Abstract
With the development of technology and accessories, the role of endoscopic ultrasound (EUS) has evolved from diagnostics to therapeutics. In order to characterise the therapeutic role of EUS, we searched Web of Knowledge database and reviewed articles associated with therapeutic EUS. There are two modalities for the therapeutic purpose: drainage and fine-needle injection. EUS-guided drainage is a promising procedure for the treatment of peripancreatic fluid collection and biliary obstruction; EUS-guided fine-needle injections such as celiac plexus neurolysis, for the purpose of pain relief for pancreatic cancer and chronic pancreatitis, has emerged as a promising procedure. The aim of the study was to perform a comprehensive and conscientious review on the techniques, complications and clinical outcomes of those EUS-based procedures.
Keywords: Endoscopic ultrasound, Therapeutic, Biliary drainage, Peripancreatic fluid collection, Celiac plexus neurolysis
Core tip: Endoscopic ultrasound (EUS)-guided interventions have gained popularity in recent years. In this review, EUS-guided peripancreatic fluid collection drainage and biliary drainage are discussed. EUS-guided fine-needle injections, including celiac plexus neurolysis, photodynamic therapy, stereotactic body radiation therapy and ethanol ablation, are also discussed.
ENDOSCOPIC ULTRASOUND-GUIDED DRAINAGE
Endoscopic ultrasound-guided peripancreatic fluid collection drainage
Background: Peripancreatic fluid collections (PFCs) are the result of different types of pathophysiological processes, such as acute and chronic pancreatitis, trauma, surgery and malignancy, that can damage the pancreatic ducts[1,2]. The different types of PFCs, which are defined by the Atlanta Classification, include acute fluid collection, pancreatic pseudocyst (PPC), pancreatic abscess and walled-off necrosis (WON)[3]. These local complications have traditionally been managed surgically. However, surgery is often associated with higher morbidity and mortality[4]. Percutaneous drainage is also effective in managing all types of PFCs but it has disadvantages, including the need for external catheters and the potential development of pancreatico-cutaneous fistulas[5]. The advantages of endoscopic ultrasound (EUS)-guided drainage include the following: (1) it is minimally invasive; (2) it avoids local complications related to percutaneous drainage; and (3) it enables real-time visualization of PFCs and a decreased bleeding rate by avoiding the interposition of blood vessels with the use of Doppler ultrasound[6,7].
The traditional indications for drainage have been largely neglected[8,9]; only those with symptoms, for example, abdominal pain, weight loss, gastric outlet obstruction, and adverse cyst-related events (biliary obstruction, infection), require medical intervention[10,11]. Patients with asymptomatic PFCs are usually only followed.
Technique: The PFC may be drained through either the transpapillary duct, or the transmural duct, or using a combination of the two routes[12,13]. The decision to choose one approach over the other depends on the size of PFC, its proximity to the wall of the stomach or duodenum, and the accessibility of the pancreatic duct and/or the area of disruption[4]. Transpapillary drainage is effective if the PFC communicates with the main pancreatic duct and is less than 6 cm in size[13]. Transpapillary drainage alone is not recommended in patients with pancreatic necrosis because of the high risk of secondary infection[13]. Because the body of the pancreas is near the stomach and duodenum, patients with central pancreatic necrosis are suitable candidates for transmural drainage. The advantage of the transpapillary approach over transmural drainage is the avoidance of the bleeding or perforation that may occur during transmural drainage[4,14]. The disadvantages are the risks involved in performing endoscopic retrograde cholangiopancreatography (ERCP). It may also have a lower success rate if the disruption is unable to be bridged, and there is a risk of retroperitoneal perforation and guidewire/stent-induced ductal damage[15].
A 19-gauge puncture needle is preferred under EUS guidance[16]. Before puncture, the PFC morphology should be evaluated by EUS, and color Doppler ultrasound is used to identify the regional vessels. A 0.025- or 0.03-inch guidewire is then introduced through the needle and coiled within the PFC under fluoroscopic guidance (Figure 1), and the tract is sequentially dilated (Figure 2). For pseudocysts, the procedure entails balloon dilation to 10 mm, generally followed by placement of two plastic pigtail stents (usually 7Fr or 8.5Fr) (Figure 3). For WON, we use progressive balloon dilation to 18-20 mm, followed by placement of two plastic pigtail stents (7 or 8.5Fr and 10Fr) or a fully-covered, self-expanding metal stent (FCSEMS) (Figure 4). Then a naso-cystic drainage tube for intermittent flushing of the WON collection is placed, or, occasionally, direct endoscopic necrosectomy is performed by inserting a gastroscope into the collection through the dilated cyst-gastrostomy or cyst-duodenostomy fistula tract, followed by careful debridement of the necrotic content[17].
Figure 1.
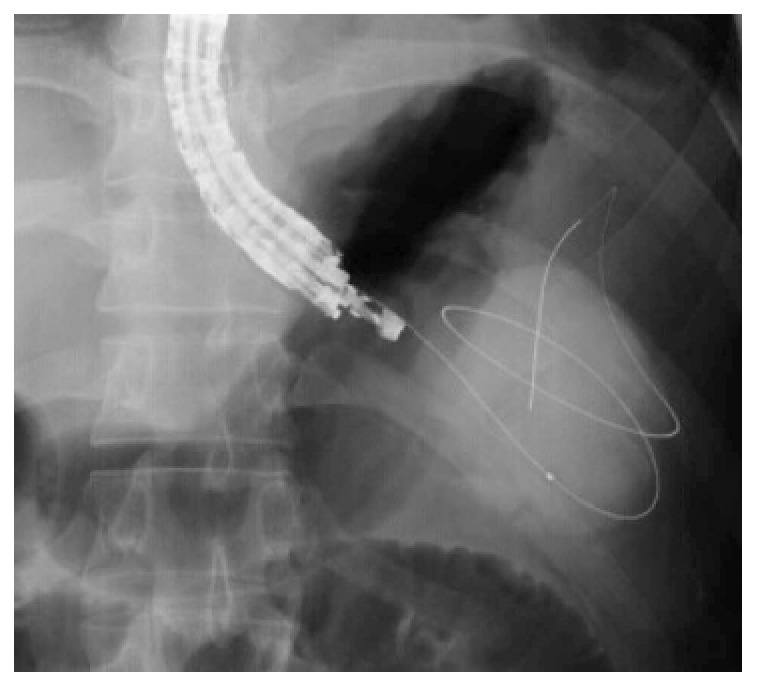
Fluoroscopic image showing a 0.035-inch guidewire coiling within a pancreatic pseudocyst. Courtesy of Prof. Lee[18] with permission.
Figure 2.
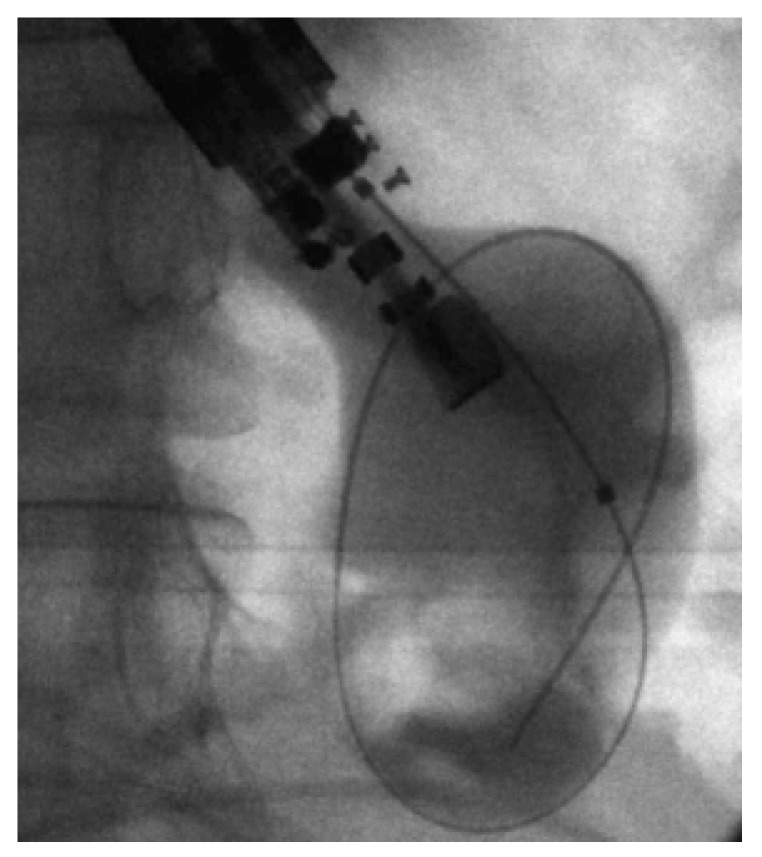
Fluoroscopic image showing dilation of the fistula tract with a balloon dilator. Courtesy of Prof. Kahaleh[16] with permission.
Figure 3.
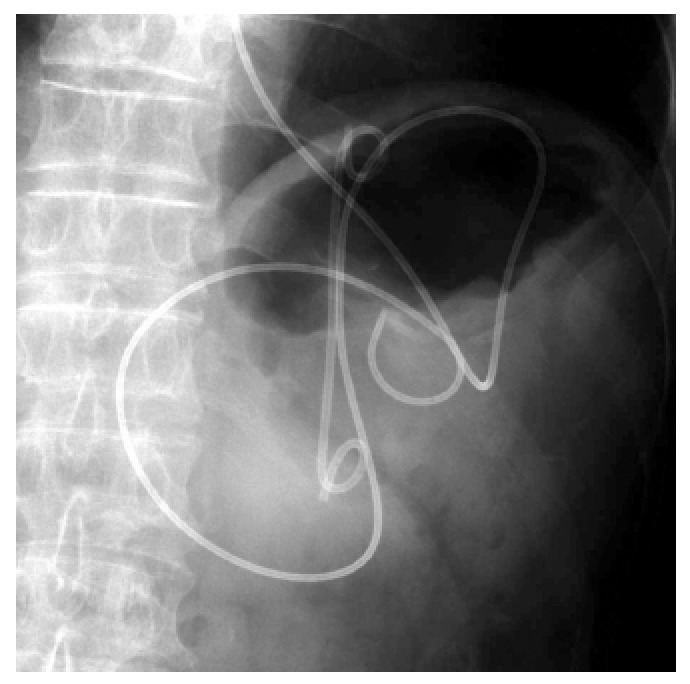
Fluoroscopic image showing placement of a 10-Fr double pig-tail plastic stent and a nasocystic catheter into a pancreatic pseudocyst. Courtesy of Prof. Itoi[37] with permission.
Figure 4.
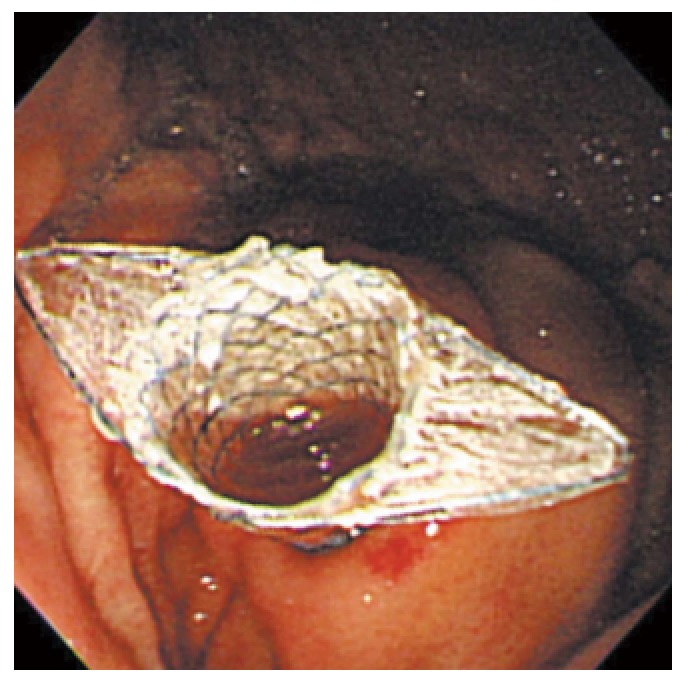
Endoscopic image of placement of a fully-covered metal stent into a pancreatic pseudocyst. Courtesy of Prof. Lee[18] with permission.
After the guidewire has been placed, it is sometimes difficult to dilate the fistula tract. In such cases, a tapered dilator, cystotome and needle knife may be used instead of a balloon dilator and dilation catheter.
Technical and clinical outcomes: EUS-guided drainage has shown a technical success rate of more than 90% and a clinical success rate of 75% to 90%[18]. A recent study reported a treatment success rate of 93.5% for pseudocysts, but only 63.2% for WON, suggesting that treatment outcome is related to type of collection[19]. Two randomized trials that compared EUS-guided PPC drainage and conventional endoscopy-guided PPC drainage demonstrated that EUS-guided transmural approach is superior to conventional endoscopy-guided drainage in terms of technical success and complications[20,21]. Several observational studies have investigated the efficacy of EUS-guided drainage of pseudocysts and abscesses. They all resulted in high technical and clinical success rates, ranging from 89% to 100% and 82% to 100%, respectively[22-24]. However, these techniques were performed by experienced endoscopists. Ng et al[25] recently demonstrated that, although EUS-guided drainage of pseudocysts was technically successful in 93% of patients, the treatment success rate was 75% and the complication rate was 5%. Varadarajulu et al[21], in a comparison of the efficacy of EUS-guided and non-EUS-guided pseudocyst drainage, found that the technical success rate was 100% with EUS, but only 33% with the non-EUS-based approach.
A recent randomized, controlled trial of EUS-guided vs surgical cystgastrostomy for pseudocyst drainage determined that there were no differences in terms of treatment success rate, complications, or recurrence, but there was a significantly shorter hospital stay (median, 2 d vs 6 d; P < 0.001) and lower costs in the endoscopic group[26]. An earlier randomized study by the same group yielded similar conclusions[27]. Therefore, the endoscopic approaches seem to be the preferable strategy for uncomplicated pseudocysts because there is no apparent advantage to surgery, and it seems to require prolonged hospitalization and higher cost.
Complications: The complication rate for EUS-guided drainage of PFCs varies widely among centers, ranging from 5% to 16%, and complications are more frequent in PFC patients with solid necrotic debris[12]. Bleeding, the most common complication of transmural drainage, has been observed in 8% to 10% of patients[4]. The bleeding may be caused by splenic artery pseudoaneurysms. Varadarajulu et al[28] reported a complication rate for transpapillary drainage of 6.2%. Hookey et al[12] found no difference in the complication rate between the transmural and transpapillary approaches.
EUS-guided biliary drainage
Background: ERCP has become the standard procedure for the management of both benign and malignant biliary obstructions. Although its success rate has been reported to be as high as 95%, biliary cannulation cannot be achieved in certain cases[29]. Therefore, EUS-guided biliary drainage (EUS-BD) has been introduced as an effective alternative for biliary decompression. Advantages of EUS-BD over percutaneous and surgical biliary drainage include a one-stage procedure, internal drainage, the avoidance of long-term external drainage, logistical and economic benefits, and a faster recovery as compared with percutaneous drainage. These advantages can significantly improve the quality of life of terminally ill patients and possibly result in lower morbidity than that with percutaneous drainage or surgery[30,31].
The widely accepted indications for EUS-BD include failed ERCP despite maximal efforts by experienced operators, altered anatomy, tumor preventing access to the biliary tree, and contraindication to percutaneous access, such as large ascites[32].
Technique: EUS-BD utilizes a variety of access approaches (intrahepatic or extrahepatic), direction of stent insertion (antegrade or retrograde), and drainage routes (transmural, transpapillary or rendezvous). The extrahepatic (EH) approach involves a needle puncture from the transduodenal (CDS) (Figure 5) or transenteral route directly into the common bile duct[29]. The intrahepatic (IH) approach involves gaining access to the left hepatic biliary system with either transesophageal or transgastric (HGS) (Figure 6) EUS guidance.
Figure 5.
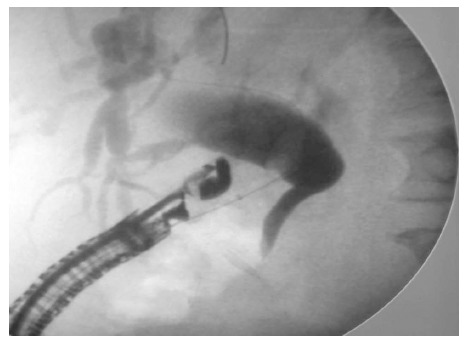
Flouroscopic image of the choledochoduodenal tract dilation with a balloon dilator. Courtesy of Prof. Altonbary[33] with permission.
Figure 6.
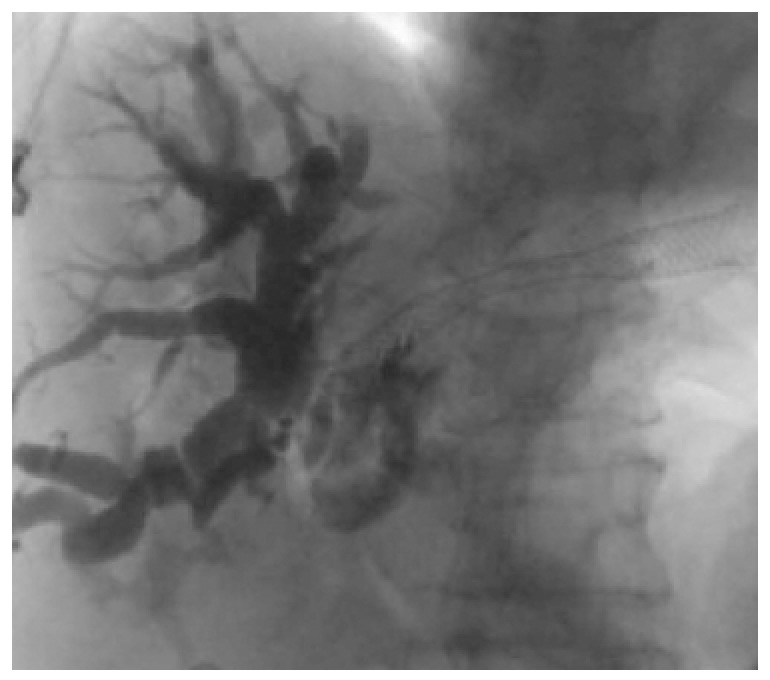
Flouroscopic image of placement of a metal stent into the hepatic biliary system. Courtesy of Prof. Kahaleh[31] with permission.
A 19-gauge EUS-FNA needle is preferred for the initial puncture, which permits easier wire manipulation. Biliary access can be confirmed with the aspiration of bile. Contrast medium is subsequently injected to perform a cholangiogram, and a 0.025- or 0.035-inch guidewire is then inserted through the FNA needle into the biliary system.
Once biliary access has been obtained, the options for drainage include rendezvous, transpapillary, or transmural decompression. In EUS-guided biliary rendezvous technique, the guidewire is placed into the intrahepatic or extrahepatic biliary duct, then passed through the papilla and retrieved by a duodenoscope for biliary intervention. The rendezvous approach is preferred if the duodenoscope can be advanced to the papilla when the guidewire has traversed the papilla in an antegrade way[33,34].
The transmural approach, which leaves a stent in the stomach or duodenum lumen, is performed when the duodenoscope cannot be advanced to the ampulla to perform the rendezvous drainage[35]. A plastic or metallic stent is subsequently deployed.
Technical and clinical outcomes: The overall cumulative success rate for EUS-BD is 84%-93%[32,36]. Gupta et al[36] reviewed studies from six experienced international centers, comparing techniques, outcomes, and complications. The success rates were similar between the EH and IH approaches (84.3% vs 90.4%, P = 0.15). No significant difference in the complication rate was noted between the EH and IH approaches (35.6% vs 32.6%, P = 0.64). Artifon et al[37] compared EUS-guided choledochoduodenostomy (EUS-CDS) and PTBD in 25 patients with distal biliary malignant obstructions. The two procedures were technically and clinically successful in both groups. The study concluded that EUS-CDS is an effective and safe alternative to PTBD, with similar success and complication rates, cost, and quality of life.
A Japanese multicenter study of EUS-BD (44 cases of EUS-CDS and 20 cases of EUS-HGS ) conducted by Kawakubo et al[38] found that the technical success rates for both EUS-CDS and EUS-HGS were 95%. The complication rate was 19%.
Complications: The overall complication rate ranges from 16% to 35%[29]. Complications associated with EUS-BD include (1) bile leakage; (2) infection (peritonitis, cholangitis, and cholecystitis); (3) pancreatitis; (4) pneumoperitoneum; (5) bleeding, (6) abdominal pain; and (7) stent migration. The most common complications are bile leakage and pneumoperitoneum[39].
Other applications of EUS-guided drainage
EUS-guided drainage can also be used in the management of pelvic and hepatic abscesses, and acute cholecystitis.
Hadithi et al[40] reported the use of EUS-guided drainage for pelvic abscesses in eight patients. The technical success rate was 100% and the clinical success rate was 100%, and no recurrence was observed in any of the patients.
EUS-guided gallbladder drainage (EUS-GBD) can be carried out through the gastric or duodenal wall, thus avoiding puncture of the liver, which is more vascular. It is therefore a safer technique for patients with coagulopathy. Widmer et al[41] analyzed published studies of EUS-GBD and found that the overall technical success rate was 96.7%. All the patients with technical successes were clinically successful as well. A total of 12.2% of the patients had complications, including pneumoperitoneum, bile peritonitis and stent migration, indicating that EUS-GBD is very challenging technically.
Singhal et al[42] reviewed seven published case series, comprising a total of seven patients, on EUS-guided drainage for hepatic abscesses. They determined that the technical and clinical success rates were both 100%; no complications were observed.
EUS-GUIDED FINE-NEEDLE INJECTION
EUS-guided fine-needle injection (EUS-FNI) is a modified technique based on the concept of needle guidance to deliver a therapeutic agent, radiation source or immune-related cells into a targeted lesion. The technique is utilized to accomplish localized therapy rather than systemic chemotherapy, which may reduce systemic toxicity, and also the cost[43,44]. EUS-FNI also includes neurolysis. Experience with this technique is still limited and intensive investigation efforts are needed.
EUS-guided celiac plexus/ganglion neurolysis
Background: The incidence of pancreatic cancer has increased over the past decade[45], and the standardized net survival at 5 years is 6% for men and 10% for women[46]. Because it frequently presents at an inoperable stage so that palliation of symptoms is a primary goal. In these situations, interventional pain-relief techniques, such as celiac plexus neurolysis (CPN) or celiac ganglion neurolysis (CGN), may be indicated.
The celiac plexus surrounds the celiac artery and the root of the mesenteric artery. Pain originating from the liver, pancreas, diaphragm, spleen, and stomach spreads through the celiac plexus and is further transmitted to the central nervous system.
The posterior approach using a fluoroscope is associated with severe side effects, such as pneumothorax and paraplegia[47]. Therefore, the anterior approach is considered a better option for CPN/CGN.
Technique: 22-G or 19-G needles are used. For a central injection, the needle is advanced above the celiac trunk, in the space between the aorta and the origin of the celiac axis. If a bilateral injection into the plexus is chosen, the echo-endoscope, which is placed above the celiac axis, should be rotated to one side until the origin of the celiac axis is no longer seen, and the procedure is repeated on the other side. If injections into the ganglia are chosen, the solution should be injected into the central part of the ganglia for those less than 1 cm in diameter or, for larger ganglia, into the deepest part[48].
Before injection, aspiration should be performed to ascertain that the needle has not been placed inside a vessel, and 3-10 mL of a local analgesic is injected initially to prevent exacerbation of transient pain by the neurolytic agent. Subsequently, 10-20 mL of 98%dehydrated alcohol is injected[45].
Clinical outcomes: Two randomized controlled trials have demonstrated improvement in quality-of-life parameters[48,49]. In a recently published retrospective study conducted by Iwata et al[50], EUS-CPN was performed in 47 patients with unresectable pancreatic cancer or non-pancreatic abdominal cancer. EUS-CPN was successful in 68.1% of the patients. Of these, 14 patients obtained complete pain relief. Wyse et al[49] found that pain relief was slightly higher in the EUS-CPN group than in the control group at 1 mo and significantly higher at 3 mo.
In a meta-analysis of these EUS-CPN studies, pain relief was observed in approximately 80% of 289 patients with pancreatic cancer[51]. Although EUS-CPN can be delivered on either or both sides of the aorta, a recent study showed that bilateral injection was more effective than a central injection[52]. In a recent randomized controlled study, CGN was more effective than CPN in pain relief (73.5% vs 45.5%, respectively, P = 0.026)[53]. A randomized, controlled trial demonstrated that CPN was effective in pain relief at 1-mo and 3-mo follow-up, but opioid usage persisted, although it was higher in the control group[49].
Complications: Complications associated with EUS-CPN, including transient diarrhea and hypotension, were observed in 9% and 10%-15% of cases, respectively, and both complications were self-limiting in most cases[54]. Recently, more serious complications, including paralysis due to spinal cord infarction[55,56], death from gastric perforation[57] and celiac artery thrombosis-induced infarction[58,59], have been observed.
EUS-guided photodynamic therapy
EUS-guided needle injection is also applied to deliver photosensitizing medication. photodynamic therapy (PDT) uses light to produce localized tissue necrosis after administration of a photosensitizing agent in the presence of oxygen. Studies have shown that PDT can induce apoptosis and necrosis by regulating the pancreatic cellular signaling pathways or modulating the structures of plasma membrane proteins[60].
EUS-guided stereotactic body radiation therapy and brachytherapy
Fiduciary markers, or fiducials, are used as reference points to target radiation beams. After identification of a tumor, a 19-G fine-needle is inserted into the target lesion, and the fiducials are deployed through the needle lumen. The position of the fiducials is confirmed via EUS and fluoroscopy[61,62]. In recent years, fiducial placement has been reported to facilitate stereotactic body radiation therapy (SBRT) in patients with pancreatic cancer[63-65]. Prospective phase I and phase II studies[66,67] and two retrospective studies[68,69] have indicated that SBRT is a safe and effective approach for treatment of patients with pancreatic cancer.
Brachytherapy involves the placement of a radioactive seed directly into the pancreatic tumor for localized therapy. Currently, the most commonly used radioactive seed is iodine-125. Studies have shown that brachytherapy is effective for the local control of malignant pancreatic tumors[70,71].
EUS-guided ethanol ablation
EUS-guided ethanol ablation is a recently developed method that has been successfully applied as a treatment for pancreatic cysts, pancreatic neuroendocrine tumors, and abdominal metastatic lesions.
A multicenter, randomized study indicated that EUS-guided ethanol injection resulted in a greater reduction in the size of pancreatic cystic tumors compared with a saline-solution injection, and the overall resolution rate of the pancreatic cystic tumors was 33.3%[72]. EUS-guided ethanol lavage with a paclitaxel injection has been introduced to improve the effect of the ethanol ablation. An initial study found that complete resolution of pancreatic cystic neoplasms was achieved in 11 of 14 patients after treatment with ethanol and paclitaxel injection[73].
A case series on EUS-guided ethanol ablation in pancreatic neuroendocrine tumors showed complete resolution after the ethanol ablation[74].
This technique has also been used for the ablation of other abdominal tumors, such as gastrointestinal stromal tumors and intra-abdominal metastatic lesions[75].
Other techniques involving EUS-FNI for the treatment of pancreatic cancer include immunotherapy and radiofrequency ablation[60].
CONCLUSION
EUS-guided drainage and fine-needle injection are promising therapeutic techniques that have shown benefits in patients with PFC, biliary obstruction or pancreatic cancer because they are minimally invasive, require only a short hospital stay, and are less costly. However, there is currently no consensus on these techniques. Participants of a 2011 consortium agreed that EUS-BD should be performed by trained pancreaticobiliary endoscopists who complete between 200 and 300 EUS and ERCP procedures annually, and have appropriate interventional radiology and surgical backup[76]. These techniques are highly challenging, which has constrained their use. To date, the data are limited because of the retrospective aspect and the small number of trials; therefore, a larger number of multicenter trials are required.
Footnotes
Conflict-of-interest statement: The authors declare no conflicts of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 26, 2015
First decision: July 19, 2015
Article in press: September 30, 2015
P- Reviewer: Amaro F S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
References
- 1.Yusuf TE, Baron TH. Endoscopic transmural drainage of pancreatic pseudocysts: results of a national and an international survey of ASGE members. Gastrointest Endosc. 2006;63:223–227. doi: 10.1016/j.gie.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 2.Varadarajulu S. Endoscopic management of pancreatic pseudocysts. J Dig Endosc. 2012;3:58. doi: 10.1016/j.gie.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 3.Bollen TL, van Santvoort HC, Besselink MG, van Leeuwen MS, Horvath KD, Freeny PC, Gooszen HG. The Atlanta Classification of acute pancreatitis revisited. Br J Surg. 2008;95:6–21. doi: 10.1002/bjs.6010. [DOI] [PubMed] [Google Scholar]
- 4.Bhasin D, Rana S. Endoscopic management of pancreatic fluid collections. J Dig Endosc. 2012;3:40. [Google Scholar]
- 5.Rana SS, Bhasin DK, Nanda M, Siyad I, Gupta R, Kang M, Nagi B, Singh K. Endoscopic transpapillary drainage for external fistulas developing after surgical or radiological pancreatic interventions. J Gastroenterol Hepatol. 2010;25:1087–1092. doi: 10.1111/j.1440-1746.2009.06172.x. [DOI] [PubMed] [Google Scholar]
- 6.Seewald S, Ang TL, Teng KC, Soehendra N. EUS-guided drainage of pancreatic pseudocysts, abscesses and infected necrosis. Dig Endosc. 2009;21 Suppl 1:S61–S65. doi: 10.1111/j.1443-1661.2009.00860.x. [DOI] [PubMed] [Google Scholar]
- 7.Yoon WJ, Brugge WR. Endoscopic ultrasound and pancreatic cystic lesions-diagnostic and therapeutic applications. Endosc Ultrasound. 2012;1:75–79. doi: 10.7178/eus.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrén-Sandberg A, Dervenis C. Pancreatic pseudocysts in the 21st century. Part II: natural history. JOP. 2004;5:64–70. [PubMed] [Google Scholar]
- 9.Singhal S, Rotman SR, Gaidhane M, Kahaleh M. Pancreatic fluid collection drainage by endoscopic ultrasound: an update. Clin Endosc. 2013;46:506–514. doi: 10.5946/ce.2013.46.5.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kahaleh M, Shami VM, Conaway MR, Tokar J, Rockoff T, De La Rue SA, de Lange E, Bassignani M, Gay S, Adams RB, et al. Endoscopic ultrasound drainage of pancreatic pseudocyst: a prospective comparison with conventional endoscopic drainage. Endoscopy. 2006;38:355–359. doi: 10.1055/s-2006-925249. [DOI] [PubMed] [Google Scholar]
- 11.Varadarajulu S, Wilcox CM, Tamhane A, Eloubeidi MA, Blakely J, Canon CL. Role of EUS in drainage of peripancreatic fluid collections not amenable for endoscopic transmural drainage. Gastrointest Endosc. 2007;66:1107–1119. doi: 10.1016/j.gie.2007.03.1027. [DOI] [PubMed] [Google Scholar]
- 12.Hookey LC, Debroux S, Delhaye M, Arvanitakis M, Le Moine O, Devière J. Endoscopic drainage of pancreatic-fluid collections in 116 patients: a comparison of etiologies, drainage techniques, and outcomes. Gastrointest Endosc. 2006;63:635–643. doi: 10.1016/j.gie.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 13.Baron TH. Endoscopic drainage of pancreatic fluid collections and pancreatic necrosis. Gastrointest Endosc Clin N Am. 2003;13:743–764. doi: 10.1016/s1052-5157(03)00100-4. [DOI] [PubMed] [Google Scholar]
- 14.Navaneethan U, Al Mohajer M, Schmulewitz N, Chauhan S, Ahmad S, Ying J, Palascak J, Gelrud A. Endoscopic management of pancreatic fluid collections - a single center experience. Am J Gastroenterol. 2008;103:S83–S83. [Google Scholar]
- 15.Bhasin DK, Rana SS, Rawal P. Endoscopic retrograde pancreatography in pancreatic trauma: need to break the mental barrier. J Gastroenterol Hepatol. 2009;24:720–728. doi: 10.1111/j.1440-1746.2009.05809.x. [DOI] [PubMed] [Google Scholar]
- 16.Rotman SR, Kahaleh M. Pancreatic fluid collection drainage by endoscopic ultrasound: new perspectives. Endosc Ultrasound. 2012;1:61–68. doi: 10.7178/eus.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teshima CW, Sandha GS. Endoscopic ultrasound in the diagnosis and treatment of pancreatic disease. World J Gastroenterol. 2014;20:9976–9989. doi: 10.3748/wjg.v20.i29.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song TJ, Lee SS. Endoscopic drainage of pseudocysts. Clin Endosc. 2014;47:222–226. doi: 10.5946/ce.2014.47.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varadarajulu S, Bang JY, Phadnis MA, Christein JD, Wilcox CM. Endoscopic transmural drainage of peripancreatic fluid collections: outcomes and predictors of treatment success in 211 consecutive patients. J Gastrointest Surg. 2011;15:2080–2088. doi: 10.1007/s11605-011-1621-8. [DOI] [PubMed] [Google Scholar]
- 20.Park DH, Lee SS, Moon SH, Choi SY, Jung SW, Seo DW, Lee SK, Kim MH. Endoscopic ultrasound-guided versus conventional transmural drainage for pancreatic pseudocysts: a prospective randomized trial. Endoscopy. 2009;41:842–848. doi: 10.1055/s-0029-1215133. [DOI] [PubMed] [Google Scholar]
- 21.Varadarajulu S, Christein JD, Tamhane A, Drelichman ER, Wilcox CM. Prospective randomized trial comparing EUS and EGD for transmural drainage of pancreatic pseudocysts (with videos) Gastrointest Endosc. 2008;68:1102–1111. doi: 10.1016/j.gie.2008.04.028. [DOI] [PubMed] [Google Scholar]
- 22.Ahn JY, Seo DW, Eum J, Song TJ, Moon SH, Park do H, Lee SS, Lee SK, Kim MH. Single-Step EUS-Guided Transmural Drainage of Pancreatic Pseudocysts: Analysis of Technical Feasibility, Efficacy, and Safety. Gut Liver. 2010;4:524–529. doi: 10.5009/gnl.2010.4.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopes CV, Pesenti C, Bories E, Caillol F, Giovannini M. Endoscopic-ultrasound-guided endoscopic transmural drainage of pancreatic pseudocysts and abscesses. Scand J Gastroenterol. 2007;42:524–529. doi: 10.1080/00365520601065093. [DOI] [PubMed] [Google Scholar]
- 24.Varadarajulu S, Tamhane A, Blakely J. Graded dilation technique for EUS-guided drainage of peripancreatic fluid collections: an assessment of outcomes and complications and technical proficiency (with video) Gastrointest Endosc. 2008;68:656–666. doi: 10.1016/j.gie.2008.03.1091. [DOI] [PubMed] [Google Scholar]
- 25.Ng PY, Rasmussen DN, Vilmann P, Hassan H, Gheorman V, Burtea D, Surlin V, Săftoiu A. Endoscopic Ultrasound-guided Drainage of Pancreatic Pseudocysts: Medium-Term Assessment of Outcomes and Complications. Endosc Ultrasound. 2013;2:199–203. doi: 10.4103/2303-9027.121245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varadarajulu S, Bang JY, Sutton BS, Trevino JM, Christein JD, Wilcox CM. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology. 2013;145:583–90.e1. doi: 10.1053/j.gastro.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 27.Varadarajulu S, Trevino J, Wilcox CM, Sutton B, Christein JD. Randomized Trial Comparing EUS and Surgery for Pancreatic Pseudocyst Drainage. Gastrointest Endosc. 2010;71:Ab116–Ab116. [Google Scholar]
- 28.Varadarajulu S, Noone TC, Tutuian R, Hawes RH, Cotton PB. Predictors of outcome in pancreatic duct disruption managed by endoscopic transpapillary stent placement. Gastrointest Endosc. 2005;61:568–575. doi: 10.1016/s0016-5107(04)02832-9. [DOI] [PubMed] [Google Scholar]
- 29.Kumta NA, Kedia P, Kahaleh M. Endoscopic ultrasound-guided biliary drainage: an update. Curr Treat Options Gastroenterol. 2014;12:154–168. doi: 10.1007/s11938-014-0011-1. [DOI] [PubMed] [Google Scholar]
- 30.Ang TL. Current status of endosonography-guided biliary drainage. Singapore Med J. 2010;51:762–766. [PubMed] [Google Scholar]
- 31.Altonbary AY, Deiab AG, Bahgat MH. Endoscopic ultrasound-guided choledechoduodenostomy for palliative biliary drainage of obstructing pancreatic head mass. Endosc Ultrasound. 2014;3:137–140. doi: 10.4103/2303-9027.131043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kedia P, Gaidhane M, Kahaleh M. Endoscopic guided biliary drainage: how can we achieve efficient biliary drainage? Clin Endosc. 2013;46:543–551. doi: 10.5946/ce.2013.46.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dhir V, Bhandari S, Bapat M, Maydeo A. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access (with videos) Gastrointest Endosc. 2012;75:354–359. doi: 10.1016/j.gie.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 34.Iwashita T, Lee JG, Shinoura S, Nakai Y, Park DH, Muthusamy VR, Chang KJ. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy. 2012;44:60–65. doi: 10.1055/s-0030-1256871. [DOI] [PubMed] [Google Scholar]
- 35.Kawakami H, Itoi T, Sakamoto N. Endoscopic ultrasound-guided transluminal drainage for peripancreatic fluid collections: where are we now? Gut Liver. 2014;8:341–355. doi: 10.5009/gnl.2014.8.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta K, Perez-Miranda M, Kahaleh M, Artifon EL, Itoi T, Freeman ML, de-Serna C, Sauer B, Giovannini M. Endoscopic ultrasound-assisted bile duct access and drainage: multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol. 2014;48:80–87. doi: 10.1097/MCG.0b013e31828c6822. [DOI] [PubMed] [Google Scholar]
- 37.Artifon EL, Aparicio D, Paione JB, Lo SK, Bordini A, Rabello C, Otoch JP, Gupta K. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768–774. doi: 10.1097/MCG.0b013e31825f264c. [DOI] [PubMed] [Google Scholar]
- 38.Kawakubo K, Isayama H, Kato H, Itoi T, Kawakami H, Hanada K, Ishiwatari H, Yasuda I, Kawamoto H, Itokawa F, et al. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci. 2014;21:328–334. doi: 10.1002/jhbp.27. [DOI] [PubMed] [Google Scholar]
- 39.Chavalitdhamrong D, Draganov PV. Endoscopic ultrasound-guided biliary drainage. World J Gastroenterol. 2012;18:491–497. doi: 10.3748/wjg.v18.i6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hadithi M, Bruno MJ. Endoscopic ultrasound-guided drainage of pelvic abscess: A case series of 8 patients. World J Gastrointest Endosc. 2014;6:373–378. doi: 10.4253/wjge.v6.i8.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widmer J, Singhal S, Gaidhane M, Kahaleh M. Endoscopic ultrasound-guided endoluminal drainage of the gallbladder. Dig Endosc. 2014;26:525–531. doi: 10.1111/den.12221. [DOI] [PubMed] [Google Scholar]
- 42.Singhal S, Changela K, Lane D, Anand S, Duddempudi S. Endoscopic ultrasound-guided hepatic and perihepatic abscess drainage: an evolving technique. Therap Adv Gastroenterol. 2014;7:93–98. doi: 10.1177/1756283X13506178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hara K, Yamao K, Hijioka S, Mizuno N, Imaoka H, Tajika M, Kondo S, Tanaka T, Haba S, Takeshi O, et al. Prospective clinical study of endoscopic ultrasound-guided choledochoduodenostomy with direct metallic stent placement using a forward-viewing echoendoscope. Endoscopy. 2013;45:392–396. doi: 10.1055/s-0032-1326076. [DOI] [PubMed] [Google Scholar]
- 44.Carrara S, Petrone MC, Testoni PA, Arcidiacono PG. Tumors and new endoscopic ultrasound-guided therapies. World J Gastrointest Endosc. 2013;5:141–147. doi: 10.4253/wjge.v5.i4.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seicean A. Celiac plexus neurolysis in pancreatic cancer: the endoscopic ultrasound approach. World J Gastroenterol. 2014;20:110–117. doi: 10.3748/wjg.v20.i1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jooste V, Grosclaude P, Remontet L, Launoy G, Baldi I, Molinié F, Arveux P, Bossard N, Bouvier AM, Colonna M. Unbiased estimates of long-term net survival of solid cancers in France. Int J Cancer. 2013;132:2370–2377. doi: 10.1002/ijc.27857. [DOI] [PubMed] [Google Scholar]
- 47.Wong GY, Schroeder DR, Carns PE, Wilson JL, Martin DP, Kinney MO, Mantilla CB, Warner DO. Effect of neurolytic celiac plexus block on pain relief, quality of life, and survival in patients with unresectable pancreatic cancer: a randomized controlled trial. JAMA. 2004;291:1092–1099. doi: 10.1001/jama.291.9.1092. [DOI] [PubMed] [Google Scholar]
- 48.LeBlanc JK, Al-Haddad M, McHenry L, Sherman S, Juan M, McGreevy K, Johnson C, Howard TJ, Lillemoe KD, DeWitt J. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. 2011;74:1300–1307. doi: 10.1016/j.gie.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 49.Wyse JM, Carone M, Paquin SC, Usatii M, Sahai AV. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541–3546. doi: 10.1200/JCO.2010.32.2750. [DOI] [PubMed] [Google Scholar]
- 50.Iwata K, Yasuda I, Enya M, Mukai T, Nakashima M, Doi S, Iwashita T, Tomita E, Moriwaki H. Predictive factors for pain relief after endoscopic ultrasound-guided celiac plexus neurolysis. Dig Endosc. 2011;23:140–145. doi: 10.1111/j.1443-1661.2010.01046.x. [DOI] [PubMed] [Google Scholar]
- 51.Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330–2337. doi: 10.1007/s10620-008-0651-x. [DOI] [PubMed] [Google Scholar]
- 52.Sahai AV, Lemelin V, Lam E, Paquin SC. Central vs. bilateral endoscopic ultrasound-guided celiac plexus block or neurolysis: a comparative study of short-term effectiveness. Am J Gastroenterol. 2009;104:326–329. doi: 10.1038/ajg.2008.64. [DOI] [PubMed] [Google Scholar]
- 53.Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, Irisawa A, Mukai T, Katanuma A, Kubota K, Ohnishi T, et al. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy. 2013;45:362–369. doi: 10.1055/s-0032-1326225. [DOI] [PubMed] [Google Scholar]
- 54.Gunaratnam NT, Sarma AV, Norton ID, Wiersema MJ. A prospective study of EUS-guided celiac plexus neurolysis for pancreatic cancer pain. Gastrointest Endosc. 2001;54:316–324. doi: 10.1067/mge.2001.117515. [DOI] [PubMed] [Google Scholar]
- 55.Fujii L, Clain JE, Morris JM, Levy MJ. Anterior spinal cord infarction with permanent paralysis following endoscopic ultrasound celiac plexus neurolysis. Endoscopy. 2012;44 Suppl 2 UCTN:E265–E266. doi: 10.1055/s-0032-1309708. [DOI] [PubMed] [Google Scholar]
- 56.Mittal MK, Rabinstein AA, Wijdicks EF. Pearls & amp; oy-sters: Acute spinal cord infarction following endoscopic ultrasound-guided celiac plexus neurolysis. Neurology. 2012;78:e57–e59. doi: 10.1212/WNL.0b013e318248df51. [DOI] [PubMed] [Google Scholar]
- 57.Loeve US, Mortensen MB. Lethal necrosis and perforation of the stomach and the aorta after multiple EUS-guided celiac plexus neurolysis procedures in a patient with chronic pancreatitis. Gastrointest Endosc. 2013;77:151–152. doi: 10.1016/j.gie.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Gimeno-García AZ, Elwassief A, Paquin SC, Sahai AV. Fatal complication after endoscopic ultrasound-guided celiac plexus neurolysis. Endoscopy. 2012;44 Suppl 2 UCTN:E267. doi: 10.1055/s-0032-1309709. [DOI] [PubMed] [Google Scholar]
- 59.Jang HY, Cha SW, Lee BH, Jung HE, Choo JW, Cho YJ, Ju HY, Cho YD. Hepatic and splenic infarction and bowel ischemia following endoscopic ultrasound-guided celiac plexus neurolysis. Clin Endosc. 2013;46:306–309. doi: 10.5946/ce.2013.46.3.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo X, Cui Z, Hu Z. Role of endoscopic ultrasound in treatment of pancreatic cancer. Endosc Ultrasound. 2013;2:181–189. doi: 10.4103/2303-9027.121238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412–417. doi: 10.1016/j.gie.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 62.DiMaio CJ, Nagula S, Goodman KA, Ho AY, Markowitz AJ, Schattner MA, Gerdes H. EUS-guided fiducial placement for image-guided radiation therapy in GI malignancies by using a 22-gauge needle (with videos) Gastrointest Endosc. 2010;71:1204–1210. doi: 10.1016/j.gie.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Park WG, Yan BM, Schellenberg D, Kim J, Chang DT, Koong A, Patalano C, Van Dam J. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc. 2010;71:513–518. doi: 10.1016/j.gie.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 64.Sanders MK, Moser AJ, Khalid A, Fasanella KE, Zeh HJ, Burton S, McGrath K. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. 2010;71:1178–1184. doi: 10.1016/j.gie.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 65.Ammar T, Coté GA, Creach KM, Kohlmeier C, Parikh PJ, Azar RR. Fiducial placement for stereotactic radiation by using EUS: feasibility when using a marker compatible with a standard 22-gauge needle. Gastrointest Endosc. 2010;71:630–633. doi: 10.1016/j.gie.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 66.Koong AC, Le QT, Ho A, Fong B, Fisher G, Cho C, Ford J, Poen J, Gibbs IC, Mehta VK, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017–1021. doi: 10.1016/j.ijrobp.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Koong AC, Christofferson E, Le QT, Goodman KA, Ho A, Kuo T, Ford JM, Fisher GA, Greco R, Norton J, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–323. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 68.Didolkar MS, Coleman CW, Brenner MJ, Chu KU, Olexa N, Stanwyck E, Yu A, Neerchal N, Rabinowitz S. Image-guided stereotactic radiosurgery for locally advanced pancreatic adenocarcinoma results of first 85 patients. J Gastrointest Surg. 2010;14:1547–1559. doi: 10.1007/s11605-010-1323-7. [DOI] [PubMed] [Google Scholar]
- 69.Rwigema JC, Parikh SD, Heron DE, Howell M, Zeh H, Moser AJ, Bahary N, Quinn A, Burton SA. Stereotactic body radiotherapy in the treatment of advanced adenocarcinoma of the pancreas. Am J Clin Oncol. 2011;34:63–69. doi: 10.1097/COC.0b013e3181d270b4. [DOI] [PubMed] [Google Scholar]
- 70.Du Y, Jin Z, Meng H, Zou D, Chen J, Liu Y, Zhan X, Wang D, Liao Z, Li Z. Long-term effect of gemcitabine-combined endoscopic ultrasonography-guided brachytherapy in pancreatic cancer. J Interv Gastroenterol. 2013;3:18–24. [Google Scholar]
- 71.Jin Z, Du Y, Li Z, Jiang Y, Chen J, Liu Y. Endoscopic ultrasonography-guided interstitial implantation of iodine 125-seeds combined with chemotherapy in the treatment of unresectable pancreatic carcinoma: a prospective pilot study. Endoscopy. 2008;40:314–320. doi: 10.1055/s-2007-995476. [DOI] [PubMed] [Google Scholar]
- 72.DeWitt J, McGreevy K, Schmidt CM, Brugge WR. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest Endosc. 2009;70:710–723. doi: 10.1016/j.gie.2009.03.1173. [DOI] [PubMed] [Google Scholar]
- 73.Oh HC, Seo DW, Lee TY, Kim JY, Lee SS, Lee SK, Kim MH. New treatment for cystic tumors of the pancreas: EUS-guided ethanol lavage with paclitaxel injection. Gastrointest Endosc. 2008;67:636–642. doi: 10.1016/j.gie.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 74.Muscatiello N, Nacchiero M, Della Valle N, Di Terlizzi F, Verderosa G, Salcuni A, Macarini L, Cignarelli M, Castriota M, D’Agnessa V, et al. Treatment of a pancreatic endocrine tumor by ethanol injection (PEI) guided by endoscopic ultrasound. Endoscopy. 2008;40 Suppl 2:E83. doi: 10.1055/s-2007-995540. [DOI] [PubMed] [Google Scholar]
- 75.Zhang WY, Li ZS, Jin ZD. Endoscopic ultrasound-guided ethanol ablation therapy for tumors. World J Gastroenterol. 2013;19:3397–3403. doi: 10.3748/wjg.v19.i22.3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kahaleh M, Artifon EL, Perez-Miranda M, Gupta K, Itoi T, Binmoeller KF, Giovannini M. Endoscopic ultrasonography guided biliary drainage: summary of consortium meeting, May 7th, 2011, Chicago. World J Gastroenterol. 2013;19:1372–1379. doi: 10.3748/wjg.v19.i9.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]


