Abstract
Local infiltration anesthesia (LIA) with anesthetics, steroids, NSAIDS, and epinephrine has been shown to be effective in reducing total knee arthroplasty (TKA) postoperative pain. This systematic review explores the functional outcomes of randomized control trials that have compared the use of LIA with and without steroids during TKA. Five studies with 412 patients met the inclusion criteria, 228 received local infiltration anesthesia with steroids (LIAS) and 184 received local infiltration anesthesia without steroids (LIAWS). The use of LIAS in management of postoperative TKA pain has been shown to decrease the length of hospital stay, time required to achieve straight leg raise, and pro-inflammatory signals in patients. Although there is no overwhelming data to suggest LIAS improves postoperative TKA pain, current literature does support its effectiveness in producing other favorable surgical outcomes.
Keywords: Local infiltration anesthesia, Steroid, Total knee arthroplasty, Systematic review, Periarticular injection
1. Introduction
Total knee arthroplasty (TKA) is a beneficial procedure to treat patients presenting with knee joint degeneration, but can be associated with postoperative pain hindering patient rehabilitation.1, 2 Recent studies demonstrate the number of knee revision procedures is expected to increase from 37,544 in 2005 to 56,918 in 2030, a 51% total increase in volume.3 Local infiltration anesthesia (LIA) is widely accepted as a means to manage pain during the post-operative period after TKA and provides relief with a decrease incidence of nausea, vomiting, urinary retention, constipation, drowsiness, and urinary catheter problems often associated with opioid use.4, 5
Studies and systematic reviews of LIA to relieve post-operative TKA pain have found it to be effective in reducing pain, increasing range of motion, and lessening opioid consumption post surgery.6, 7, 8, 9, 10 Although LIA for TKA has demonstrated positive results in reducing patient pain postoperatively, there is still very high variability in what medications are included in an LIA and in what dosages.9
Steroids are inconsistently included in LIA formulas.9, 10 Corticosteroids have been shown to decrease post-surgical pain by reducing production of prostaglandins and increasing vasodilation when injected directly into the surgical wound.11, 12 Although there are numerous studies that claim local infiltration anesthesia with steroids (LIAS) is effective in reducing post-surgical pain, there are few studies comparing LIAS and local infiltration anesthesia without steroids (LIAWS). In addition, no studies were found comparing the effect of different types of steroids in LIAS on patient pain relief. This systematic review provides a collective summary of existing TKA LIA studies that evaluate whether or not LIAS decreases pain and increases functional outcomes for patients versus LIAWS. To our knowledge, there are currently no published reviews examining LIAS and its role exclusively in TKA recovery.
2. Methods
2.1. Search methods
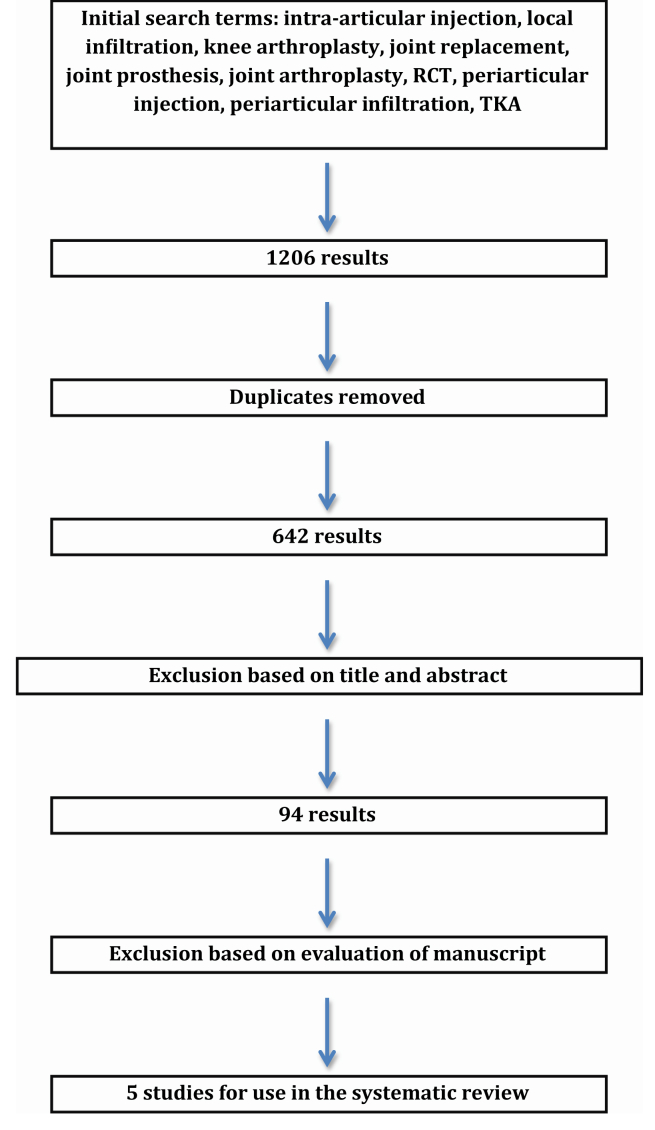
Relevant articles were found via the search methodology outlined in Fig. 1. A comprehensive search was conducted using PubMed, EMBASE, and the Cochrane Library for any study that involved the use of LIAS to relieve pain after TKA up until May 1, 2014. The initial search utilized the following key terms: intra-articular injection, local infiltration, knee arthroplasty, joint replacement, joint prosthesis, joint arthroplasty, RCT, periarticular injection, periarticular infiltration, and TKA. Using various combinations of these key terms, 1206 studies were determined relevant for further analysis. Removal of duplicates then yielded 642 results. Exclusion based on title and abstract reduced the studies for analysis to 94. After full evaluation of the 94 manuscripts, 5 studies were deemed appropriate for inclusion in this systematic review and detailed analysis (Fig. 1).13, 14, 15, 16, 17 Any studies that utilized LIAS during unicondylar knee arthroplasty were not included. Evaluation and inclusion of selected studies was performed in accordance with the Cochrane Handbook for Systematic Reviews.
Fig. 1.
Flow diagram showing details of literature search.
2.2. Data extraction
Data from the following fields were extracted from each study for further analysis: contents of LIA, pain visual analog scores (VAS), daily consumption of morphine post surgery, days to achieve straight leg raise (SLR), knee society score, range of motion (ROM), length of stay (LOS), C-reactive protein (CRP), and IL-6. In order to properly compare between studies, all steroids included in LIAS were converted into equivalents of dexamethasone (Table 1).
Table 1.
Steroids administered in each study and dexamethasone equivalents.
| Author | Steroid administered (mg) | Equivalents in dexamethasone of administered steroid (mg) |
|---|---|---|
| Christensen14 | Methylprednisolone acetate (40) | 7.5 |
| Sean13 | Betamethasone (6) | 7.5 |
| Ikeuchi15 | Dexamethasone (6.6) | 6.6 |
| Chia17 | Triamcinolone acetate (40) and Triamcinolone acetate (80) |
7.5 15 |
| Yue16 | Triamcinolone (40) | 7.5 |
2.3. Statistical analysis
Review Manager 5.3 was used to analyze extracted data and produce meta-analyses when possible. The overall effect for continuous data was evaluated using mean difference with a 95% confidence interval (CI). The I2 statistic was used to evaluate heterogeneity. If the I2 statistic was less than 50%, heterogeneity was considered to be insignificant and a random effect model was used. If the I2 statistic was greater than 50% a fixed-effect model was applied for the meta-analysis.18, 19
2.4. Functional outcome
The results produced by the 5 chosen studies in the following categories were used to evaluate the efficacy of LIAS: VAS scores, daily consumption of morphine post surgery, days to achieve straight leg raise, knee society score (KSS), range of motion score, length of hospital stay, and CRP.13, 14, 15, 16, 17
Of all of the evaluated categories, 4 were significant and not conflicting with the data of other studies: LOS, CRP, IL-6, and SLR. LOS represents hospital length of stay after surgery. CRP is an acute-phase protein synthesized by the liver in response to inflammation.20 IL-6 is a pro-inflammatory cytokine released into the bloodstream by neutrophil granulocytes and macrophages during inflammation and trauma.21 SLR is a test-conducted post TKA to gauge how high a patient is able to elevate his/her leg off of an exam table and it reflects pain control as well as muscle strength recovery.
3. Results
3.1. Population characteristics
Five papers met the inclusion criteria for this study and data on the cohort ages of all studies was recorded (Table 2). 412 patients were analyzed in total with an overall average age of 69.0 years (range of averages: 65.5–76.5 years).13, 14, 15, 16, 17 228 patients received LIAS while 184 patients received LIAWS during TKA.13, 14, 15, 16, 17 The average age of patients receiving LIAS was 69.7 (range of study averages 65.8–77) and the average age of patients receiving an LIAWS was 68.1 (range of study averages: 65.09–76).13, 14, 15, 16, 17 Due to variable age reporting, certain studies did not provide exact age ranges of patients selected for trials.
Table 2.
Age and cohort size of each study examined.
| Author | Steroid group number | Average age steroid group | Non-steroid group number | Average age non-steroid group | Overall average age of study | Total Patients |
|---|---|---|---|---|---|---|
| Christensen14 | 38 | 65.8 | 37 | 65.2 | 65.5 | 76 |
| Sean13 | 50 | 67.9 | 50 | 65.4 | 66.65 | 100 |
| Ikeuchi15 | 20 | 77 | 20 | 76 | 76.5 | 40 |
| Chia17 | 84 | 67.855 | 41 | 65.09 | 66.4725 | 125 |
| Yue16 | 36 | 70.2 | 36 | 69.3 | 69.75 | 72 |
| Total: | Average: | Total: | Average: | Average: | Total: | |
| 228 | 69.751 | 184 | 68.198 | 68.9745 | 413 |
3.2. Outcome overview
Patients undergoing LIAS were able to achieve SLR earlier and had shorter LOS.13, 14, 15 CRP and IL-6 levels were found to be lower in LIAS receiving patients compared to LIAWS patients.15 In addition, there are no studies with conflicting data claiming SLR, LOS, and CRP-IL6 did not improve with LIAS.13, 14, 15, 16, 17 Although VAS, morphine consumption, KSS, and ROM all had 1 or 2 studies claiming significant improvement for the LIAS cohort, there was at least 1 study included in this analysis that presented significant conflicting data.13, 14, 15, 16, 17
3.3. Length of hospital stay
Of the 5 chosen studies 2 found significant differences in LOS between LIAS and LIAWS groups. Sean et al report the mean LOS of the steroid group to be 5.2 days while the mean LOS of the non-steroid group to be 6.8 days (p = 0.022).13 Christensen et al also found a decrease in LOS between LIAS and LIAWS groups: 2.6 days for the steroid group as compared to 3.5 days for the non-steroid group (p = 0.01).14 All other studies included in this systematic review did not utilized LOS as an evaluation of LIAS versus LIAWS (Table 3).15, 16, 17 Sean et al did not provide standard deviations for reported values to complete the attempted meta-analysis.13
Table 3.
Summary of studies included  : Value is significantly higher for LIA steroid group compared to LIA without steroid group;
: Value is significantly higher for LIA steroid group compared to LIA without steroid group;  : Value is significantly lower for LIA steroid group compared to LIA without steroid group; ND: No significant differences detected between values of LIA steroid group compared to LIA without steroid group; NR: Value not reported; ★: Indicates a study in which all values are either significant or have not been reported.
: Value is significantly lower for LIA steroid group compared to LIA without steroid group; ND: No significant differences detected between values of LIA steroid group compared to LIA without steroid group; NR: Value not reported; ★: Indicates a study in which all values are either significant or have not been reported.
3.4. C-reactive protein and IL-6
Of all the studies chosen, only one measured the levels of serum CRP and IL6 postoperatively. On post-operative day 3 (POD3), LIAS treated patients had a serum CRP level of 3.9 mg/dl and LIAWS patients had 10.4 mg/dl (p < 0.0001).15 Fluid drained from the post-surgical knee of LIAS patients contained an IL-6 concentration of 26.7 ng/ml. The fluid from LIAWS patients contained an IL-6 concentration of 87.1 ng/ml, greater than that of the LIAS patients (p = 0.0005).15 All other studies included in this systematic review did not record serum CRP concentration and IL-6 concentration in fluid drained from the post-surgical wound (Table 3).13, 14, 16, 17
3.5. Straight leg raise
Of the 5 studies analyzed 2 reported significant decreases in days required to achieve SLR for the LIAS group as compared to the LIAWS group. Sean et al reported it took the LIAS cohort an average of 2.3 days to achieve SLR as opposed to an average of 2.8 days for the LIAWS cohort (p = 0.042).13 Similarly, Ikeuchi et al reports 15/20 (75%) of LIAS patients as compared to 5/20 (25%) of LIAWS patients were able to achieve SLR on POD1 (p = 0.002).15 All other studies included in this systematic review did not utilized SLR as an evaluation of LIAS effectiveness (Table 3).14, 16, 17 Neither Sean et al or Ikeuchi et al provided sufficient data to conduct a meta-analysis on SLR.13, 15
3.6. Conflicting outcomes
Pain VAS was only found to decrease in the study by Ikeuchi et al at POD1 and POD3 (p < 0.0001, p = 0.0048).15 Yue et al found no statistical significant difference between the VAS score of LIAS and LIAWS cohorts at POD1 and POD3.16 Three of the remaining 5 studies did not record a specific value for pain VAS at these time points.13, 14, 17
Demands for parenteral morphine at 18, 24, and 36 h and cumulative morphine consumption for the first 42 h postoperatively were significantly reduced in the LIAS group versus the LIAW group in the Sean et al study (p = 0.007).13 Yue et al found no significant morphine consumption difference at 24, 48, and 72 h postoperatively.16 Chia et al report no difference in total morphine equivalents of morphine consumed over the first two weeks postoperatively and Christensen et al found no significant difference in mean narcotic consumption on any day postoperatively (p = 0.79).14, 17 Ikeuchi et al did not utilize morphine consumption as a measure for postoperative outcome.15 No meta-analysis was conducted for morphine consumption because Sean et al, Chia et al, and Yue et al did not provide standard deviations.
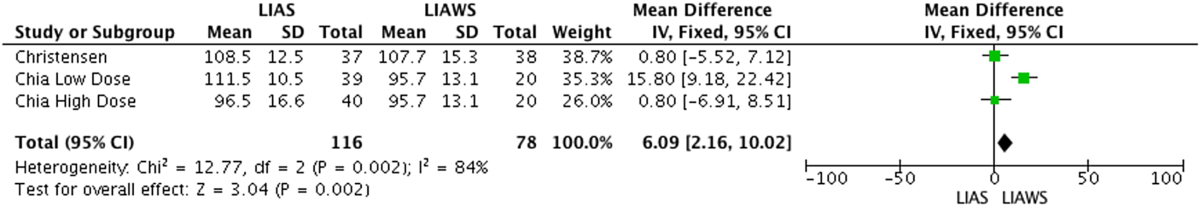
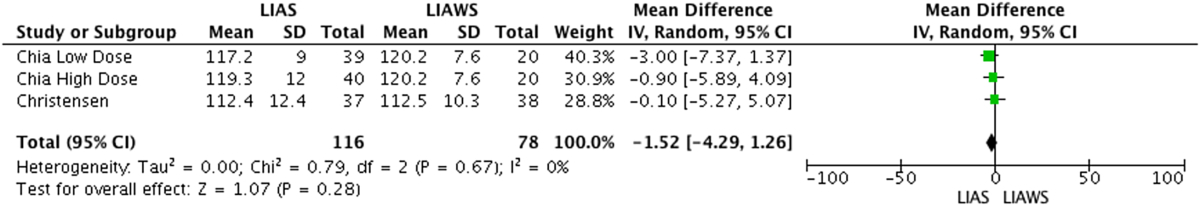
Sean et al found a higher ROM score for LIAS versus LIAW on each day between and including the 2nd and 5th days postoperatively (p = 0.01).13 Christensen et al reported no difference in ROM score between LIAS and LIAW groups.14 Chia et al reported no difference in an average of the ROM score for patients on any of the postoperative days in the first 2 weeks and no difference at 6 and 12 week time point measurements postoperatively (p = 0.82, p = 0.71, p = 0.67).17 Ikeuchi et al and Yue et al did not record ROM values.15, 16 Meta-analyses of ROM scores was conducted for measurements at 6 and 12 weeks postoperatively and demonstrated no significant difference between LIAS and LIAWS at either time point (p = 0.25, p = 0.28) (Table 4a, Table 4ba and b).14, 17
Table 4a.
LIAS versus LIAWS Range of Motion at 6 Weeks Forest Plot. Control patients for Chia were split into 2 groups of 20 patients. One group was assigned to the high dose steroid study Chia performed and the other group to the low dose steroid study Chia performed.
Table 4b.
LIAS versus LIAWS Range of Motion at 12 Weeks Forest Plot. Control patients for Chia were split into 2 groups of 20 patients. One group was assigned to the high dose steroid study Chia performed and the other group to the low dose steroid study Chia performed.
Yue et al reported the postoperative KSS to be better in LIAS patients versus LIASW patients at 1 month (p < 0.0045) and 3 months (p < 0.0027) – this reported difference, however, is not present before 1 month and disappears 6 months postoperatively.16 Christensen et al found no difference in KSS between LIAS and LIASW patients at 1.5 months and 3 months.2 Chia et al found no difference in KSS between LIAS and LIASW patients but no specific time points for KSS measurement were reported.17 Seat et al and Ikeuchi et al did not measure KSS.13, 15
4. Discussion
Glucocorticoids are highly effective in reducing inflammation and are often prescribed to treat chronic inflammatory diseases such as rheumatoid arthritis and lupus.22 Glucocorticoids act in a variety of pathways to decrease inflammation including: activation of glucocorticoid response elements on glucocorticoid-responsive genes, up regulation of transcription of anti-inflammatory gene proteins, inhibition of many inflammatory genes, and alteration of chromatin structure.22 Due to these anti-inflammatory effects of steroids, it is postulated that LIAS should better prevent postoperative inflammation in TKA patients and help facilitate a rapid recovery with less pain.
The efficacy of local infiltration anesthesia following TKA has been confirmed by multiple studies but none of these studies have included steroids in their LIA formulas.23, 24, 25, 26 Little is known as to whether or not the inclusion of a steroid in the LIA formula would be beneficial to patients, but past research on steroid use seems to indicate a potential benefit.22
The key findings reported in the literature are that LIAS decreases LOS, serum CRP concentration, surgical wound drainage fluid IL-6 concentration, and days required for SLR in postoperative TKA patients.13, 14, 15, 16, 17 Trends in the data collected in our study indicate that the benefits of LIAS are fully realized in the early postoperative period despite inconsistencies in LIAS formula and data collection time points by the included studies. Overall, steroids likely play a positive role in facilitating patient recovery from TKA, but more comprehensive randomized clinical studies focusing on TKA LIAS versus LIAWS need to be completed to confirm this hypothesis.
Of the outcome categories containing significant results and no conflicting data, 3 do not directly measure post-operative pain: LOS, serum CRP, and synovial fluid IL-6. The decrease in LOS for LIAS cohorts could be indicative of the patient feeling overall less pain and ready to begin rehabilitation earlier or more aggressively. Such a conclusion would be consistent with Sean et al findings of a decreased time to achieve SLR and increased ROM score for patients in the early postoperative period.13 Christenson et al measurements of ROM in their study were taken during POD1, the day of discharge, week 6, and week 12 and found no difference between ROM between LIAS and LIAWS.14 Unlike Sean et al, however, Christensen and colleagues did not continue to take ROM measurements after POD1 for the early postoperative period, a key period in which the administered steroids could have allowed patients to perform better on physical activities such as knee ROM.14 A relationship between LOS and patient functional ability post TKA could exist. Future studies need to evaluate LOS and extensively measure patient reported and objective outcomes on a daily schedule in order to better evaluate the benefit of including steroids in the LIA formula.
Ikeuchi et al found significantly lower levels of CRP and IL6 at POD 1 and 3 but did not find significant differences at POD7 and POD14.15 These findings suggest the clinical benefit of steroids is realized within the early postoperative period. Lower levels of CRP and IL6 could indicate lower levels of inflammation in the knee and that steroid inclusion is providing the desired anti-inflammatory effects.
Sean et al and Ikeuchi et al found LIAS patients were able to achieve complete SLR quicker in the first week postoperatively as compared to LIAWS patients.13, 15 This again suggests the clinical benefits of steroids for patients are within the early postoperative period.
Although Ikeuchi and colleagues were able to claim a significant decrease in VAS scores in LIAS patients on postoperative day 1 (p < 0.0001) and day 3 (p = 0.0048), both Yue et al and Chia et al reported no significant difference in VAS Score between LIAS and LIAWS cohorts at these time points.15, 16, 17 Yue et al included 20 mg of ropivacaine and 7.5 mg equivalents of dexamethasone in the LIAS given and Chia et al included 22.5 mg of ropivacaine and 15 mg equivalents of dexamethasone (high dosage group) (Table 1).16, 17 Ikeuchi et al only administered 15 mg of ropivacaine and 6.6 mg of dexamethasone equivalents in the LIAS (Table 1).15 In addition, the average age of all patients in Ikeuchi et al's study was 76.5 compared to the 69.75 and 66.47 of Yue and Chia.15, 16, 17 It is unclear as to why Ikeuchi's younger patients experienced decrease VAS on POD 1 and POD 3 when they received less anesthetics as well as less steroid in the LIAS administered.15 This may be attributed partly to administration techniques of the periarticular injections. These results suggest there is high variability in results of RCTs studying LIAS. There could be a role for LIAS following TKA if optimum formulas and techniques for LIAS can be developed. Future LIA studies involving steroids should investigate the effect of varying amounts of anesthetic and steroid used whilst keeping other ingredients in the LIA constant.
Although patterns can be drawn from the data presented on TKA and LIAS, there still exists conflicting results between the studies examined here. Analysis of morphine consumption, KSS, and ROM, yielded conflicting results. Whereas Yue et al showed that KSS is improved in LIAS patients at 1 and 3 months, Christensen et al showed there is no difference at 6 weeks and 12 weeks.14, 16 The inconsistency in results highlight the need for additional studies examining whether or not LIAS is more beneficial than LIAWS. A meta-analysis was able to be conducted comparing the ROM results from 6 to 12 weeks between Christensen et al and Chia et al, but the results demonstrated no significant difference between LIAS and LIAW at both time points14, 17 (Table 4a, Table 4ba and b). The inconsistency in results highlight the need for additional studies examining whether or not LIAS is more beneficial than LIAWS.
This work had a few limitations; the first is the inherent limitation in a systemic review work, although the search was performed with use of all major databases (Fig. 1).13, 14, 15, 16, 17 Another limitation of this systematic review is the lack of a meta-analysis of significant study outcome measures (LOS, CRP, IL-6, and SLR) demonstrating increased patient benefit from LIAS. A meta-analysis of the listed outcome measurements was attempted, however, but due to lack of data presented by and inconsistent measurement methods of the selected studies no results were generated. In addition, there was a lack in uniformity of what drugs were and were not included in LIAS given to patients across all studies examined. Although a few studies used the same steroid and/or anesthetic making comparison possible, no two studies had the exact same LIAS formula. Finally, no study reported on the exact site of LIAS delivery during the TKA, a potential confounding factor to this systematic review.
In conclusion, LIAS could provide increased benefits to patients in the early postoperative period when compared to patients receiving LIAWS after TKA. 4 outcome criteria with no conflicting data from the included 5 studies were found to significantly be improved in LIAS patients as compared to LIAWS patients: LOS, CRP, IL-6, and SLR. Significant data on the aforementioned categories was recorded within the first few hours and days following the TKA. Based on this systematic review, future RCTs studying the effect of steroids in LIA following TKA should concentrate efforts on data collection and outcome analysis on the first hours and days following the TKA and its effect on patient reported outcomes. Although there seems to be data supporting the benefits of LIAS, higher power studies need to be conducted before we can definitively claim LIAS is beneficial for patients post TKA.
Conflicts of interest
All authors have none to declare.
References
- 1.Jones C.A., Pohar S. Health-related quality of life after total joint arthroplasty: a scoping review. Clin Geriatr Med. 2012 Aug;28:395–429. doi: 10.1016/j.cger.2012.06.001. Review. PubMed PMID: 22840305. [DOI] [PubMed] [Google Scholar]
- 2.Bonica J.J. Postoperative pain. In: Bonica J.J., editor. 2nd ed. vol. I. Lea & Febiger; Philadelphia: 1990. pp. 461–480. (The Management of Pain). [Google Scholar]
- 3.Lavernia C., Lee D.J., Hernandez V.H. The increasing financial burden of knee revision surgery in the United States. Clin Orthop Relat Res. 2006 May;446:221–226. doi: 10.1097/01.blo.0000214424.67453.9a. PubMed PMID: 16672891. [DOI] [PubMed] [Google Scholar]
- 4.Singelyn F.J., Deyaert M., Joris D., Pendeville E., Gouverneur J.M. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998 Jul;87:88–92. doi: 10.1097/00000539-199807000-00019. PubMed PMID: 9661552. [DOI] [PubMed] [Google Scholar]
- 5.DeWeese F.T., Akbari Z., Carline E. Pain control after knee arthroplasty: intraarticular versus epidural anesthesia. Clin Orthop Relat Res. 2001 Nov;392:226–231. doi: 10.1097/00003086-200111000-00028. PubMed PMID: 11716387. [DOI] [PubMed] [Google Scholar]
- 6.Parvataneni H.K., Shah V.P., Howard H., Cole N., Ranawat A.S., Ranawat C.S. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007 Sep;22(6 suppl 2):33–38. doi: 10.1016/j.arth.2007.03.034. Epub 2007 Jul 26. PubMed PMID: 17823012. [DOI] [PubMed] [Google Scholar]
- 7.Andersen L.O., Kehlet H. Analgesic efficacy of local infiltration analgesia in hip and knee arthroplasty: a systematic review. Br J Anaesth. 2014 Sep;113:360–374. doi: 10.1093/bja/aeu155. Epub 2014 Jun 17. Review. PubMed PMID: 24939863. [DOI] [PubMed] [Google Scholar]
- 8.Busch C.A., Shore B.J., Bhandari R. Efficacy of periarticular multimodal drug injection in total knee arthroplasty. A randomized trial. J Bone Joint Surg Am. 2006 May;88:959–963. doi: 10.2106/JBJS.E.00344. PubMed PMID: 16651569. [DOI] [PubMed] [Google Scholar]
- 9.Jiang J., Teng Y., Fan Z., Khan M.S., Cui Z., Xia Y. The efficacy of periarticular multimodal drug injection for postoperative pain management in total knee or hip arthroplasty. J Arthroplasty. 2013 Dec;28:1882–1887. doi: 10.1016/j.arth.2013.06.031. Epub 2013 Aug 1. Review. PubMed PMID: 23910819. [DOI] [PubMed] [Google Scholar]
- 10.Kehlet H., Andersen L.Ø. Local infiltration analgesia in joint replacement: the evidence and recommendations for clinical practice. Acta Anaesthesiol Scand. 2011 Aug;55:778–784. doi: 10.1111/j.1399-6576.2011.02429.x. Epub 2011 Apr 4. Review. PubMed PMID: 21463261. [DOI] [PubMed] [Google Scholar]
- 11.Mirzai H., Tekin I., Alincak H. Perioperative use of corticosteroid and bupivacaine combination in lumbar disc surgery: a randomized controlled trial. Spine (Phila Pa 1976) 2002 Feb 15;27:343–346. doi: 10.1097/00007632-200202150-00003. PubMed PMID: 11840097. [DOI] [PubMed] [Google Scholar]
- 12.Glasser R.S., Knego R.S., Delashaw J.B., Fessler R.G. The perioperative use of corticosteroids and bupivacaine in the management of lumbar disc disease. J Neurosurg. 1993 Mar;78:383–387. doi: 10.3171/jns.1993.78.3.0383. PubMed PMID: 8433138. [DOI] [PubMed] [Google Scholar]
- 13.Sean V.W., Chin P.L., Chia S.L., Yang K.Y., Lo N.N., Yeo S.J. Single-dose periarticular steroid infiltration for pain management in total knee arthroplasty: a prospective, double-blind, randomised controlled trial. Singapore Med J. 2011 Jan;52:19–23. PubMed PMID: 21298236. [PubMed] [Google Scholar]
- 14.Christensen C.P., Jacobs C.A., Jennings H.R. Effect of periarticular corticosteroid injections during total knee arthroplasty. A double-blind randomized trial. J Bone Joint Surg Am. 2009 Nov;91:2550–2555. doi: 10.2106/JBJS.H.01501. PubMed PMID: 19884426. [DOI] [PubMed] [Google Scholar]
- 15.Ikeuchi M., Kamimoto Y., Izumi M. Effects of dexamethasone on local infiltration analgesia in total knee arthroplasty: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc. 2014 Jul;22:1638–1643. doi: 10.1007/s00167-013-2367-5. Epub 2013 Jan 11. PubMed PMID: 23306715. [DOI] [PubMed] [Google Scholar]
- 16.Yue D.B., Wang B.L., Liu K.P., Guo W.S. Efficacy of multimodal cocktail periarticular injection with or without steroid in total knee arthroplasty. Chin Med J Engl. 2013 Oct;126:3851–3855. PubMed PMID: 24157144. [PubMed] [Google Scholar]
- 17.Chia S.K., Wernecke G.C., Harris I.A., Bohm M.T., Chen D.B., Macdessi S.J. Peri-articular steroid injection in total knee arthroplasty: a prospective, double blinded, randomized controlled trial. J Arthroplasty. 2013 Apr;28:620–623. doi: 10.1016/j.arth.2012.07.034. Epub 2012 Oct 26. PubMed PMID: 23107810. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002 Jun 15;21:1539–1558. doi: 10.1002/sim.1186. PubMed PMID: 12111919. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P.T., Green S., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009] 2009. The Cochrane Collaboration. [Google Scholar]
- 20.Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J Clin Invest. Jun 15, 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011 May;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. Epub 2011 Feb 4. Review. PubMed PMID: 21296109. [DOI] [PubMed] [Google Scholar]
- 22.Barnes P.J. Glucocorticosteroids: current and future directions. Br J Pharmacol. 2011 May;163:29–43. doi: 10.1111/j.1476-5381.2010.01199.x. Review. PubMed PMID: 21198556; PubMed Central PMCID: PMC3085866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr D.R., Kohan L. Local infiltration analgesia: a technique for the control of acute postoperative pain following knee and hip surgery: a case study of 325 patients. Acta Orthop. 2008 Apr;79:174–183. doi: 10.1080/17453670710014950. PubMed PMID: 18484242. [DOI] [PubMed] [Google Scholar]
- 24.Lombardi A.V., Jr., Berend K.R., Mallory T.H., Dodds K.L., Adams J.B. Soft tissue and intra-articular injection of bupivacaine, epinephrine, and morphine has a beneficial effect after total knee arthroplasty. Clin Orthop Relat Res. 2004 Nov;428:125–130. doi: 10.1097/01.blo.0000147701.24029.cc. PubMed PMID: 15534532. [DOI] [PubMed] [Google Scholar]
- 25.Badner N.H., Bourne R.B., Rorabeck C.H., MacDonald S.J., Doyle J.A. Intra-articular injection of bupivacaine in knee-replacement operations. Results of use for analgesia and for preemptive blockade. J Bone Joint Surg Am. 1996 May;78:734–738. doi: 10.2106/00004623-199605000-00013. PubMed PMID: 8642030. [DOI] [PubMed] [Google Scholar]
- 26.Vendittoli P.A., Makinen P., Drolet P. A multimodal analgesia protocol for total knee arthroplasty. A randomized, controlled study. J Bone Joint Surg Am. 2006;2:282. doi: 10.2106/JBJS.E.00173. [DOI] [PubMed] [Google Scholar]