Abstract
The objective of the present study is to investigate the confounding effects, if any, of beta-cyclodextrins (βCDs) on corneal permeability coefficients obtained from in vitro transmembrane diffusion studies. Transcorneal permeability studies were carried out with 2-hydroxypropyl-beta-cyclodextrin (HPβCD) and randomly methylated-beta-cyclodextrin (RMβCD) at 5 and 2.5%w/v in isotonic phosphate-buffered solution (IPBS) (pH 7.4). Rabbit corneas received from Pel-Freez Biologicals® were used for these studies. Propranolol hydrochloride (PHCl) (1 mg/mL) was used as the paracellular permeability marker. A series of permeation studies were carried out with IPBS as the control, with CDs on the donor side only, CDs on the receiver side only, and CDs on both the donor and receiver sides. At the end of 1 or 3 h, corneas were collected and fixed using a solution containing 2%v/v glutaraldehyde + 2%w/v paraformaldehyde + IPBS and histological examinations were performed (Excalibur Pathology, Inc). The order of transcorneal permeability of PHCl was found to be CDs on the receiver side > control (no CDs) ≈ CDs on both the receiver and donor sides > CDs on the donor side. Histology studies revealed that the corneal epithelial and endothelial layers remained intact in the control sets. Damage to the cornea was observed in the order of CDs on the receiver side > CDs on the donor side > CDs on both sides > control. The use of CDs in solutions for in vitro permeation experiments with rabbit corneas needs to be carefully considered to avoid confounding effects in the data obtained.
KEY WORDS: corneal histology, cyclodextrins, in vitro transcorneal permeability, morphology, propranolol hydrochloride
INTRODUCTION
Cyclodextrins (CDs) are a group of cyclic oligosaccharides with a hydrophobic inner core and a hydrophilic outer surface. Based on the number of glucopyranose units in the structure, they are classified into alpha (α), beta (β), and gamma (γ) CDs (6, 7, and 8 units, respectively). Over the last few decades, CDs have emerged as an important pharmaceutical excipient for solubility enhancement of lipophilic drugs and permeability improvement across biological membranes (1). CDs act as penetration enhancers by increasing the availability of drug molecules at the surface of the biological membrane barrier. Because of their aqueous solubility improving characteristics, suitable cavity size, and drug complexation efficiency (1), beta-cyclodextrins (βCDs; Fig. 1a) such as 2-hydroxypropyl-beta-cyclodextrin (HPβCD; Fig. 1b) and randomly methylated-beta-cyclodextrin (RMβCD; Fig. 1c) are widely used in the field of formulation and drug delivery.
Fig. 1.
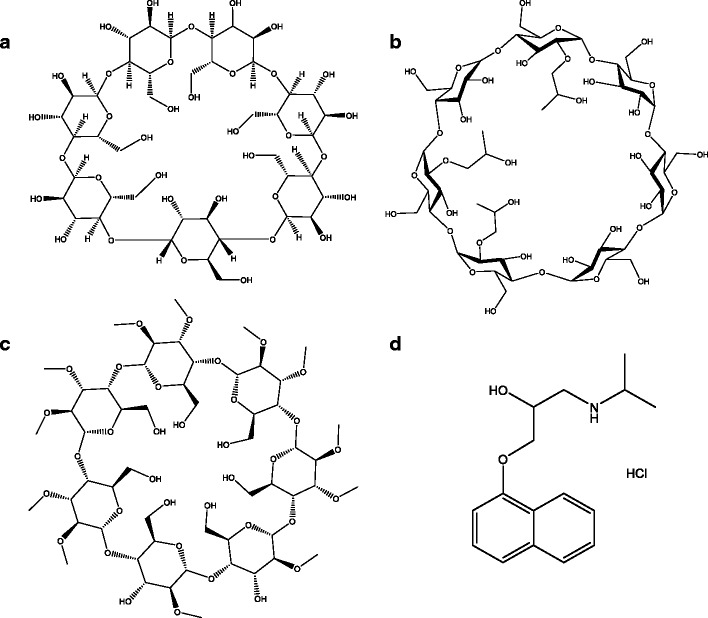
Chemical structure of a general structure of beta cyclodextrin (β-CD), b hydroxypropyl-βeta-cyclodextrin (HPβCD), c randomly methylated-βeta-cyclodextrin (RMβCD), and d propranolol (PHCl)
It is only recently that the mechanism of permeability enhancement across biological membranes, such as the skin, buccal mucosa, and cornea, have been discussed. CDs interact with cholesterol present in the phospholipid monolayers of the cell membranes, thus exchanging them with the drugs/drug candidates held in the hydrophobic CD core (Fig. 2). In addition to their utility as transcorneal permeability enhancers, by virtue of their ability to extract lipophilic components like cholesterol and phospholipids from the corneal membrane (2), CDs are often added to the receiver solutions to maintain sink conditions during in vitro transcorneal permeability studies of lipophilic molecules (3). Unknowingly damaging the cornea (epithelium, stroma lamellae, or endothelium), however, may lead to altered permeability of the drug/drug candidates as a result of the receiver solution characteristics rather than formulation or drug/prodrug candidate properties. This can lead to misinterpretation of the results and overestimation of the permeability enhancement of the formulation/drug candidate if adequate controls were not included in the experiment design. Thus, an understanding of the effect of CDs on the corneal permeability characteristics, when added as a solubilizer in the receiver solution, is very important and has not been analyzed as yet. The aim of the present study is to evaluate the effect of CD concentration, duration, and type on the morphological characteristics and barrier properties, using propranolol hydrochloride (PHCl; Fig. 1d) as a paracellular diffusion marker, of isolated rabbit cornea in vitro.
Fig. 2.
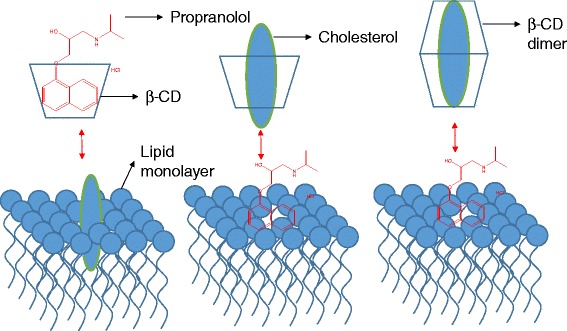
Extraction of cholesterol and the exchange of active moiety by cyclodextrins from the phospholipid cell membrane
MATERIALS AND METHODS
Chemicals
Propranolol hydrochloride (PHCl), 2-hydroxypropyl-beta-cyclodextrin (HPβCD), randomly methylated-beta-cyclodextrin (RMβCD), and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Animal Tissues
Whole eye globes of New Zealand albino rabbits were purchased from Pel-Freez Biologicals® (Rogers, AK), shipped overnight in hanks balanced salt solution (HBSS) over wet ice. Corneas excised from the whole eye globes were used immediately on the day of receipt.
Phase Solubility Studies
Complexation of PHCl with HPβCD and RMβCD was evaluated using phase solubility studies according to the method of Higuchi and Connors (4). Excess amount of PHCl was added to 1 mL IPBS, containing increasing concentrations of CDs. The concentrations ranged from 2.5–10%w/v. The resulting suspensions were shaken at 25°C for 24 h in a reciprocating water bath. Following equilibration, the suspensions were centrifuged at 13,000 rpm for 20 min at 25°C and the supernatant thus obtained was analyzed using an HPLC-UV method.
Phase solubility profile was obtained by plotting the solubility of PHCl against the concentration of CDs used. The binding constants (K1:1) and complexation efficiencies (CE) for the PHCl-CD complex were calculated from the linear region of the solubility curves using Eq. 1 and 2:
| 1 |
| 2 |
where K1:1 is the binding constant, So is the saturation concentration of PHCl in pure water, and slope denotes the slope of the straight line.
In vitro Transcorneal Permeability
A series of in vitro transcorneal permeability studies were carried out using a 9-mm side-by-side diffusion apparatus (PermeGear Inc., Hellertown, PA). Freshly excised rabbit whole eye globes received from Pel-Freez Biologicals® were used for these studies. Briefly, corneas were excised by making a brief incision, about 2 mm from the corneal-scleral junction and cutting radially along the sclera. The excised corneas were immediately mounted between the diffusion cells. The half-cell facing the epithelial layer was termed as the donor compartment, and the other half towards the endothelium was termed as the receiver chamber. The nomenclature is based on the addition of PHCl to the epithelial side half-cell. A circulating water bath was used to maintain the temperature at 34°C during the transport studies.
In vitro transcorneal permeability studies were carried out with 2.5 and 5%w/v HPβCD and RMβCD in IPBS (pH 7.4) for 1 or 3 h at 34°C. PHCl (1 mg/mL) was used as the paracellular marker (PHCl; pKa = 9.4). PHCl solution was always added on the donor side. Permeation studies were performed with IPBS as control; with CDs on the donor side only, CDs on the receiver side only, and CDs on both the donor and receiver sides as represented in Table I. Aliquots, 600 μL, were withdrawn at predetermined time points for 1 or 3 h and replaced with an equal volume of the receiver solution. Samples were analyzed by HPLC-UV system.
Table I.
In vitro Transcorneal Permeability Coefficients of PHCl at Different Concentrations of CDs. (I) IPBS without CDs (Control; 1), (II) CDs in the Donor Solution (3 and 6), (III) CDs in the Receiver Solution (2 and 5), and (IV) CDs in Both the Donor and Receiver Solutions (4 and 7). Experiments were Carried out for 1 or 3 h
| S. No | Donor | Receiver | Permeability × 106 cm/s | |
|---|---|---|---|---|
| 3 ml | 3.2 ml | 5% w/v CDs | 2.5% w/v CDs | |
| 1 | IPBS | IPBS | 27.0 ± 1.3 | 21.3 ± 1.0 |
| 2 | IPBS | HPβCD | 40.8 ± 5.7 | 32.2 ± 4.5 |
| 3 | HPβCD | IPBS | 8.1 ± 1.2 | 6.4 ± 1.0 |
| 4* | HPβCD | HPβCD | 17.6 ± 1.1 | 13.8 ± 0.9 |
| 5 | IPBS | RMβCD | 52.1 ± 8.4 | 41.3 ± 6.6 |
| 6 | RMβCD | IPBS | 14.5 ± 5.2 | 11.4 ± 4.1 |
| 7a | RMβCD | RMβCD | 24.5 ± 3.4 | 19.2 ± 2.7 |
CDs cyclodextrins, IPBS isotonic phosphate-buffered solution, HPβCD 2-hydroxypropyl-beta-cyclodextrin, RMβCD randomly methylated-beta-cyclodextrin
aExperiments were carried out for 1 and 3 h. Permeability coefficients were calculated only for a 1-h experiment.
Corneal Histology
Histological evaluation was carried out at Excalibur Pathology Inc. (Oklahoma City, OK). At the end of each study, corneas were fixed in 2%w/v paraformaldehyde and 2%v/v gluteraldehyde in IPBS. Corneas embedded in paraffin were sliced into 5-μm cross sections using a microtome (American Optical® 820 Rotary Microtome). These sections were mounted on a slide and dried overnight in an oven. The slide was washed with xylene to remove the paraffin and washed with alcohol and water to hydrate the tissue. This was then stained with nuclear dye Gill III hematoxylin (StatLab medical) for 10 min and rinsed, and then counterstained with eosin. These slides were then washed in reverse manner (running water, alcohol, and xylene), cover slipped, and examined under microscope (Chromavision ACIS II).
Analytical Method for Measurement of PHCl
Waters HPLC system with 600E pump controller, 717 plus autosampler, and 2487 UV detector was used. Data handling was carried out using an Agilent 3395 integrator. Mobile phase consists of 160 mL of water, 180 mL of methanol, 70 mL of acetonitrile, 2.5 mL of acetic acid, and 125 μL of triethylamine (v/v). The pH of the whole mixture was adjusted to 3.4. Phenomenex Luna® 5 μm C18 100 Å, 250 × 4.6 mm column was used at a flow rate of 0.5 mL/min and detection wavelength of 291 nm.
RESULTS
Phase Solubility Studies
Binding constants of PHCl with HPβCD and RMβCD were very low (278.1 and 326.4 μM−1, respectively). Complexation efficiency of PHCl with HPβCD and RMβCD were 0.94 and 1.1, respectively. RMβCD was observed to have slightly higher CE with PHCl than HPβCD. Low-binding constant values demonstrate that only a low fraction of PHCl was bound to either of the CDs and that there was more free concentrations of CDs and PHCl in the solution.
CDs on the Receiver Side
With 5%w/v HPβCD and RMβCD on the receiver side, permeability coefficient of PHCl at the end of 1 h was found to be 40.8 ± 5.7 × 10−6 and 52.1 ± 8.4 × 10−6 cm/s, respectively (1.5-fold and 1.9-fold higher than the control) (Table I). Similar results were encountered with the use of 2.5% w/v HPβCD and RMβCD on the receiver side at the end of 1 h (32.2 ± 4.9 × 10−6 and 41.3 ± 6.6 × 10−6 cm/s, respectively).
Histological studies at the end of 1 h revealed damage to both the endothelium and epithelium in the case of 5%w/v HPβCD on the receiver side. Stroma lamellae were found to be disrupted at several points on the cornea. When 5% w/v RMβCD was used on the receiver side, less damage was caused to the epithelium, and little or no damage to the endothelium was observed. HPβCD at 2.5% w/v caused significant rupture of the corneal epithelium, but the endothelium was found to be intact. At the same concentration, RMβCD showed little damage to the epithelium and none to the endothelium. These studies revealed that more damage was caused when HPβCD is used in the receiver solution in comparison to RMβCD (both 5% w/v and 2.5% w/v) (Figs. 3 and 4).
Fig. 3.
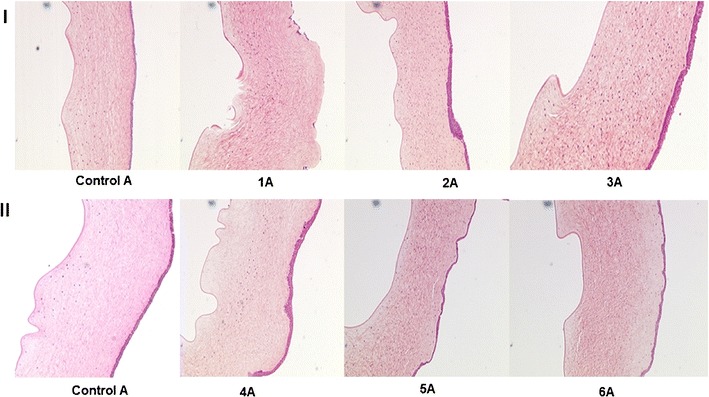
Hematoxylin-eosin-stained corneal cross-sections 1 h postexposure to 1 IPBS: control A, 1A 5% w/v HPβCD in the receiver solution, 2A 5% w/v HPβCD in the donor solution, 3A 5% w/v HPβCD in the receiver and donor solutions, 4A 5% w/v RMβCD in the receiver solution, 5A 5% w/v RMβCD in the donor solution, and 6A 5% w/v RMβCD in the receiver and donor solutions
Fig. 4.
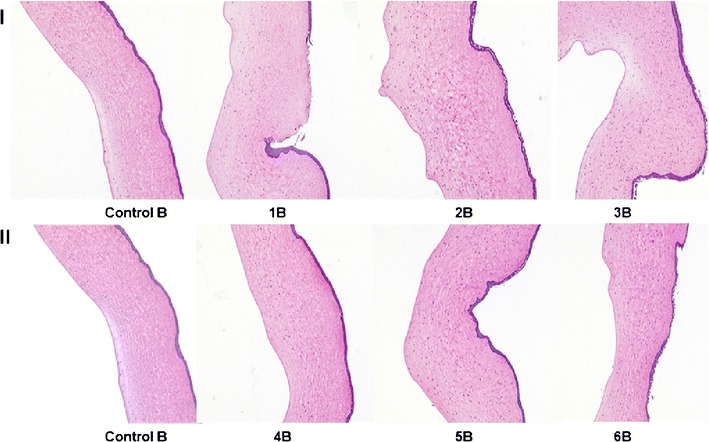
Hematoxylin-eosin-stained corneal cross-sections 1 h postexposure to 1 IPBS: Control B, 1B 2.5% w/v HPβCD in the receiver solution, 2B 2.5% w/v HPβCD in the donor solution, 3B 2.5% w/v HPβCD in the receiver and donor solutions, 4B 2.5% w/v RMβCD in the receiver solution, 5B 2.5% w/v RMβCD in the donor solution, and 6B 2.5% w/v RMβCD in the receiver and donor solutions
CDs on the Donor Side
The permeability coefficients of PHCl at the end of 1 h with 5%w/v HPβCD and RMβCD were found to be 8.1 ± 1.2 × 10−6 and 14.5 ± 5.2 × 10−6 cm/s. Permeability coefficient of PHCl in IPBS (control) was found to be 27.02 ± 1.2 × 10−6 cm/s. Permeability coefficients of PHCl were also observed to be lower than the control in case of 2.5% w/v HPβCD and RMβCD (Table I).
Histological studies revealed rupture of the corneal epithelium with the use of 5% w/v and 2.5% w/v HPβCD in the donor solution. With 5% w/v and 2.5% w/v RMβCD on the donor side, little or no damage was caused to the corneal epithelium. In the case of the control group, corneal morphology was found to be intact. In all the studies, HPβCD and RMβCD in the donor chamber, at the concentrations used, did not cause any damage to the endothelium (Figs. 3 and 4).
CDs in Both the Donor and Receiver Chamber
Surprisingly, use of 5%w/v and 2.5%w/v RMβCD in both the donor and receiver chambers simultaneously did not show significant difference compared to the control. Permeability coefficients of PHCl in the control, 5%w/v HPβCD and 5%w/v RMβCD solutions on the donor and the receiver sides were observed to be 27.0 ± 1.3 × 10−6, 17.6 ± 1.1 × 10−6, and 24.5 ± 3.4 × 10−6 cm/s, respectively. Permeability coefficients of PHCl in the control, 2.5%w/v HPβCD and 2.5%w/v RMβCD solutions on the donor and the receiver sides were observed to be 21.3 ± 1.03 × 10−6, 13.8 ± 0.9 × 10−6, and 19.2 ± 2.7 × 10−6 cm/s, respectively. On an average, a 20% drop in the permeability was observed as a function of 50% drop in CD concentrations.
No damage was observed to the corneal epithelia with 5% w/v and 2.5% w/v RMβCD. At the end of 1 h, when 5% w/v and 2.5% w/v HPβCD solution was used in both the donor and receiver chambers, the corneal epithelia were found to be damaged. In all cases, endothelia were found to be intact without any significant damage compared to the control (Figs. 3 and 4). When corneas were exposed to 2.5% w/v HPβCD on both sides for 3 h, complete erosion of the epithelium was observed, exposing the stroma. But no significant damage to the endothelium was noted (Fig. 5).
Fig. 5.
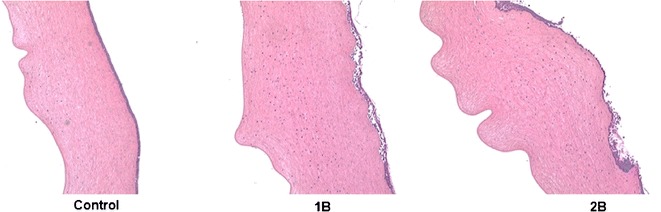
Hematoxylin-eosin-stained corneal cross-sections 3 h postexposure to 1 IPBS: control, 1B 2.5% w/v HPβCD in the receiver and donor solutions, and 2B 2.5% w/v RMβCD in the receiver and donor solutions
DISCUSSION
CDs have become important pharmaceutical excipients due to their ability to improve solubility of lipophilic molecules and to enhance permeability across biological membranes. It is considered that CDs increase permeability across biological membranes through their surface activity, membrane partitioning, and cholesterol extraction characteristics (5,6).
In the present study, we used rabbit corneas because of their similarities to human corneal ultra-structure (7) and common use in in vitro studies evaluating corneal drug penetration. We selected HPβCD and RMβCD because of their widespread use in the ocular formulations and permeability studies. Permeability coefficients of PHCl were in the order of CDs on the receiver side > control > CDs on both sides > CDs on the donor side. Corneal histology revealed the damage to the membranes in the order of CDs on the receiver side > CDs on the donor side > CDs on both sides > control. CDs after forming inclusion complexes are known to improve permeability of compounds across the biological membranes. Thus, we observed higher permeability coefficients with the RMβCD-PHCl complex which could be because of the higher CE of PHCl with RMβCD compared to HPβCD.
RMβCD produced less corneal damage than HPβCD. This is in agreement with previous literature reports. Savolainen et al. studied toxicity/irritation caused by CDs on human corneal epithelial cell lines and observed that the cytotoxic effect was in the order of αCD > dimethyl-βCD > SBEβCD = HPβCD > γCD (9). In another study, Lopez et al. performed a computational simulation of CDs interacting with cholesterol (8). It was reported that within 10 ns of the initial interaction, CDs orient in a perpendicular direction to the surface of the phospholipid monolayer and the hydroxyl groups of membrane cholesterol penetrate into the inner core of the CD. On an average, rate of extraction of one cholesterol molecule per CD was found to be 0.5 μs. Based on the stoichiometric ratios, it was observed that one HPβCD is required to extract one molecule of cholesterol or membrane lipid forming a 1:1 soluble complex (9). In some cases, two molecules of CDs interact with one another in a head-head, head-tail, or tail-tail fashion to form a dimer (10). Both head-tail and tail-tail interactions are less stable than head-head interactions due to their spontaneous dissociation in water. Tsamaloukas et al. reported that extraction of one molecule of cholesterol/membrane lipid requires two molecules of RMβCD stoichiometrically (cholesterol: RMβCD::1:2), since the formation of 1:1 complex is not a spontaneous process (11). These reports suggest that at a given concentration, the number of cholesterol molecules extracted per RMβCD is less compared to HPβCD. This could probably explain why RMβCD caused less damage to the corneal membranes.
Besides the type of CDs used, the duration of contact and concentration used are other important parameters. Duckner et al. studied the effect of HPβCD concentration on porcine corneal endothelium (12). It was observed that use of 10%w/v HPβCD caused high grade of endothelial damage followed by minimal damage with 1%w/v HPβCD and none with 0.1%w/v HPβCD within 3 h of the study. Similar observations were recorded in our studies also. In the current report, use of 5%w/v HPβCD caused severe damage to the corneal epithelium and endothelium layers within 3 h of exposure. Disruptions in the stromal lamellae were also observed when CDs were employed in the receiver media. Damage to the corneal layers was observed to be minimum with the use of 2.5%w/v CDs. In our study, we observed that 2.5%w/v RMβCD causes minimal damage compared to HPβCD, but only after 3 h of exposure.
In the current study, permeability coefficients of PHCl were found to be higher when CDs were taken on the receiver side rather than on the donor side. This was attributed to the fact that the corneal endothelium is more permeable when compared to the epithelium (13). Due to the concentration gradient, CDs incorporated in the receiver side probably cross the endothelium and stroma and interacts with basal cells and tight junctions of the corneal epithelium, thus causing more damage to the epithelia and endothelia. In contrast, CDs on the donor side are unable to pass through the non-keratinized squamous cells of the epithelia; thus causing less damage to the cornea. When CDs were employed on both the donor and receiver side, due to the absence of net concentration gradient, little or no change was observed in the corneal integrity. But prolonged exposure (3 h) to CD solutions leads to the loss of the ZO-1, initiating significant damage and complete erosion of the corneal epithelium. As a result, we observed severe damage of the corneal epithelia after 3-h exposure of the cornea to both HPβCD and RMβCD.
Several studies in the literature, including some from our own laboratory, have used CDs in the receiver solution during in vitro transcorneal permeability evaluation (14–20). In view of the findings from this study, it is apparent that use of CDs as solubilizers in the receiver solution could significantly impact transcorneal flux. Thus, it is very important to understand and use proper controls and experimental design during in vitro transcorneal permeability studies using CDs.
CONCLUSION
The effect of CDs on the corneal membrane in vitro was observed to be dependent on factors such as type of CDs employed, type of inclusion complex formed, CD concentration, and the duration of exposure to the biological membrane. Thus, the use of CDs in in vitro transcorneal permeation experiments as solubilizers in the receiving medium, especially when used only on one side of the tissue, needs to be carefully considered to avoid confounding effects in the permeability data obtained due to damage to the corneal ultra-structure.
ACKNOWLEDGEMENTS
This work was supported by the National Institute of General Medical Sciences, National Institutes of Health Grant P20GM104932 and SBAHQ-10-I-0309. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Loftsson T, Brewster ME. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J Pharm Sci. 1996;85(10):1017–25. doi: 10.1021/js950534b. [DOI] [PubMed] [Google Scholar]
- 2.Siefert B, Keipert S. Influence of alpha-cyclodextrin and hydroxyalkylated beta-cyclodextrin derivatives on the in vitro corneal uptake and permeation of aqueous pilocarpine-HCl solutions. J Pharm Sci. 1997;86(6):716–20. doi: 10.1021/js960389h. [DOI] [PubMed] [Google Scholar]
- 3.Masson M, Loftsson T, Masson G, Stefansson E. Cyclodextrins as permeation enhancers: some theoretical evaluations and in vitro testing. J Control Release. 1999;59(1):107–18. doi: 10.1016/S0168-3659(98)00182-5. [DOI] [PubMed] [Google Scholar]
- 4.Higuchi T, Connors KA. Phase-solubility techniques. Advan Anal Chem. 1965;4:117–212. [Google Scholar]
- 5.Salem LB, Bosquillon C, Dailey LA, Delattre L, Martin GP, Evrard B, et al. Sparing methylation of beta-cyclodextrin mitigates cytotoxicity and permeability induction in respiratory epithelial cell layers in vitro. J Control Release. 2009;136(2):110–6. doi: 10.1016/j.jconrel.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 6.Duchene D, Wouessijewe D, Poelman MC. New Trends in Cyclodextrins and Derivatives. Paris 1991.
- 7.Van Der Bijl P, Engelbrecht AH, Van Eyk AD, Meyer D. Comparative permeability of human and rabbit corneas to cyclosporin and tritiated water. J Ocul Pharmacol Ther. 2002;18(5):419–27. doi: 10.1089/10807680260362704. [DOI] [PubMed] [Google Scholar]
- 8.Lopez CA, de Vries AH, Marrink SJ. Computational microscopy of cyclodextrin mediated cholesterol extraction from lipid model membranes. Sci Rep. 2013;3:2071. doi: 10.1038/srep02071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams RO, 3rd, Mahaguna V, Sriwongjanya M. Characterization of an inclusion complex of cholesterol and hydroxypropyl-beta-cyclodextrin. Eur J Pharm Biopharm. 1998;46(3):355–60. doi: 10.1016/S0939-6411(98)00033-2. [DOI] [PubMed] [Google Scholar]
- 10.Lopez CA, de Vries AH, Marrink SJ. Molecular mechanism of cyclodextrin mediated cholesterol extraction. PLoS Comput Biol. 2011;7(3):e1002020. doi: 10.1371/journal.pcbi.1002020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsamaloukas A, Szadkowska H, Slotte PJ, Heerklotz H. Interactions of cholesterol with lipid membranes and cyclodextrin characterized by calorimetry. Biophys J. 2005;89(2):1109–19. doi: 10.1529/biophysj.105.061846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncker G, Reichelt J. Effects of the pharmaceutical cosolvent hydroxypropyl-beta-cyclodextrin on porcine corneal endothelium. Graefes Arch Clin Exp Ophthalmol. 1998;236(5):380–9. doi: 10.1007/s004170050094. [DOI] [PubMed] [Google Scholar]
- 13.Huang AJ, Tseng SC, Kenyon KR. Paracellular permeability of corneal and conjunctival epithelia. Invest Ophthalmol Vis Sci. 1989;30(4):684–9. [PubMed] [Google Scholar]
- 14.Hippalgaonkar K, Adelli GR, Repka MA, Majumdar S. Indomethacin-loaded solid lipid nanoparticles for ocular delivery: development, characterization, and in vitro evaluation. J Ocul Pharmacol Ther. 2013;29(2):216–28. doi: 10.1089/jop.2012.0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hippalgaonkar K, Gul W, ElSohly MA, Repka MA, Majumdar S. Enhanced solubility, stability, and transcorneal permeability of delta-8-tetrahydrocannabinol in the presence of cyclodextrins. AAPS PharmSciTech. 2011;12(2):723–31. doi: 10.1208/s12249-011-9639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loftssona T, Jarvinen T. Cyclodextrins in ophthalmic drug delivery. Adv Drug Deliv Rev. 1999;36(1):59–79. doi: 10.1016/S0169-409X(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 17.Reer O, Bock TK, Muller BW. In vitro corneal permeability of diclofenac sodium in formulations containing cyclodextrins compared to the commercial product voltaren ophtha. J Pharm Sci. 1994;83(9):1345–9. doi: 10.1002/jps.2600830928. [DOI] [PubMed] [Google Scholar]
- 18.Tirucherai GS, Mitra AK. Effect of hydroxypropyl beta cyclodextrin complexation on aqueous solubility, stability, and corneal permeation of acyl ester prodrugs of ganciclovir. AAPS PharmSciTech. 2003;4(3):E45. doi: 10.1208/pt040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siefert B, Pleyer U, Muller M, Hartmann C, Keipert S. Influence of cyclodextrins on the in vitro corneal permeability and in vivo ocular distribution of thalidomide. J Ocul Pharmacol Ther. 1999;15(5):429–38. doi: 10.1089/jop.1999.15.429. [DOI] [PubMed] [Google Scholar]
- 20.Bozkir A, Denli ZF, Basaran B. Effect of hydroxypropyl-beta-cyclodextrin on the solubility, stability and in-vitro release of ciprofloxacin for ocular drug delivery. Acta Pol Pharm. 2012;69(4):719–24. [PubMed] [Google Scholar]


