Abstract
The long-term consequences of exposure to excess stress, particularly during sensitive developmental windows, on the initiation and progression of many complex, common physical and mental disorders that confer a major global burden of disease are well established. The period of intrauterine life represents among the most sensitive of these windows, at which time the effects of stress may be transmitted inter-generationally from a mother to her as-yet-unborn child. As explicated by the concept of fetal, or developmental, programming of health and disease susceptibility, a growing body of evidence supports the notion that health and disease susceptibility is determined by the dynamic interplay between genetic makeup and environment, particularly during intrauterine and early postnatal life. Except in extreme cases, an adverse intrauterine exposure may not, per se, ‘cause’ disease, but, instead, may determine propensity for disease(s) in later life (by shaping phenotypic responsivity to endogenous and exogenous disease-related risk conditions). Accumulating evidence suggests that maternal psychological and social stress during pregnancy represents one such condition that may adversely affect the developing child, with important implications for a diverse range of physical and mental health outcomes.
In this paper we review primarily our own contributions to the field of maternal stress during pregnancy and child mental and physical health-related outcomes. We present findings on stress-related maternal-placental-fetal endocrine and immune/inflammatory processes that may mediate the effects of various adverse conditions during pregnancy on the developing human embryo and fetus. We enunciate conceptual and methodological issues related to the assessment of stress during pregnancy and discuss potential mechanisms of intergenerational transmission of the effects of stress. Lastly, we describe on-going research and some future directions of our program.
Keywords: prenatal stress, fetal programming, pregnancy, development, health, disease risk
1. Introduction
The origins of health and disease susceptibility for many complex, common disorders that confer a major burden of disease in not only developed but also other societies in rapid transition can be traced back to the intrauterine period of life. Development is a plastic process, wherein a range of different phenotypes can be expressed from a given genotype. The unfolding of developmental processes from genotype to phenotype is context-dependent, wherein the developing embryo/fetus responds to, or is acted upon by, conditions in the internal or external environment during sensitive periods of cellular proliferation, differentiation and maturation, resulting in structural and functional changes in cells, tissues and organ systems. These changes may, in turn, either independently or through interactions with subsequent developmental processes and environments, have short- and/or long-term consequences for health and disease susceptibility. These concepts have variously been referred to as the fetal or developmental origins of health and disease (Gluckman and Hanson, 2004; Wadhwa et al., 2009).
Rationale for considering a role for stress in fetal programming
The rationale for considering a role for stress and stress biology in fetal programming of health and disease risk derives, in part, from concepts in evolutionary biology and developmental plasticity (Entringer, 2013; Entringer et al., 2012a; Entringer et al., 2010b). From conception onwards the mother and her developing fetus both play an obligatory, active role in all aspects of development. Based on the consideration that the two fundamental processes that are believed to shape evolutionary selection and developmental plasticity are variation in energy substrate availability (nutrition) and challenges that have the potential to impact the structural or functional integrity and survival of the organism (stress), it is likely and plausible that prenatal stress represents an important aspect of the intrauterine environment that would be expected to influence many, if not all, developmental outcomes (Wadhwa et al., 2011).
To date, the majority of human studies on fetal programming have focused on energy substrate and nutrition (e.g., effects on central and peripheral organ systems of under- or over-nutrition and of specific macro or micro nutrients such as excess fat or protein intake). We suggest that studies of stress and stress biology in gestation and early postnatal life may be relevant even in the context of nutrition and its programming effects. Growing evidence supports the concept of a bidirectional interaction between nutrition and stress, such that the effects of nutrition on health may vary as a function of stress, or that the effects of stress on health may vary as a function of nutritional status. For example, several experimental studies in animals have demonstrated that nutritional manipulations, particularly in the preconception or early pregnancy period, may produce their effects on maternal and fetal outcomes via alterations in stress biology (cortisol, inflammatory cytokines (Bispham et al., 2003; Lingas and Matthews, 2001). Conversely, studies in animals and humans of stress induction (by exposure to laboratory-based stressors or endocrine stress analogues) have demonstrated effects on feeding behavior, food choice (high calorie dense food preference) and the metabolic fate of food in target tissues (Epel et al., 2001; Hitze et al., 2010).
Thus, we submit the application of a prenatal stress and stress biology framework offers an excellent model system for the study of intrauterine development and associated developmental, birth and subsequent health-related phenotypes because it is increasingly apparent that the developing fetus acquires and incorporates information about the nature of its environment in part via the same biological systems that in an already-developed individual mediate adaptation and central and peripheral responses to endogenous and exogenous stress (i.e., the maternal-placental-fetal neuroendocrine and immune systems (Wadhwa, 2005)). In this context, stress-related endocrine and immune/inflammatory mediators may serve as important signals or cues of a wide range of maternal, placental and/or fetal conditions including but not limited to nutrient availability, oxygen availability, presence of obstetric complications such as preeclampsia and infection, and other important conditions that can sculpt fetal development (Fowden and Forhead, 2009).
The following sections summarize findings on the association of prenatal stress and stress biology with neurodevelopmental and physical health outcomes. We note that the majority of the published studies reviewed here on prenatal stress and child neurodevelopmental outcomes were conducted by a research group led by Curt Sandman and Elysia Davis, in which one of us (Buss) was a collaborator, whereas the studies on physical health outcomes were conducted by our own research program.
2. Prenatal stress exposure and brain development
The fetal brain is highly plastic and is not only receptive to but in fact requires signals or cues from its environment in order to develop. Brain development is a product of the dynamic, bi-directional interplay between the individual’s genotype, acquired at conception, and the nature of the early environment. The ontogeny of brain development is considerably longer than that of other organ systems. It extends from the fetal period of life into the neonatal, infancy, childhood and adolescence years. Given that one of the principles of developmental programming is that organs undergoing rapid developmental changes are especially vulnerable to organizing and disorganizing influences of environmental conditions, the brain is a prominent target for such influences. The fetal developmental period is particularly important because this is the developmental stage when growth and differentiation of major brain structures occur. Since brain development is a cascade of bidirectional interactions with its environment, even small or subtle alterations in brain structure or function during fetal life can become progressively and substantially magnified over time to produce long-lasting or permanent deficits (Buss et al., 2012c).
There is substantial empirical evidence from epidemiological studies suggesting that exposure to excess stress in intrauterine life does have the potential to adversely impact short- and long-term neurodevelopmental outcomes. Stress-related maternal-placental-fetal (MPF) biological processes appear to play a tripartite role as key sensors, transducers and effectors of maternal stress on the developing fetus. While endocrine and immune stress mediators play a critical, obligatory role in neuronal and glial cell migration, differentiation, synaptic maturation, and many other important aspects of brain development, inappropriate levels of these biological mediators can produce detrimental effects on the developing brain (Buss et al., 2012d).
Most of what is known about fetal programming of the brain stems from animal studies. The studies summarized here have contributed to a better understanding of prenatal programming of fetal brain development in humans. Initially, using a retrospective study design, we (Buss et al) studied the interactive effects of pre- and postnatal risk factors on adult hippocampal volume and showed that only those individuals who had been small for gestational age at birth and also reported low maternal care during childhood had smaller hippocampal volumes (Buss et al., 2007). This finding thus suggested that the consequences of prenatal risk conditions can be modified by the quality of the postnatal environment, and that ideally studies should be designed in a way that allows testing the independent as well as interactive effects of pre- and postnatal conditions.
In the following section, a summary is provided on findings related to neurodevelopmental outcomes that were assessed in middle childhood, examining associations with length of gestation, maternal psychosocial state during pregnancy (pregnancy anxiety and depression), and biological processes during pregnancy (maternal endogenous cortisol concentrations and synthetic glucocorticoid administration).
Several studies have shown short- and long-term effects on brain development in the context of adverse gestational outcomes such as preterm birth and low birth weight (Salmaso et al., 2014). The potential effects of variation in birth outcomes such as gestational length within the normal range have been less frequently studied. Findings suggest that length of gestation is positively associated with gray matter development (Davis et al., 2011) and brain network efficiency in middle childhood (Kim et al., 2014). Interestingly, the beneficial effects of longer gestation were apparent even among infants born after the conventional cut-off for term delivery (37 weeks). The results suggest that even if neurodevelopmental studies include only term born individuals, they do not represent a homogenous group, and variation in length of gestation should be considered as a contributing/confounding factor to variation in neurodevelopmental outcomes.
Adverse birth outcomes (i.e., low birth weight, preterm birth) are often considered and used as indicators of suboptimal intrauterine conditions (e.g. maternal stress and others). Until recently, no human studies had directly examined the neurodevelopmental consequences in the offspring of maternal stress during pregnancy. The first study examined the association of maternal stress assessed during pregnancy on her child’s brain morphology and cognitive function and found a link between maternal emotional state during pregnancy and changes in offspring brain morphology at 7 years of age. Children born to mothers who experienced high levels of pregnancy anxiety in the second trimester of pregnancy had region-specific reductions in gray matter volume. Specifically, independent of postnatal stress, pregnancy anxiety at 19 weeks gestation was associated with gray matter volume reductions in the prefrontal cortex, the premotor cortex, the medial temporal lobe, the lateral temporal cortex, the postcentral gyrus as well as the cerebellum extending to the middle occipital gyrus and the fusiform gyrus (Buss et al., 2010). Furthermore, pregnancy anxiety was associated with impaired executive function in a larger subset of the same cohort of children (Buss et al., 2011), which is interesting in light of the gray matter reductions in prefrontal cortical regions in association with pregnancy anxiety. The second study examined cortical changes in middle childhood in association with maternal depressive symptoms during pregnancy and found evidence for maternal depressive symptoms during pregnancy to be associated with cortical changes in her child at 7 years of age. Significant reductions in cortical thickness were observed in children primarily in the right frontal lobes in association with exposure to prenatal maternal depression. Furthermore a significant association between prenatal maternal depression and child externalizing behavior was found, which was mediated by reduced cortical thickness in prefrontal areas of the right hemisphere (Sandman et al., 2015).
Cortisol is one of the primary biomarkers of physiological stress because its production, bioavailability and activity is altered by all adverse conditions that have been shown to program the developing brain (Entringer et al., 2011b). Therefore, cortisol has been suggested as one important mediator of the effects of maternal emotional state and stress on the developing brain. It is known that changes in maternal cortisol concentrations (as may occur in the context of stress and depression) affect fetal cortisol concentrations. Direct fetal exposure to maternal cortisol is regulated by the placental enzyme, 11 beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2), which oxidizes cortisol to its inactive form cortisone (Beitins et al., 1973; Brown et al., 1996). Because placental 11beta-HSD2 serves only as a partial barrier, some proportion of active maternal cortisol does pass through the placenta into the fetal compartment (Benediktsson et al., 1997). Interestingly, many adverse intrauterine conditions that have been associated with impaired fetal brain development also have been associated with a down-regulation of placental 11beta-HSD2 activity, including high maternal anxiety (O’Donnell et al., 2012), severe infection (Johnstone et al., 2005), high levels of pro-inflammatory cytokines (Kossintseva et al., 2006) and alcohol exposure (Liang et al., 2011). Another plausible pathway by which maternal cortisol can produce elevations in fetal cortisol is by stimulating and thereby increasing the production of placental corticotrophin-releasing hormone (CRH, Cheng et al., 2000; Rehman et al., 2007; Sandman et al., 2006), which is known to act on the fetal HPA axis and stimulate adrenal steroid biosynthesis.
The third study examined the association between maternal cortisol concentrations during pregnancy and child amygdala and hippocampus volumes as well as affective problems. After adjusting for postnatal maternal depression and other potentially confounding factors, higher maternal cortisol concentration in early but not later gestation was associated with larger right amygdala volumes in girls but not in boys. Maternal cortisol concentrations were not associated with the size of the left amygdala or the left or right hippocampus in either girls or boys. Mediation analyses show that the association between high maternal cortisol concentrations and higher prevalence of child affective problems was partially mediated by the larger amygdala volumes (Buss et al., 2012a). In addition to studying the programming influence of variation in endogenous maternal cortisol concentrations, the next study contributed to a better understanding of the neurodevelopmental consequences of exogenous glucocorticoid administration during pregnancy. It is standard clinical practice to give betamethasone to pregnant women when they present with preterm labor to promote fetal lung maturation. Evaluating the neurodevelopmental consequences of this pharmacological intervention has been challenging though because the majority of empirical evidence available was confounded by the fact that many of the children whose mothers had received betamethasone were born preterm, which is a risk factor for neurodevelopmental alterations itself (Salmaso et al., 2014). Therefore only children born at term (after 37 weeks gestation) exposed to betamethasone in utero were included in the study. Bilateral reductions in cortical thickness were observed. The largest differences between betamethasone exposed and non-exposed children were in the rostral anterior cingulate cortex (rACC) with 30% of the rACC being thinner among children with fetal glucocorticoid exposure. Furthermore, a thinner left rACC was associated with more affective problems (Davis et al., 2013).
It is well-established that a fetus developing in a pro-inflammatory milieu is significantly more susceptible to subsequently developing various neurodevelopmental disorders (Bilbo and Schwarz, 2009). This increased susceptibility is assumed to be a consequence of specific alterations in brain anatomy and connectivity that result from excessive exposure of the fetal brain to pro-inflammatory cytokines (Buss et al., 2012d). The next study showed that chorioamnionitis, inflammation of the fetal membranes due to a bacterial infection, is associated with widespread cortical and subcortical changes in children born prematurely (Hatfield et al., 2011). One particularly potent condition that produces an increased inflammatory milieu during gestation is maternal obesity (Huda et al., 2010), which has been associated with deficits in neurodevelopmental outcomes during childhood and adulthood, including attention-deficit/hyperactivity disorder (ADHD, Van Lieshout et al., 2011). The next study addressed the question which neurocognitive alterations underlie the association between maternal obesity and increased risk for child ADHD. For these analyses, in addition to controlling for key potential confounding variables, children whose mother and/or father had an ADHD diagnosis were excluded from the analyses, in order to limit the likelihood that the results represent genetic transmission of ADHD risk. Results suggested that maternal pre-pregnancy BMI, but not weight gain during pregnancy, was associated with subsequent child ADHD symptoms, as assessed with the Child Behavior Checklist (CBCL, Achenbach and Rescorla, 2001) in the child at about 7 years age. There was a 2.8-fold increase in the prevalence of ADHD among children of obese compared to those of non-obese mothers (Buss et al., 2012b). Maternal pre-pregnancy BMI, but not weight gain during pregnancy, also was associated with child executive function at 7 years age (Buss et al., 2012b). Impaired child executive function mediated the association between maternal pre-pregnancy obesity and child ADHD symptoms. This suggests that maternal obesity is associated with an altered trajectory of brain development, which may be mediated by a higher inflammatory milieu in obese mothers.
Among the studies summarized above there is some evidence for sex of the child to moderate the effects of the prenatal environment on neurodevelopmental outcomes. Specifically, the results summarized here suggest greater susceptibility of the developing brain for programming by maternal stress in females than in males (Buss et al., 2012a; Buss et al., 2012b; Buss et al., 2007). These findings are in agreement with several findings in the animal and human literature that suggest many prenatal insults produce outcome-specific sexually dimorphic developmental consequences (Bale, 2011; Behan et al., 2011; Zohar and Weinstock, 2011). Mechanisms that have been discussed in this context include the presence of sex-specific placental adaptation to stress exposure (Clifton, 2010) and the notion of increased susceptibility of the female brain to its milieu given the more rapid neurodevelopmental trajectory in females compared to males (Buss et al., 2009a; Nathanielsz et al., 2003).
Taken together, the findings support the notion that preconceptional and prenatal conditions may impact child brain development, with specific empirical evidence for outcomes related to cognitive and affective function and the size of limbic and frontal brain structures. These neurodevelopmental alterations may, in turn, have implications for subsequent risk of neuropsychiatric disorders. The findings suggest most significant offspring brain alterations being associated with variation in environmental conditions in either early or mid gestation. This may be related to the ontogeny of different brain regions, assuming that during periods of most rapid developmental change, the susceptibility to organizing and disorganizing environmental influences is largest. But these results are not conclusive because it has to be acknowledged that observational studies are not ideally suited for studying sensitive time windows due to the limitation that conditions and exposures are often not restricted to specific circumscribed time periods but are rather consistent across time.
3. Prenatal stress exposure and physical health-related outcomes
A large number of epidemiological studies across the world have reported associations between markers of an individual’s birth phenotype such as low birth weight or small body size and subsequent risk of disease in adult life, including but not limited to changes in the hypothalamus-pituitary-adrenal (HPA) axis response to psychosocial stress (Wust et al., 2005). These associations are independent of adult size and other established risk factors. It is, however, unlikely that birth phenotype per se plays a causal role in increasing risk of adult disease. Instead, birth phenotype is more likely a crude reflection of developmental processes in intrauterine life that may also influence the structure and function of physiological systems that underlie health and disease risk in later life.
We and others have proposed that prenatal stress exposure represents an adverse environmental condition that may contribute to variation in both birth phenotype and the physiology of the developing organism (Entringer et al., 2010b). Experimental studies in animals suggest maternal exposure to stress during gestation can independently exert long-term effects simultaneously on several physiological systems in the offspring, and that titration of the prenatal stress exposure dose can produce significant long-term effects without altering the birth phenotype (Bailey et al., 2004; Coe et al., 2007).
Our first set of studies on the long-term consequences of prenatal stress exposure on physical health outcomes employed a retrospective case-control design in a sample of healthy young adults born to mothers with healthy pregnancies. One half of the study population of young adults was born to mothers who had experienced a major stressful life event during the index pregnancy (prenatal stress group; PS), whereas the other half was a sociodemographically-matched population with no history of maternal exposure to prenatal stress (comparison group; CG). We selected a study population of younger as opposed to older adults in order to focus on pre-disease markers of physiological dysregulation of metabolic, endocrine and immune systems as early predictors of disease susceptibility. The potential effects of other established obstetric, newborn and childhood risk factors on adult health were controlled using a stringent set of exclusionary criteria. Maternal and child medical records were obtained and screened to exclude presence of any maternal acute or chronic diseases, obstetric complications (e.g., gestational diabetes, hypertension/preeclampsia, infection), unhealthy behaviors (smoking), adverse birth outcomes (preterm birth, low birth weight), newborn complications, and history of any major childhood or current diseases (obesity, diabetes, asthma, and adverse neurodevelopmental or psychiatric conditions). Study assessments were performed to quantify health and physiological markers of disease risk, including (i) body composition and glucose-insulin metabolism (BMI and percent fat mass; basal and post-oral glucose tolerance test levels of glucose, insulin, leptin, adiponectin; fasting lipid profile), (ii) endocrine function (basal and post behavioral/pharmacological stress levels of pituitary-adrenal stress hormones, chronobiological regulation of adrenal function, and assessment of HPA-axis feedback sensitivity), (iii) immune function (immune cell trafficking and lipopolysaccharide (LPS)-stimulated production of pro- and anti-inflammatory and TH1/TH2 cytokines), and (iv) cognitive function (working memory under basal and hydrocortisone conditions). Because subtle physiological differences in disease susceptibility are often not detected in basal state we employed appropriate challenge tests to quantify the function of these systems under stimulated conditions (e.g., oral glucose challenge, ACTH stimulation test, LPS-stimulated immune responses).
Our results indicate that the young adults exposed during intrauterine life to maternal psychosocial stress consistently exhibited significant dysregulation of all these key physiological parameters, thereby placing them at increased risk for developing clinical disorders. Specifically, individuals in the PS group exhibited higher BMI and percent body fat, primary insulin resistance, and a lipid profile consistent with the metabolic syndrome (Entringer et al., 2008b); altered immune function with a TH2 shift in the TH1/TH2 balance (consistent with increased risk of asthma and autoimmune disorders (Entringer et al., 2008a); altered endocrine function, with an increased ACTH and reduced cortisol levels during pharmacological and psychological stimulation paradigms (consistent with the high-risk endocrine profile exhibited by individuals exposed to early life abuse (Heim et al., 2000)); and impaired prefrontal cortex (PFC)-related cognitive performance (impairments in working memory performance after hydrocortisone administration (Entringer et al., 2009)). Stress-related changes in PFC function are believed to play a role in alterations of hypothalamic energy balance homeostasis circuits and obesity risk (Alonso-Alonso and Pascual-Leone, 2007; Mietus-Snyder and Lustig, 2008).
Experimental studies in animals (e.g., administration of glucocorticoids or of a 11beta-hydroxysteroid dehydrogenase (11beta-HSD) inhibitor to the pregnant mother) and in-vitro studies (exposure of pre-adipocytes to glucocorticoids) provide further support for the biological plausibility of programming an obesity-prone and hyperinsulinemic phenotype in the offspring through exposure to biological stress mediators during intrauterine development (summarized in (Entringer, 2013)).
It is noteworthy that our above-described finding on body composition is consistent with recent reports in large, national cohort samples linking pre-pregnancy and prenatal stress exposure related to maternal bereavement to risk of offspring overweight, obesity and risk for type-2 diabetes (Hohwu et al., 2014; Li et al., 2010; Virk et al., 2012), and our finding on immune function is consistent with another recent report linking prenatal maternal anxiety with infant illnesses and antibiotic use (Beijers et al., 2010).
Taken together, our findings suggest that in utero exposure to prenatal psychosocial stress may confer increased long-term risk of a range of negative health-related outcomes in humans; these effects are independent from those of other established obstetric and childhood risk factors; and these long-term effects are not necessarily mediated by unfavorable birth outcomes.
4. Telomere biology and developmental programming of health and disease risk
The observation that adverse intrauterine conditions such as prenatal stress simultaneously influence a diverse set of disease risk-related phenotypes, coupled with the fact that the majority of these phenotypes are implicated in increased risk of common age-related disorders, raises the possibility that prenatal stress may exert effects via some common underlying mechanisms (i.e., that are common across different cells, tissues and phenotypes). In this context, we have proposed that telomere biology represents a candidate mechanism of particular interest. Telomere biology, which refers to the structure and function of two entities – telomeres, and the activity of the enzyme telomerase, plays a central and very fundamental role in maintaining the integrity of the genome and cell. Telomeres are highly evolutionarily-conserved TTAGGG′ non-coding tandem DNA repeats at the ends of linear (eukaryote) chromosomes across all vertebrates that, together with shelterin protein complexes, form a protective cap (Blackburn, 2005). Telomeres lose base pairs with each cell division, due in part to incomplete chromosomal end replication (Blackburn, 2005). As somatic cells divide, telomeres eventually reach a critical short length, resulting in decreased ability to recruit shelterin proteins, and thus leading to cellular senescence or apoptosis.(Stewart and Weinberg, 2006) Loss of telomere function causes chromosomal fusion, activation of DNA damage checkpoint responses, genome instability, and impaired stem cell function. After cells become senescent, they produce inflammatory mediators that also affect neighboring cells, leading to further damage within organs and tissues that accumulates over the life course. Thus, as individuals age, they acquire more senescent cells, accompanied by various age-related pathologies (e.g., arteriosclerosis). This is how a reduction of telomere length (TL) and a steeper telomere attrition rate not only relates to longevity, but also to earlier onset and more rapid progression of common chronic diseases. The enzyme telomerase can add back telomeric repeats after cell devision (Shore and Bianchi, 2009). If the telomere shortening represents the clock ticking forward on the limited lifespan of cells, telomerase can reverse or slow this clock (Chan and Blackburn, 2003), making the two an intricately inter-dependent, dynamic system.
We believe that telomere biology may represent an import common underlying mechanism in the context of fetal programming of health and disease risk, because a) of its established role in health and disease risk across a range of common disorders, including cardiovascular disease, diabetes, obesity, psychiatric diseases like depression, dementia, and schizophrenia (Armanios and Blackburn, 2012; Haycock et al., 2014; Ma et al., 2011; Zhao et al., 2013) and earlier mortality (Cawthon et al., 2003; Kimura et al., 2008), b) recognition of the importance of the initial (newborn) setting of this system (Aviv, 2008; Entringer et al., 2012b), and c) strong biological plausibility for the concept that this initial setting is plastic and modulated by conditions in early life (Entringer et al., 2012b).
Importance of newborn and infant telomere biology for long-term health
Adult telomere length (TL) at any given age is a joint function of a) the initial (newborn) setting of TL, and b) TL attrition over time/age. Although there are no human studies that have prospectively tracked TL from birth until old age with characterization of age-related phenotypes, two lines of evidence support the importance of the initial setting of the system. First, findings from animal studies of telomere dynamics over the life span and across generations suggest that initial TL and TL attrition rate in early life is a substantially better predictor of realized life span than TL and TL attrition rate in later life (Asghar et al., 2015; Bateson et al., 2015; Heidinger et al., 2012), and the effects of early life TL persist over and above those of risk exposures in later life (Asghar et al., 2015; Bateson et al., 2015; Heidinger et al., 2012). Second, human studies across different adult populations that have longitudinally assessed TL attrition have found almost no within-individual rank change (Benetos et al., 2013), and therefore concluded that TL in early life is one of the main determinants of inter-individual differences in TL throughout the human life course, and that the relationship of TL with longevity and age-related disease risk appears to originate in the initial (early life) setting of TL. Moreover, a recent human study found that newborns with reduced TL already exhibit more DNA damage than those with longer TL(Moreno-Palomo et al., 2014).
Determinants of the initial setting of the telomere system
Based, in part, on findings related to the relatively high heritability of telomere length but the relatively small contribution of known genetic variation (from candidate gene as well as GWAS approaches) to explaining variation in telomere length (e.g., Codd et al., 2010; Levy et al., 2010; Prescott et al., 2011), we argue for a potential role of maternal systemic and intrauterine effects in the initial setting of the telomere system. Furthermore, mother-offspring correlation in TL is substantially larger than the father-offspring correlation (regardless of the sex of the offspring, Broer et al., 2013). Several experimental and observational studies in animals and humans (that demonstrate adverse intrauterine conditions such as dietary manipulations and obstetric complications are associated with shorter offspring TL at birth and in adult life (Entringer et al., 2012b; Shalev et al., 2014)) provide biological plausibility for this hypothesis.
The links between psychological stress exposure, accelerated cellular aging, and shorter TL are well established in adults (Shalev et al., 2013), however, there is relatively little empirical literature on these relationships during early (intrauterine) life. We published the first human study that established an effect of maternal prenatal psychosocial stress (assessed retrospectively) on adult offspring’s TL(Entringer et al., 2011c). We found a significant association with leukocyte TL in young adult offspring (Entringer et al., 2011c). The effect equated approximately to an additional 3.5 years of cell aging in prenatally-stressed offspring, was more pronounced in women, and was unchanged after adjusting for potential confounders (subject characteristics, birth weight percentile, and early-life and concurrent stress level). We more recently replicated this finding prospectively with newborn TL(Entringer et al., 2013). Other recent animal studies have found that prenatal administration of the stress hormone cortisol or prenatal exposure to maternal infection produced shorter offspring TL, thus providing the first experimental evidence of a ‘programming’ effect of prenatal stress biology on the telomere system (Asghar et al., 2015; Haussmann et al., 2012).
We recently extended our research program to study intrauterine effects on offspring telomere biology by also including maternal nutrition-related factors in our studies. In this context, we were particularly interested in the effects of maternal folate concentrations during pregnancy on offspring telomere length for the following reasons: 1) Folate is essential for DNA synthesis and maintenance; 2) The maternal compartment is the only source of folate for the developing fetus; 3)In cross-sectional studies in adults effects of folate on telomere length have been reported. We, therefore, tested the hypothesis that variation in maternal folate during pregnancy is associated with newborn TL. We found a protective effect of maternal folate concentrations in early pregnancy on offspring telomere length. The median TL in newborns of mother in the lowest quartile of total folate levels was approximately 10% shorter than that of newborns of mothers in the highest folate quartile (Entringer et al., 2015).
Taken together our findings suggest that fetal TL exhibits developmental plasticity, and provide evidence that maternal stress, maternal-placental-fetal endocrine factors and nutrition during pregnancy may exert a “programming” effect on this system. Given the established role of telomere biology in health and disease risk across a range of common mental and physical disorders, we submit that telomere biology may represent an important and novel mechanism underlying the observed effects of suboptimal intrauterine exposures on subsequent health and disease risk phenotypes of interest.
5. Issues and considerations regarding the assessment of stress and stress biology during pregnancy
A question that frequently comes up with respect to the putative effects of maternal stress on fetal development concerns the elucidation of the aspects or domains of maternal stress that may be particularly salient (Wadhwa et al., 2011). Most human studies of prenatal stress have used measures of major life event stress (e.g. death of a family member), catastrophic community-wide disasters (e.g. earthquakes, acts of terrorism), chronic stress, daily hassles, perceived stress, symptoms of depression or general anxiety, and pregnancy-specific anxiety (Dunkel Schetter, 2011). Although it is difficult to draw firm conclusions, it appears from this and other reviews that stressors such as major negative life events and catastrophes confer greater risk for preterm birth (a commonly studied outcome in the context of maternal stress during pregnancy) than less severe chronic stressful exposures and depressive symptoms (see, e.g., Khashan et al., 2009; Xiong et al., 2008; Zhu et al., 2010). Moreover, measures of stress that focus on pregnancy-related concerns (pregnancy-specific anxiety) seem to be stronger predictors of preterm birth risk (Dole et al., 2003; Kramer et al., 2009; Lobel et al., 2008; Roesch et al., 2004; Wadhwa et al., 1993) and also offspring neurodevelopmental outcomes (Buss et al., 2011) than measures of general stress and anxiety.
We submit that two key limitations to current approaches for the characterization and assessment of maternal stress in pregnancy relate to a) the problem of retrospective recall measures, and b) the lack of consideration of the issue of psychobiological stress reactivity (Wadhwa et al., 2011).
The problem of retrospective recall measures
The overwhelming majority of human studies on the effects of stress during pregnancy (including prospective studies) have relied almost exclusively on self-reported retrospective-recall measures of stress (Wadhwa et al., 2011). Respondents are typically asked to rate how stressed they have felt over the past week/month/since the beginning of their pregnancy. These traditional, self-report recall measures are prone to numerous systematic biases (e.g., recency effect, availability heuristic) that undermine their validity. Furthermore, emotional states and arousal at the time of encoding and recall affect memories. Recent technological and methodological advances now afford the opportunity to address many of these issues and limitations through the application of the ecological monetary (EMA) approach to the study of maternal stress and stress biology in human pregnancy. EMA methods emphasize the longitudinal, repeated collection of information about respondents’ momentary or current state, affect, experience, behavior, and physiology in real time and natural settings. Our findings suggest that EMA derived measures of maternal psychosocial stress are better predictors of cortisol in pregnancy than traditional recall measures, and that EMA-derived measures of cortisol during pregnancy are better predictors of birth outcomes (length of gestation) than cortisol assessed in the clinical setting (Buss et al., 2009b; Entringer et al., 2011a).
Neglect of the concept of psychobiological stress reactivity
It is well-established that for any given individual, the likelihood of occurrence of a stress-related adverse health outcome is a joint function of not only the amount of stress exposure over time, but also of that individual’s biological propensity to respond to stress. The concept of psychobiological stress reactivity refers to characteristics of the biological response (e.g., magnitude, duration) to a unit of stress exposure. Individuals who display exaggerated physiological responses to acute or emotional or stressful states are at greater risk of developing stress-related disorders (al’Absi and Bongard, 2006). However, only a very small number of studies have used this approach in studies of stress and stress processes in human pregnancy (e.g., Entringer et al., 2010a; McCubbin et al., 1996) and see (de Weerth and Buitelaar, 2005) for a review. We conducted a longitudinal study of psychological and biological responses to a standardized laboratory stressor at 2 time points in a population of pregnant and non-pregnant women. Our findings suggest there is a progressive attenuation of not only maternal biological but also psychological responses to stress over the course of gestation. Furthermore, as pregnancy advances, the cortisol response to awakening progressively decreases (Entringer et al., 2010a), and a larger cortisol awakening response in late pregnancy and reduced attenuation of this response from early to late gestation is significantly associated with shorter gestational length after accounting for the effects of other established risk factors (Buss et al., 2009b). Thus, we submit that the use of standardized behavioral or psychological probes, using appropriate and ecologically-valid stimuli, to assess individual differences in the responsivity of stress-related maternal or fetal biological systems at various time points over the course of gestation may prove useful in identifying individuals who may be particularly susceptible to stress.
6. Conclusion, on-going studies and outlook
Taken together, and based on the empirical findings presented here, we suggest it is important to consider the potential role of intrauterine stress and stress biology in arriving at a better understanding of the developmental programming of susceptibility for a range of different mental and physical health and disease-related outcomes.
In our currently ongoing prospective, longitudinal studies, we follow-up infants born to mothers who have been extensively characterized during pregnancy with EMA-derived measures of maternal psychosocial stress, accompanied by biological stress measures (continuous monitoring of maternal heart rate and frequent collection of saliva samples for assessment of cortisol concentrations). This will allow us to compute measures of an individual’s propensity to biologically respond to varying psychosocial stress levels, which we will use jointly with measures of maternal stress as predictors of infant and child outcomes. Furthermore, repeated measures of the maternal-placental-fetal endocrine and immune/inflammatory milieu are being collected.
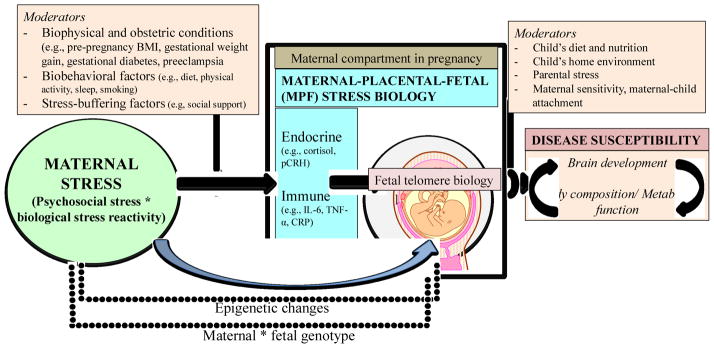
Simultaneously, we are also collecting detailed measures of maternal nutrition (based on 24-h nutritional recall interviews conducted in each pregnancy trimester and on nutrition-related metabolomics) because as discussed above, there is the potential for bi-directional interactive effects between maternal nutrition and stress during pregnancy on fetal development. Because the prenatal and postnatal environments are known to exert both independent as well as interactive effects on mental and physical health related outcomes, the first infant assessment is conducted during the newborn period, in order to examine the influence of the intrauterine environment without the confounding effects of the postnatal environment. In order to study the influence of intrauterine factors on outcomes related to brain development, we acquire multimodal MRI scans for characterization of brain anatomy as well as structural and functional brain connectivity during the newborn period. The infants are then serially tested with the same MRI protocol at 12 and 24 months age. MRI assessments are accompanied by age-appropriate developmental tests, which provides the opportunity of assessing functional correlates of brain anatomy and connectivity changes. To study risk for overweight and obesity and metabolic dysfunction in the same infants, we are using state-of-the art methodology to assess body composition and fat deposition in newborns and during infancy (with dual energy X-ray absorptiometry; DXA to assess percent body fat and magnetic resonance imaging (MRI) to quantify visceral fat depots, especially intra-hepatic fat). We have recently developed an MRI-based protocol to quantify brown adipose tissue depots in human neonates and infants (Rasmussen et al., 2013). Brown adipose tissue seems to play a protective role against obesity/adiposity risk and metabolic dysfunction. In order to examine whether energy balance homeostasis set points mediate the effects of intrauterine stress on newborn and infant body composition, we are assessing energy expenditure with the doubly labeled water method (DLW). We expect this trans-disciplinary, comprehensive approach to provide further insight about whether prenatal stress exposure induces changes in newborn and infant brain circuits related to energy balance and appetite regulation. During the infant follow-up period, the quality of the postnatal environment is being assessed with serial measures of child diet and nutrition, parental stress, maternal sensitivity, maternal-child attachment, the child’s home environment, which will enable tests of interactive and cumulative pre- and postnatal influences on these different outcomes. Our conceptual framework to study the effects of maternal stress during pregnancy on disease susceptibility is presented in Figure 1.
Figure 1.
Conceptual framework to study the effects of maternal stress during pregnancy on disease susceptibility.
We submit it may be important to adopt a life-course perspective in considering the issue of the contribution of maternal stress to programming of health and disease risk of the offspring (Fox et al., 2015). It is, for example, well established that exposure to stressful or traumatic conditions in early life may produce adverse psychological and somatic health consequences, which may endure over the entire lifespan (Heim and Binder, 2012). Emerging evidence now suggests that among women exposed to childhood trauma, its consequences may also be transmitted to their children and confer increased risk for adverse neurodevelopmental (Rijlaarsdam et al., 2014) and other physical health outcomes (Roberts et al., 2014). In one of our ongoing studies we seek to extend the prevailing paradigm that posits such intergenerational transmission likely occurs after her child’s birth and suggest that intrauterine life represents a particularly sensitive time period when the effects of maternal childhood trauma may be transmitted to her offspring; and that transmission occurs via the psychological and behavioral consequences of maternal childhood trauma on aspects of maternal-placental-fetal gestational biology that participate in the process of fetal programming of health and disease risk.
Our future directions include a multi-level approach that extends current questions and incorporates additional approaches and/or methods. These plans include a) assessments of targeted epigenetic mechanisms in genes related on the one hand to stress and on the other to obesity, the effects of telomere as well as mitochondrial biology on child neurodevelopmental outcomes in order to obtain a more comprehensive characterization of the mechanisms underlying stress-related developmental programming; b) studies of the role of biological stress mediators on the differentiation of human mesenchymal and neural stem cell lineages (in tissue culture systems), in order to simulate the effects of this process of interest in an ex vivo model system that has direct relevance to embryonic and fetal development; c) studies of the effects of prenatal stress and other intrauterine processes on the maternal and child microbiome, in order to examine whether prenatal stress-induced changes in the microbiome mediate the association between intrauterine stress exposure and outcomes related to child adiposity and brain development; and d) studies of the effects of prenatal stress on age-related changes in DNA methylation (“epigenetic clock”) and mitochondrial function, to examine whether prenatal stress effects on cellular aging are also reflected in cellular energetics. We suggest these studies will set the stage for translational research with implications for early identification of risk/vulnerable populations, and will thereby inform the subsequent development of primary and secondary intervention strategies.
Highlights.
Prenatal stress exposure can have long-lasting impacts on health and disease susceptibility.
We review here primarily our own contributions to the field of maternal stress during pregnancy and child health-related outcomes.
We enunciate methodological issues related to the assessment of stress during pregnancy.
Potential mechanisms of intergenerational transmission of the effects of stress are discussed.
Acknowledgments
Role of the funding source
Preparation of this manuscript was supported, in part, by US PHS (NIH) grants RO1 HD-065825, RO1 HD-06028, R01 MH-091351 and R21 RDK-098765. The funding source had no involvement in any activities related to study design, collection, analysis and interpretation of data presented in this manuscript, writing of the reportor in the decision to submit the article for publication.
Footnotes
Contributors
E and CB wrote the paper. SE, CB and PDW conceptualized the theoretical framework. SE, CB and PDW had primary responsibility for final content. All authors read and approved the final manuscript.
Potential Conflicts of Interest
The authors have no conflict of interest relevant to this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms and profiles. Burlington: University of Vermont, Research Centre for Children, Youth and Families; 2001. [Google Scholar]
- al’Absi M, Bongard S. Neuroendocrine and behavioral mechanisms mediating the relationship between anger expression and cardiovascular risk: assessment considerations and improvements. J Behav Med. 2006;29:573–591. doi: 10.1007/s10865-006-9077-0. [DOI] [PubMed] [Google Scholar]
- Alonso-Alonso M, Pascual-Leone A. The right brain hypothesis for obesity. JAMA. 2007;297:1819–1822. doi: 10.1001/jama.297.16.1819. [DOI] [PubMed] [Google Scholar]
- Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar M, Hasselquist D, Hansson B, Zehtindjiev P, Westerdahl H, Bensch S. Chronic infection. Hidden costs of infection: chronic malaria accelerates telomere degradation and senescence in wild birds. Science. 2015;347:436–438. doi: 10.1126/science.1261121. [DOI] [PubMed] [Google Scholar]
- Aviv A. The epidemiology of human telomeres: faults and promises. J Gerontol A Biol Sci Med Sci. 2008;63:979–983. doi: 10.1093/gerona/63.9.979. [DOI] [PubMed] [Google Scholar]
- Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr. 2004;38:414–421. doi: 10.1097/00005176-200404000-00009. [DOI] [PubMed] [Google Scholar]
- Bale TL. Sex differences in prenatal epigenetic programing of stress pathways. Stress. 2011;14:348–356. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- Bateson M, Brilot BO, Gillespie R, Monaghan P, Nettle D. Developmental telomere attrition predicts impulsive decision-making in adult starlings. Proc Biol Sci. 2015;282:20142140. doi: 10.1098/rspb.2014.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behan AT, van den Hove DL, Mueller L, Jetten MJ, Steinbusch HW, Cotter DR, Prickaerts J. Evidence of female-specific glial deficits in the hippocampus in a mouse model of prenatal stress. Eur Neuropsychopharmacol. 2011;21:71–79. doi: 10.1016/j.euroneuro.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Beijers R, Jansen J, Riksen-Walraven M, de Weerth C. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics. 2010;126:e401–409. doi: 10.1542/peds.2009-3226. [DOI] [PubMed] [Google Scholar]
- Beitins IZ, Bayard F, Ances IG, Kowarski A, Migeon CJ. The metabolic clearance rate, blood production, interconversion and transplacental passage of cortisol and cortisone in pregnancy near term. Pediatr Res. 1973;7:509–519. doi: 10.1203/00006450-197305000-00004. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Calder AA, Edwards CR, Seckl JR. Placental 11 beta-hydroxysteroid dehydrogenase: a key regulator of fetal glucocorticoid exposure. Clin Endocrinol (Oxf) 1997;46:161–166. doi: 10.1046/j.1365-2265.1997.1230939.x. [DOI] [PubMed] [Google Scholar]
- Benetos A, Kark JD, Susser E, Kimura M, Sinnreich R, Chen W, Steenstrup T, Christensen K, Herbig U, von Bornemann Hjelmborg J, Srinivasan SR, Berenson GS, Labat C, Aviv A. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell. 2013;12:615–621. doi: 10.1111/acel.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front Behav Neurosci. 2009;3:14. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, Broughton Pipkin F, Stephenson T, Symonds ME. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology. 2003;144:3575–3585. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett. 2005;579:859–862. doi: 10.1016/j.febslet.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, Albrecht E, Amin N, Beekman M, de Geus EJ, Henders A, Nelson CP, Steves CJ, Wright MJ, de Craen AJ, Isaacs A, Matthews M, Moayyeri A, Montgomery GW, Oostra BA, Vink JM, Spector TD, Slagboom PE, Martin NG, Samani NJ, van Duijn CM, Boomsma DI. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21:1163–1168. doi: 10.1038/ejhg.2012.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW, Diaz R, Robson AC, Kotelevtsev YV, Mullins JJ, Kaufman MH, Seckl JR. The ontogeny of 11 beta-hydroxysteroid dehydrogenase type 2 and mineralocorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–797. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- Buss C, Davis EP, Class QA, Gierczak M, Pattillo C, Glynn LM, Sandman CA. Maturation of the human fetal startle response: Evidence for sex-specific maturation of the human fetus. Early Hum Dev. 2009a;85:633–638. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Hobel CJ, Sandman CA. Maternal pregnancy-specific anxiety is associated with child executive function at 6–9 years age. Stress. 2011;14:665–676. doi: 10.3109/10253890.2011.623250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Muftuler LT, Head K, Sandman CA. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology. 2010;35:141–153. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Davis EP, Shahbaba B, Pruessner JC, Head K, Sandman CA. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc Natl Acad Sci U S A. 2012a;109:E1312–1319. doi: 10.1073/pnas.1201295109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Davis EP, Hobel CJ, Swanson JM, Wadhwa PD, Sandman CA. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS ONE. 2012b;7:e37758. doi: 10.1371/journal.pone.0037758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Reyes JF, Chicz-Demet A, Sandman CA, Waffarn F, Wadhwa PD. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol. 2009b;201:398e391–398. doi: 10.1016/j.ajog.2009.06.063. [DOI] [PubMed] [Google Scholar]
- Buss C, Entringer S, Swanson JM, Wadhwa PD. The Role of Stress in Brain Development: The Gestational Environment’s Long-Term Effects on the Brain. Cerebrum. 2012c:4. [PMC free article] [PubMed] [Google Scholar]
- Buss C, Entringer S, Wadhwa PD. Fetal Programming of Brain Development: Role of Intrauterine Stress in Susceptibility to Psychopathology. Science Signaling. 2012d;5:pt7. doi: 10.1126/scisignal.2003406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss C, Lord C, Wadiwalla M, Hellhammer DH, Lupien SJ, Meaney MJ, Pruessner JC. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM, Smith KR, O’Brien E, Sivatchenko A, Kerber RA. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361:393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- Chan SW, Blackburn EH. Telomerase and ATM/Tel1p protect telomeres from nonhomologous end joining. Mol Cell. 2003;11:1379–1387. doi: 10.1016/s1097-2765(03)00174-6. [DOI] [PubMed] [Google Scholar]
- Cheng YH, Nicholson RC, King B, Chan EC, Fitter JT, Smith R. Glucocorticoid stimulation of corticotropin-releasing hormone gene expression requires a cyclic adenosine 3′,5′-monophosphate regulatory element in human primary placental cytotrophoblast cells. J Clin Endocrinol Metab. 2000;85:1937–1945. doi: 10.1210/jcem.85.5.6552. [DOI] [PubMed] [Google Scholar]
- Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl):S33–39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Codd V, Mangino M, van der Harst P, Braund PS, Kaiser M, Beveridge AJ, Rafelt S, Moore J, Nelson C, Soranzo N, Zhai G, Valdes AM, Blackburn H, Mateo Leach I, de Boer RA, Kimura M, Aviv A, Goodall AH, Ouwehand W, van Veldhuisen DJ, van Gilst WH, Navis G, Burton PR, Tobin MD, Hall AS, Thompson JR, Spector T, Samani NJ. Common variants near TERC are associated with mean telomere length. Nat Genet. 2010;42:197–199. doi: 10.1038/ng.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr Res. 2007;61:520–524. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- Davis EP, Buss C, Muftuler LT, Head K, Hasso A, Wing DA. Children’s brain development benefits from longer gestation. Front Psychol. 2011;2:1–7. doi: 10.3389/fpsyg.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, Buss C, Wing DA, Head K. Fetal Glucocorticoid Exposure is Associated with Preadolescent Brain Development. Biol Psychiatry. 2013;74:647–655. doi: 10.1016/j.biopsych.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy--a review. Neurosci Biobehav Rev. 2005;29:295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Dole N, Savitz DA, Hertz-Picciotto I, Siega-Riz AM, McMahon MJ, Buekens P. Maternal stress and preterm birth. Am J Epidemiol. 2003;157:14–24. doi: 10.1093/aje/kwf176. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C. Psychological science on pregnancy: stress processes, biopsychosocial models, and emerging research issues. Annu Rev Psychol. 2011;62:531–558. doi: 10.1146/annurev.psych.031809.130727. [DOI] [PubMed] [Google Scholar]
- Entringer S. Impact of stress and stress physiology during pregnancy on child metabolic function and obesity risk. Curr Opin Clin Nutr Metab Care. 2013;16:320–327. doi: 10.1097/MCO.0b013e32835e8d80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Andersen J, Chicz-DeMet A, Wadhwa PD. Ecological momentary assessment of maternal cortisol profiles over a multiple-day period predicts the length of human gestation. Psychosom Med. 2011a;73:469–474. doi: 10.1097/PSY.0b013e31821fbf9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Kumsta R, Hellhammer DH, Wadhwa PD, Wust S. Prenatal psychosocial stress exposure is associated with subsequent working memory performance in young women. Behav Neurosci. 2009;123:886–893. doi: 10.1037/a0016265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Shirtcliff EA, Cammack AL, Yim IS, Chicz-DeMet A, Sandman CA, Wadhwa PD. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010a;13:258–268. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Swanson JM, Cooper DM, Wing DA, Waffarn F, Wadhwa PD. Fetal programming of body composition, obesity, and metabolic function: the role of intrauterine stress and stress biology. J Nutr Metab. 2012a;2012:632548. doi: 10.1155/2012/632548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr Opin Endocrinol Diabetes Obes. 2010b;17:507–516. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress and developmental programming of human health and disease risk: concepts and integration of empirical findings. Curr Opin Endocrinol Diabetes Obes. 2011b;17:507–516. doi: 10.1097/MED.0b013e3283405921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. Prenatal stress, telomere biology, and fetal programming of health and disease risk. Sciene Signaling. 2012b;5:pt12. doi: 10.1126/scisignal.2003580. [DOI] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, Wust S, Wadhwa PD. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proc Natl Acad Sci U S A. 2011c;108:E513–518. doi: 10.1073/pnas.1107759108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Blackburn EH, Buss C, Shahbaba B, Gillen DL, Venkataramanan R, Simhan HN, Wadhwa PD. Maternal Folate Concentration in Early Pregnancy and Newborn Telomere Length. Ann Nutr Metab. 2015;66:202–208. doi: 10.1159/000381925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH, Simhan HN, Wadhwa PD. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol. 2013;208:134e131–137. doi: 10.1016/j.ajog.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Kumsta R, Nelson EL, Hellhammer DH, Wadhwa PD, Wust S. Influence of prenatal psychosocial stress on cytokine production in adult women. Dev Psychobiol. 2008a;50:579–587. doi: 10.1002/dev.20316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Wust S, Kumsta R, Layes IM, Nelson EL, Hellhammer DH, Wadhwa PD. Prenatal psychosocial stress exposure is associated with insulin resistance in young adults. Am J Obstet Gynecol. 2008b;199:498e491–497. doi: 10.1016/j.ajog.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Forhead AJ. Endocrine regulation of feto-placental growth. Horm Res. 2009;72:257–265. doi: 10.1159/000245927. [DOI] [PubMed] [Google Scholar]
- Fox M, Entringer S, Buss C, DeHaene J, Wadhwa PD. Intergenerational transmission of the effects of acculturation on health in Hispanic americans: a fetal programming perspective. Am J Public Health. 2015;105(Suppl 3):S409–423. doi: 10.2105/AJPH.2015.302571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Hatfield T, Wing DA, Buss C, Head K, Muftuler LT, Davis EP. Magnetic resonance imaging demonstrates long-term changes in brain structure in children born preterm and exposed to chorioamnionitis. Am J Obstet Gynecol. 2011;205:384e381–388. doi: 10.1016/j.ajog.2011.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc Biol Sci. 2012;279:1447–56. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycock PC, Heydon EE, Kaptoge S, Butterworth AS, Thompson A, Willeit P. Leucocyte telomere length and risk of cardiovascular disease: systematic review and meta-analysis. BMJ. 2014;349:g4227. doi: 10.1136/bmj.g4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A. 2012;109:1743–1748. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol. 2012;233:102–111. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Hitze B, Hubold C, van Dyken R, Schlichting K, Lehnert H, Entringer S, Peters A. How the selfish brain organizes its supply and demand. Front Neuroenergetics. 2010;2:7. doi: 10.3389/fnene.2010.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohwu L, Li J, Olsen J, Sorensen TI, Obel C. Severe maternal stress exposure due to bereavement before, during and after pregnancy and risk of overweight and obesity in young adult men: a Danish National Cohort Study. PLoS One. 2014;9:e97490. doi: 10.1371/journal.pone.0097490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huda SS, Brodie LE, Sattar N. Obesity in pregnancy: prevalence and metabolic consequences. Semin Fetal Neonatal Med. 2010;15:70–76. doi: 10.1016/j.siny.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Johnstone JF, Bocking AD, Unlugedik E, Challis JR. The effects of chorioamnionitis and betamethasone on 11beta hydroxysteroid dehydrogenase types 1 and 2 and the glucocorticoid receptor in preterm human placenta. J Soc Gynecol Investig. 2005;12:238–245. doi: 10.1016/j.jsgi.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Khashan AS, McNamee R, Abel KM, Mortensen PB, Kenny LC, Pedersen MG, Webb RT, Baker PN. Rates of preterm birth following antenatal maternal exposure to severe life events: a population-based cohort study. Hum Reprod. 2009;24:429–437. doi: 10.1093/humrep/den418. [DOI] [PubMed] [Google Scholar]
- Kim DJ, Davis EP, Sandman CA, Sporns O, O’Donnell BF, Buss C, Hetrick WP. Longer gestation is associated with more efficient brain networks in preadolescent children. Neuroimage. 2014;100:619–627. doi: 10.1016/j.neuroimage.2014.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M, Hjelmborg JV, Gardner JP, Bathum L, Brimacombe M, Lu X, Christiansen L, Vaupel JW, Aviv A, Christensen K. Telomere length and mortality: a study of leukocytes in elderly Danish twins. Am J Epidemiol. 2008;167:799–806. doi: 10.1093/aje/kwm380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossintseva I, Wong S, Johnstone E, Guilbert L, Olson DM, Mitchell BF. Proinflammatory cytokines inhibit human placental 11beta-hydroxysteroid dehydrogenase type 2 activity through Ca2+ and cAMP pathways. Am J Physiol Endocrinol Metab. 2006;290:E282–288. doi: 10.1152/ajpendo.00328.2005. [DOI] [PubMed] [Google Scholar]
- Kramer MS, Lydon J, Seguin L, Goulet L, Kahn SR, McNamara H, Genest J, Dassa C, Chen MF, Sharma S, Meaney MJ, Thomson S, Van Uum S, Koren G, Dahhou M, Lamoureux J, Platt RW. Stress pathways to spontaneous preterm birth: the role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;169:1319–1326. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- Levy D, Neuhausen SL, Hunt SC, Kimura M, Hwang SJ, Chen W, Bis JC, Fitzpatrick AL, Smith E, Johnson AD, Gardner JP, Srinivasan SR, Schork N, Rotter JI, Herbig U, Psaty BM, Sastrasinh M, Murray SS, Vasan RS, Province MA, Glazer NL, Lu X, Cao X, Kronmal R, Mangino M, Soranzo N, Spector TD, Berenson GS, Aviv A. Genome-wide association identifies OBFC1 as a locus involved in human leukocyte telomere biology. Proc Natl Acad Sci U S A. 2010;107:9293–9298. doi: 10.1073/pnas.0911494107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Olsen J, Vestergaard M, Obel C, Baker JL, Sorensen TI. Prenatal stress exposure related to maternal bereavement and risk of childhood overweight. PLoS One. 2010;5:e11896. doi: 10.1371/journal.pone.0011896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Chen M, Pan XL, Zheng J, Wang H. Ethanol-induced inhibition of fetal hypothalamic-pituitary-adrenal axis due to prenatal overexposure to maternal glucocorticoid in mice. Exp Toxicol Pathol. 2011;63:607–611. doi: 10.1016/j.etp.2010.04.015. [DOI] [PubMed] [Google Scholar]
- Lingas RI, Matthews SG. A short period of maternal nutrient restriction in late gestation modifies pituitary-adrenal function in adult guinea pig offspring. Neuroendocrinology. 2001;73:302–311. doi: 10.1159/000054647. [DOI] [PubMed] [Google Scholar]
- Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol. 2008;27:604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- Ma H, Zhou Z, Wei S, Liu Z, Pooley KA, Dunning AM, Svenson U, Roos G, Hosgood HD, 3rd, Shen M, Wei Q. Shortened telomere length is associated with increased risk of cancer: a meta-analysis. PLoS One. 2011;6:e20466. doi: 10.1371/journal.pone.0020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCubbin JA, Lawson EJ, Cox S, Sherman JJ, Norton JA, Read JA. Prenatal maternal blood pressure response to stress predicts birth weight and gestational age: a preliminary study. Am J Obstet Gynecol. 1996;175:706–712. doi: 10.1053/ob.1996.v175.a74286. [DOI] [PubMed] [Google Scholar]
- Mietus-Snyder ML, Lustig RH. Childhood obesity: adrift in the “limbic triangle”. Annu Rev Med. 2008;59:147–162. doi: 10.1146/annurev.med.59.103106.105628. [DOI] [PubMed] [Google Scholar]
- Moreno-Palomo J, Creus A, Marcos R, Hernandez A. Genomic instability in newborn with short telomeres. PLoS One. 2014;9:e91753. doi: 10.1371/journal.pone.0091753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanielsz PW, Berghorn KA, Derks JB, Giussani DA, Docherty C, Unno N, Davenport A, Kutzlers M, Koenen S, Visser GH, Nijland MJ. Life before birth: effects of cortisol on future cardiovascular and metabolic function. Acta Paediatr. 2003;92:766–772. [PubMed] [Google Scholar]
- O’Donnell KJ, Bugge Jensen A, Freeman L, Khalife N, O’Connor TG, Glover V. Maternal prenatal anxiety and downregulation of placental 11beta-HSD2. Psychoneuroendocrinology. 2012;376:818–826. doi: 10.1016/j.psyneuen.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Prescott J, Kraft P, Chasman DI, Savage SA, Mirabello L, Berndt SI, Weissfeld JL, Han J, Hayes RB, Chanock SJ, Hunter DJ, De Vivo I. Genome-wide association study of relative telomere length. PLoS One. 2011;6:e19635. doi: 10.1371/journal.pone.0019635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen JM, Entringer S, Nguyen A, van Erp TG, Guijarro A, Oveisi F, Swanson JM, Piomelli D, Wadhwa PD, Buss C, Potkin SG. Brown adipose tissue quantification in human neonates using water-fat separated MRI. PLoS One. 2013;8:e77907. doi: 10.1371/journal.pone.0077907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman KS, Sirianni R, Parker CR, Jr, Rainey WE, Carr BR. The regulation of adrenocorticotrophic hormone receptor by corticotropin-releasing hormone in human fetal adrenal definitive/transitional zone cells. Reprod Sci. 2007;14:578–587. doi: 10.1177/1933719107307908. [DOI] [PubMed] [Google Scholar]
- Rijlaarsdam J, Stevens GW, Jansen PW, Ringoot AP, Jaddoe VW, Hofman A, Ayer L, Verhulst FC, Hudziak JJ, Tiemeier H. Maternal Childhood Maltreatment and Offspring Emotional and Behavioral Problems: Maternal and Paternal Mechanisms of Risk Transmission. Child Maltreat. 2014;19:67–78. doi: 10.1177/1077559514527639. [DOI] [PubMed] [Google Scholar]
- Roberts AL, Galea S, Austin SB, Corliss HL, Williams MA, Koenen KC. Women’s experience of abuse in childhood and their children’s smoking and overweight. Am J Prev Med. 2014;46:249–258. doi: 10.1016/j.amepre.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesch SC, Dunkel-Schetter C, Woo G, Hobel C. Modeling the types and timing of stress in pregnancy. Anxiety, Stress & Coping. 2004;17:87–102. [Google Scholar]
- Salmaso N, Jablonska B, Scafidi J, Vaccarino FM, Gallo V. Neurobiology of premature brain injury. Nat Neurosci. 2014;17:341–346. doi: 10.1038/nn.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Buss C, Head K, Davis EP. Fetal Exposure to Maternal Depressive Symptoms Is Associated With Cortical Thickness in Late Childhood. Biol Psychiatry. 2015;77:324–334. doi: 10.1016/j.biopsych.2014.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, Hobel C. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Shalev I, Caspi A, Ambler A, Belsky DW, Chapple S, Cohen HJ, Israel S, Poulton R, Ramrakha S, Rivera CD, Sugden K, Williams B, Wolke D, Moffitt TE. Perinatal complications and aging indicators by midlife. Pediatrics. 2014;134:e1315–1323. doi: 10.1542/peds.2014-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, Epel ES. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013;38:1835–42. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore D, Bianchi A. Telomere length regulation: coupling DNA end processing to feedback regulation of telomerase. EMBO J. 2009;28:2309–2322. doi: 10.1038/emboj.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart SA, Weinberg RA. Telomeres: cancer to human aging. Annu Rev Cell Dev Biol. 2006;22:531–557. doi: 10.1146/annurev.cellbio.22.010305.104518. [DOI] [PubMed] [Google Scholar]
- Van Lieshout RJ, Taylor VH, Boyle MH. Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obes Rev. 2011;12:e548–559. doi: 10.1111/j.1467-789X.2010.00850.x. [DOI] [PubMed] [Google Scholar]
- Virk J, Li J, Vestergaard M, Obel C, Kristensen JK, Olsen J. Prenatal exposure to bereavement and type-2 diabetes: a Danish longitudinal population based study. PLoS One. 2012;7:e43508. doi: 10.1371/journal.pone.0043508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Entringer S, Buss C. The contribution of maternal stress to preterm birth: Issues and considerations. Clinics in Perinatology. 2011;38:351–384. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol. 1993;169:858–865. doi: 10.1016/0002-9378(93)90016-c. [DOI] [PubMed] [Google Scholar]
- Wust S, Entringer S, Federenko IS, Schlotz W, Hellhammer DH. Birth weight is associated with salivary cortisol responses to psychosocial stress in adult life. Psychoneuroendocrinology. 2005;30:591–598. doi: 10.1016/j.psyneuen.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Xiong X, Harville EW, Mattison DR, Elkind-Hirsch K, Pridjian G, Buekens P. Exposure to Hurricane Katrina, post-traumatic stress disorder and birth outcomes. Am J Med Sci. 2008;336:111–115. doi: 10.1097/MAJ.0b013e318180f21c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Miao K, Wang H, Ding H, Wang DW. Association between telomere length and type 2 diabetes mellitus: a meta-analysis. PLoS One. 2013;8:e79993. doi: 10.1371/journal.pone.0079993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Tao F, Hao J, Sun Y, Jiang X. Prenatal life events stress: implications for preterm birth and infant birthweight. Am J Obstet Gynecol. 2010;203:34e31–38. doi: 10.1016/j.ajog.2010.02.023. [DOI] [PubMed] [Google Scholar]
- Zohar I, Weinstock M. Differential effect of prenatal stress on the expression of cortiocotropin releasing hormone and its receptors in the hypothalamus and amygdala in male and female rats. J Neuroendocrinol. 2011;23:320–328. doi: 10.1111/j.1365-2826.2011.02117.x. [DOI] [PubMed] [Google Scholar]