optical microscopy. The arrow shows a true micronucleus (magnification, 1,000×).
The frequency of erythrocytes with MN inductions was 0.19 ± 0.05% in the negative control group; 0.27 ± 0.18%, 0.30 ± 0.10%, and 0.38 ± 0.10% in the 100 mg/kg, 200 mg/kg, and 400 mg/kg allyl chloride-treated groups, respectively; and 1.19 ± 0.22% in the positive control group. The ratio of PCEs within the total number of erythrocytes was 63.03 ± 3.21% in the negative control group; 46.10 ± 5.60%, 53.89 ± 5.98%, and 46.74 ± 4.23% in the 100 mg/kg, 200 mg/kg, and 400 mg/kg allyl chloride-treated groups, respectively; and 45.07 ± 9.03% in the positive control group. There were no statistically significant changes in comparison to the negative control group (Table 5).
Table 5.
Results of the main micronucleus test with allyl chloride (for 24 hours)
| Groups | PCE observed | MNPCE observed | MNPCE frequency (%) | PCE + NCE counted | PCE counted | PCE/(PCE + NCE) (%) |
|---|---|---|---|---|---|---|
| Negative control | 2,033.83 ± 19.05 | 3.83 ± 0.98 | 0.19 ± 0.05 | 516.83 ± 13.11 | 325.83 ± 20.07 | 63.03 ± 3.21 |
| 100 mg/kg b.w. | 2,045.50 ± 63.98 | 5.50 ± 3.56 | 0.27 ± 0.18 | 520.00 ± 15.49 | 240.33 ± 36.13 | 46.10 ± 5.60 |
| 200 mg/kg b.w. | 2,048.33 ± 40.90 | 6.17 ± 1.94 | 0.30 ± 0.10 | 510.17 ± 5.81 | 275.17 ± 33.11 | 53.89 ± 5.98 |
| 400 mg/kg b.w. | 2,023.33 ± 13.02 | 7.67 ± 2.07 | 0.38 ± 0.10 | 512.83 ± 14.72 | 239.50 ± 19.69 | 46.74 ± 4.23 |
| Positive control (MMC, 0.5 mg/kg b.w.) |
2,027.00 ± 15.84 | 24.17 ± 4.45 | 1.19 ± 0.22 | 517.50 ± 28.48 | 234.83 ± 59.44 | 45.07 ± 9.03 |
Dats are presented as mean ± the standard deviation.
b.w., body weight; MMC, mitomycin C; MNPCE, micronucleated polychromatic erythrocyte; NCE, normochromatic erythrocyte; PCE, polychromatic erythrocyte.
In this study, we performed in vivo MN tests based on the results of a dose range-finding assay. The maximum dose was estimated at 400 mg/kg, based on regulatory guidelines. Bone marrow was extracted, and at least 2,000 PCEs per animal were analyzed for the frequency of micronuclei. Cytotoxicity was assessed by scoring the number of PCEs and normochromatic erythrocytes (NCEs) in at least the first 500 erythrocytes for each animal. Allyl chloride did not induce signs of clinical toxicity in the animals treated at the highest dose level (based on regulatory guidelines). The chemical also did not induce statistically significant increases in micronucleated PCEs at any dose. It was also not cytotoxic to the bone marrow (i.e., it did not produce statistically significant decreases in the PCE:NCE ratio) at any dose.
When a bone marrow erythroblast develops into a PCE, the main nucleus is extruded. Any MN that has been formed may remain behind in an otherwise anucleated cytoplasm. Visualization of the micronuclei is facilitated in these cells because they lack a main nucleus. An increase in the frequency of MNPCEs in treated animals is an indication of induced chromosomal damage. Statistical significance should not be the only determining factor for a positive response: positive results in a MN test indicate that a substance induces micronuclei because of chromosomal damage or damage to the mitotic apparatus in the erythroblasts of the test species. We evaluated and interpreted these results according to OECD guidelines [12]. The experimental and control MN frequency for each specimen within and between different mice strains were compared. There was no requirement for verification of a clear positive or clear negative response.
Table 6 shows the in vitro and in vivo genetic toxicity assays that optimize the standard battery for genetic toxicology recommended by the International Conference on Harmonisation (ICH; Geneva, Switzerland) [15]. Allyl chloride was tested for mutagenicity in a battery of in vitro and in vivo assays. In the older in vitro assays negative results were obtained, possibly because of the vaporization of allyl chloride. Adequate mutagenicity assays with S. typhimurium were positive with and without metabolic activation. The mutagenicity greatly decreased in the presence of an exogenous activating system. In a spot test with Escherichia coli and in tests with Streptomyces coelicolor positive results were obtained with and without metabolic activation. Tests with A. nidulans were negative for allyl chloride. The substance induces gene conversions in Saccharomyces cerevisiae and somatic segregation in A. nidulans. No significant compound-related chromosome damage was observed in RL1 cells. Allyl chloride induces unscheduled DNA synthesis in human HeLa S3 cells, but not in human embryonic intestinal cells. No increase in chromosomal aberrations was observed in a cytogenetic test with rats exposed to allyl chloride by inhalation. The substance was negative in a dominant lethal assay with rats and in a sperm abnormality test with mice. Allyl chloride did not increase sex-linked recessive lethal mutations in Drosophila melanogaster. Based on all available mutagenicity data, it can be concluded that allyl chloride is mutagenic to bacteria and yeast and it induces unscheduled DNA synthesis in human HeLa cells but not in embryonic testinal cells. Allyl chloride did not cause chromosome aberrations in vitro in mammalian cells. Negative results were obtained in the available in vivo tests (Table 3).
Table 6.
In vitro and in vivo genetic toxicity assays that optimize the standard battery for genetic toxicology recommended by the International Conference on Harmonisation
| Test name ∗ | No./Chapter No. |
|||
|---|---|---|---|---|
| OECD | EPA-OCSP | FDA Redbook 2000 | ||
| In vitro | Bacterial reverse mutation test (Ames test) | 471 | 870.5100 | IV.C.1.a. |
| In vitro mammalian chromosome aberration assay | 473 | 870.5375 | IV.C.1.b. | |
| In vitro mammalian cell micronucleus test | 487 | None | None | |
| In vitro mammalian cell gene mutation test | 476 | 870.5300 | IV.C.1.c. (only MLA) | |
| In vivo | Mammalian micronucleus test | 474 | 870.5395 | IV.C.1.d. (only erythrocyte) |
| Mammalian bone marrow chromosome aberration test | 475 | 870.5385 | None | |
| Unscheduled DNA synthesis (UDS) test with mammalian liver cells in vivo | 486 | None | None | |
| Transgenic mouse mutation assay | 488 | None | None | |
| In vivo comet assay | None | None | None | |
| Alkaline elution assay | None | None | None | |
| In vivo DNA covalent binding assay | None | None | None | |
EPA, Environmental Protection Agency; FDA, Food and Drug Administration; MLA, methyllycaconitine; OCSP, the Office of Chemical Safety and Pollution Prevention; OECD, Organisation for Economic Co-operation and Development.
Note. From R.D. Harbison, M.M. Bourgeois, and G.T. Johnson, Hamilton and Hardy's Industrial Toxicology, 6th ed, p. 1183. Copyright 2015, Hoboken (NJ): John Wiley & Sons, Inc. Adapted with permission.
The recommended International Conference on Harmonisation standard test battery.
A stepwise tiered approach is applied in regulatory mutagenicity testing [16]. In the first step, in vitro assays with a high sensitivity are used to identify test compounds that have high intrinsic genotoxic activity. In the second step, specific in vivo tests are performed to determine the relevance of the in vitro results for the in vivo situation. These in vivo mutagenicity studies are also included because some genotoxicants can only be detected in vivo after metabolic activation [17]. Compared to regulatory carcinogenicity testing, mutagenicity testing is relatively cheap and fast. Compounds without genotoxic liability can proceed first into clinical trials in humans. The carcinogenic potential is assessed later in the full developmental phase of drug development; however, the regulatory test strategy consists of a battery of core and ancillary tests for identifying the three forms of mutagenicity (i.e., gene mutations, clastogenicity, and aneugenicity), which cannot be detected in one single test.
Genotoxicity tests are used to detect genetic damage by various mechanisms in in vitro and in vivo systems. Several regulatory guidelines have been developed to provide various assays that are conducted for testing the genotoxicity. To date, most regulatory agencies and international authorities recommend a test scheme consisting of in vitro and in vivo methods to detect genotoxicity/mutagenicity induced by substances. The ICH recommends a standard battery test for pharmaceuticals to detect their genotoxicity. The ICH guidance optimizes the standard battery test for genetic toxicology and provides guidelines on the interpretation of results (Table 6) [15]. These guidelines help improve risk characterization for carcinogenic effects. In the following sections, some regulatory agencies or organizations are briefly described with regard to their own guidelines.
The Ames assay has a relatively high specificity, compared to other in vitro mutagenicity tests (Table 7). The sensitivity, specificity, and predictivity of the Ames assay calculated by Kirkland et al [18] were 58.8%, 73.9%, and 62.5%, respectively.
Table 7.
The sensitivity, specificity, and predictivity of the assays of the standard regulatory test battery for the assessment of carcinogenicity [18], [19]
| Assay | Sensitivity (%) | Specificity (%) | Predictivity (%) |
|---|---|---|---|
| Ames test | 58.8 | 73.9 | 62.5 |
| Chromosome aberration test | 65.6 | 44.9 | 59.8 |
| Mouse lymphoma TK test | 73.1 | 39.0 | 62.9 |
| Micronucleus test in vitro | 78.7 | 30.8 | 67.8 |
| Micronucleus test in vivo | 40.0 | 75.0 | 48.0 |
TK, thymidine kinase.
Note. From P. Steinberg (editor), High-throughput Screening Methods in Toxicity Testing, p. 213–69, Copyright 2013, Hoboken (NJ): John Wiley & Sons, Inc., Adapted with permission.
The chromosome aberration test is performed in vitro in cultured mammalian cells. It is also performed in the presence and in the absence of the S9 mixture [19], [20]. Scoring the test requires specialized training and experience. The sensitivity and predictivity of carcinogenicity for this test are 65.6% and 59.8%, respectively. The specificity of this test is low (44.9%).
The sensitivity and predictivity of the mouse lymphoma thymidine kinase assay is 73.1% and 62.9%, respectively. As for the chromosome aberration assay, the specificity of this assay is low (39%).
The fourth regulatory mutagenicity assay is the MN test. The sensitivity, specificity, and predictivity of the in vitro MN assay are 78.7%, 30.8%, and 67.8%, respectively. The specificity of the in vivo MN assay in bone marrow is much higher (75%). The sensitivity of the in vivo test is lower (40%) and the predictivity is 48% [21], [22].
In this paper, a regulatory test battery to determine the genotoxic potential of industrial chemicals has been described. The validation data for these higher throughput assays show that bacterial mutagenicity (i.e., gene mutations) and mammalian mutagenicity (i.e., chromosome damage) can be predicted early. To develop a strategy for applying the novel mutagenicity assays in the lead optimization phase, several combinations of assays must be evaluated. Further application of these assays may prove useful in future development strategies of chemicals.
The toxicological relevance of the MN test is well defined: it is a multitarget genotoxic endpoint; it assesses clastogenic and aneugenic events; and it assesses some epigenetic effects, which is simple to score, accurate, and applicable in different cell types. In addition, it is predictive for cancer, amenable for automation, and allows good extrapolation for potential limits of exposure or thresholds. It is easily measured in experimental in vitro and in vivo systems. Implementation of in vitro micronucleus (IVMN) assays in the battery of tests for hazard and risk assessment of potential mutagens/carcinogens is therefore fully justified. The final draft of the OECD guideline is available for this test [20].
The presence of MN in cultured human cells was reported as early as the 1960s [21] and 1970s [22]. The in vitro micronucleus test (IVMNT) has evolved into a robust quantitative assay of chromosome damage by the development of the cytokinesis-block technique that eliminated the confounding effects on MN expression by the cytostatic effects caused by poor culture conditions, treatment effects, cell senescence, and variability in mitogen response in the lymphocyte test system [23].
In the cytokinesis-block micronucleus (CBMN) assay, the scoring of MN discriminates between the accumulation of once-divided cells that appear binucleated and mononucleated cells that did not divide during the in vitro culturing period [24]. In recent years, the IVMNT has become an attractive tool for mutagenicity testing because of its capacity to detect clastogenic and aneugenic events and some epigenetic effects and because of the simplicity of scoring; its accuracy, wide applicability in different cell types; and its amenability to automation. More recently, the final draft of the OECD guideline 478 has become available [24]. The initial recommendations for this guideline came from two workshops [by the International Workshops on Genotoxicity Testing (IWGT)], which proposed an internationally harmonized protocol designed for human primary lymphocytes and for cell lines [25], [26]. The European Centre for the Validation of Alternative Methods (ECVAM) validated the methodology by retrospectively examining the existing published data on the IVMNT [27], [28], [29], [30], [31] using the modular approach for validation [32]. The ECVAM confirmed that the IVMNT is reliable, reproducible, transferable, and predictive [33], and ECVAM Scientific Advisory Committee endorsed the IVMNT [34], [35]. The final step before acceptance by the OECD consists of an interlaboratory exercise to evaluate different measures of cytotoxicity/cytostasis that can be applied when the IVMNT is performed in the absence of cytochalasin B [34]. The use of the IVMNT within a battery of tests will be defined by various regulatory bodies responsible for developing such test strategies. The advantages of the IVMNT are well defined and discussed by Bonassi et al [36], Decordier et al [37], and Elhajouji et al [38]: it is a multitarget genotoxic endpoint and predictive for cancer [39], it is amenable for automation [38], and it allows good extrapolation for potential limits of exposure or thresholds [38]. In addition, the MN can be scored and easily measured in a variety of in vitro and in vivo systems [39]. Implementation of IVMN assays in the battery of tests for hazard and risk assessment of potential mutagens/carcinogens is therefore fully justified.
Many findings have been used to determine the mutagenicity of allyl chloride; however, no GLP tests have been performed, except for our previous study [8]. It is nevertheless classified as a Category 2 germ cell notified mutagen and labeled according to CLP criteria on the evidence of these non-GLP dataset. A Public Notice of ministry of employment and labor (MoEL), Korea was also classified it as a category 2 germ cell mutagen in accordance wtih the classification of the European Union Classification, Labelling, and Packaging (EU-CLP). Therefore, the MN assay accorded by GLP guidelines [12] was needed to determine exactly its mutagenicity and propose it to a regulatory body such as the MoEL of Korea. Based on these results and discussion, allyl chloride did not induce micronuclei, as reflected by the MN test of the bone marrow cells of mice. Allyl chloride should be categorized as “not classified” as to its mutagenicity, according to GHS.
Nevertheless, it is anticipated that allyl chloride will trigger health problems of workers as occupational diseases. To predict risks for workers' health, especially occupational cancers, and to improve the assessment of hazardous effects, we recommend additional studies that focus on lung exposure and the long-term effects of these low-level contaminants with inhalation. Allyl chloride is practically regulated by Occupational Safety and Health Act (OSHAct) in the MoEL of Korea with its carcinogenicity (Category 2; Table 1).
Despite the use of large amounts of allyl chloride, the available data for the mutagenicity of this chemical remains controversial. We believe this is the first study to involve an in vivo MN assay with a mouse bone marrow cells. In conclusion, the test substance allyl chloride did not show any evidence of inducing MN under the conditions of this study.
In this paper, a regulatory test battery has been described with a medium or high throughput to determine the genotoxic potential of chemicals at work. The validation data for these higher throughput assays show that an early prediction is possible for bacterial mutagenicity (i.e., gene mutations) and mammalian mutagenicity (i.e., chromosomal damage). Several combinations of assays must be evaluated to develop a strategy to apply the novel mutagenicity assays in the lead optimization phase. Further application of these assays may prove useful in future development strategies of hazard evaluations of industrial chemicals.
Many findings have been used to determine the mutagenicity of allyl chloride; however, no GLP tests have been performed, except in our previous study [8]. Allyl chloride is nevertheless classified as a Category 2 germ cell notified mutagen and labeled according to EU-CLP criteria on the evidence of these non-GLP dataset. The public notice of the MoEL of Korea also classified it as a category 2 germ cell mutagen in accordance with the classification of the EU-CLP. However, based on these results and discussion, the mutagenicity of allyl chloride should be “not classified” according to GHS.
This study was performed with GLP guidelines to determine exactly its mutagenicity and propose it to a regulatory body such as the MoEL of Korea. This study also should help to improve the testing of this chemical by commonly used mutagenicity testing methods and investigations on the underlying mechanisms and could be applicable for workers' health issues, which include occupational cancers.
Conflicts of interest
The authors have no potential conflicts of interest to report relevant to this article.
Acknowledgments
This work was supported by the Korea Occupational Safety and Health Agency (KOSHA; Ulsan, Korea), Ministry of Employment and Labor (MoEL; Sejong, Korea), and a Grant-in-Aid for chemical hazard evaluation (2014).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc-nd/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Morita T., Hayashi M., Nakajima M., Tanaka N., Tweats D.J., Morikawa K., Sofuni T. Practical issues on the application of the GHS classification criteria for germ cell mutagens. Regul Toxicol Pharmacol. 2009;55:52–68. doi: 10.1016/j.yrtph.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.O’Neil M.J. 13th ed. Merck and Co. Inc.; Whitehouse Station (NJ): 2001. The Merck index—an encyclopedia of chemicals, drugs, and biologicals; p. 54. [Google Scholar]
- 3.International Labour Office (ILO) 3rd ed. ILO; Geneva (Switzerland): 1983. Encyclopedia of occupational health and safety; pp. 128–136. Chapters 1 and 2. [Google Scholar]
- 4.Hawley G.G. 9th ed. Van Nostrand Reinhold Co.; New York (NY): 1977. The condensed chemical dictionary; p. 29. [Google Scholar]
- 5.High production volume (HPV) challenge program . Office of Pollution Prevention and Toxics; 2005 Dec. 1-Propene, 3-chloro-(107-05-1) [Internet]. Washington (DC): environmental Protection Agency (EPA)http://www.epa.gov/hpv/pubs/general/opptsrch.htm [cited 2015 Jun 17]. Available from: [Google Scholar]
- 6.International safety cards . National Institute for Occupational Safety and Health (NIOSH); Atlanta (GA): 2005. Allyl chloride. 107-05-1 [Internet]http://www.cdc.gov/niosh/ipcsneng/neng0010.html [cited 2014 Feb]. Available from: [Google Scholar]
- 7.Bignami M., Conti G., Conti L., Crebelli R., Misuraca F., Puglia A.M., Randazzo R., Sciandrello G., Carere A. Mutagenicity of halogenated aliphatic hydrocarbons in Salmonella typhimurium, Streptomyces coelicolor and Aspergillus nidulans. Chem Biol Interact. 1980;30:9–23. doi: 10.1016/0009-2797(80)90110-6. [DOI] [PubMed] [Google Scholar]
- 8.Rim K.T., Kim S.J. In vitro mammalian chromosomal aberration test of allyl chloride for workers' health. J Korean Soc Occup Env Hyg. 2014;24:160–168. [Google Scholar]
- 9.Scott D., Evans H.J. X-ray-induced chromosomal aberrations in vicia faba: changes in response during the cell cycle. Mutat Res. 1967;4:579–599. doi: 10.1016/0027-5107(67)90044-9. [DOI] [PubMed] [Google Scholar]
- 10.European Chemical Agency (ECHA) ECHA; Helsinki (Finland): 2015. Summary of Classification and Labelling [Internet] Available from: http://echa.europa.eu/web/guest/regulations/clp/understanding-clp [cited 2015 Apr 24] [Google Scholar]
- 11.Exposure limits for chemical substances and physical agents [Internet] Ministry of Employment Labor (MoEL); Sejong (Korea): 2013. [cited 2015 Jun 18]. Available from: http://www.moel.go.kr. [Google Scholar]
- 12.Organisation for Economic Co-operation and Development (OECD) OECD; Paris (France): 1997. OECD Guidelines for the Testing of Chemicals. TG 474. [Google Scholar]
- 13.Hayashi M. Japan Scientist Press Co., Ltd.; Tokyo (Japan): 1991. The micronucleus test, monograph series no. 2. [Google Scholar]
- 14.Heddle J.A., Cimino M.C., Hayashi M., Romagna F., Shelby M.D., Tucker J.D., Vanparys P., MacGregor J.T. Micronuclei as an index of cytogenetic damage: past, present, and future. Environ Mol Mutagen. 1991;18:277–291. doi: 10.1002/em.2850180414. [DOI] [PubMed] [Google Scholar]
- 15.International Conference on Harmonisation (ICH) ICH; Geneva (Switzerland): 2012. International Conference on Harmonisation (ICH) of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH S2 R1: Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use. [Google Scholar]
- 16.Elliott B.M. Genotoxicity testing strategies. Toxicol In Vitro. 1994;8:871–872. doi: 10.1016/0887-2333(94)90090-6. [DOI] [PubMed] [Google Scholar]
- 17.Tweats D.J., Blakey D., Heflich R.H., Jacobs A., Jacobsen S.D., Morita T., Nohmi T., O'Donovan M.R., Sasaki Y.F., Sofuni T., Tice R., IWGT Working Group Report of the IWGT working group on strategy/interpretation for regulatory in vivo tests II. Identification of in vivo-only positive compounds in the bone marrow micronucleus test. Mutat Res. 2007;627:92–105. doi: 10.1016/j.mrgentox.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirkland D., Aardema M., Henderson L., Müller L. Evaluation of the ability of a battery of three in vitro genotoxicity tests to discriminate rodent carcinogens and non-carcinogens I. Sensitivity, specificity and relative predictivity. Mutat Res. 2005;584 doi: 10.1016/j.mrgentox.2005.02.004. 1–256. [DOI] [PubMed] [Google Scholar]
- 19.Benigni R., Bossa C., Worth A. Structural analysis and predictive value of the rodent in vivo micronucleus assay results. Mutagenesis. 2010;25:335–341. doi: 10.1093/mutage/geq010. [DOI] [PubMed] [Google Scholar]
- 20.Mammalian cell micronucleus test (MNvit) Organisation for Economic Co-operation and Development; Paris (France): 2010. OECD guideline for testing of chemicals no. 487 [Internet]http://www.oecd.org/env/testguidelines [cited 2014 Feb]. Available from: [Google Scholar]
- 21.Elston R.N. Nuclear budding and MN formation in human bone marrow, skin and fascia lata cells in vitro and in oral mucosa cells in vivo. Acta Pathol Microbiol Scand. 1963;59:195–199. doi: 10.1111/j.1699-0463.1963.tb01788.x. [DOI] [PubMed] [Google Scholar]
- 22.Countryman P.I., Heddle J.A. The production of MN from chromosome aberrations in irradiated cultures of human lymphocytes. Mutat Res. 1976;41:321–332. doi: 10.1016/0027-5107(76)90105-6. [DOI] [PubMed] [Google Scholar]
- 23.Fenech M., Morley A. Solutions to the kinetic problem in the micronucleus assay. Cytobios. 1985;43:233–246. [PubMed] [Google Scholar]
- 24.Fenech M., Morley A. Cytokinesis-block micronucleus method in human lymphocytes: effect of in vivo ageing and low dose X-irradiation. Mutat Res. 1986;161:193–198. doi: 10.1016/0027-5107(86)90010-2. [DOI] [PubMed] [Google Scholar]
- 25.Kirsch-Volders M., Sofuni T., Aardema M., Albertini S., Eastmond D., Fenech M., Ishidate M., Jr., Lorge E., Norppa H., Surrallés J., von der Hude W., Wakata A. Report from the in vitro micronucleus assay working group. Environ Mol Mutagen. 2000;35:167–172. doi: 10.1002/(sici)1098-2280(2000)35:3<167::aid-em3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Kirsch-Volders M., Sofuni T., Aardema M., Albertini S., Eastmond D., Fenech M., Ishidate M., Jr., Kirchner S., Lorge E., Morita T., Norppa H., Surrallés J., Vanhauwaert A., Wakata A. Report from the In vitro Micronucleus Assay Working Group. Mutat Res. 2003;540:153–163. doi: 10.1016/j.mrgentox.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Lorge E., Thybaud V., Aardema M.J., Oliver J., Wakata A., Lorenzon G., Marzin D. SFTG international collaborative study on in vitro micronucleus test I. General conditions and overall conclusions of the study. Mutat Res. 2006;607:13–36. doi: 10.1016/j.mrgentox.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Oliver J., Meunier J.R., Awogi T., Elhajouji A., Ouldelhkim M.C., Bichet N., Thybaud V., Lorenzon G., Marzin D., Lorge E. SFTG international collaborative study on in vitro micronucleus test V. Using L5178Y cells. Mutat Res. 2006;607:125–152. doi: 10.1016/j.mrgentox.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Aardema M.J., Snyder R.D., Spicer C., Divi K., Morita T., Mauthe R.J., Gibson D.P., Soelter S., Curry P.T., Thybaud V., Lorenzon G., Marzin D., Lorge E. SFTG international collaborative study on in vitro micronucleus test III. Using CHO cells. Mutat Res. 2006;607:61–87. doi: 10.1016/j.mrgentox.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Wakata A., Matsuoka A., Yamakage K., Yoshida J., Kubo K., Kobayashi K., Senjyu N., Itoh S., Miyajima H., Hamada S., Nishida S., Araki H., Yamamura E., Matsui A., Thybaud V., Lorenzon G., Marzin D., Lorge E. SFTG international collaborative study on in vitro micronucleus test IV. Using CHL cells. Mutat Res. 2006;607:88–124. doi: 10.1016/j.mrgentox.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Clare M.G., Lorenzon G., Akhurst L.C., Marzin D., van Delft J., Montero R., Botta A., Bertens A., Cinelli S., Thybaud V., Lorge E. SFTG international collaborative study on in vitro micronucleus test II. Using human lymphocytes. Mutat Res. 2006;607:37–60. doi: 10.1016/j.mrgentox.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Hartung T., Bremer S., Casati S., Coecke S., Corvi R., Fortaner S., Gribaldo L., Halder M., Hoffmann S., Roi A.J., Prieto P., Sabbioni E., Scott L., Worth A., Zuang V. A modular approach to the ECVAM principles on test validity. Altern Lab Anim. 2004;32:467–472. doi: 10.1177/026119290403200503. [DOI] [PubMed] [Google Scholar]
- 33.Corvi R., Albertini S., Hartung T., Hoffmann S., Maurici D., Pfuhler S., van Benthem J., Vanparys P. ECVAM retrospective validation of in vitro micronucleus test (MNT) Mutagenesis. 2008;23:271–283. doi: 10.1093/mutage/gen010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.European Centre for the Validation of Alternative Methods (ECVAM) ESAC 25th meeting; Ispra (Italy): 2006 Nov 16–17. Statement by the ECVAM Scientific Advisory Committee (ESAC) on the scientific validity of the in vitro micronucleus test as an alternative to the in vitro chromosome aberration assay for genotoxicity testing. [Google Scholar]
- 35.ECVAM Scientific Advisory Committee (ESAC) European Centre for the Validation of Alternative Methods; Ispra (Italy): 2006 Nov 16–17. ESAC Peer Review. Retrospective Validation of the In vitro Micronucleus Test. Summary and Conclusions of the Peer Review Panel. [Google Scholar]
- 36.Bonassi S., El-Zein R., Barale R., Fenech M. Associations of micronucleus frequency with cancer risk. Mutagenesis. 2010;26:93–100. Ispra (Italy):European Union Reference Laboratory for alternatives to animal testing. [Google Scholar]
- 37.Decordier I., Papine A., Vande Loock K., Plas G., Soussaline F., Kirsch-Volders M. Automated image analysis of MN by IMSTAR for biomonitoring. Mutagenesis. 2011;26:163–168. doi: 10.1093/mutage/geq063. Ispra (Italy):European Centre for the Validation of Alternative Methods Scientific Advisory Committee. [DOI] [PubMed] [Google Scholar]
- 38.Elhajouji A., Lukamowicz M., Cammerer Z., Kirsch-Volders M. Potential thresholds for genotoxic effects by MN scoring. Mutagenesis. 2010;26:199–204. doi: 10.1093/mutage/geq089. [DOI] [PubMed] [Google Scholar]
- 39.Hayashi M., MacGregor J.T., Gatehouse D.G., Blakey D.H., Dertinger S.D., Abramsson-Zetterberg L., Krishna G., Morita T., Russo A., Asano N., Suzuki H., Ohyama W., Gibson D., In vivoMicronucleus Assay Working Group, IWGT In vivo erythrocyte micronucleus assay III. Validation and regulatory acceptance of automated scoring and the use of rat peripheral blood reticulocytes, with discussion of non-hematopoietic target cells and a single dose-level limit test. Mutat Res. 2007;627:10–30. doi: 10.1016/j.mrgentox.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Dean B.J., Brooks T.M., Hodson-Walker G., Hutson D.H. Genetic toxicology testing of 41 industrial chemicals. Mutat Res. 1985;153:57–77. doi: 10.1016/0165-1110(85)90005-3. [DOI] [PubMed] [Google Scholar]
- 41.McCoy E.C., Burrows L., Rosenkranz H.S. Genetic activity of allyl chloride. Mutat Res. 1978;57:11–15. [PubMed] [Google Scholar]
- 42.Neudecker T., Henschler D. Mutagenicity of chloro-olefins in the Salmonella/mammalian microsome test. I. Allyl chloride mutagenicity re-examined. Mutat Res. 1985;157:145–148. doi: 10.1016/0165-1218(85)90109-0. [DOI] [PubMed] [Google Scholar]
- 43.Eder E., Neudecker T., Lutz D., Henschler D. Mutagenic potential of allyl and allylic compounds. Structure-activity relationship as determined by alkylating and direct in vitro mutagenic properties. Biochem Pharmacol. 1980;29:993–998. doi: 10.1016/0006-2952(80)90161-6. [DOI] [PubMed] [Google Scholar]
- 44.Crebelli R., Conti G., Conti L., Carere A. Induction of somatic segregation by halogenated aliphatic hydrocarbons in Aspergillus nidulans. Mutat Res. 1984;138:33–38. doi: 10.1016/0165-1218(84)90082-x. [DOI] [PubMed] [Google Scholar]
- 45.Schiffmann D., Eder E., Neudecker T., Henschler D. Induction of unscheduled DNA synthesis in HeLa cells by allylic compounds. Cancer Lett. 1983;20:263–269. doi: 10.1016/0304-3835(83)90023-x. [DOI] [PubMed] [Google Scholar]
- 46.McGregor D.B. NIOSH; Atlanta (GA): 1981. IRI report to The National Institute for Occupational Safety and Health NTIS no. PB86-239845, Jul 1, 1981. [Google Scholar]