Abstract
Background
In France the most recent data on drug use by the elderly living at home were published in 2000. Since then the available drugs and their use have changed.
Objective
We compared data collected in 2011 with the 2000 data to evaluate how drug use has changed in France.
Methods
The study analysed retrospectively the 2011 data collected prospectively in France from a sample of 600,000 people representative (1/97th) of the French population. All prescribed drugs reimbursed by the French national health insurance were recorded. Due to the reimbursement procedure the unit of analysis was the trimester. The drugs were coded using the Anatomical Therapeutic Chemical (ATC) Classification System.
Results
Data from 580,989 patients were analysed (133,411 (23.0 %) aged ≥60 years, 32,314 (5.6 %) ≥80 years). The percentage of patients who used medication increased from 55.9 % for patients in their fourth decade to 88.6 % for patients in their eighth decade, remained stable till 90 years of age and decreased to 26.3 % in centenarians. The median number of drugs prescribed was five (IQR: 3–8) in those aged under 80 years and ten (IQR: 7–14) in those aged over 80 years. Cardiovascular drugs were the most used, by 70.9, 78.1, and 69.6 % of patients aged 70–79, 80–89, and 90–99 years, respectively. Analgesics, non-steroidal anti-inflammatory drugs, and antibiotics were prescribed in almost half of the patients.
Conclusion
Polypharmacy is common among the elderly in France. Although this may be explained by the multiple co-morbidities, our results suggest an overuse of drugs for which the risk–benefit ratio is unknown in these age ranges. Consequently, numerous elderly patients are exposed to iatrogenic risks without the certainty of therapeutic benefits.
Electronic supplementary material
The online version of this article (doi:10.1007/s40801-015-0041-6) contains supplementary material, which is available to authorized users.
Key Points
| The percentage of medication users in France increases with age, with almost 90 % of octogenarians receiving drug therapy. |
| The number of drugs used also increases with age, with patients over the age of 80 years receiving a median of ten drugs. |
| This elderly population may be exposed to a high risk of adverse drug reactions resulting from polypharmacy. |
Introduction
According to the Organisation for Economic Co-operation and Development, France had one of the highest pharmaceutical expenditures in the world in 2001 [1], which has risen more than 50 % in the past decade [2], the elderly being the greatest consumers [3]. State of health is the main determinant of the use of pharmaceuticals among the elderly and multiple illnesses logically lead to polypharmacy, defined as the use of multiple medications and/or more medications than are clinically indicated [4–6] and to increased drug use as health declines [7].
We examined the state of drug use among the elderly in France and how it has changed in recent years. The most recent data on elderly French people living at home were published in 2000 by the Institut de Recherche et Documentation en Economie de la Santé (IRDES; Institute for Research and Information in Health Economics). Over the last decade, the French population has increased and with it the consumption of drugs [8]. The availability of drugs has also changed as some of them have been withdrawn or delisted, and new drugs have became available on the market. Lastly, guidelines for good geriatric clinical practice have been published (e.g., [9–11]). By updating the data on drug use by the elderly and comparing them with the most recent published data, the aim of this study was to provide information for the authorities involved in elderly care in order to adapt the future health strategies appropriately.
Methods
Source Data, Population and Experimental Plan
We retrospectively analysed the EGB sample (Echantillon Généraliste de Bénéficiaires), which is representative of French health insurance beneficiaries, data on whom are collected prospectively by the public health insurance authority (Assurance Maladie). The EGB is a representative and anonymous sample of beneficiaries of the three main French health insurance schemes, which ensures approximately 86 % representativity of the French population [12, 13]. It results from a 1/97th sampling of beneficiaries, whether or not they have been reimbursed for medical expenses (drugs included). It currently includes nearly 670,000 people. This database includes sociodemographic information on beneficiaries plus data on reimbursement of their medical expenses. The population living in nursing homes is excluded.
All EGB cohort members present throughout 2011 were selected for the study. They were considered medical-care consumers if they had been reimbursed for the purchase of prescribed drugs at least once in the year.
Classification of Drugs
All drugs purchased during 2011 were included. In the EGB cohort, reimbursed drugs were coded using the Anatomical Therapeutic Chemical (ATC) Classification System, which divides drugs into different groups according to the organ or system on which they act and/or their therapeutic and chemical characteristics. In France nearly 3,000 active substances are included in the composition of medicinal products. We therefore decided to divide them into 74 clinically relevant classes, starting from the ATC sections and highlighting some drugs or family of drugs. This methodological choice was made by a working group comprising a clinician (OSJ), two pharmacists (JFH and BS) and a statistician (EL). We made a consensus decision when there were differing views. For example, we divided analgesics into three distinct classes: paracetamol, opioid drugs and other analgesics, or we selected the ATC family ‘cardiovascular system’ but chose to highlight statins or diuretics in addition.
These 74 classes were then divided into ten large classes so as to summarise the results in a clinically meaningful manner from a geriatric viewpoint (cardiovascular and related drugs, drugs for degenerative diseases, vaccines and antibiotics, psychoactive drugs, diabetes drugs, anti-ulcer agents, nonsteroidal anti-inflammatory drugs (NSAIDs) and drugs for rheumatological diseases and the musculoskeletal system, analgesics, drugs for the respiratory system, drugs for thyroid disorders). This first division in 74 classes was made to have more precise data about interesting medicines or drug families (see supplementary material). It would have been impossible to get a clear and clinically significant synthesis if we had kept these 74 classes, so they were clustered in ten large classes that the multidisciplinary group deemed to be clinically interesting from a geriatric viewpoint. That is why, for example, NSAIDs are grouped separately, because of the serious side effects they produce, particularly in the elderly. Drugs fitting none of these categories were classed as ‘Other’.
Qualitative Analyses
The population was analysed in 10-year age ranges and we focused on people aged 65 years and over. The percentage of drug users and the number of drugs reimbursed were calculated for 3-month periods to take account of medication that can be prescribed for 3 months. A sensitivity analysis comparing these two results for the four quarters of 2011 revealed stable use during the year, except for vaccines, use of which was seasonal because of the influenza immunization programme, and antibiotics, due to the greater frequency of respiratory problems during winter. It was decided to present the results for the fourth quarter of the year.
The number of drugs corresponded to the number of different active substances reimbursed by the French health insurance system. Thus, a prescription for two identical drugs or for different dosages of the same drug was only taken into account once for a given cohort member. Conversely, a combination therapy was considered as including two distinct drugs. Only the drug users were considered in the analysis of the number of drugs.
Results
Characteristics of the Population
In 2011, 594,317 beneficiaries were covered by at least one of the three main insurance schemes included in the EGB cohort. Of them, 1.5 % (9,039) were lost to follow-up (switched to another health insurance scheme) and 0.7 % (4,289) died during that year. Drug use was analysed in 580,989 people (50.7 % women; mean age 40.1 ± 23.7 years). Of cohort members, 16.9 % (98,052) were over 65 years of age, 5.6 % (32,314) were over 80 years of age and 541 were centenarians.
Overall Active Drugs
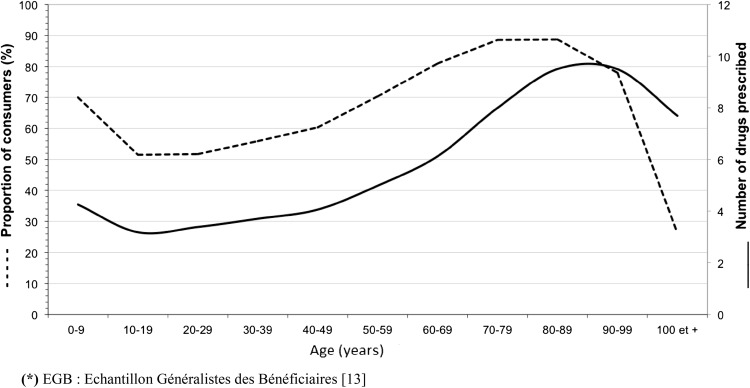
The percentage of cohort members who used medicinal products at least once in the year (Fig. 1) varied according to age: from 10 to 80 years, it rose linearly from 51.5 to 88.6 %, then levelled off, and declined sharply to 26.3 % among centenarians. The greatest use of medicinal products was seen among the 70- to 89-year-olds.
Fig. 1.
Percentage of cohort members (dotted line) who bought a drug and the number of drugs (solid line) bought (Echantillon Généraliste de Bénéficiaires [13], fourth quarter of 2011), according to age
As with the percentage of drug users, the number of active substances reimbursed increased with age (Fig. 1), from 3.2 drugs at 10–19 years to 9.2 drugs between 80 and 99 years of age. Only 14 % of drug users aged 65 years or more were reimbursed for fewer than four drugs for the 3-month period and over 25 % of them used more than 12 different drugs.
Drug Use by Class
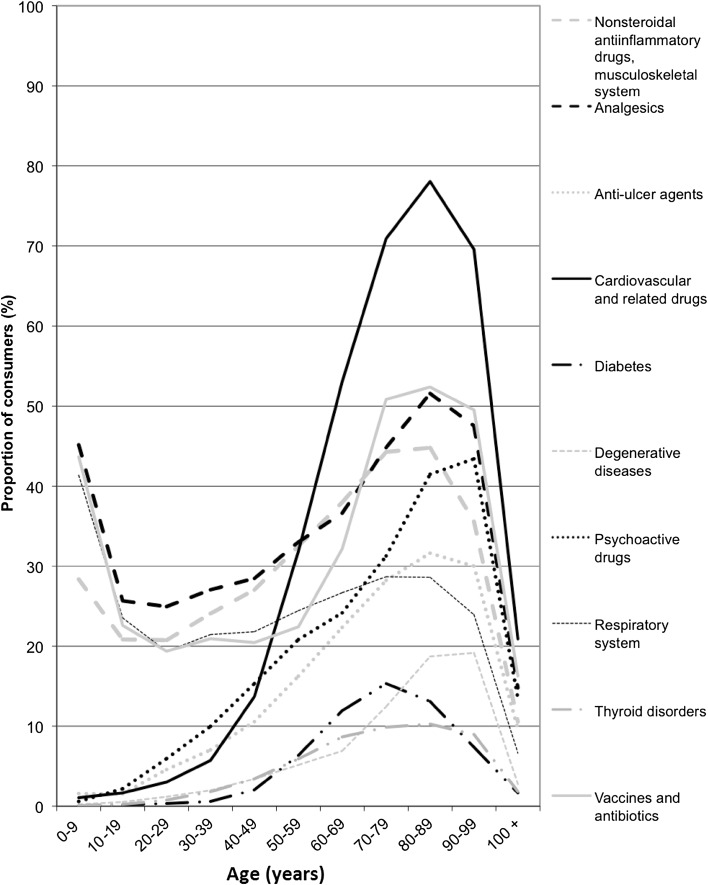
In people aged 65 years and over, 21 % of drugs reimbursed were for cardiovascular conditions (Fig. 2). Cardiovascular drugs were used the most, by 70.9, 78.1 and 69.6 % of patients aged 70–79, 80–89 and 90–99 years, respectively. Analgesics were used by 36–51 % of elderly drug users, depending on age class. Vaccines and antibiotics were also widely used (32–52 % of drug users). From 11 to 45 % of the elderly used NSAIDs or drugs for rheumatological and bone diseases, which reflects the prevalence of musculoskeletal disorders (e.g. osteoarthritis, osteoporosis) in older people. Psychoactive drugs were used by 14 % of centenarians and 43 % of nonagenarians. Anti-ulcer drugs, mostly proton pump inhibitors, were used by 26 % of the elderly (10 % of centenarians and 32 % of octogenarians).
Fig. 2.
Proportion of consumers by age range and drug class
Comparison with 2000 Data
Comparing our 2011 findings (Table 1) with data from the 2000 CREDES/IRDES health and social protection survey (ESPS), it was found that analgesics, notably paracetamol, remained in first place among the drugs most used by the elderly. The 2000 CREDES/IRDES results, presented in terms of brand drugs, placed Di-Antalvic (paracetamol plus dextropropoxyphene), Doliprane, Dafalgan and Efferalgan (paracetamol alone) in first, third, fifth and sixth positions, respectively. Ten years on, paracetamol was still in first place. Dextropropoxyphene, the major ingredient of Di-Antalvic and Di-Algirex (18th place in 2000), was withdrawn in 2009 and tramadol climbed to 15th place. Twelve drugs, delisted or withdrawn, had disappeared from the new classification.
Table 1.
Comparison of the 30 most sold drugs among those aged over 65 years: 2000 vs. 2011
| Rank | 2000 (CREDES, ESPS survey) Commercial name (DCI) |
Rank | 2011 (EGB) DCI |
|---|---|---|---|
| 1 | # Di-Antalvic® (dextropropoxyphene, paracetamol) | 1 | Paracetamol |
| 2 | Kardégic® (acetylsalicylic acid) | 2 | Acetylsalicylic acid |
| 3 | Doliprane® (paracetamol) | 3 | Hydrochlorothiazide |
| 4 | # Vastarel® (trimetazidine) | 4 | Sodium levothyroxin |
| 5 | Dafalgan® (paracetamol) | 5 | Furosemide |
| 6 | # Efferalgan® (paracetamol) | 6 | Influenza vaccine |
| 7 | # Tanakan® (ginkgo biloba extract) | 7 | Metformin |
| 8 | # Endotélon® (grapeseed extract) | 8 | Bisoprolol |
| 9 | # Fonzylane® (buflomedil) | 9 | Atorvastatin |
| 10 | # Gaviscon® (sodium alginate) | 10 | Colecalciferol |
| 11 | Stilnox® (zolpidem tartrate) | 11 | Clopidogrel |
| 12 | Corvasal® (molsidomine) | 12 | Diclofenac |
| 13 | Lasilix® (furosemide) | 13 | Omeprazole |
| 14 | Amlor® (amlodipine) | 14 | Amlodipine |
| 15 | # Daflon® (flavonoïc) | 15 | Tramadol |
| 16 | Aspégic® (acetylsalicylic acid) | 16 | Simvastatin |
| 17 | Mopral® (omeprazole) | 17 | Rosuvastatin |
| 18 | # Dialgirex® (dextropropoxyphene, paracetamol) | 18 | Calcium in combination |
| 19 | Zocor® (simvastatin) | 19 | Perindopril |
| 20 | Previscan® (fluendione) | 20 | Allopurinol |
| 21 | Zyloric® (allopurinol) | 21 | Pravastatin |
| 22 | # Praxilène® (naftudrofuryl) | 22 | Influenza antigen vaccine |
| 23 | # Veinamitol® (troxerutine) | 23 | Valsartan |
| 24 | Diamicron® (glyclazide) | 24 | Vitamin K antagonist |
| 25 | Sotalex® (sotalol) | 25 | Irbesartan |
| 26 | Cozaar® (losartan) | 26 | Pantoprazole |
| 27 | Sectral® (acebutolol) | 27 | Lercanidipine |
| 28 | # Piasclédine® (soybean extract) | 28 | Fenofibrate |
| 29 | Lipanthyl® (fenofibrate) | 29 | Esomeprazole |
| 30 | Témesta® (lorazepam) | 30 | Candesartan |
# Withdrawn or delisted
CREDES = Centre de Recherche d'Étude et de Documentation en Économie de la Santé, DCI = Dénomination Commune Internationale, EGB = Echantillon Generaliste de Beneficiaires, EsPs = IRDES health and social protection survey, IRDES = institute for research and information in health economics
Discussion
Our study shows that the percentage of drug use in the EGB cohort increased from age 30 to age 65 years, peaked at 90 % among octogenarians, and then declined, with a sharp drop among centenarians. The curve of percentage use was superimposable on the number of drugs reimbursed per person over the year, which increased from 3.2 drugs from 10–19 years of age to 9.2 drugs in octogenarians and nonagenarians before decreasing to 7.9 drugs among centenarians. The three large classes of most prescribed drugs in those aged 65 years or over were cardiovascular (64 % of drug users), analgesics (42 %) and vaccines and antibiotics (42 %). Psychoactive drugs were also widely used (31 %).
Quantitatively, our findings are similar to those published 10 years ago. The disappearance of some drugs from the 2011 classification is explained by the fact that the French national health authority (Haute Autorité de Santé) delisted certain drugs, the therapeutic benefit of which was deemed insufficient [14, 15]. The health authority also followed the recommendations of the European Medicines Agency in 2009 on the progressive discussions withdrawal in Europe of certain drugs that carry risks in the event of overdosage [16].
Cardiovascular drugs such as platelet inhibitors, diuretics, agents acting on the renin-angiotensin system, beta-blockers and statins were more common in the 2011 classification, with 17 of the 30 most used drugs, compared with ten in 2000. This could be explained by the spread of recommendations concerning care of cardiovascular diseases [17]. The widespread use of the influenza vaccine is due to the methodological choice to consider the fourth quarter of the year, during which the influenza immunisation programme takes place. This frequent use of influenza vaccines shows that the elderly population is now highly vaccinated, in line with the recommendations of the French Public Health Council (Haut Conseil de la Santé Publique). Proton pump inhibitors are still widely used.
The efficacy of the drugs used in 2011 has been validated by the health authorities according to strict criteria, which was not the case in 2000 when some marketed drugs were of uncertain efficacy. However, this recognised efficacy is based on studies including few if any very elderly subjects, so there is some doubt regarding drug efficacy and risk-benefit ratio in such patients. However, studies with population-based cohorts of patients are published and will outline tolerance and treatment efficacy (e.g. [18]).
The literature shows that in France those aged 65 years or over in 2000 used on average three to five different drugs a day, with those aged 80 years or over using 4.4 [19], as reported in other studies [20, 21]. The updated data from our study, the methodology of which differed as it was not a survey, highlight a substantial increase over the last 10 years. This is a limitation of our study. In 2011, 70- to 79-year-olds used on average between six and seven different drugs, and those aged over 80 years used more than eight. The number of active substances used by the elderly has therefore virtually doubled in the last 10 years. This rise in drug use with age, characterised 20 years ago as ‘relatively recent’ [22], has become more pronounced over the last decade.
Studies from other countries report similar findings. In Sweden in 2011, octogenarians used 5.3 drugs per person, nonagenarians 5.7, and centenarians 5.1 [23], showing a reduction in the very elderly. In a 2010 Polish study [24], 12 % of centenarians used at least one drug, not far from the 20 % of French centenarians. However, the average number of prescribed drugs in this population in Poland was 1.9 (±2.2) as it reached seven on average in France.
The first results of a comparative study of drug sales in seven European countries between 2000 and 2011 [25] show that France, once the country that consumed the most anti-ulcer drugs, antibiotics, antidepressants, anxiolytics, drugs for asthma, hypertension and diabetes, and lipid-lowering drugs, is now around the European average (except for antibiotics and anxiolytics, where France is still the leading consumer). This convergence between countries from 2000 to 2011 is due to the fact that over this period the countries with the generally highest growth in drug use were those whose sales levels in 2000 were the lowest (UK, Germany and Italy). The reasons for this increased use in Europe have not been analysed, but national public health programmes play a part [26].
In our study, the observed tendency to decreased drug use in the very elderly is paradoxical and hard to explain. One hypothesis is that the number of people with multiple morbidities decreases as the population ages: the sickest old people die and the survivors are relatively healthier and take fewer drugs. Another hypothesis is that an increase in iatrogenic complications results in the death of those patients taking the most drugs. Lastly, it could be that the oldest and therefore sickest patients, who are the greatest users of medicinal products, are institutionalised and so excluded from the sample analysed.
The EGB cohort is representative of the population covered by the three main French health insurance systems and therefore does not include those on minority or supplementary insurance schemes. In addition, nursing home patients are excluded. This can easily explain the decrease in drug use above 95 years of age, as more than 50 % of those aged over 95 years are institutionalised. Moreover, neither self-medication nor the taking of prescribed drugs that are not reimbursed can be measured. Conversely, the fact that a medication is purchased and reimbursed does not necessarily mean that it is taken by the patient [27].
Conclusion
Our study shows that drug use by the elderly in France has evolved over the last 10 years. Already among the highest in Europe and the world, it shows no sign of abating, whereas life expectancy in good health is levelling off. As the number of sick people increases, so too does the need for medication. This polypharmacy, which is justified by the large number of symptoms and diseases affecting the elderly, raises the problem of adverse drug reactions, complex and unpredictable interactions, and the adaptation of clinical recommendations to geriatric patients. The elderly are particularly exposed to iatrogenic effects, most of which involve cardiovascular drugs and psychoactive drugs. Our study also shows that the drug classes most used are influenced by delisting and by public health policy recommendations. The results of this study were communicated to the French Health Ministry in April 2015.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors thank the CNAM-TS for making data from the EGB cohort freely available.
Author contributions
JFH: study concept, analysis and preparation of paper
EL: methods and analysis
JL: study concept and preparation of paper
BS: study concept, analysis and preparation of paper
GC: study concept, analysis and preparation of paper
OSJ: study concept, analysis and preparation of paper
Compliance with Ethical Standards
Funding
This study received no funding.
Conflict of interest
JFH, EL, JL, GC, BS and OSJ report that they have no conflicts of interest to declare.
Ethical approval
Ethical approval was not required.
Contributor Information
Julien LeGuen, Phone: + 33 156093310, Email: julien.leguen@egp.aphp.fr.
Olivier Saint-Jean, Phone: + 33 156093313, Email: olivier.saint-jean@egp.aphp.fr.
References
- 1.Organisation for Economic Co-operation and Development (OECD). Health at a Glance: Europe 2003 [Internet]. 2003. Available from: http://www.oecd-ilibrary.org/social-issues-migration-health/health-at-a-glance-2003_health_glance-2003-en. Cited 26 May 2015.
- 2.Organisation for Economic Co-operation and Development (OECD). Health at a Glance: Europe 2014 [Internet]. 2014. Available from: http://www.oecd.org/health/health-at-a-glance-europe-23056088.htm. Cited 26 May 2015.
- 3.Haut Conseil pour l’Avenir de l’Assurance Maladie. Rapport du Haut Conseil pour l’Avenir de l’Assurance Maladie; 2006.
- 4.Hajjar ER, Cafiero AC, Hanlon JT. Polypharmacy in elderly patients. Am J Geriatr Pharmacother. 2007;5(4):345–351. doi: 10.1016/j.amjopharm.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Shalini M, Joshi M. Study of polypharmacy and associated problems among elderly patients. Internet J Med Update. 2012;7:35–39. [Google Scholar]
- 6.Patterson SM, Hughes C, Kerse N, Cardwell CR, Bradley MC. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2012;5:CD008165. doi: 10.1002/14651858.CD008165.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Auvray L, Dumesnil S, Le Fur P, Centre de Recherche d’Etude et de Documentation en Economie de la Santé. Santé, soins et protection sociale en 2000. 2001. https://www.google.fr/search?q=Auvray+L,+Dumesnil+S,+Le+Fur+P,+Centre+de+Recherche+d%E2%80%99Etude+et+de+Documentation+en+Economie+de+la+Sant%C3%A9.+Sant%C3%A9,+soins+et+protection+sociale+en+2000.+2001S&ie=utf-8&oe=utf-8&gws_rd=cr&ei=TzoSVv7CJsP6ygPG5LvQAQ#q=Auvray+L%2C+Dumesnil+S%2C+Le+Fur+P%2C+Centre+de+Recherche+d%E2%80%99Etude+et+de+Documentation+en+Economie+de+la+Sant%C3%A9.+Sant%C3%A9%2C+soins+et+protection+sociale+en+2000.+2001. Accessed 5 Oct 2015.
- 8.LEEM. Bilan économique des entreprises du médicament-édition 2014 [Internet]. 2014. Available from: http://www.leem.org/bilan-economique-des-entreprises-du-medicament-edition-2014. Cited 27 May 2015.
- 9.Fick DM, Semla TP. 2012 american geriatrics society beers criteria: new year, new criteria, new perspective. J Am Geriatr Soc. 2012;60(4):614–615. doi: 10.1111/j.1532-5415.2012.03922.x. [DOI] [PubMed] [Google Scholar]
- 10.Haute Autorité de Santé. Modalités d’arrêt des benzodiazépines et médicaments apparentés chez le patient âgé [Internet]. 2007. Available from: http://www.has-sante.fr/portail/upload/docs/application/pdf/arret_des_bzd_-_argumentaire.pdf. Cited 27 May 2015.
- 11.Haute Autorité de Santé. Prise en charge de la maladie d’Alzheimer et des maladies apparentées: interventions médicamenteuses et non médicamenteuses [Internet]. Available from: http://www.omedit-hautenormandie.fr/Files/maladie_dalzheimer__synthese___interventions_medicamenteuses_et_non_medicamenteuses.pdf. Cited 27 May 2015.
- 12.Assurance Maladie. L’échantillon généraliste de bénéficiaire [Internet]. 2009. Available from: http://www.ameli.fr/l-assurance-maladie/statistiques-et-publications/points-de-repere/n-25-l-echantillon-generaliste-de-beneficiaires.php. Cited 28 Nov 2012.
- 13.Roquefeuil LD, Studer A, Neumann A, Merlière Y. L’échantillon généraliste de bénéficiaires: représentativité, portée, et limites. Pratiques et Organisation des Soins. 2009;40(3):213–223. doi: 10.3917/pos.403.0213. [DOI] [Google Scholar]
- 14.Haute Autorité de Santé. Réévaluation du Service Médical Rendu (SMR)—1991–2001 [Internet]. 2001. Available from: http://www.has-sante.fr/portail/jcms/c_451925/reevaluation-du-service-medical-rendu-smr-1999-2001?xtmc=&xtcr=33. Accessed 5 Oct 2015.
- 15.Ministère du travail, de l’emploi et de la santé, Journal Officiel de la République. Arrêté du 24 janvier 2012 portant radiation de spécialités pharmaceutiques de la liste mentionnée au premier alinéa de l’article L. 162-17 du code de la sécurité sociale. 2012. http://www.legifrance.gouv.fr/eli/arrete/2012/1/24/ETSS1129357A/jo/texte/fr. Accessed 5 Oct 2015.
- 16.European Medicines Agency. European Medicines Agency recommends withdrawal of dextropropoxyphene-containing medicines [Internet]. 2009. Available from: http://www.emea.europa.eu/. Cited 14 Apr 2013.
- 17.Haute Autorité de Santé. Prévention vasculaire après un infarctus cérébral ou un accident ischémique transitoire [Internet]. 2008. Available from: http://www.has-sante.fr/portail/jcms/c_1252051/fr/prevention-vasculaire-apres-un-infarctus-cerebral-ou-un-accident-ischemique-transitoire. Accessed 5 Oct 2015. [DOI] [PubMed]
- 18.Joppi R, Cinconze E, Mezzalira L, Pase D, Poggiani C, Rossi E, et al. Hospitalized patients with atrial fibrillation compared to those included in recent trials on novel oral anticoagulants: a population-based study. Eur J Intern Med. 2013;24(4):318–323. doi: 10.1016/j.ejim.2013.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Auvray L, Sermet C. Consommations et prescriptions pharmaceutiques chez les personnes âgées—Un état des lieux. Gérontologie et Société. 2002;4(103):13–27. doi: 10.3917/gs.103.0013. [DOI] [Google Scholar]
- 20.Union Régionale des Caisses d’Assurance Maladie de Haute-Normandie et Union Régionale des Médecins libéraux. Personnes âgées et médicaments. 2002. http://www.bdsp.ehesp.fr/Base/260836/. Accessed 5 Oct 2015.
- 21.Salles-Montaudon N, Fourrier A, Dartigues JF, Rainfray M, Emeriau JP. Evolution of drug treatments in the aged living at home. Rev Méd Intern. 2000;21(8):664–671. doi: 10.1016/S0248-8663(00)80021-1. [DOI] [PubMed] [Google Scholar]
- 22.Lecomte T, Centre de Recherche, d’Etudes et de Documentation en Economie de la Santé. La consommation pharmaceutique en 1991. 1994. http://www.bdsp.ehesp.fr/Base/88339/. Accessed 5 Oct 2015.
- 23.Wastesson J, Parker M, Fastbom J, Thorslund M, Johnell K. Drug use in centenerians compared with nonagenarians and octogenarians in Sweden: a nationwide register-based study. Age Ageing. 2011;2012(41):218–224. doi: 10.1093/ageing/afr144. [DOI] [PubMed] [Google Scholar]
- 24.Rajska-Neumann A, Mossakowska M, Wieczorowska K. Drug consumption among Polish centenarians. Arch Gerontol Geriatrics. 2011;53:29–32. doi: 10.1016/j.archger.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 25.LIR—ESSEC. Evolution comparée des ventes de médicaments dans 7 pays européens (2000–2011). 2012. http://www.lir.asso.fr/. Accessed 5 Oct 2015.
- 26.The Dartmouth Institute for Health Policy and Clincal Practice. The Dartmouth Atlas of Medicare Prescription Drug Use. 2013. http://www.dartmouthatlas.org/publications/reports.aspx. Accessed 5 Oct 2015. [PubMed]
- 27.Observatoire Régional de la Santé Franche Comté, URCAM Franche Comté. L’automédication et l’observance thérapeutique chez les personnes âgées de plus de 70 ans. 2003. http://www.bdsp.ehesp.fr/Base/304961/. Accessed 5 Oct 2015.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.