Abstract
Monoclonal antibody biotherapeutics are often administered by subcutaneous (SC) injection. Due to dose requirements and formulation limitations, SC injections >1 mL are often required. We used a viscous placebo buffer (5 cP), characteristic of a high-concentration antibody formulation, to investigate the effect of dose volume and injection rate on the tolerability of higher-volume SC injections. In this randomized, crossover, single-center study, 48 healthy adults received one 1.2-mL bolus injection over 5 s and three 3.5-mL injections over 1, 4, and 10 min in different abdominal quadrants, with each injection separated by approximately 2 h. The primary objective was to compare pain scores associated with the injections, immediately after administration and 1 h later, using a 100-mm visual analog scale (VAS). Secondary objectives included assessment of adverse events, including injection site reactions and swelling. Mean age was 38.4 (11.6) years and 20 subjects (42%) were female. Lowest mean VAS score was for the 10-min (6.83 mm) and highest for the 1-min injection (19.13 mm). One hour after administration, mean VAS scores were <3.5 mm for all injections. Swelling was similar among the three 3.5-mL injections. After needle removal, leakage occurred following 14 (29%) 1.2-mL injections, eight (17%) 4-min injections, five (10%) 1-min injections, and four (8%) 10-min injections. Fifteen subjects (31%) experienced an adverse event, none of which was serious, fatal, or led to study discontinuation. All injection durations were well tolerated, suggesting a single large-volume SC injection of a biotherapeutic agent could be used instead of multiple injections.
KEY WORDS: placebo buffer, subcutaneous injections, tolerability, viscous
INTRODUCTION
Biotherapeutics are increasingly being used to treat a wide range of serious illnesses requiring chronic drug administration [1–4]. Biotherapeutics offer several attractive features, including high selectivity and specificity, good solubility and stability, long persistence in the body, and low risk for bioconversion to toxic metabolites [5]. However, most have a practical disadvantage, namely administration by injection. Antibodies cannot be administered orally because of degradation in the gastrointestinal tract, and they have limited capacity for diffusion or convection through the gastrointestinal epithelium [5]. Subcutaneous (SC) administration is hampered by the extracellular matrix, which offers a barrier to the delivery of many drugs, limiting both the volume that can be injected at a single site and the amount that can reach the vascular compartment [6]. Therefore, research has focused on methods that permit delivery of larger volumes into the SC space, such as recombinant human hyaluronidase to temporarily degrade interstitial hyaluronan [6, 7]. Recombinant human hyaluronidase is associated with mild, local injection site reactions and is incompatible with some drugs, including furosemide, benzodiazepines, and phenytoin [8].
The requirement for injection of biologics presents a challenge in patients with chronic conditions, who have markedly lower rates of drug adherence and persistence than patients with acute conditions [9–12]. Biotherapeutics that require regular self-injection are used by patients with various inflammatory diseases [2, 13, 14], osteoporosis [15], multiple sclerosis [1], and diabetes mellitus [16, 17]. Insulin pumps, which deliver a continuous SC infusion, offer an alternative to multiple daily injections for patients with diabetes and have been shown to improve glycemic control and quality of life and reduce vascular complications [16].
Most biologics are administered by SC injection, commonly in volumes not exceeding 1 mL [18], but there is no definitive evidence to support this limitation on volume. Newer biologics, including glucagon-like peptide-1 agonists, monoclonal antibodies, and antisense DNA, may require injection of volumes >1 mL due to a combination of factors, including potency, protein aggregation, and increased viscosity at higher concentrations. For volumes >2 mL, multiple injections are typically used [5, 19, 20], but this approach may increase the attrition rate in clinical trials or reduce patient adherence. In primary immunodeficiency, SC administration of high-dose immunoglobulin in volumes of up to 5 mL at a single injection site [20, 21], or a self-administered bolus up to 20 mL given over 5–20 min [22], may be acceptable to some patients. Therefore, single-site, larger-volume injections without hyaluronidase represent a potential alternative to multiple injections, if the formulation and delivery rate can be shown to be safe and well tolerated.
The effect of dose volume, together with speed of injection, on the tolerability of higher-volume SC injections with viscosities characteristic of some concentrated antibody formulations remains unknown. To address this, we developed a placebo buffer formulation with viscosity (5 cP), osmolality, and pH similar to those of some protein/antibody formulations. We tested a large volume (3.5 mL) to reduce the number of injections necessary to achieve a therapeutic dose and varied the rate of injection to determine whether slower SC delivery was tolerated.
Pain assessments were performed with 100-mm visual analog scales (VASs), which offer a simple, reliable, and validated method for measuring pain, and correlate well with categorical pain scales [23–25]. VASs are widely used in clinical assessment of pain as they have greater sensitivity to changes in pain than other instruments, such as verbal rating scales, and allow generation of continuous variable output [26]. However, pain is a subjective experience and any rating scale will reflect that, with the potential for wide ranges of ratings. VAS scores ranged from 0 (no pain at all) to 100 (a lot of pain) in 96 patients presenting to the emergency department with pain [23], from 0 to 100 in 48 patients with acute pain from trauma [25], and from 5 to 98 in 98 patients with osteoarthritis of the hip [26]. The minimum clinically meaningful difference in acute pain using VAS determined in two of these cohorts was 13 and 16 mm, respectively [23, 25], and determined in 101 patients presenting to the emergency department with acute abdominal pain was 13 mm [24].
This study was designed to compare VAS scores associated with various SC injection rates of a 3.5-mL viscous placebo buffer immediately after administration.
MATERIALS AND METHODS
Study Design
This randomized, crossover, single-center, phase 0 study was carried out in 48 healthy subjects. Subjects received one injection of 1.2 mL over 5 s and three injections of 3.5 mL over 1, 4, and 10 min of placebo buffer in different quadrants of their abdomen, each separated by approximately 2 h. This was expected to be long enough to eliminate the potential for a carry-over effect from the previous administration as injection site pain is not expected to last long since fluid distribution in the SC space should occur quickly. The 1-h assessment was included to understand the duration of pain. Subjects were randomized (1:1:1:1) to one of four different injection sequences. The study was conducted in accordance with the International Conference on Harmonisation Good Clinical Practice regulations and guidelines.
Endpoints
The primary endpoint was pain (VAS) score assessed immediately after administration. Secondary endpoints included subject incidence of adverse events related to SC injection, including administration site reactions and swelling; subjects’ perception of pain as assessed by ranking; and VAS scores obtained 1 h after administration. Leakage from the administration site was an exploratory endpoint.
Subjects
Subjects were male or female, aged 18–55 years, with a body mass index of 18–32 kg/m2, thus excluding subjects with severe or moderate obesity. Subjects had to be in good general health and have no clinically significant laboratory abnormalities at screening (Day –21). Vital signs were recorded on Day –21 and study day (before first and after last injection). All subjects provided written informed consent; consent form and study protocol were approved by the Institutional Review Board. Exclusion criteria included conditions that could interfere with delivery of placebo buffer or interpretation of assessments or significantly impair pain perception; susceptibility to bleeding; clinically significant skin allergies or active dermatologic disorders; sensitivity to buffer ingredients; and pain medications (non-steroidal anti-inflammatory drugs within 5 days before study day, steroids within 7 days before study day, and antihistamines or analgesics within 48 h prior to injection).
Investigators could prescribe any concomitant medications or treatments deemed necessary to provide adequate supportive care. However, administration of any analgesic medication before the final assessment on day 1 resulted in withdrawal of the subject. Subjects could be replaced at the discretion of the investigator in consultation with Amgen, with the exception of withdrawals due to adverse events.
Interventions
The placebo buffer (acetate), which contained sodium carboxymethyl cellulose (Na-CMC; 7 mg/mL), matched the osmolality (250–350 mOsm/kg) and pH (5.0) for biologic parenteral products. Sodium chloride was not added to the buffer.
The study was conducted at a large Phase 1 clinical center with nursing staff experienced in SC drug administration. An experienced, qualified, and trained staff member performed SC placement of the needle for all injections. Subjects were isolated from other subjects throughout the study.
Subjects were randomized 1:1:1:1 to receive one of four sequences using the Williams design [27]. Two injections (1.2 mL over 5 s and 3.5 mL over 1 min) were delivered manually and two (3.5 mL over 4 min and 3.5 mL over 10 min) were delivered using an infusion pump (Razel A99-EJM, Razel Scientific Instruments, VT, USA). Since SC injections of 1–1.2 mL are common, 1.2 mL/5 s was included as the reference. For the 3.5 mL injections, a 5-mL syringe was connected to approximately 3 ft of tubing with a butterfly catheter and a 27 G needle. The setup was primed with placebo buffer before needle insertion. The needle was inserted at 90° to the skin, such that the wings of the butterfly were flush with the skin. The butterfly was secured to skin using transparent adhesive.
Using the umbilicus as a center point, the abdomen was divided into quadrants. Each quadrant was injected once, starting with the right upper quadrant and progressing in a clockwise manner to the left upper quadrant, left lower quadrant, and right lower quadrant. Each injection was separated by approximately 2 h.
Pain Assessments
Subjects assessed their injection site pain using 100-mm VAS scores (0 mm = no pain at all; 100 mm = a lot of pain) immediately after each administration (before needle removal) and 1 h (±5 min) after the initiation of administration. Immediately after the fourth administration, subjects were asked to rank administrations from least [1] to most [4] painful.
Safety Evaluation
Adverse events, including administration site reactions and swelling, were recorded throughout the study, with follow-up 24 h after the last injection via telephone. The person who administered the injections observed the subjects for adverse events. The Common Terminology Criteria for Adverse Events V 4.0 were used [28]. The study was not blinded, except that patients could not see the rate of infusion shown on the pump.
Swelling due to fluid collection in the SC space was measured immediately after needle removal by measuring the long and short axes of the swelling (using calipers) and determining bump height (using a ruler). Swelling height, swelling diameter (mean of the long and short axes), spread ratio (swelling diameter/swelling height), and swelling index (100/spread ratio) were used to quantify swelling after the 3.5-mL injections. Leakage during injection and after needle removal was noted as present or absent.
Statistical Analyses
All subjects who received at least one SC administration were included in the safety analyses. Descriptive statistics (means and standard deviations [SDs] for continuous variables; frequency counts and percentages for categorical variables) are provided for selected demographic data, baseline characteristics, and safety data. Least squares means (SD) are provided for swelling height, swelling diameter, spread ratio, and swelling index.
VAS scores were analyzed using a repeated measures analysis of variance (ANOVA). Volume/rate (treatment), time (immediately or 1 h after initiation of administration), treatment by time period, and sequence were independent variables, while subject within sequence was a random effect. For each treatment-by-time combination, the least squares mean is provided. The primary comparison was pair-wise comparisons of VAS scores assessed immediately after administration for 1.2 mL/5 s versus 3.5-mL/1 min, 3.5-mL/4 min, and 3.5-mL/10 min. Corresponding 95% confidence intervals (CIs) of the mean differences are provided. No adjustment was made for multiple comparisons. If leakage was observed, the associated VAS score was not included in the repeated measures ANOVA model. All analyses were conducted using SAS version 9.2 (SAS Institute Inc, Cary, NC).
The study was powered to detect a 15-mm difference in VAS assuming a within-subject SD of 21 mm based on previous experience with this scale. A study with 44 subjects (11 per sequence) would have 90% power to detect a 15-mm difference between treatments (α = 0.05, 2-sided). Allowing one extra subject per sequence for potential dropout, 48 healthy subjects were enrolled.
RESULTS
Twelve healthy volunteers were randomized to each of the four sequences. All 48 subjects completed the study. In one patient, 3.5 mL/10 min was given over 15 min, instead of the 10 min specified by the protocol. Mean age of subjects was 38.4 (11.6) years, range 19–55 years, 20 subjects (42%) were female, and most subjects were white (58%) (Table I).
Table I.
Subject Characteristics
| Characteristic | Sequence number | Total (n = 48) | |||
|---|---|---|---|---|---|
| 1 (n = 12) | 2 (n = 12) | 3 (n = 12) | 4 (n = 12) | ||
| Female, n (%) | 6 (50) | 8 (67) | 2 (17) | 4 (33) | 20 (42) |
| Race, n (%) | |||||
| White | 5 (42) | 9 (75) | 7 (58) | 7 (58) | 28 (58) |
| Black (or African American) | 5 (42) | 0 (0) | 5 (42) | 5 (42) | 15 (31) |
| Mixed race | 1 (8) | 3 (25) | 0 (0) | 0 (0) | 4 (8) |
| Asian | 1 (8) | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Age, mean (SD), years | 37.3 (11.0) | 38.2 (13.1) | 42.5 (12.2) | 35.8 (10.3) | 38.4 (11.6) |
| Body mass index, mean (SD), kg/m2 | 26.0 (2.9) | 25.7 (3.3) | 27.2 (3.8) | 26.5 (3.0) | 26.3 (3.1) |
SD standard deviation.
Order of injection administration: Sequence 1: 1.2 mL/5 s, 3.5 mL/1 min, 3.5 mL/4 min, 3.5 mL/10 min; Sequence 2: 3.5 mL/1 min, 3.5 mL/4 min, 1.2 mL/5 s, 3.5 mL/10 min; Sequence 3: 3.5 mL/4 min, 3.5 mL/10 min, 3.5 mL/1 min, 1.2 mL/5 s; Sequence 4: 3.5 mL/10 min, 1.2 mL/5 s, 3.5 mL/4 min, 3.5 mL/1 min.
Visual-Analog Scores and Pain Rankings
Immediately after SC administration and before needle removal, mean VAS scores were <20 mm for all four injections, with values ranging from 0 to 89 mm (Table II and Fig. 1). The lowest mean VAS score was reported for 3.5 mL/10 min (6.8 mm); slightly higher scores were reported for 1.2 mL/5 s and 3.5 mL/4 min (12.4 and 11.9 mm, respectively); and the highest score (19.1 mm) was associated with 3.5 mL/1 min.
Table II.
Summary of Visual Analog Scale Pain Scores and Pair-wise Comparisons of Least Square Mean Visual-Analog Scale Values for 3.5 mL Injected Over 1, 4, and 10 min to 1.2 mL Injected Over 5 s
| 1.2 mL/5 s | 3.5 mL/1 min | 3.5 mL/4 min | 3.5 mL/10 min | |
|---|---|---|---|---|
| Immediately after administration, before removal of needle | ||||
| No. of subjects | 48 | 48 | 48 | 46* |
| Mean (SD) (mm) | 12.4 (16.1) | 19.1 (19.7) | 11.9 (15.8) | 6.8 (9.2) |
| Comparison versus 1.2 mL/5 s† | ||||
| LS mean (SE) mm | – | 6.8 (1.9) | −0.5 (1.9) | −5.6 (1.9) |
| 95% CI (p value‡) | – | 3.0, 10.5 (<0.001) | −4.3, 3.2 (0.78) | −9.4, −1.8 (0.004) |
| One hour after initiation of administration | ||||
| No. of subjects | 48 | 48 | 48 | 48 |
| Mean (SD) (mm) | 1.6 (4.0) | 2.7 (3.7) | 3.1 (4.5) | 2.2 (3.4) |
| Comparison vs 1.2 mL/5 s† | ||||
| LS mean (SE) mm | – | 1.0 (1.9) | 1.5 (1.9) | 0.6 (1.9) |
| 95% CI (p value‡) | – | −2.7, 4.8 (0.58) | −2.2, 5.3 (0.43) | −3.2, 4.3 (0.77) |
SD standard deviation; LS least square; CI confidence interval; SE standard error.
*The score was omitted for two subjects in whom leakage occurred during injection.
† n = 48 for each comparison.
‡By analysis of variance.
Fig. 1.
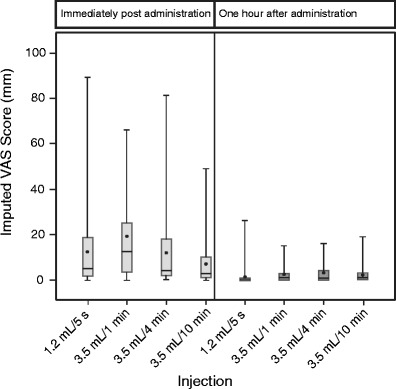
Visual-analog scale pain scores by treatment, immediately and 1 h after administration. Dots represent means, boxes represent Q1 to Q3 with lines showing medians, and whiskers indicate ranges
VAS scores measured 1 h after administration were significantly lower (<3.5 mm for all four injections) than those measured immediately after administration (Table II and Fig. 1).
Immediately after administration, 3.5 mL/1 min was reported to be more painful than 1.2 mL/5 s (6.8 [1.9] mm, p < 0.001) whereas 3.5 mL/10 min was less painful (−5.6 [1.9] mm, p = 0.004) (Table II). By 1 h after administration, no differences were apparent in VAS scores relative to 1.2 mL/5 s.
Three-quarters (n = 36) of subjects ranked pain associated with 3.5 mL/10 min as 1 or 2 (Fig. 2); this figure decreased to 56% (n = 27) of subjects for 1.2 mL/5 s, 48% (n = 23) for 3.5 mL/4 min, and 21% (n = 10) for 3.5 mL/1 min. The lowest overall mean (SD) pain ranking was 1.9 (1.0) for 3.5 mL/10 min, followed by 2.3 (1.3) for 1.2 mL/5 s, 2.6 (0.9) for 3.5 mL/4 min, and 3.1 (0.9) for 3.5 mL/1 min.
Fig. 2.
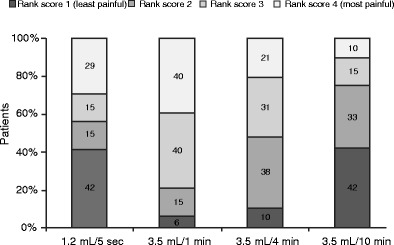
Pain rankings by treatment, after the fourth administration
Safety Data
Swelling height, diameter, spread ratios, and swelling indexes were similar among the three 3.5-mL injections (Table III). During administration, leakage was observed in two subjects, both during injection of 3.5 mL/10 min. After needle removal, leakage occurred in 31 (65%) subjects: 14 (29%) following 1.2 mL/5 s, eight (17%) following 3.5 mL/4 min, five (10%) following 3.5 mL/1 min, and four (8%) following 3.5 mL/10 min.
Table III.
Summary of Swelling after Needle Removal
| 3.5 mL/1 min(n = 48) | 3.5 mL/4 min(n = 48) | 3.5 mL/10 min(n = 48) | |
|---|---|---|---|
| Subjects with swelling, n | 16 | 15 | 12 |
| Swelling height (mm) | |||
| Mean (SD) | 1.7 (1.2) | 1.6 (0.8) | 2.2 (1.2) |
| Median (Q1, Q3) | 1.0 (1.0, 2.5) | 2.0 (1.0, 2.0) | 2.0 (1.3, 3.0) |
| Range | 0.3–5.0 | 0.5–3.0 | 0.5–5.0 |
| Swelling diameter (mm) | |||
| Mean (SD) | 20.7 (6.2) | 19.5 (6.5) | 22.9 (5.0) |
| Median (Q1, Q3) | 20.2 (15.8, 23.1) | 19.3 (15.0, 23.1) | 22.8 (20.1, 27.1) |
| Range | 10.8–31.8 | 6.6–34.1 | 15.0–31.3 |
| Spread ratio | |||
| Mean (SD) | 18.9 (17.7) | 15.4 (9.2) | 15.6 (13.5) |
| Median (Q1, Q3) | 14.5 (10.5, 17.6) | 10.7 (9.3, 23.1) | 11.0 (9.3, 19.8) |
| Range | 5.9–74.3 | 6.6–34.1 | 3.0–53.6 |
| Swelling index | |||
| Mean (SD) | 8.0 (4.4) | 8.5 (3.9) | 10.6 (8.5) |
| Median (Q1, Q3) | 6.9 (5.7, 9.5) | 9.3 (4.3, 10.8) | 9.1 (5.1, 10.7) |
| Range | 1.4–17.1 | 2.9–15.2 | 1.9–33.4 |
LS least square; Q quartile; SD standard deviation; spread ratio = swelling diameter/swelling height; swelling index = 100/spread ratio.
Of the 48 subjects, 15 (31%) experienced one or more treatment-emergent adverse events: one following 1.2 mL/5 s, nine following 3.5 mL/1 min, and five each following 3.5 mL/4 min and 3.5 mL/10 min. None of the adverse events was serious, fatal, or led to discontinuation from the study. The most common adverse events were erythema (eight subjects; 17%), headache (three subjects; 6%), and abdominal distension (two subjects; 4%) or contusion (two subjects; 4%). All cases of erythema were considered treatment related and occurred during injection of 3.5 mL over 1, 4, and 10 min (4, 4, and 3 subjects, respectively). No cases occurred after injection of 1.2 mL/5 s.
DISCUSSION
This single-center, crossover study was designed to compare VAS scores associated with three 3.5-mL SC injection durations to that associated with a 1.2-mL SC bolus injection and to investigate tolerability, swelling, and leakage from the injection site immediately after administration and 1 h later. As anticipated, immediately after administration, the range of mean VAS scores indicated that least pain was associated with the longest injection (3.5 mL/10 min) and most pain was associated with the shortest injection (3.5 mL/1 min). When comparing the mean VAS scores for the 3.5-mL injections with that for the 1.2-mL injection, statistically significant differences were apparent for two injections, with more pain associated with 3.5 mL/1 min and less pain associated with 3.5 mL/10 min. However, the differences were small (6.8 and 5.6 mm, respectively), and may not be clinically meaningful, since the minimum clinically meaningful difference on a VAS is 13 mm [23–25]. At 1 h after treatment, all VAS scores were close to zero, indicating that the injection pain experienced upon administration was transient.
The data from this study indicated that all four injections have acceptable tolerability. No subjects developed a serious adverse event. The most common adverse event was erythema, in 17% of subjects, which was associated with all three 3.5-mL injections but not with the 1.2-mL injection. There was no correlation between rate of 3.5-mL SC injection and incidence of erythema, so erythema may be a general response to the placebo formulation. Na-CMC was included in the formulation to achieve the required viscosity. While it is generally recognized as a safe polymer, is used as a general purpose food additive and is incorporated into several marketed drugs [29], rare cases of anaphylactic reactions have been reported in patients receiving drugs containing Na-CMC [30, 31]. These data on tolerability at different delivery rates are in general accord with findings in the SC treatment of primary immunodeficiency [32].
The study investigated the effect of infusion duration on leakage of clear fluid or blood at the injection site, which is common during bolus SC injection. Leakage assessments were observational in nature and were conducted to gain a high-level understanding of the impact of 3.5-mL injections on distribution of the viscous placebo in the SC space and any potential leakage from the injection site due to the large volume administered. Similar values for spread ratio and swelling index for the three 3.5-mL injection durations indicated that the distribution of fluid in the SC space was similar irrespective of injection rate. Leakage of clear fluid during the injection was seen in two subjects (4%) who received the 10-min injection. After removal of the needle, leakage was most frequently observed after the 1.2-mL injection, and the incidence of leakage decreased with increasing duration of injection. Together, these data indicate that SC injections of up to 3.5 mL are feasible, even when administered as fast as 1 min.
Studies on large-volume SC therapy for primary immunodeficiency indicate that patients tend to prefer SC over intravenous injection, as it provides them with more autonomy, causes fewer adverse systemic reactions, and obviates the need for vascular access [33]. In a retrospective study in primary immunodeficiency, patients were given the choice of SC immunoglobulin replacement therapy either by infusion pump or by rapid push administration. Of the 104 patients, 71% chose the rapid push, with a volume per site of 3 to 20 mL, administered over 5–30 min [22]. The results from a US phase 3 non-comparative extension study in primary immunodeficiency found that health-related quality of life remained largely unchanged and treatment satisfaction was high in patients previously treated with intravenous immunoglobulin therapy who switched to a self-administered SC equivalent [34]. This analysis was, however, conducted in a very small subset of patients (n = 16), and the findings require confirmation in a larger trial. In patients with breast cancer, the monoclonal antibody trastuzumab given as a 5-mL SC injection of 600 mg trastuzumab plus 10,000 units human recombinant DNA-derived hyaluronidase enzyme by slow manual push over 5 min was found to be non-inferior to the intravenous standard of care [35]. Trastuzumab administered by the SC route offers a faster and less invasive mode of administration, with equivalent efficacy, compared with the intravenous formulation, and is preferred by patients [21].
Increasingly, biotherapeutic medications are being developed for SC delivery [36], and methods for facilitating the injection of the requisite large volumes are the subject of investigation. To address this challenge, we needed to evaluate the tolerability of SC administration of >1 mL of a formulation that has a viscosity, osmolality, and pH mimicking those of protein formulations. Since the volume studied in this trial was 3.5 mL, it would not be feasible to administer it as a short bolus push; hence, a constant infusion over various durations was employed. We also excluded the use of excipients such as hyaluronidase, as this recombinant cannot be used in association with several commonly used medications.
Potential limitations of this study include that the 1.2 mL bolus control was chosen based on experience and not on published literature and that study personnel who assessed adverse events were not blinded to treatment.
CONCLUSION
Subcutaneous injection of 3.5 mL of a viscous placebo buffer, with the characteristics of a typical protein formulation, administered over 1 min was associated with more pain than a 1.2-mL bolus injection, but administered over 10 min was associated with less pain than the bolus injection, but the differences were not considered clinically meaningful. This suggests that it may be possible to reduce the number of injections per biotherapeutic treatment, through the injection of larger SC dose volumes using a prefilled syringe, autoinjector, or other personal injection device.
Acknowledgments
This study was sponsored by Amgen. Merrill Goldenberg helped to provide the formulation and select the infusion pump. Lucy Hyatt, PhD (Amgen) and Sophie Rushton-Smith, PhD (Medlink Healthcare Communications Ltd, UK) provided writing support, funded by Amgen. J Greg Slatter (Amgen) provided comments on the draft.
REFERENCES
- 1.Anderson G, Meyer D, Herrman CE, Sheppard C, Murray R, Fox EJ, et al. Tolerability and safety of novel half milliliter formulation of glatiramer acetate for subcutaneous injection: an open-label, multicenter, randomized comparative study. J Neurol. 2010;257(11):1917–23. doi: 10.1007/s00415-010-5779-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun J, Deodhar A, Inman RD, van der Heijde D, Mack M, Xu S, et al. Golimumab administered subcutaneously every 4 weeks in ankylosing spondylitis: 104-week results of the GO-RAISE study. Ann Rheum Dis. 2012;71(5):661–7. doi:10.1136/ard.2011.154799. [DOI] [PMC free article] [PubMed]
- 3.Davies M, Heller S, Sreenan S, Sapin H, Adetunji O, Tahbaz A, et al. Once-weekly exenatide versus once- or twice-daily insulin detemir: randomized, open-label, clinical trial of efficacy and safety in patients with type 2 diabetes treated with metformin alone or in combination with sulfonylureas. Diabetes Care. 2013;36(5):1368–76. doi: 10.2337/dc12-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE-TIMI 57): a randomised, placebo-controlled, dose-ranging, phase 2 study. Lancet. 2012;380(9858):2007–17. doi: 10.1016/S0140-6736(12)61770-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84(5):548–58. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 6.Frost GI. Recombinant human hyaluronidase (rHuPH20): an enabling platform for subcutaneous drug and fluid administration. Expert Opin Drug Deliv. 2007;4(4):427–40. doi: 10.1517/17425247.4.4.427. [DOI] [PubMed] [Google Scholar]
- 7.Wynne C, Harvey V, Schwabe C, Waaka D, McIntyre C, Bittner B. Comparison of subcutaneous and intravenous administration of trastuzumab: a phase I/Ib trial in healthy male volunteers and patients with HER2-positive breast cancer. J Clin Pharmacol. 2013;53(2):192–201. doi: 10.1177/0091270012436560. [DOI] [PubMed] [Google Scholar]
- 8.Hylenex prescribing information. 2012 [12 April 2014]; Available from: http://www.hylenex.com/files/HylenexPackageInsertSep2012.pdf.
- 9.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 10.Jackevicius CA, Mamdani M, Tu JV. Adherence with statin therapy in elderly patients with and without acute coronary syndromes. JAMA. 2002;288(4):462–7. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 11.Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 12.Benner JS, Glynn RJ, Mogun H, Neumann PJ, Weinstein MC, Avorn J. Long-term persistence in use of statin therapy in elderly patients. JAMA. 2002;288(4):455–61. doi: 10.1001/jama.288.4.455. [DOI] [PubMed] [Google Scholar]
- 13.Oldfield V, Plosker GL. Golimumab: in the treatment of rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. BioDrugs. 2009;23(2):125–35. doi: 10.2165/00063030-200923020-00005. [DOI] [PubMed] [Google Scholar]
- 14.Keystone EC, Kavanaugh AF, Sharp JT, Tannenbaum H, Hua Y, Teoh LS, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400–11. doi: 10.1002/art.20217. [DOI] [PubMed] [Google Scholar]
- 15.Black DM, Bilezikian JP, Greenspan SL, Wuster C, Munoz-Torres M, Bone HG, et al. Improved adherence with PTH(1-84) in an extension trial for 24 months results in enhanced BMD gains in the treatment of postmenopausal women with osteoporosis. Osteoporos Int. 2013;24(4):1503–11. doi:10.1007/s00198-012-2098-3. [DOI] [PMC free article] [PubMed]
- 16.Bode BW, Sabbah HT, Gross TM, Fredrickson LP, Davidson PC. Diabetes management in the new millennium using insulin pump therapy. Diabetes/Metab Res Rev. 2002;18(Suppl 1):S14–20. doi: 10.1002/dmrr.205. [DOI] [PubMed] [Google Scholar]
- 17.Diamant M, Van Gaal L, Stranks S, Northrup J, Cao D, Taylor K, et al. Once weekly exenatide compared with insulin glargine titrated to target in patients with type 2 diabetes (DURATION-3): an open-label randomised trial. Lancet. 2010;375(9733):2234–43. doi: 10.1016/S0140-6736(10)60406-0. [DOI] [PubMed] [Google Scholar]
- 18.Allen LV Jr, Popovich NG, Ansel HC. Pharmaceutical Dosage Forms and Drug Delivery Systems. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004.
- 19.Pfizer. Prescribing information for XOLAIR® (omalizumab) for injection, for subcutaneous use. 2014; Available from: http://www.pharma.us.novartis.com/product/pi/pdf/Xolair.pdf.
- 20.Kukreti K, Ng P, Incekol D, et al. Tolerability of Velcade (bortezomib) subcutaneous administration using a maximum 3 mL per injection site. Clin Lymphoma Myeloma Leuk. 2013;13:S232. [DOI] [PubMed]
- 21.Pivot X, Gligorov J, Muller V, Barrett-Lee P, Verma S, Knoop A, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14(10):962–70. doi: 10.1016/S1470-2045(13)70383-8. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro R. Subcutaneous immunoglobulin therapy by rapid push is preferred to infusion by pump: a retrospective analysis. J Clin Immunol. 2010;30(2):301–7. doi: 10.1007/s10875-009-9352-2. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher EJ, Liebman M, Bijur PE. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann Emerg Med. 2001;38(6):633–8. doi: 10.1067/mem.2001.118863. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher JE, Bijur PE, Latimer C, Silver W. Reliability and validity of a Visual Analog Scale for acute abdominal pain in the ED. USA: Elsevier Science; 2002. [DOI] [PubMed] [Google Scholar]
- 25.Todd KH, Funk KG, Funk JP, Bonacci R. Clinical significance of reported changes in pain severity. Ann Emerg Med. 1996;27(4):485–9. doi: 10.1016/S0196-0644(96)70238-X. [DOI] [PubMed] [Google Scholar]
- 26.Averbuch M, Katzper M. Assessment of visual analog versus categorical scale for measurement of osteoarthritis pain. J Clin Pharmacol. 2004;44(4):368–72. doi: 10.1177/0091270004263995. [DOI] [PubMed] [Google Scholar]
- 27.Williams EJ. Environmental designs balanced for the estimation of residual effects of treatments. Aust J Sci Res. 1949;2(2):149–68. [Google Scholar]
- 28.U.S. department of health and human services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. 2009; Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- 29.FDA. Database of Select Committee on GRAS Substances (SCOGS) Reviews. Carboxymethyl cellulose. FDA; 1973.
- 30.Dumond P, Franck P, Morisset M, Sainte Laudy J, Kanny G, Moneret-Vautrin DA. Pre-lethal anaphylaxis to carboxymethylcellulose confirmed by identification of specific IgE–review of the literature. Eur Ann Allergy Clin Immunol. 2009;41(6):171–6. [PubMed] [Google Scholar]
- 31.Hamada T, Horiguchi S. Allergic contact dermatitis due to sodium carboxymethyl cellulose. Contact Dermatitis. 1978;4(4):244. doi: 10.1111/j.1600-0536.1978.tb03809.x. [DOI] [PubMed] [Google Scholar]
- 32.Hansen S, Gustafson R, Smith CI, Gardulf A. Express subcutaneous IgG infusions: decreased time of delivery with maintained safety. Clin Immunol. 2002;104(3):237–41. doi: 10.1006/clim.2002.5215. [DOI] [PubMed] [Google Scholar]
- 33.Berger M. Subcutaneous immunoglobulin replacement in primary immunodeficiencies. Clin Immunol. 2004;112(1):1–7. doi: 10.1016/j.clim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Jones CA, Rojavin M, Baggish JS. Patients with primary immunodeficiency receiving subcutaneous immune globulin Hizentra maintain health-related quality of life and treatment satisfaction in a multicentre extension study of efficacy, tolerability and safety. J Pharm Health Serv Res. 2012;3(1):41–7. doi: 10.1111/j.1759-8893.2011.00076.x. [DOI] [Google Scholar]
- 35.Ismael G, Hegg R, Muehlbauer S, Heinzmann D, Lum B, Kim SB, et al. Subcutaneous versus intravenous administration of (neo)adjuvant trastuzumab in patients with HER2-positive, clinical stage I-III breast cancer (HannaH study): a phase 3, open-label, multicentre, randomised trial. Lancet Oncol. 2012;13(9):869–78. doi: 10.1016/S1470-2045(12)70329-7. [DOI] [PubMed] [Google Scholar]
- 36.Kang DW, Oh DA, Fu GY, Anderson JM, Zepeda ML. Porcine model to evaluate local tissue tolerability associated with subcutaneous delivery of protein. J Pharmacol Toxicol Methods. 2013;67(3):140–7. doi: 10.1016/j.vascn.2013.01.011. [DOI] [PubMed] [Google Scholar]


