Abstract
The kinetics of photolysis of riboflavin (RF) in water (pH 7.0) and in organic solvents (acetonitrile, methanol, ethanol, 1-propanol, 1-butanol, ethyl acetate) has been studied using a multicomponent spectrometric method for the assay of RF and its major photoproducts, formylmethylflavin and lumichrome. The apparent first-order rate constants (kobs) for the reaction range from 3.19 (ethyl acetate) to 4.61 × 10−3 min−1 (water). The values of kobs have been found to be a linear function of solvent dielectric constant implying the participation of a dipolar intermediate along the reaction pathway. The degradation of this intermediate is promoted by the polarity of the medium. This indicates a greater stabilization of the excited-triplet states of RF with an increase in solvent polarity to facilitate its reduction. The rate constants for the reaction show a linear relation with the solvent acceptor number indicating the degree of solute–solvent interaction in different solvents. It would depend on the electron-donating capacity of RF molecule in organic solvents. The values of kobs are inversely proportional to the viscosity of the medium as a result of diffusion-controlled processes.
KEY WORDS: dielectric constant, kinetics, photolysis, riboflavin, solvent effect, viscosity
INTRODUCTION
The influence of solvents on the rates of degradation of drugs is an important consideration for the formulation chemist. The effects of dielectric constant and viscosity of the medium may be significant on the stability of pharmaceutical formulations. Theoretical basis of the effects of solvent on the rates and mechanism of chemical reactions has been extensively dealt by many workers (14,18,21,28,37,47,56,65). The effect of dielectric constant on the degradation kinetics and stabilization of chloramphenicol (40), barbiturates (31), methanamine (59), ampicillin (29), prostaglandin E2 (48), chlorambucil (43), 2-tetrahydropyranyl benzoate (30), indomethacin (24), aspirin (16), phenoxybenzamine (2), azathioprine (55), polypeptides (17), neostigmine (64), triprolidine (39), 10-methylisoalloxazine (12), formylmethylflavin (7), levofloxacin (6), and moxifloxacin (4) has been reported. The viscosity of the medium may also affect the stability of a drug. A linear relation has also been found between the rate constant and the inverse of solvent viscosity for the photodegradation of 10-methylisoalloxazine (12), formylmethylflavin (9), levofloxacin (6), and moxifloxacin (4) in organic solvents.
Some kinetic studies of the photolysis of riboflavin (RF) in carboxylic acids (34,58), alcoholic solvents (32,42,50,57), and pyridine (36) have been conducted. However, the method used for the determination of RF is based on the measurement of absorbance at 445 nm without any consideration of the interference caused by photoproducts formed during degradation. Thus, the kinetic data obtained may not be accurate, and specific methods may be required for assay (10,13). Studies on the photolysis of formylmethylflavin (FMF), a major intermediate in the photolysis sequence of the RF, in organic solvents have been conducted (7,9). Solvent effects on flavin electron transfer reactions have been found to be significant (12,51). The present work involves a detailed study of the kinetics of photolysis of RF in a wide range of organic solvents using specific multicomponent spectrometric method for the assay of RF and photoproducts (10,13,52) and to develop correlations between the kinetic data and solvent parameters such as dielectric constant and viscosity. These considerations are important in the formulation of drugs with different polar characters using cosolvents and those whose oxidation is viscosity dependent to achieve their stabilization.
MATERIALS AND METHODS
RF, lumichrome (LC), and lumiflavin (LF) were obtained from Sigma Chemical Co., St. Louis, MO, USA. Formylmethylflavin (FMF) and carboxymethylflavin (CMF) were synthesized by the previously reported methods (22,23). All solvents and reagents were of analytical grade from Merck & Co., Whitehouse Station, NJ, USA.
The methods of photolysis, chromatography, and assay are the same as previously described for FMF in organic solvents (7,9) and in aqueous solution (8). These are briefly described below.
Photolysis
A 3 × 10−5 M solution of RF (100 ml) was prepared in water (pH 7.0, 0.005 M phosphate buffer) and in organic solvents in a volumetric flask (Pyrex) and immersed in a water bath maintained at 25 ± 1°C. The solution was exposed to a Philips HPL-N 125 W high-pressure mercury lamp (emission bands at 405 and 435 nm; the later band overlaps the 445 nm band of RF (13)), fixed at a distance of 25 cm from the center of the flask for a period of 2–3 h depending upon the nature of the solvent used. Samples of photolyzed solution were withdrawn at a various time intervals for thin-layer chromatography and spectrometric assay.
pH Measurements
The pH measurements of solutions were performed on an Elmetron pH meter (Model—CP501, sensitivity ±0.01 pH units, Poland) using a combination pH electrode. The electrode was automatically calibrated using phthalate (pH 4.008), phosphate (pH 6.865), and disodium tetraborate (pH 9.180) buffer solutions.
Thin-Layer Chromatography
The thin-layer chromatography (TLC) of the photolyzed solutions of RF in aqueous and organic solvents was carried out on 250 μm cellulose plates using the following solvent systems: (a) 1-butanol–acetic acid–water (40:10:50, v/v, organic phase) and (b) 1-butanol–1-propanol–acetic acid–water (50:30:2:18, v/v) (11). The compounds were detected by their characteristic fluorescence on exposure to UV (365 nm) light; RF, LF, FMF, CMF (yellow green), LC (sky blue).
Spectrometric Assay
A 5-ml aliquot of the photolyzed solution of RF was evaporated to dryness under reduced pressure at room temperature and the residue dissolved in 0.2 M KCl–HCl buffer solution (pH 2.0). The solution was extracted with 3 × 5 ml of chloroform, the chloroform was evaporated and the residue dissolved in 0.2 M acetate buffer solution (pH 4.5). The absorption of this solution was measured at 356 nm to determine the concentration of LC. The aqueous phase (pH 2.0) was used to determine the concentrations of RF and FMF in degradation solutions by a two-component spectrometric assay at 385 and 445 nm according to the method of Ahmad and Rapson (10).
Determination of Light Intensity
The intensity of the Philips HPL-N 125 W lamp was determined using potassium ferrioxalate actinometry (25) as 1.21 ± 0.10 × 1017 quanta s−1.
RESULTS
Photoproducts of RF
TLC of the photolyzed solutions of RF in organic solvents using solvent systems (a) and (b) showed the presence of FMF and LC as the main photoproducts of this reaction. CMF was also detected as a minor oxidation product of FMF in these solvents (7,9). These products were identified by comparison of their fluorescence emission and Rf values with those of the authentic compounds. FMF and LC as the main photoproducts of RF in organic solvents have previously been reported (7,9,34). The formation of LC in organic solvents may take place through FMF as an intermediate in the photolysis of RF as observed in the case of aqueous solutions (7–10). The fluorescence intensity of the photoproducts on TLC plates is an indication of the extent of their formation in a particular solvent during the irradiation period. In aqueous solutions (pH 7.0), LF is also formed in addition to FMF and LC as previously observed (8,57).
Spectral Characteristics
RF exhibits absorption maxima in organic solvents in the region of 440–450, 344–358, and 270–271 nm (35). A typical set of absorption spectra for the photolysis of RF in methanol is shown in Fig. 1. There is a gradual loss of absorbance around 445 nm with a shift of the peak at 358 to 350 nm, with time, due to the formation of LC (λmax in methanol, 339 nm) (54), the major photoproduct of RF in organic solvents; LC is formed through the mediation of FMF, an intermediate in the photolysis of RF (57). FMF has an absorption spectrum similar to that of RF, and, therefore, it could not be distinguished from the absorption spectrum of RF in organic solvents.
Fig. 1.
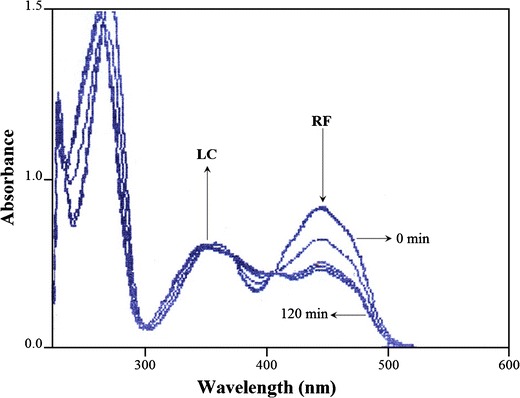
Absorption spectra of RF photolyzed in methanol at 0, 30, 60, 90, and 120 min
Assay of RF and Photoproducts
The photolyzed solutions of RF have been assayed at pH 2.0 by extraction of LC with chloroform and its determination at pH 4.5 at 356 nm. The aqueous phase was used to determine RF and FMF by a two-component assay at 385 and 445 nm corresponding to the absorption maxima of these compounds. The molar concentrations of RF and its photoproducts, FMF and LC, determined in a photolysis reaction (10) carried out in methanol are reported in Table I. The assay method shows uniformly increasing values of FMF and LC with an almost constant molar balance, with time, indicating a good reproducibility of the method. CMF, a minor oxidation product of FMF in organic solvents (7), accounting to less than 1% (9), does not interfere with the assay method.
Table I.
Concentrations of RF and Photoproducts in Methanol
| Time (min) | RF (M × 105) | FMF (M × 105) | LC (M × 105) | Total (M × 105) |
|---|---|---|---|---|
| 0 | 3.00 | 00 | 00 | 3.00 |
| 30 | 2.55 | 0.36 | 0.15 | 3.06 |
| 60 | 2.15 | 0.58 | 0.29 | 3.02 |
| 90 | 2.01 | 0.71 | 0.32 | 3.04 |
| 120 | 1.91 | 0.79 | 0.37 | 3.07 |
RF riboflavin, FMF formylmethylflavin, LC lumichrome
Kinetics of Photolysis
The photolysis of RF in aqueous solution (3,8,57) and in organic solvents (36,57) follows first-order kinetics. A kinetic plot for the photolysis of RF in methanol (Fig. 2) shows that LC is the final product in this reaction as observed by previous workers (32,42). The first-order rate constants, (kobs), determined for the photolysis reactions in organic solvents and water range from 3.19 (ethyl acetate) to 4.61 × 10−3 min−1 (water) (correlation coefficients 0.997–0.999) (Table II). The values of kobs increase with an increase in the dielectric constant showing the influence of solvent on the rate of reaction. The value for the photolysis of RF in aqueous solution (pH 7.0, 0.005 M phosphate buffer) is also included for comparison. A plot of kobs for the photolysis of RF as a function of solvent dielectric constant is presented in Fig. 3. It shows that the rate constants are linearly dependent upon the solvent dielectric constant. Similarly, a linear relation has been found between the values of kobs and the solvent acceptor number indicating the degree of solute–solvent interaction (Fig. 4). In order to observe the effect of viscosity on the rate of photolysis, a plot of kobsversus inverse of viscosity was constructed (Fig. 5). It showed a linear relation between the two values indicating the influence of solvent viscosity on the rate of reaction. These results are supported by the fact that a plot of dielectric constant versus inverse of viscosity of organic solvents is linear. However, the values of kobs for RF in ethyl acetate and water do not fit in the plot probably due to different behaviors of RF in acetate (compared to alcohols) and water (e.g., degree of hydrogen bonding).
Fig. 2.
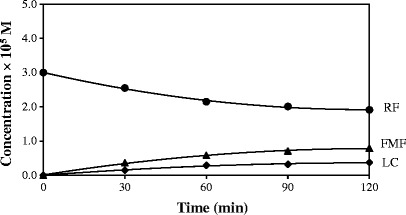
A kinetic plot for the photolysis of RF in methanol
Table II.
Apparent First-Order Rate Constants for the Photolysis of Riboflavin (k obs) in Organic Solvents and Water
| Solvents | Acceptor number | Dielectric constant (ϵ) (25°C) | Inverse viscosity (mPa.s−1) (25°C) | k obs × 103 min−1 ± SD |
|---|---|---|---|---|
| Ethyl acetate | 17.1 | 6.02 | 2.268 | 3.19 ± 0.14 |
| 1-Butanol | 36.8 | 17.8 | 0.387 | 3.28 ± 0.13 |
| 1-Propanol | 37.3 | 20.1 | 0.514 | 3.34 ± 0.16 |
| Ethanol | 37.1 | 24.3 | 0.931 | 3.45 ± 0.15 |
| Methanol | 41.3 | 32.6 | 1.828 | 3.64 ± 0.17 |
| Acetonitrile | 18.9 | 38.5 | 2.898 | 3.81 ± 0.16 |
| Water | 54.8 | 78.5 | 1.123 | 4.61 ± 0.25 |
SD standard deviation
Fig. 3.
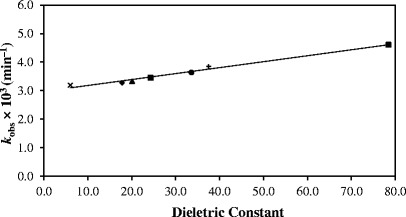
Plot of k obs for the photolysis of RF versus dielectric constant; (letter x) ethyl acetate, (black diamond) 1-butanol, (black triangle) 1-propanol, (black square) ethanol, (black circle) methanol, (cross sign) acetonitrile, (asterisk) water
Fig. 4.
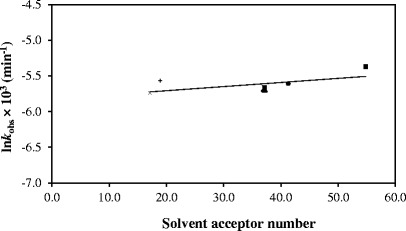
Plot of lnk obs for the photolysis of RF versus acceptor number. Symbols are as in Fig. 3
Fig. 5.
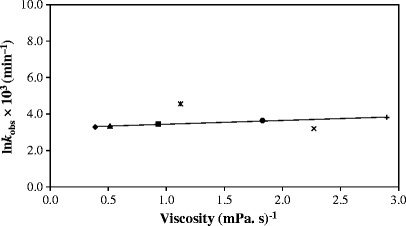
Plot of k obs for the photolysis of RF versus inverse of viscosity. Symbols are as in Fig. 3
DISCUSSION
Effect of Solvent
It is known that solvents could influence the degradation of drugs depending on the solute–solvent interaction. Solvents may alter the rate and mechanism of chemical reactions (1,15,38,44,46,51) and thus play a significant role in the stabilization of pharmaceutical products (21). Pharmaceutical formulations of ionizable compounds such as RF may be stabilized by an alteration in the solvent characteristics. A suppression of the ionization of a drug susceptible to degradation in water may be achieved by the addition of a cosolvent (e.g., alcohol). This would result in the destabilization of the polar excited state and, therefore, a decrease in the rate of reaction as observed in the case of many drugs (65). The use of organic solvents as cosolvent can have a photostabilizing effect on the product as a result of a change in the polarity and viscosity of the medium (61). These considerations are important in the formulation of drugs with different polar characters and those whose oxidation is viscosity dependent. These aspects with respect to the photolysis of RF as a model compound used in the clinical treatment of neonatal jaundice (60) keratoconus (19) and HIV infection (41) would now be considered, and correlations would be developed between the solvent characteristics and the rate of reaction.
Effect of Dielectric Constant
The rate of degradation reactions between ions and dipoles in solution depends on bulk properties of the solvent such as the dielectric constant. Any change in the dielectric constant of a solvent can lead to variation in the energy of activation (∆G) and hence in the rate constants (65). This can be applied to the degradation of RF since its rate of photolysis is a linear function of dielectric constant. This can be explained on the basis of the participation of a polar intermediate in the reaction pathway to facilitate the reaction (7,12). The rate of RF photolysis is affected by solvent polarity probably due to changes in the conformation of the ribityl side chain in different solvents (42). Quenching of flavin excited-triplet state [3FL] by oxygen during the reaction has been suggested (7,33), and this may affect the rate of photolysis. However, under the present reaction conditions (i.e., solvents in equilibrium with the air), first-order plots are linear for RF solutions photolyzed up to 30%, and the values of kobs are relative to these conditions. The electron-donating capacity of a molecule (e.g., fluoroquinolone, RF) is affected by the nature of the solvent (5,45) and hence its rate of degradation. The acceptor number is a measure of the ability of solvents to share electron pairs from suitable donors (49,63), and this could affect the rate of photolysis. The results obtained and degradation behavior of RF in organic solvents suggest that the stability of such polar drugs can be improved by alteration of dielectric constant of the medium.
Effect of Viscosity
The viscosity of the medium can also influence the rate of degradation, particularly of an oxidizable drug. The photolysis of RF involves oxidation of the ribityl side chain (42) and thus may be affected by the solvent viscosity. The values of kobs for RF in ethyl acetate and water do not follow the relation (Fig. 5) probably due to its different structural orientation (42) and degree of hydrogen bonding (53) compared to those of the organic solvents. The behavior of RF in organic solvents indicates that the viscosity of the medium suppresses the rate of photolysis, probably as a result of solute diffusion-controlled processes (12,62). It has been observed that [3RF] quenching depends on solvent viscosity (12) that would affect the rate of reaction. Similar effects of viscosity have been observed on the photooxidative degradation of formylmethylflavin (9) and fluoroquinolones (4–6).
Mode of Photolysis
The photochemistry of RF has widely been studied by several workers, and the various modes of its photodegradation reactions (i.e., intramolecular and intermolecular photoreduction, photodealkylation, and photoaddition) have been discussed (7,9,13,20,26,27,51). The pathway of RF degradation in organic solvents appears to be similar to that of the aqueous solution involving intramolecular photoreduction followed by side-chain cleavage (13). However, the rate of the reaction is solvent dependent due to the participation of a dipolar intermediate (12) whose degradation is promoted by polar environment and suppressed by nonpolar media. It has been observed by laser flash photolysis that the reduction of [3FL] in organic solvents proceeds through the mediation of the dipolar intermediate according to the following reaction (12).
| 1 |
The flavin semiquinone radical [FLH●] undergoes further reactions to give the final products shown by Eqs. (2) and (3).
| 2 |
| 3 |
The extent of the reaction to form radicals is controlled by the degree of solute–solvent interaction. The polar character of the reaction intermediate would determine the rate of reaction, and the rate would be higher in solvents of greater polarity. Thus, the solvent characteristics play an important role in determining the rate of RF degradation. An appropriate combination of water–alcohol mixture would be a suitable medium for the stabilization of RF and drugs of similar character.
Conclusion
Solvent characteristics are an important factor in the stabilization of pharmaceutical formulations. The choice of a solvent or cosolvent would depend on the chemical nature, polar character, and the behavior of the drug in a particular medium. In the present study, it has been demonstrated that solvent characteristics, such as dielectric constant and viscosity, may alter the rate of degradation of a drug to achieve stabilization. In the case of RF, it has been found that the rate of photolysis is linearly dependent on solvent polarity and is inversely dependent on solvent viscosity. This is reflected in the values of kobs obtained for the photolysis of RF in different solvents. The value of kobs in water (ϵ 78.5) is nearly one and half times that of ethyl acetate (ϵ 6.0) indicating a prominent effect of dielectric constant on the rate of reaction. Similarly, the value of kobs increases with a decrease in solvent viscosity. Thus, a change in the medium on the basis of solvent characteristics could improve the stability of a drug and prolong its shelf life. A rational approach in this direction and the use of appropriate cosolvents with water would enable the formulator to achieve better stabilization of a drug.
References
- 1.Abraham MH. Solvent effects on reaction rates. Pure Appl Chem. 1985;57:1055–64. doi: 10.1351/pac198557081055. [DOI] [Google Scholar]
- 2.Adams WP, Kostenbauder HB. Phenoxybenzamine stability in aqueous ethanolic solutions. II. Solvent effects on kinetics. Int J Pharm. 1985;25:313–27. doi: 10.1016/0378-5173(85)90171-1. [DOI] [Google Scholar]
- 3.Ahmad I, Anwar Z, Iqbal K, Ali SA, Mirza T, Khurshid A, et al. Effect of acetate and carbonate buffers on the photolysis of riboflavin in aqueous solution: a kinetic study. AAPS PharmSciTech. 2014;15:550–9. doi: 10.1208/s12249-013-0067-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmad I, Bano R, Musharraf SG, Ahmed S, Sheraz MA, Arfeen QU, et al. Photodegradation of moxifloxacin in aqueous and organic solvents: a kinetic study. AAPS PharmSciTech. 2014;15:1588–97. doi: 10.1208/s12249-014-0184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahmad I, Bano R, Musharraf SG, Sheraz MA, Ahmed S, Tahir H, et al. Photodegradation of norfloxacin in aqueous and organic solvents: a kinetic study. J Photochem Photobiol A Chem. 2015;302:1–10. doi: 10.1016/j.jphotochem.2015.01.005. [DOI] [Google Scholar]
- 6.Ahmad I, Bano R, Sheraz MA, Ahmed S, Mirza T, Ansari SA. Photodegradation of levofloxacin in aqueous and organic solvents: a kinetic study. Acta Pharm. 2013;63:221–7. doi: 10.2478/acph-2013-0011. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad I, Fasihullah Q, Vaid FHM. Photolysis of formylmethylflavin in aqueous and organic solvents. Photochem Photobiol Sci. 2006;5:680–5. doi: 10.1039/b602917e. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad I, Fasiullah Q, Noor A, Ansari IA, Ali QNM. Photolysis of riboflavin in aqueous solution: a kinetic study. Int J Pharm. 2004;280:199–208. doi: 10.1016/j.ijpharm.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad I, Mirza T, Iqbal K, Ahmed S, Sheraz MA, Vaid FHM. Effect of pH, buffer and viscosity on the photolysis of formylmethylflavin: a kinetic study. Aust J Chem. 2013;66:579–85. [Google Scholar]
- 10.Ahmad I, Rapson HDC. Multicomponent spectrophotometric assay of riboflavin and photoproducts. J Pharm Biomed Anal. 1990;8:217–23. doi: 10.1016/0731-7085(90)80029-O. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad I, Rapson HDC, Heelis PF, Phillips GO. Alkaline hydrolysis of 7, 8-dimethyl-10(formylmethyl)-isoalloxazine. A kinetic study. J Org Chem. 1980;45:31–33. doi: 10.1021/jo01292a040. [DOI] [Google Scholar]
- 12.Ahmad I, Tollin G. Solvent effects on flavin electron transfer reactions. Biochemistry. 1981;20:5925–8. doi: 10.1021/bi00523a042. [DOI] [PubMed] [Google Scholar]
- 13.Ahmad I, Vaid FHM. Photochemistry of flavins in aqueous and organic solvents. In: Silva E, Edwards AM, editors. Flavins photochemistry and photobiology. Cambridge: Royal Society of Chemistry; 2006. pp. 13–40. [Google Scholar]
- 14.Amis ES, Hinton JF. Solvent effects on chemical phenomena. New York: Academic; 1973. [Google Scholar]
- 15.Amis ES, Hinton JF. Solvent effect on chemical phenomena. New York: Academic; 1973. [Google Scholar]
- 16.Baker SK, Niazi S. Stability of aspirin in different media. J Pharm Sci. 1983;72:1024–6. doi: 10.1002/jps.2600720914. [DOI] [PubMed] [Google Scholar]
- 17.Brennan TV, Clarke S. Spontaneous degradation of polypeptides at aspartyl and asparaginyl residues. Effects of solvent dielectric. Protein Sci. 1993;2:331–8. doi: 10.1002/pro.5560020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buncel E, Stairs RA, Wilson H. The role of the solvent in chemical reactions. 3. New York: Oxford University Press; 2003. [Google Scholar]
- 19.Caporossi A, Mazzotta C, Baiocchi S, Tomaso C. Long-term results of riboflavin ultraviolet a corneal collagen cross-linking for keratoconus in Italy: the Siena eye cross study. Am J Opthal. 2010;149:585–93. doi: 10.1016/j.ajo.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Choe E, Huang R, Min DB. Chemical reactions and stability of riboflavin in food. J Food Sci. 2005;70:R28–R36. doi: 10.1111/j.1365-2621.2005.tb09055.x. [DOI] [Google Scholar]
- 21.Connors KA, Amidon GL, Stella VJ. Chemical stability of pharmaceuticals a handbook for the pharmacist. 2. New York: Wiley; 1986. pp. 38–41. [Google Scholar]
- 22.Fall HH, Petering HG. Metabolic inhibitors. 1. 6,7-Dimethyl-9-formylmethylisoalloxazine, 6,7-dimethyl-9-(12-hydroxyethyl)-isoalloxazine and derivatives. J Am Chem Soc. 1956;78:377–81. doi: 10.1021/ja01583a035. [DOI] [Google Scholar]
- 23.Fukumachi C, Sakurai Y. Vitamin B2 photolysis. V. The photolytic formation of 6, 7-dimethylflavin-9-acetic acid ester from riboflavin. Vitamins (Kyoto) 1954;7:939–43. doi: 10.5925/jnsv1954.1.3_217. [DOI] [PubMed] [Google Scholar]
- 24.Ghanem AH, Hassan ES, Hamdi AA. Stability of indomethacin solubilized system. Pharmazie. 1979;34:406–7. [PubMed] [Google Scholar]
- 25.Hatchard CG, Parker CA. A new sensitive chemical actinometer. II. Potassium ferrioxalate as a standard chemical actinometer. Proc Roy Soc (Lond) 1956;A235:518–36. doi: 10.1098/rspa.1956.0102. [DOI] [Google Scholar]
- 26.Heelis PF. The photophysical and photochemical properties of flavin (isoalloxazines) Chem Soc Rev. 1982;11:15–39. doi: 10.1039/cs9821100015. [DOI] [Google Scholar]
- 27.Heelis PF. The photochemistry of flavins. In: Muller F, editor. Chemistry and biochemistry of flavoenzymes. Boca Raton: CRC Press; 1991. pp. 171–93. [Google Scholar]
- 28.Heitele H. Dynamic solvent effects on electron transfer reactions. Angew Chem Int Ed Engl. 1993;32:359–77. doi: 10.1002/anie.199303591. [DOI] [Google Scholar]
- 29.Hou JP, Poole JW. β-lactam antibiotics: their physicochemical properties and biological activities in relation to structure. J Pharm Sci. 1969;60:503–32. doi: 10.1002/jps.2600600402. [DOI] [PubMed] [Google Scholar]
- 30.Hussain A, Truelove J. Effect of hydroxyl group substituents on pyran ring on hydrolysis rate of benzoates: 2-tetrahydropyranyl benzoate. J Pharm Sci. 1979;65:235–66. doi: 10.1002/jps.2600680229. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda K. Studies on decomposition and stabilization of drugs in solution. IV. Effect of dielectric constant on the stabilization of barbiturate in alcohol-water mixtures. Chem Pharm Bull. 1960;8:504–9. doi: 10.1248/cpb.8.504. [DOI] [Google Scholar]
- 32.Insinska-Rak M, Golczak A, Sikorski M. Photochemistry of riboflavin derivatives in methanolic solutions. J Phys Chem. 2012;116:1199–207. doi: 10.1021/jp2094593. [DOI] [PubMed] [Google Scholar]
- 33.Insinska-Rak M, Sikorski M. Riboflavin interactions with oxygen-survey from the photochemical perspective. Chem Eur J. 2014;20:15280–91. doi: 10.1002/chem.201403895. [DOI] [PubMed] [Google Scholar]
- 34.Koziol J. Studies on flavins in organic solvents–II. Photodecomposition of riboflavin in the presence of oxygen. Photochem Photobiol. 1966;5:55–62. doi: 10.1111/j.1751-1097.1966.tb05759.x. [DOI] [Google Scholar]
- 35.Koziol J. Studies on flavins in organic solvents–I. Spectral characteristics of riboflavin, riboflavin tetrabutyrate and lumichrome. Photochem Photobiol. 1966;5:41–54. doi: 10.1111/j.1751-1097.1966.tb05759.x. [DOI] [PubMed] [Google Scholar]
- 36.Kurtin WE, Latino MA, Song PS. A study of photochemistry of flavins in pyridine and with a donor. Photochem Photobiol. 1967;6:247–59. doi: 10.1111/j.1751-1097.1967.tb08812.x. [DOI] [PubMed] [Google Scholar]
- 37.Laidler KJ. Chemical kinetics. 3. New York: Harper & Row; 1987. pp. 183–95. [Google Scholar]
- 38.Laidler KJ. Chemical kinetics. 3. New York: Harper & Row; 1987. pp. 279–280. [Google Scholar]
- 39.Mao YP, Tao XL, Lipsky PE. Analysis of the stability and degradation products triptolide. J Pharm Pharmcol. 2000;52:3–12. doi: 10.1211/0022357001773625. [DOI] [PubMed] [Google Scholar]
- 40.Marcus AD, Taraszka AJ. A kinetic study of the specific hydrogen ion catalyzed solvolysis of chloramphenicol in water-propylene glycol systems. J Pharm Sci. 1959;48:77–84. doi: 10.1002/jps.3030480202. [DOI] [PubMed] [Google Scholar]
- 41.Montessori V, Press N, Harris M, Akagi L, Montaner JSG. Adverse effect of antiretroviral therapy for HIV infection. CMAJ. 2004;170:229–38. [PMC free article] [PubMed] [Google Scholar]
- 42.Moore WM, Ireton RC. The photochemistry of riboflavin, V. The photodegradation of isoalloxazines in alcoholic solvents. Photochem Photobiol. 1977;25:347–56. doi: 10.1111/j.1751-1097.1977.tb07354.x. [DOI] [PubMed] [Google Scholar]
- 43.Owen WR, Stewart PJ. Kinetics and mechanism of chlorambucil hydrolysis. J Pharm Sci. 1979;68:992–6. doi: 10.1002/jps.2600680819. [DOI] [PubMed] [Google Scholar]
- 44.Parker AJ. Protic-dipolar aprotic solvent effects on rates of bimolecular reactions. Chem Rev. 1969;69:1–32. doi: 10.1021/cr60257a001. [DOI] [Google Scholar]
- 45.Peng Z, HaiXia L, SiDe Y, WenFeng W. Effect of pH and polarity on the excited states of norfloxacin and its 4-N-acetyl derivative: a steady state and time-resolved study. Sci China Chem. 2014;57:409–16. doi: 10.1007/s11426-013-5012-8. [DOI] [Google Scholar]
- 46.Reichardt C. Solvent effects on chemical reactivity. Pure Appl Chem. 1982;54:1867–84. doi: 10.1351/pac198254101867. [DOI] [Google Scholar]
- 47.Reichardt C. Solvents and solvent effects in organic chemistry. 2. New York: VCH Publishers; 1988. [Google Scholar]
- 48.Roseman TJ, Sims B, Stehle RG. Stability of prostaglandins. Am J Hosp Pharm. 1973;30:236–9. [PubMed] [Google Scholar]
- 49.Schmid R, Sapunov VN. Non-formal kinetics: in search of chemical reactions pathways (monograph in modern chemistry) Weinheim: Verlag Chemie; 1982. pp. 123–54. [Google Scholar]
- 50.Schmidt WC. Light-induced redox cycles of flavins in various alcohol/acetic acid mixtures. Photochem Photobiol. 1982;36:699–703. doi: 10.1111/j.1751-1097.1982.tb09491.x. [DOI] [Google Scholar]
- 51.Sheraz MA, Kazi SH, Ahmed S, Anwar Z, Ahmad I. Photo, thermal and chemical degradation of riboflavin. Beilstein J Org Chem. 2014;10:1999–2012. doi: 10.3762/bjoc.10.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheraz MA, Kazi SH, Ahmed S, Qadeer K, Khan MF. Multicomponent spectrometric analysis of riboflavin and photoproducts and their kinetic applications. Cent Eur J Chem. 2014;12:635–42. doi: 10.2478/s11532-014-0527-1. [DOI] [Google Scholar]
- 53.Sikorska E, Koziolowa A, Sikorski M, Siemiarczuk A. The solvent effect on the excited state proton transfer of lumichrome. J Photochem Photobiol A Chem. 2003;157:5–14. doi: 10.1016/S1010-6030(03)00078-9. [DOI] [Google Scholar]
- 54.Sikorski E, Worrall DR, Bourdelande JI, Sikroski M. Photophysics of lumichrome and its analogs. Polish J Chem. 2003;77:65–73. [Google Scholar]
- 55.Singh S, Gupta RI. Dielectric constant effects on degradation of azothioprine in solution. Int J Pharm. 1988;46:267–70. doi: 10.1016/0378-5173(88)90088-9. [DOI] [Google Scholar]
- 56.Sinko PJ. Chemical kinetics and stability. In: Martin’s Physical Pharmacy and Pharmaceutical Sciences, 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2006. p. 413–6.
- 57.Song PS, Metzler DE. Photochemical degradation of Flavins–IV. Studies of the anaerobic photolysis of riboflavin. Photochem Photobiol. 1967;6:691–709. doi: 10.1111/j.1751-1097.1967.tb08735.x. [DOI] [PubMed] [Google Scholar]
- 58.Szezesma V, Koziol J. Photolysis of flavin in carboxylic acids. In: Ostrowski W, editor. Flavins and flavoproteins. Physiochemical properties and functions. Warsaw: Polish Scientific Publishers; 1977. pp. 117–26. [Google Scholar]
- 59.Tada H. Decomposition reaction of hexamine by acid. J Am Chem Soc. 1960;82:255–63. doi: 10.1021/ja01487a001. [DOI] [Google Scholar]
- 60.Tan KL. Phototherapy for neonatal jaundice. Acta Paediatr. 1996;85:277–9. doi: 10.1111/j.1651-2227.1996.tb14014.x. [DOI] [PubMed] [Google Scholar]
- 61.Tonnesen HH. Formulation and stability testing of photolabile drugs. Int J Pharm. 2001;225:1–14. doi: 10.1016/S0378-5173(01)00746-3. [DOI] [PubMed] [Google Scholar]
- 62.Turro NJ, Ramamurthy V, Scaierno JC. Modern molecular photochemistry of organic molecules. Sausalito: University Science; 2010. pp. 469–74. [Google Scholar]
- 63.Wypych G. Hand book of solvents. 2. Toronto: Chem Tec Publishing; 2001. pp. 577–81. [Google Scholar]
- 64.Yeh MK. Degradation kinetics of neostigmine in solution. Drug Dev Ind Pharm. 2000;26:1221–6. doi: 10.1081/DDC-100100996. [DOI] [PubMed] [Google Scholar]
- 65.Yoshioka S, Stella VJ. Stability of drugs and dosage forms. New York: Kluwer Academic/Plenum Publishers; 2000. pp. 102–4. [Google Scholar]


