Abstract
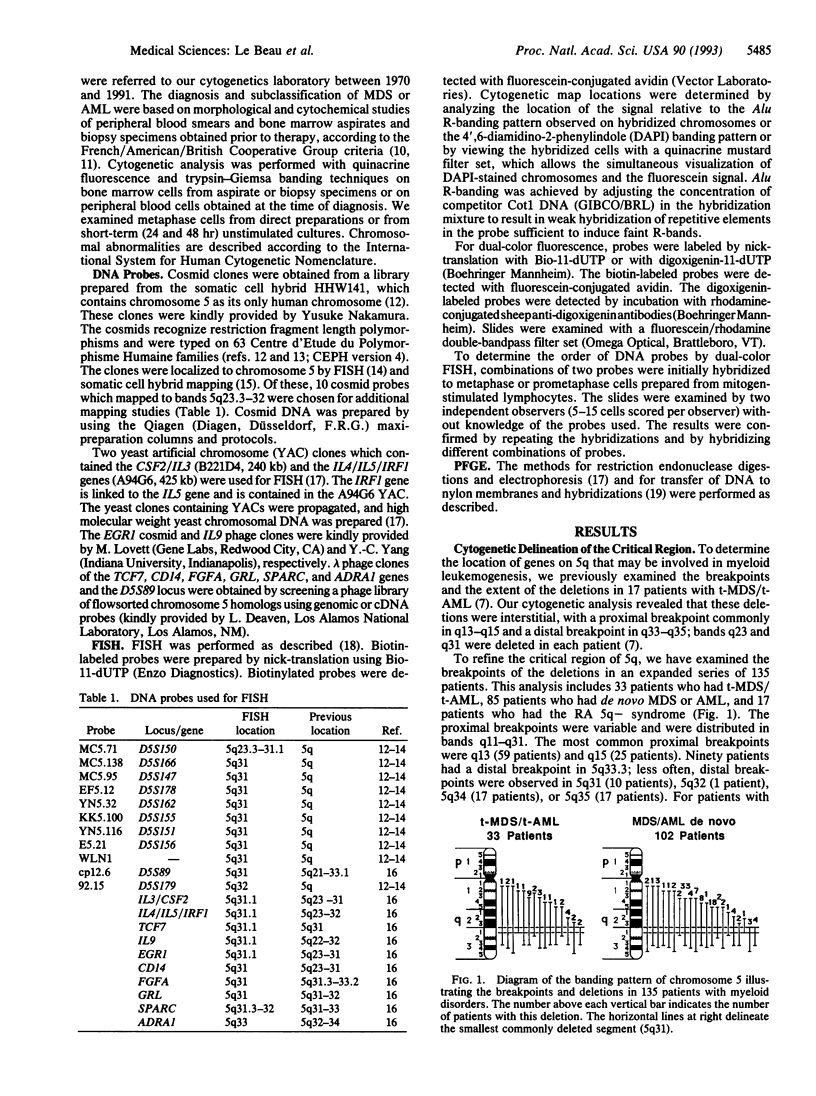
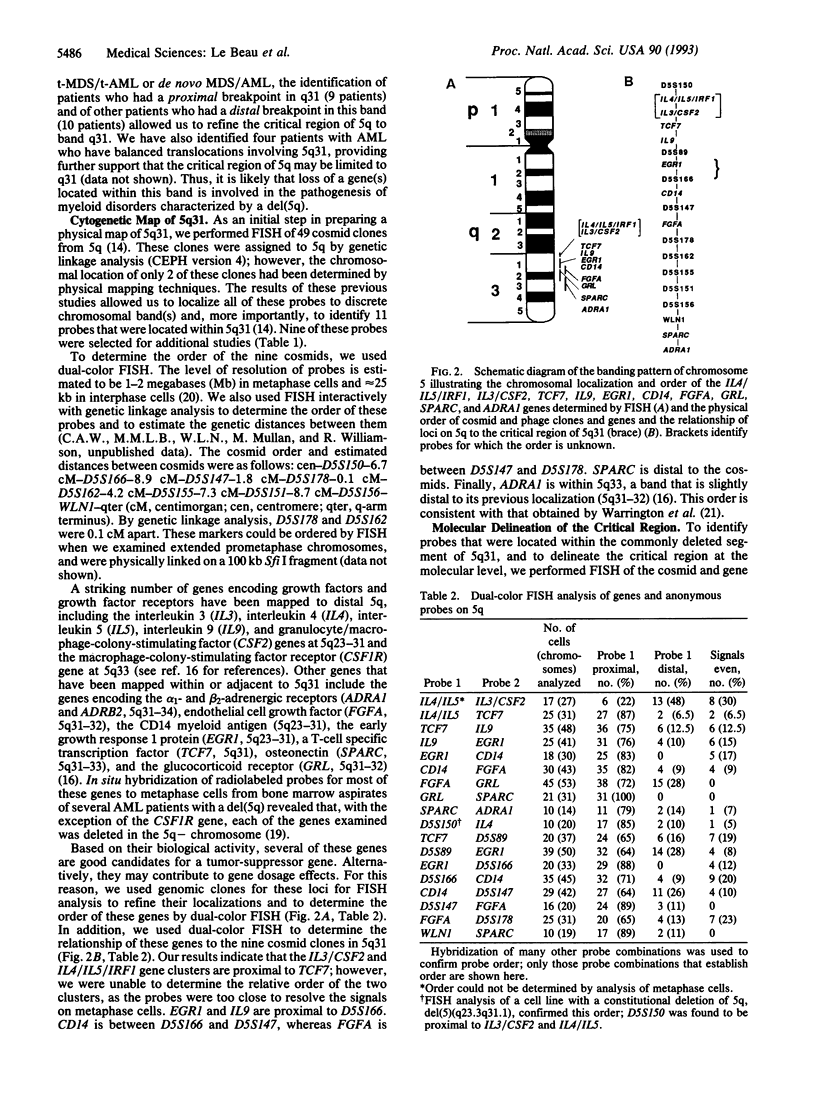
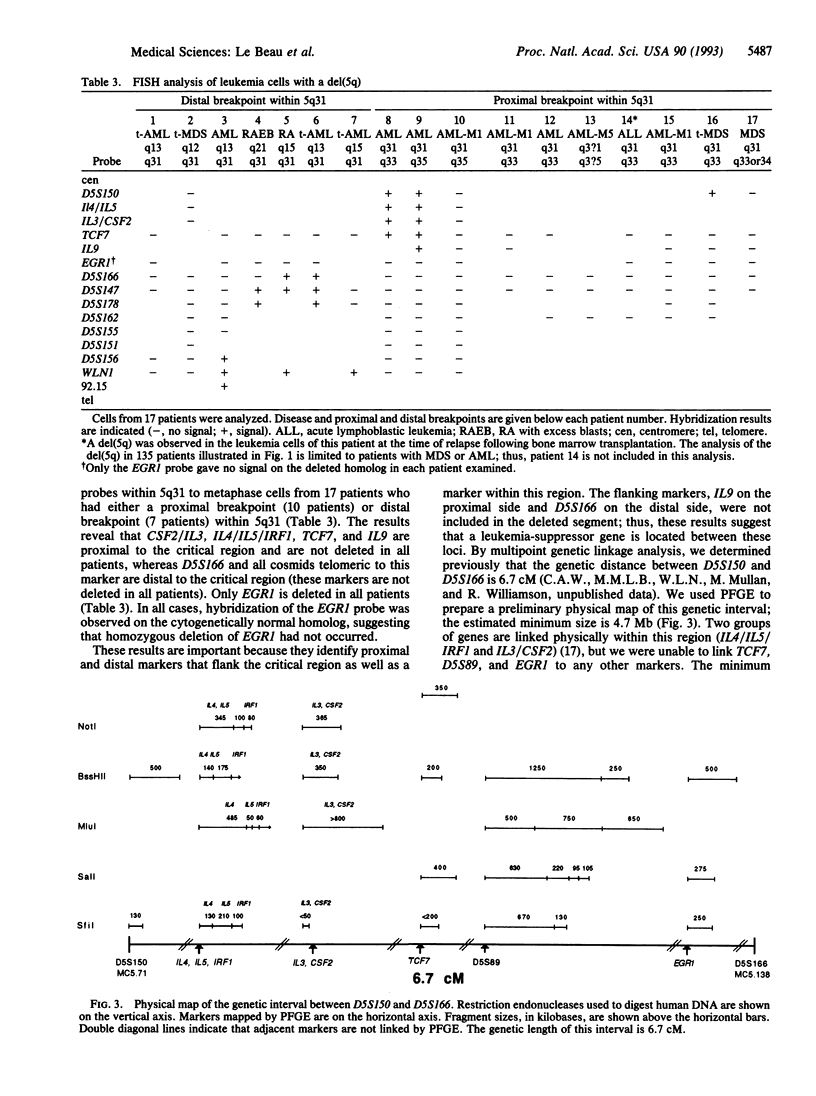
Loss of a whole chromosome 5 or a deletion of its long arm (5q) is a recurring abnormality in malignant myeloid neoplasms. To determine the location of genes on 5q that may be involved in leukemogenesis, we examined the deleted chromosome 5 homologs in a series of 135 patients with malignant myeloid diseases. By comparing the breakpoints, we identified a small segment of 5q, consisting of band 5q31, that was deleted in each patient. This segment has been termed the critical region. Distal 5q contains a number of genes encoding growth factors, hormone receptors, and proteins involved in signal transduction or transcriptional regulation. These include several genes that are good candidates for a tumor-suppressor gene, as well as the genes encoding five hematopoietic growth factors (CSF2, IL3, IL4, IL5, and IL9). By using fluorescence in situ hybridization, we have refined the localization of these genes to 5q31.1 and have determined the order of these genes and of other markers within 5q31. By hybridizing probes to metaphase cells with overlapping deletions involving 5q31, we have narrowed the critical region to a small segment of 5q31 containing the EGR1 gene. The five hematopoietic growth factor genes and seven other genes are excluded from this region. The EGR1 gene was not deleted in nine other patients with acute myeloid leukemia who did not have abnormalities of chromosome 5. By physical mapping, the minimum size of the critical region was estimated to be 2.8 megabases. This cytogenetic map of 5q31, together with the molecular characterization of the critical region, will facilitate the identification of a putative tumor-suppressor gene in this band.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett J. M., Catovsky D., Daniel M. T., Flandrin G., Galton D. A., Gralnick H. R., Sultan C. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982 Jun;51(2):189–199. [PubMed] [Google Scholar]
- Klein G. The approaching era of the tumor suppressor genes. Science. 1987 Dec 11;238(4833):1539–1545. doi: 10.1126/science.3317834. [DOI] [PubMed] [Google Scholar]
- Le Beau M. M., Albain K. S., Larson R. A., Vardiman J. W., Davis E. M., Blough R. R., Golomb H. M., Rowley J. D. Clinical and cytogenetic correlations in 63 patients with therapy-related myelodysplastic syndromes and acute nonlymphocytic leukemia: further evidence for characteristic abnormalities of chromosomes no. 5 and 7. J Clin Oncol. 1986 Mar;4(3):325–345. doi: 10.1200/JCO.1986.4.3.325. [DOI] [PubMed] [Google Scholar]
- Lithotripsy. Health and Public Policy Committee, American College of Physicians. Ann Intern Med. 1985 Oct;103(4):626–629. [PubMed] [Google Scholar]
- Nakamura Y., Lathrop M., Leppert M., Dobbs M., Wasmuth J., Wolff E., Carlson M., Fujimoto E., Krapcho K., Sears T. Localization of the genetic defect in familial adenomatous polyposis within a small region of chromosome 5. Am J Hum Genet. 1988 Nov;43(5):638–644. [PMC free article] [PubMed] [Google Scholar]
- Neuman W. L., Le Beau M. M., Farber R. A., Lindgren V., Westbrook C. A. Somatic cell hybrid mapping of human chromosome band 5q31: a region important to hematopoiesis. Cytogenet Cell Genet. 1992;61(2):103–106. doi: 10.1159/000133381. [DOI] [PubMed] [Google Scholar]
- Neuman W. L., Westbrook C. A., Dixon M., Espinosa R., 3rd, Patel Y. D., Nakamura Y., Weiffenbach B., Le Beau M. M. Physical localization of 70 polymorphic markers to human chromosome 5 by fluorescence in situ hybridization. Cytogenet Cell Genet. 1993;62(4):207–210. doi: 10.1159/000133477. [DOI] [PubMed] [Google Scholar]
- Nguyen H. Q., Hoffman-Liebermann B., Liebermann D. A. The zinc finger transcription factor Egr-1 is essential for and restricts differentiation along the macrophage lineage. Cell. 1993 Jan 29;72(2):197–209. doi: 10.1016/0092-8674(93)90660-i. [DOI] [PubMed] [Google Scholar]
- Nimer S. D., Golde D. W. The 5q- abnormality. Blood. 1987 Dec;70(6):1705–1712. [PubMed] [Google Scholar]
- Pedersen-Bjergaard J., Philip P., Larsen S. O., Jensen G., Byrsting K. Chromosome aberrations and prognostic factors in therapy-related myelodysplasia and acute nonlymphocytic leukemia. Blood. 1990 Sep 15;76(6):1083–1091. [PubMed] [Google Scholar]
- Rowley J. D., Diaz M. O., Espinosa R., 3rd, Patel Y. D., van Melle E., Ziemin S., Taillon-Miller P., Lichter P., Evans G. A., Kersey J. H. Mapping chromosome band 11q23 in human acute leukemia with biotinylated probes: identification of 11q23 translocation breakpoints with a yeast artificial chromosome. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9358–9362. doi: 10.1073/pnas.87.23.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley J. D., Golomb H. M., Vardiman J. W. Nonrandom chromosome abnormalities in acute leukemia and dysmyelopoietic syndromes in patients with previously treated malignant disease. Blood. 1981 Oct;58(4):759–767. [PubMed] [Google Scholar]
- Stock W., Chandrasekharappa S. C., Neuman W. L., Le Beau M. M., Brownstein B. H., Westbrook C. A. Characterization of yeast artificial chromosomes containing interleukin genes on human chromosome 5. Cytogenet Cell Genet. 1992;61(4):263–265. doi: 10.1159/000133417. [DOI] [PubMed] [Google Scholar]
- Sukhatme V. P., Cao X. M., Chang L. C., Tsai-Morris C. H., Stamenkovich D., Ferreira P. C., Cohen D. R., Edwards S. A., Shows T. B., Curran T. A zinc finger-encoding gene coregulated with c-fos during growth and differentiation, and after cellular depolarization. Cell. 1988 Apr 8;53(1):37–43. doi: 10.1016/0092-8674(88)90485-0. [DOI] [PubMed] [Google Scholar]
- Trask B. J., Massa H., Kenwrick S., Gitschier J. Mapping of human chromosome Xq28 by two-color fluorescence in situ hybridization of DNA sequences to interphase cell nuclei. Am J Hum Genet. 1991 Jan;48(1):1–15. [PMC free article] [PubMed] [Google Scholar]
- Van den Berghe H., Vermaelen K., Mecucci C., Barbieri D., Tricot G. The 5q-anomaly. Cancer Genet Cytogenet. 1985 Jul;17(3):189–255. doi: 10.1016/0165-4608(85)90016-0. [DOI] [PubMed] [Google Scholar]
- Warrington J. A., Bailey S. K., Armstrong E., Aprelikova O., Alitalo K., Dolganov G. M., Wilcox A. S., Sikela J. M., Wolfe S. F., Lovett M. A radiation hybrid map of 18 growth factor, growth factor receptor, hormone receptor, or neurotransmitter receptor genes on the distal region of the long arm of chromosome 5. Genomics. 1992 Jul;13(3):803–808. doi: 10.1016/0888-7543(92)90156-m. [DOI] [PubMed] [Google Scholar]
- Willman C. L., Sever C. E., Pallavicini M. G., Harada H., Tanaka N., Slovak M. L., Yamamoto H., Harada K., Meeker T. C., List A. F. Deletion of IRF-1, mapping to chromosome 5q31.1, in human leukemia and preleukemic myelodysplasia. Science. 1993 Feb 12;259(5097):968–971. doi: 10.1126/science.8438156. [DOI] [PubMed] [Google Scholar]



