Abstract
AIM: To investigate the safety and efficacy of the Cyberknife treatment for patients with advanced or terminal stage hepatocellular carcinoma (HCC).
METHODS: Patients with HCC with extrahepatic metastasis or vascular or bile duct invasion were enrolled between May 2011 and June 2015. The Cyberknife was used to treat each lesion. Treatment response scores were based on Response Evaluation Criteria in Solid Tumors v1.1. The trends of tumor markers, including alpha fetoprotein (AFP) and proteins induced by vitamin K absence II (PIVKA II) were assessed. Prognostic factors for tumor response and tumor markers were evaluated with Fisher’s exact test and a logistic regression model. Survival was evaluated with the Kaplan-Meier method and multivariate analysis was performed using the Cox proportional hazards model.
RESULTS: Sixty-five patients with 95 lesions were enrolled. Based on the Barcelona Clinic Liver Cancer classification, all patients were either in the advanced or terminal stage of the disease. The target lesions were as follows: 52 were bone metastasis; 9, lung metastasis; 7, brain metastasis; 9, portal vein invasion; 4, hepatic vein invasion; 4, bile duct invasion; and 10 other lesion types. The response rate and disease control rate were 34% and 53%, respectively. None of the clinical factors correlated significantly with tumor response. Fiducial marker implantation was associated with better control of both AFP (HR = 0.152; 95%CI: 0.026-0.887; P = 0.036) and PIVKA II (HR = 0.035; 95%CI: 0.003-0.342; P = 0.004). The median survival time was 9 mo (95%CI: 5-15 mo). Terminal stage disease (HR = 9.809; 95%CI: 2.589-37.17, P < 0.001) and an AFP of more than 400 ng/mL (HR = 2.548; 95%CI: 1.070-6.068, P = 0.035) were associated with worse survival. A radiation dose higher than 30 Gy (HR = 0.274; 95%CI: 0.093-0.7541, P = 0.012) was associated with better survival. In the 52 cases of bone metastasis, 36 patients (69%) achieved pain relief. One patient had cerebral bleeding and another patient had an esophageal ulcer after treatment.
CONCLUSION: The Cyberknife can be safely administered to patients with advanced or terminal stage HCC. High AFP levels were associated with worse survival, but a higher radiation dose improved the survival.
Keywords: Hepatocellular carcinoma, Stereotactic body radiotherapy, Cyberknife, Neoplasm metastasis/therapy, Liver radiotherapy
Core tip: Due to an aging of hepatocellular carcinoma (HCC) patient population, a growing number of patients are ineligible for conventional therapy. The Cyberknife® system delivers stereotactic body radiation therapy (SBRT), which offers minimally invasive treatment with high doses of radiation. There has been an increase in the number of successful reports of using SBRT against liver-confined HCC. We found that the Cyberknife can safely be administered even in patients with advanced or terminal stage HCC. Our results suggest that SBRT may have the potential to increase the overall survival for advanced stage HCC patients. High alpha fetoprotein levels were associated with worse survival, but a higher radiation dose improved the survival.
INTRODUCTION
Hepatocellular carcinoma treatment strategy
Hepatocellular carcinoma (HCC) is the third cause of cancer-related deaths worldwide[1] and is one of the leading causes of death in patients with hepatic cirrhosis[2]. The Barcelona Clinic Liver Cancer (BCLC) classification, which evaluates both tumor stage and patient condition, has commonly been used to determine the course of treatment[3,4]. Based on this staging system, patients with “very early” and “early” stage HCC are candidates for curative treatment such as surgery, percutaneous alcohol injection or radiofrequency ablation (RFA). However, less than 30% of patients are eligible for these radical treatments due to advanced disease stage, poor liver function, or other medical complications[5]. For patients with intermediate or advanced stage HCC, treatment options include transarterial chemoembolization (TACE)[6,7], sorafenib[8], or palliative care. However, patients remain incurable, and consequently have a poor prognosis. As a result, there has been a need for a highly effective and less invasive treatment option for these HCC patients
History of the Cyberknife
Although HCC is a radiosensitive tumor[9], the use of radiation therapy for HCC has been limited due to the poor tolerance of the entire liver to irradiation. Doses are required to be less than 30-35 Gy, and there is a risk of developing radiation induced liver disease (RILD)[10]. Originally, RILD was defined as having anicteric hepatomegaly, ascites, and an elevated alkaline phosphatase level typically occurring 2-12 mo after therapy[11]. In contrast to this “classic” RILD, a “non-classic RILD” has been proposed. Patients with underlying chronic liver diseases such as cirrhosis or viral hepatitis may present with liver dysfunction, including jaundice or markedly elevated serum transaminases (more than 5 times the upper limit of normal) within 3 mo after radiation[12]. Over the past two decades, thanks to advancements in computer and imaging technologies, this weakness has been overcome, and radiation therapy has evolved to be a safe and feasible option for HCC, with RILD rates of less than 5%[13].
Stereotactic body radiotherapy (SBRT) is a technique that enables the delivery of highdose radiation (usually 8-12 Gy/fraction) to the tumor with extreme accuracy, while minimizing the damage to normal surrounding tissue in 1-10 fractions. The major advantages of SBRT are the promising radiobiological efficacy of the administration of such large radiation doses to tumor tissues, the short treatment course achieved by a small number of fractions, and the minimal invasiveness of the therapy, which can also be given to patients with a poor performance status. SBRT was initiated in the 1950s for the treatment of intracranial malignancies and resulted in extremely high local control rates (greater than 80%-90%)[14]. However, its use in extracranial tumors has been limited because of the movement caused by the respiratory cycle. The Cyberknife® (Accuray Incorporated, Sunnyvale, California, United States) is a robotic image guided system that delivers SBRT, tracks tumors during respiration, and automatically adjusts treatment for any patient movement. The Cyberknife has been used to treat a broad range of tumors throughout the body, including prostate, lung, spine, liver, pancreas, kidney, and other tumors. Currently, there have been increasing numbers of successful reports of using SBRT against HCC and other liver tumors. Four prospective studies and several retrospective studies have suggested that SBRT can be used safely, and that this method has been associated with high local control rates, mostly in the range of 70%-100% at 1-2 years[15-37]. However, studies focusing on patients with advanced or terminal stage HCC are still scarce. Here, we report the treatment outcomes, safety and efficacy of Cyberknife SBRT for patients with advanced or terminal stage HCC at our institution.
MATERIALS AND METHODS
Patients
PPatients with HCC who were unsuitable for surgery, TACE, RFA, or other therapies were eligible for Cyberknife treatment and enrolled after careful discussion between the patients and their treating physicians. We selected tumors for Cyberknife treatment if they met the following eligibility criteria: intrahepatic tumors invading the hepatic vessels or bile duct without other viable lesions, single extrahepatic tumors, or bone metastases causing pain. In principle, patients with multiple metastases were eligible only if they had bone lesions.
All the patients submitted a written consent form before treatment. This retrospective, single-institution study was approved by the institutional research ethics board.
The diagnosis of HCC was based on histological confirmation, or the characteristic radiological appearance on a dynamic computed tomography (CT) scan or a dynamic contrast-enhanced magnetic resonance imaging (MRI) scan. The presence of risk factors, such as cirrhosis, HBV, or HCV infection was also taken into account. For metastatic lesions, we assumed that HCC was the primary tumor if the patient had previously been diagnosed with HCC and had metastasis.
Treatment
All patients were treated as inpatients, except for 4 patients who adamantly chose to be treated as outpatients. All patients were treated with the Cyberknife.
Real-time tracking of tumor movements was performed with the MultiPlan® (Accuray) treatment planning software and the Synchrony® (Accuray) respiratory tracking system. A gold marker was introduced beside the tumor for those who needed respiratory synchronization. For tumors invading the hepatic vessels or bile duct, Visicoil® (Sceti, Medical Labo K. K., Tokyo, Japan), a helical gold linear fiducial marker 0.75 mm in diameter by 5 mm in length was percutaneously implanted under ultrasound-guidance near the tumor. For lung metastasis, a spherical fiducial marker 1.5 mm in diameter (Olympus, Tokyo, Japan) was inserted by bronchoscopy.
Patients were immobilized in a vacuum cushion or plastic shell in the treatment position to reduce any motion caused by breathing. A spiral CT scan with and without contrast and a slice thickness of 1 mm was obtained for planning. MRI was also used for spine or brain lesion planning. The gross tumor volume (GTV) for intrahepatic lesions was defined as the arterial enhancing site with washout on the venous or delayed phase CT. The GTV for extrahepatic lesions was defined depending on the characteristic radiologic aspects of the metastases. The planned target volume (PTV) for intrahepatic lesions and lung metastases was defined as the GTV with a 2-5 mm margin in all directions. Because the lesions inside the lung are particularly vulnerable to respiratory movement, the margins for these lesions were estimated based on CT scans obtained during both the inhalation and exhalation phases. For spinal lesions, the PTV was defined as the GTV with a 2 mm margin because these lesions are less subject to respiratory movement. For brain lesions, no margin was applied for the GTV because the surrounding brain tissue is considered critical. A total dose of 8-50 Gy in 1-10 fractions was prescribed to the 80% isodose line (95% PTV coverage) and delivered to the PTV for 1-7 consecutive working days. Dose constraints for organs at risk were applied based on a previous report[38].
Evaluation
Each patient had a clinical and biological evaluation during and after the completion of treatment and every 1 to 3 mo thereafter until death unless they were lost to follow-up, or until death. Patients underwent CT scans 1-3 mo following the completion of SBRT, and radiological follow-up was performed by CT scan or MRI every 3 mo thereafter.
Tumor response was classified according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1[39] as follows: complete response (CR), complete disappearance of the irradiated tumor; partial response (PR), > 30% reduction in tumor size; stable disease (SD), < 30% reduction or < 20% increase in tumor size; and progressive disease (PD), > 20% increase in tumor size. Although the modified RECIST (mRECIST) has recently been used to evaluate treatment response in HCC, the RECIST version 1.1 still seems to be commonly used in evaluating radiotherapy responsiveness, as seen in previous reports[17,40]. Tumor markers, including alpha fetoprotein (AFP) and proteins induced by vitamin K absence II (PIVKA II) were evaluated within in one week prior to the treatment and one month after the treatment. For bone metastases, the efficacy of treatment was also evaluated by symptom relief. The response was self-assessed by subjective pain score and was classified into the following categories: pain relief, exacerbation, or no symptomatic change. Toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0[41]. Dose limiting toxicity (DLT) was any CTCAE grade 4 or 5 for hepatic, thrombocytopenic, or GI toxicity occurring within 1 mo of SBRT or RILD requiring treatment in the absence of disease progression within 3 mo of SBRT.
Statistical analysis
Prognostic factors for tumor response and tumor markers were evaluated with Fisher’s exact test and a logistic regression model. Survival was evaluated with the Kaplan-Meier method and multivariate analysis with the Cox proportional hazards model. All statistical analyses were performed using the R statistical package “cmprsk” in version 3.2.0. Differences were considered to be statistically significant at P < 0.05.
RESULTS
Patients and treatment
Between May 2011 and June 2015, a total of 65 patients with 95 lesions were treated with SBRT using the Cyberknife system. Fifty-one were male and 14 were female with a median age of 71 (range: 26-93) years. Underlying liver disease included hepatitis C in 35 patients (54%), hepatitis B in 9 patients (14%), and other causes in 21 patients. The patients included in the study had Eastern Cooperative Oncology Group performance scores of less or equal to 2, except for 6 patients with a score of 3. Pre-treatment Child-Pugh score ranged from 5A to 8B, except for 2 patients who had scores of 11C and 12C respectively. Based on the BCLC classification of HCC, 59 patients and 6 patients had advanced and terminal stage disease, respectively. All the patients had previously been treated for HCC, including 24 patients who received surgery, 28 patients who received RFA, 49 patients who received TACE, and 7 patients who received radiation therapy other than SBRT previously. Seven patients with 15 lesions were treated in combination with sorafenib administration. Six patients had been previously treated with sorafenib but discontinued therapy due to side effects. Other patients were not eligible for sorafenib treatment due to contraindications such as poor liver function or brain metastasis. The target lesions were represented as follows: 52 were bone metastasis (mostly spine); 9, lung metastasis; 7, brain metastasis; 9, portal vein invasion; 4, hepatic vein invasion; 4, bile duct invasion; and 10 other lesions (pleura, cavernous sinus, and lymph node metastases).
For tumors invading the hepatic vessels or bile duct, the median tumor size was 29 (range: 12-54) mm and the median prescribed dose was 35 (range: 28-50) Gy in 3-10 fractions. For extrahepatic lesions, the median tumor size was 23 (range: 10-53) mm and the median prescribed dose was 25 (range: 6-48) Gy in 1-6 fractions.
The median follow-up period was 4 (range: 1-33) mo. Of the 65 patients, 35 patients were referred from other institutions and were followed-up after treatment at the referring hospital, and 15 patients were lost to follow-up. Treatment was completed by all patients. The characteristics of the patients are presented in Table 1.
Table 1.
Patient characteristics n (%)
| Characteristics | Parameter | Patients |
| No. of patients | 65 (100) | |
| Sex | ||
| Male | 51 (78) | |
| Female | 14 (22) | |
| Age, yr | ||
| Median | 71 | |
| Minimum-Maximum | 26-93 | |
| Viral hepatitis | ||
| HCV | 35 (54) | |
| HBV | 9 (14) | |
| None | 21 (32) | |
| Child-Pugh classification | ||
| A | 38 (58) | |
| B | 24 (37) | |
| C | 2 (3) | |
| NA | 1 (2) | |
| ECOG perfomance status | ||
| 0 | 16 (25) | |
| 1-2 | 43 (66) | |
| 3 | 6 (9) | |
| Previous treatments | ||
| Surgery | 24 (37) | |
| RFA | 28 (43) | |
| TACE | 49 (75) | |
| Sorafenib | 13 (20) | |
| Radiation | 7 (11) | |
| BCLC stage | ||
| C | 59 (91) | |
| D | 6 (9) | |
| AFP (ng/mL) | ||
| Median | 256 | |
| Minimum-Maximum | 1-240700 | |
| PIVKA II (mAU/mL) | ||
| Median | 1431 | |
| Minimum-Maximum | 8-316400 |
ECOG: Eastern Cooperative Oncology Group; BCLC: Barcelona Clinic Liver Cancer staging system; AFP: Alpha fetoprotein; PIVKA II: Proteins induced by vitamin K absence; HCV: Hepatitis C virus; HBV: Hepatitis B virus; RFA: Radiofrequency ablation; TACE: Transarterial chemoembolization; NA: Not available.
Tumor response
The efficacy of the therapy was as follows; CR was observed in 7 lesions, PR in 25 lesions, SD in 19 lesions, and PD in 16 lesions; 28 lesions were not evaluated because of patient death or the loss of a patient to follow-up. Actual tumor responses are shown in Figure 1. The response rate (RR) and disease control rate (DCR) of all the lesions were 34% and 53%, respectively. After excluding the unevaluated cases, the RR and DCR were 48% and 76%, respectively. For tumors invading the hepatic vessels or bile duct, the RR and DCR of the evaluated cases were 50% and 80%, respectively. Lesions and treatment outcomes are summarized in Table 2.
Figure 1.
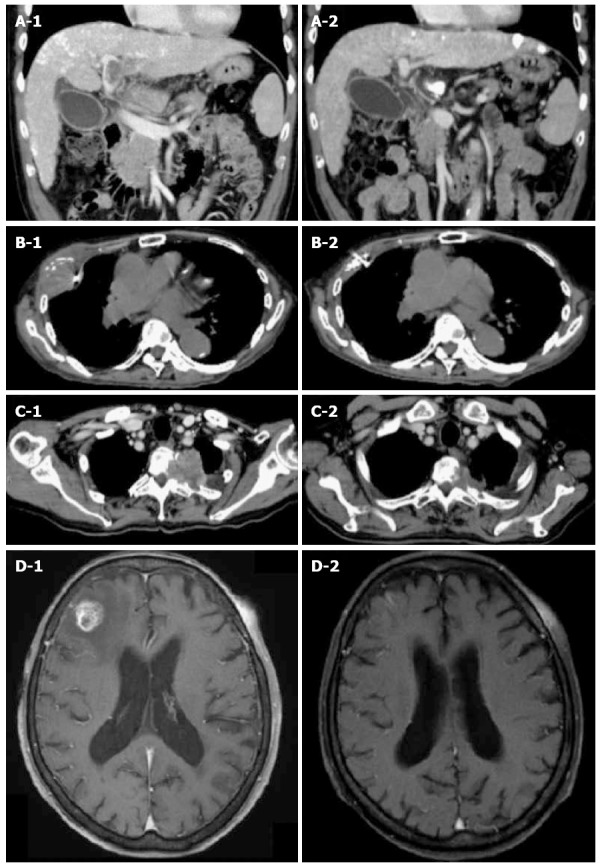
Tumor responses treated with Cyberknife. A1: CT scan of 59-year-old male with portal vein tumor thrombosis. The tumor is invading the portal vein from the main trunk to the 1st branch. The tumor diameter was 46 mm. A fiducial marker was implanted nearby; A2: Three months after irradiation with 35 Gy/5 fractions. The portal vein tumor thrombosis disappeared completely and the patient achieved CR; B1: CT scan of 85-year-old male with pleural HCC metastasis. The tumor diameter was 53 mm. A fiducial marker was implanted nearby; B2: Three months after irradiation with 30 Gy/5 fractions. The tumor disappeared completely and the patient achieved CR; C1: CT scan of 72-year-old male with thoracic spine HCC metastasis. Tumor is invading the left side of the thoracic spine at T2-3 causing bone destruction. The tumor diameter was 52 mm; C2: Three months after irradiation with 30 Gy/5 fractions. The tumor decreased to 33 mm (37% reduction in size) and the patient achieved PR; D1: T1-weighted MRI of 83-year-old female with brain HCC metastasis. There is a right frontal lobe lesion with gadolinium enhancement. The tumor diameter was 19 mm; D2: One month after irradiation of 20 Gy/1 fraction. The tumor disappeared completely and the patient achieved CR. CR: Complete response; CT: Computed tomography; HCC: Hepatocellular carcinoma.
Table 2.
Lesions and treatment outcomes
| Variables | Total lesions (n = 95) |
Size (mm) |
Radiation (Gy) |
Response |
|||||||
| n | Median | Range | Dose | Range | Fraction | CR | PR | SD | PD | NA | |
| Liver | |||||||||||
| Portal vein | 9 | 34.5 | (15-54) | 36 | (28-50) | (3-6) | 2 | 2 | 1 | 4 | |
| Hepatic vein | 4 | 38 | (20-54) | 32.1 | (28-36) | (4-10) | 1 | 1 | 1 | 1 | |
| Bile duct | 4 | 19.5 | (12-29) | 38.5 | (28-45) | (5-7) | 1 | 1 | 2 | ||
| Bone | 52 | 24.5 | (10-52) | 21.5 | (8-33) | (1-6) | 1 | 13 | 16 | 11 | 11 |
| Lung | 9 | 19 | (18-48) | 40 | (27-48) | (3-4) | 4 | 1 | 4 | ||
| Brain | 7 | 23.5 | (12-38) | 22 | (14-30) | (1-3) | 2 | 2 | 3 | ||
| Others | 10 | 31 | (15-53) | 30 | (16-48 | (1-6) | 1 | 6 | 3 | ||
CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease. NA: Not available.
Univariate analysis was performed but none of the clinical factors were statistically significant for tumor response (Table 3).
Table 3.
Prognostic factors for tumor response n (%)
| Prognostic factors | Response (+) | Response (-) | P value |
| CR, PR, SD | PD | ||
| Gender | 0.716 | ||
| Female | 8 (73) | 3 (27) | |
| Male | 43 (77) | 13 (23) | |
| Age (yr) | 1.000 | ||
| < 70 | 23 (77) | 7 (23) | |
| ≥ 70 | 28 (76) | 9 (24) | |
| AFP (ng/mL) | 0.123 | ||
| < 400 | 25 (69) | 11 (31) | |
| ≥ 400 | 23 (88) | 3 (12) | |
| BCLC | 1.000 | ||
| Advanced | 46 (75) | 15 (25) | |
| Terminal | 5 (83) | 1 (17) | |
| Child-Pugh | 0.363 | ||
| < 7 | 36 (80) | 9 (20) | |
| ≥ 7 | 15 (68) | 7 (32) | |
| Diameter (mm) | 0.401 | ||
| < 30 | 29 (81) | 7 (19) | |
| ≥ 30 | 22 (71) | 9 (29) | |
| Dose (Gy) | 0.119 | ||
| < 30 | 33 (70) | 14 (30) | |
| ≥ 30 | 18 (90) | 2 (10) | |
| Dose/fraction (Gy) | 0.137 | ||
| < 8 | 22 (88) | 3 (12) | |
| ≥ 8 | 29 (69) | 13 (31) | |
| Lesion | 0.274 | ||
| Intrahepatic | 10 (91) | 1 (9) | |
| Extrahepatic | 41 (73) | 15 (27) | |
| Fiducial | 0.126 | ||
| (-) | 34 (71) | 14 (29) | |
| (+) | 17 (89) | 2 (11) | |
| Sorafenib | 0.460 | ||
| (-) | 43 (78) | 12 (22) | |
| (+) | 8 (67) | 4 (33) |
Fisher's exact test was used to evaluate prognostic factors for tumor response. Complete response (CR), Partial response (PR) and stable disease (SD) were categorized into response (+), progressive disease (PD) was categorized into response (-).
Trends in AFP and PIVKA II levels were available in 53 lesions. Thirty patients (57%) presented with decreases in AFP, and 28 patients (53%) presented with decreases in PIVKA II. In the univariate analysis, radiation dose (≥ 30 Gy) and fiducial marker implantation were appeared to be factors associated with both AFP and PIVKA II reductions. In multivariate analysis, fiducial marker implantation remained to be associated with better control of both AFP [(HR = 0.152; 95%CI: 0.026-0.887, P = 0.036) and PIVKA II (HR = 0.035; 95%CI: 0.003-0.342, P = 0.004)]. The results are shown in Tables 4 and 5.
Table 4.
Prognostic factors for alpha fetoprotein response n (%)
| Prognostic factors |
Univariate analysis |
Multivariate analysis |
||||
|
AFP |
P value | Odds ratio | 95%CI | P value | ||
| Decrease | Increase | |||||
| Gender | 0.478 | |||||
| Female | 4 (44) | 5 (56) | ||||
| Male | 26 (59) | 18 (41) | ||||
| Age (yr) | < 0.001 | |||||
| < 70 | 11 (37) | 19 (63) | ||||
| ≥ 70 | 19 (83) | 4 (17) | 0.116 | 0.029-0.460 | 0.002 | |
| AFP (ng/mL) | 0.570 | |||||
| < 400 | 20 (61) | 13 (39) | ||||
| ≥ 400 | 10 (50) | 10 (50) | ||||
| BCLC | 1.000 | |||||
| Advanced | 27 (57) | 20 (43) | ||||
| Terminal | 3 (50) | 3 (50) | ||||
| Child-Pugh | 0.151 | |||||
| < 7 | 22 (65) | 12 (35) | ||||
| ≥ 7 | 8 (42) | 11 (58) | ||||
| Diameter (mm) | 0.054 | |||||
| < 30 | 11 (42) | 15 (58) | ||||
| ≥ 30 | 19 (70) | 8 (30) | 0.286 | 0.073-1.120 | 0.072 | |
| Dose (Gy) | 0.013 | |||||
| < 30 | 18 (46) | 21 (54) | ||||
| ≥ 30 | 12 (86) | 2 (14) | 0.992 | 0.093-10.50 | 0.995 | |
| Dose/fraction (Gy) | 0.555 | |||||
| < 8 | 11 (65) | 6 (35) | ||||
| ≥ 8 | 19 (53) | 17 (47) | ||||
| Lesion | 0.270 | |||||
| Intrahepatic | 7 (78) | 2 (22) | ||||
| Extrahepatic | 23 (52) | 21 (48) | ||||
| Fiducial | 0.025 | |||||
| (-) | 19 (48) | 21 (52) | ||||
| (+) | 11 (85) | 2 (15) | 0.152 | 0.026-0.887 | 0.036 | |
| Sorafenib | 0.738 | |||||
| (-) | 23 (55) | 19 (45) | ||||
| (+) | 7 (64) | 4 (36) | ||||
Fisher's exact test and a logistic regression model were used to evaluate prognostic factors for AFP response. AFP: Alpha fetoprotein.
Table 5.
Prognostic factors for PIVKA II response n (%)
| Prognostic factors |
Univariate analysis |
Multivariate analysis |
||||
|
PIVKA II |
P value | Odds ratio | 95%CI | P value | ||
| Decrease | Increase | |||||
| Gender | 0.278 | |||||
| Female | 3 (33) | 6 (67) | ||||
| Male | 25 (57) | 19 (43) | ||||
| Age (yr) | 0.052 | |||||
| < 70 | 12 (40) | 18 (60) | ||||
| ≥ 70 | 16 (70) | 7 (30) | 0.359 | 0.093-1.390 | 0.139 | |
| AFP (ng/mL) | 0.738 | |||||
| < 400 | 18 (55) | 15 (45) | ||||
| ≥ 400 | 10 (50) | 10 (50) | ||||
| BCLC | 0.404 | |||||
| Advanced | 26 (55) | 21 (45) | ||||
| Terminal | 2 (33) | 4 (67) | ||||
| Child-Pugh | 0.267 | |||||
| < 7 | 20 (59) | 14 (41) | ||||
| ≥ 7 | 8 (42) | 11 (58) | ||||
| Diameter (mm) | 0.056 | |||||
| < 30 | 10 (38) | 16 (62) | ||||
| ≥ 30 | 18 (67) | 9 (33) | 0.185 | 0.047-0.730 | 0.016 | |
| Dose (Gy) | < 0.001 | |||||
| < 30 | 15 (38) | 24 (62) | ||||
| ≥ 30 | 13 (93) | 1 (7) | 0.270 | 0.021-3.40 | 0.312 | |
| Dose/Fraction (Gy) | 0.769 | |||||
| < 8 | 8 (47) | 9 (53) | ||||
| ≥ 8 | 20 (56) | 16 (44) | ||||
| Lesion | 0.026 | |||||
| Intrahepatic | 8 (89) | 1 (11) | ||||
| Extrahepatic | 20 (45) | 24 (55) | 0.000 | 0.00-∞ | 0.994 | |
| Fiducial | 0.001 | |||||
| (-) | 16 (40) | 24 (60) | ||||
| (+) | 12 (92) | 1 (8) | 0.035 | 0.003-0.342 | 0.004 | |
| Sorafenib | 1.000 | |||||
| (-) | 22 (52) | 20 (48) | ||||
| (+) | 6 (55) | 5 (45) | ||||
Fisher's exact test and a logistic regression model were used to evaluate prognostic factors for PIVKA II response.
For the 52 cases of bone metastases, the efficacy of treatment was also assessed in terms of pain control. Thirty-six patients (69%) achieved pain relief, 10 patients had no symptomatic change, 1 patient had worse pain, and 4 patients were not evaluated.
Overall survival
AAt the time of the analysis, 26 patients had died; each died of cancer. The overall 1-year survival rate was 49%. The median survival times for all the patients, advanced stage patients, and terminal stage patients were 9.0 mo (95%CI: 5.0-15.0), 13.0 mo (95%CI: 7.0-15.0) and 1.0 mo (95%CI: 1.0-NA) respectively. The Kaplan-Meier curve for overall survival is presented in Figure 2. Univariate and multivariate analyses were performed to account for the factors associated with survival. In univariate analysis, AFP (≥ 400 ng/mL), BCLC terminal stage, Child-Pugh score (≥ 7) and radiation dose (< 30 Gy) were appeared to be factors associated with worse survival. In multivariate analysis, BCLC terminal stage (HR = 9.809; 95%CI: 2.589-37.17, P < 0.001) and AFP (≥ 400 ng/mL) (HR = 2.548; 95%CI: 1.070-6.068; P = 0.035) were associated with worse survival. Radiation dose (≥ 30 Gy) (HR = 0.274; 95%CI: 0.093-0.7541, P = 0.012) was associated with improved survival in multivariate analysis. Prognostic factors associated with overall survival are shown in Table 6.
Figure 2.

Kaplan Meier curves for overall survival. The median survival times for the advanced stage patients and terminal stage patients were 13 mo and 1 mo respectively.
Table 6.
Univariate and multivariate analysis for overall survival
| Prognostic factors |
Univariate analysis |
Multivariate analysis |
||||
| Hazard ratio | 95%CI | P value | Hazard ratio | 95%CI | P value | |
| Gender (male) | 0.968 | 0.387-2.419 | 0.945 | |||
| Age (≥ 70 yr) | 0.770 | 0.355-1.670 | 0.508 | |||
| AFP (≥ 400 ng/mL) | 2.662 | 1.181-6.001 | 0.018 | 2.548 | 1.070-6.068 | 0.035 |
| BCLC (terminal) | 7.022 | 2.442-20.19 | < 0.001 | 9.809 | 2.589-37.17 | < 0.001 |
| Child-Pugh (≥ 7) | 3.031 | 1.258-7.301 | 0.013 | 1.364 | 0.510-3.645 | 0.536 |
| Diameter (≥ 30 mm) | 0.654 | 0.285-1.500 | 0.316 | |||
| Dose (≥ 30 Gy) | 0.302 | 0.114-0.804 | 0.017 | 0.274 | 0.093-0.7541 | 0.012 |
| Dose/fraction (≥ 8 Gy) | 1.889 | 0.790-4.516 | 0.153 | |||
| Lesion (extrahepatic) | 1.789 | 0.665-4.817 | 0.250 | |||
| Fiducial (+) | 0.491 | 0.195-1.238 | 0.132 | 0.783 | 0.264-2.321 | 0.659 |
| Sorafenib (+) | 1.068 | 0.247-4.618 | 0.930 | |||
Cox proportional hazards model was used to evaluate the prognostic factors for overall survival. AFP: Alpha fetoprotein; BCLC: Barcelona Clinic Liver Cancer.
Adverse effects
Overall, the treatments were well-tolerated by patients. No patient complained of changes in subjective symptoms, such as abdominal pain, nausea, fatigue, or joint pain, and no patients had a toxicity greater than or equal to grade 2. No hematologic complications, significant liver enzyme elevations, or classic RILD were observed during treatment.
One patient had a grade 4 cerebral hemorrhage 2 h after radiation for brain metastasis. The patient recovered well after craniotomy and hematoma removal, but died of liver failure 45 d after therapy. This was the second case of hemorrhage that presented on the first day of the SBRT therapy experienced at our institution.
Another patient presented with a grade 2 esophageal ulcer following treatment that resulted in a digestive hemorrhage. For this patient, the treatment target was in the hepatic vessels, and CR was achieved in that lesion. The maximum dose with which the esophagus was irradiated was 31.2 Gy in 4 fractions and occurred 16 d after therapy. The patient recovered well with conservative management, including a proton pump inhibitor, mucoprotective agents, and 5-aminosalicylic acid administration.
DISCUSSION
To date, radiation therapy has not been established as a standard therapy for HCC[42]. This modality has not even been included as a treatment option in the BCLC staging system. However, a growing number of patients who are not eligible for conventional therapy have been treated with radiation therapy with promising results. Furthermore, this therapy modality can be used not only as curative treatment but also for palliative care.
The treatment of advanced HCC with invasion of the major hepatic vessels or the bile duct can be challenging. The majority of available liver-directed therapies are generally contraindicated for such cases. Additionally, these HCC lesions are associated with a worse prognosis for overall survival. Our results showed that RR and DCR for these lesions at follow-up were 50% and 80%, respectively. Kang et al[43] reported that RR for portal vein tumor thrombosis treated by SBRT alone was 66.7%, and could be improved up to 73.5% if combined with TACE. Compared with these results, our data are almost equivalent. We believe the small difference between the above results can be partly explained by the higher prescribed dose (median 40.2 Gy) in the previous study compared with our study (median 35 Gy). Previous reports underline a significant relationship between total prescribed dose and local tumor control[10,17,40]. Although our study could not significantly certify the prognostic factors for tumor response, radiation dose (≥ 30 Gy) had a favorable tendency regarding tumor response (OR = 0.266; 95%CI: 0.027-1.370, P = 0.119). In terms of overall survival, radiation dose (≥ 30 Gy) and AFP (≥ 400 ng/mL) were significant prognostic factors, as found in previous reports. Due to limited information regarding optimal treatment indication, doses, and methods remains limited, further studies are required to maximize the efficacy of SBRT.
In our study, all the patients had either advanced or terminal stage disease based on their BCLC classification. Remarkably, all of our patients were able to complete treatment, although most of them were in poor condition, had a poor performance status or other medical complications. All patients were able to complete treatment because SBRT has the advantage of enabling a short treatment course while allowing the administration of a large radiation dose in a small number of fractions.
Furthermore, our patients were mainly comprised those who were not eligible for sorafenib due to its side effects or contraindications to therapy such as poor liver function or brain metastasis. Radu et al[44] reported that inadequate treatment for advanced stage HCC patients, undertreatment results in a decreased survival (3 mo vs 4 mo) and that overtreatment may increase survival (28 mo vs 4 mo) compared with standard therapy. Therefore, SBRT may be a hopeful option for patients who are not eligible for other treatments.
To assess the overall disease control, the trends of AFP and PIVKA II were evaluated. Thirty-five patients (57%) presented with decreases in AFP, and 28 patients (53%) presented with decreases in PIVKA II. In multivariate analysis, fiducial marker implantation was associated with better control of both AFP and PIVKA II. This was most likely because fiducial marker implantation was performed against lesions that were the largest burden for patients without other coexisting viable HCC. We assume that the reason that all patients did not achieve tumor marker improvement was because some patients had other coexisting lesions that were left untreated (especially in bone metastases cases). We believe that there was a therapeutic effect with respect to the lesions irradiated, and that SBRT can also be used for palliative care. In terms of SBRT for bone metastases, 69% of the patients achieved pain relief without complications. We conclude that SBRT can be safely and successfully administered to palliate bone metastasis symptoms.
There were several limitations to this study. A major limitation was its retrospective design and consequent lack of a control group. Additionally, this study involved only one institution and our sample size was small. However, based on previous studies, the median survival times of advanced stage and terminal stage BCLC are generally reported to be 4-7 mo and 1-3 mo respectively[44-46]. In our study, in spite of some patients being lost to follow-up, the median survival times for advanced stage and terminal stage were 13 mo and 1 mo respectively. This result suggests that SBRT may have the potential to increase the overall survival for advanced stage HCC patients, and compares favorably with the best supportive care and with sorafenib (4.2 to 7.9 mo and 6.5 to 10.7 mo, respectively[5,8]), which is the only other potentially available therapy for these patients. Further prospective studies are expected to define the role of the Cyberknife in the management of HCC.
In conclusion, this report is pioneering because it focused on Cyberknife SBRT in patients with advanced or terminal stage HCC. Our results suggest that the Cyberknife may be less invasive than other therapies and is useful for local tumor control, palliative care and increasing survival for those who have no other treatment options.
COMMENTS
Background
The Cyberknife® system delivers stereotactic body radiation therapy (SBRT). SBRT is a technique that enables the delivery of high-dose radiation (usually 8-12 Gy/fraction) to the tumor with extreme accuracy in 1-10 fractions, while minimizing the damage to normal surrounding tissue. The major advantages of SBRT are the promising radiobiological efficacy of the administration of such large radiation doses to tumor tissues, the short treatment course achieved by a small number of fractions, and the minimally invasiveness of the therapy, which can also be given to patients with poor performance status. Currently, successful reports of SBRT studies against hepatocellular carcinoma (HCC) and other liver tumors have suggested that SBRT can be used safely and this method has been associated with high local control rates, mostly in the range of 70%-100% at 1-2 years. The authors report treatment outcomes, safety and efficacy of Cyberknife SBRT for patients with advanced or terminal stage HCC at our institution to clarify its safety and efficacy.
Research frontiers
Most studies published about SBRT for HCC have focused only on liver confined tumors, with a few articles discussing SBRT use for extrahepatic HCC. The authors present the largest study on Cyberknife treatment for patients with advanced and terminal stage HCC.
Innovations and breakthroughs
In this study, the authors found that the Cyberknife can safely be administered even in patients with advanced or terminal stage HCC. The median survival time was 9 mo (95%CI: 5-15 mo). Terminal stage disease (HR = 9.809; 95%CI: 2.589-37.17, P < 0.001) and an AFP of more than 400 ng/mL (HR = 2.548; 95%CI: 1.070-6.068, P = 0.035) were associated with worse survival. A radiation dose higher than 30 Gy (HR = 0.274; 95%CI: 0.093-0.7541, P = 0.012) was associated with better survival.
Applications
Present results revealed that SBRT may have the potential to increase the overall survival in advanced stage HCC patients. High AFP levels were associated with worse survival, but a higher radiation dose improved the survival.
Terminology
Cyberknife system: A non-invasive alternative to surgery for the treatment of both cancerous and non-cancerous tumors anywhere in the body. It delivers high-dose beams of high dose radiation to tumors with extreme accuracy. SBRT: A technique that enables the delivery of high radiation doses (usually 8-12 Gy/fraction) to the tumor with extreme accuracy in 1-10 fractions, while minimizing the damage to normal surrounding tissue.
Peer-review
This study describes the treatment outcome of CyberKnife SBRT for primary or metastatic lesions in patients with advanced or terminal HCC according to the Barcelona Clinic Liver Cancer classification.
Footnotes
Institutional review board statement: The study was reviewed and approved by the institutional review board of the Japanese Red Cross Medical Center. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: We have no financial relationships to disclose.
Data sharing statement: The statistical code and dataset are available from the corresponding author at hiroyuki.kato.911@gmail.com. Consent for data sharing was not obtained but the presented data are anonymized and risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 5, 2015
First decision: September 9, 2015
Article in press: October 26, 2015
P- Reviewer: Chiou JF S- Editor: Yu J L- Editor: A E- Editor: Liu XM
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–1310. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 4.Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43–58. doi: 10.1148/radiol.11110144. [DOI] [PubMed] [Google Scholar]
- 5.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 7.Oliveri RS, Wetterslev J, Gluud C. Transarterial (chemo)embolisation for unresectable hepatocellular carcinoma. Cochrane Database Syst Rev. 2011;(3):CD004787. doi: 10.1002/14651858.CD004787.pub2. [DOI] [PubMed] [Google Scholar]
- 8.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 9.Wigg AJ, Palumbo K, Wigg DR. Radiotherapy for hepatocellular carcinoma: systematic review of radiobiology and modeling projections indicate reconsideration of its use. J Gastroenterol Hepatol. 2010;25:664–671. doi: 10.1111/j.1440-1746.2009.06126.x. [DOI] [PubMed] [Google Scholar]
- 10.Ursino S, Greco C, Cartei F, Colosimo C, Stefanelli A, Cacopardo B, Berretta M, Fiorica F. Radiotherapy and hepatocellular carcinoma: update and review of the literature. Eur Rev Med Pharmacol Sci. 2012;16:1599–1604. [PubMed] [Google Scholar]
- 11.Ingold JA, Reed GB, Kaplan HS, Bagshaw MA. Radiation hepatitis. Am J Roentgenol Radium Ther Nucl Med. 1965;93:200–208. [PubMed] [Google Scholar]
- 12.Guha C, Kavanagh BD. Hepatic radiation toxicity: avoidance and amelioration. Semin Radiat Oncol. 2011;21:256–263. doi: 10.1016/j.semradonc.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng M, Ben-Josef E. Radiation therapy for hepatocellular carcinoma. Semin Radiat Oncol. 2011;21:271–277. doi: 10.1016/j.semradonc.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Young RF. The role of the gamma knife in the treatment of malignant primary and metastatic brain tumors. CA Cancer J Clin. 1998;48:177–188. doi: 10.3322/canjclin.48.3.177. [DOI] [PubMed] [Google Scholar]
- 15.Cárdenes HR, Price TR, Perkins SM, Maluccio M, Kwo P, Breen TE, Henderson MA, Schefter TE, Tudor K, Deluca J, et al. Phase I feasibility trial of stereotactic body radiation therapy for primary hepatocellular carcinoma. Clin Transl Oncol. 2010;12:218–225. doi: 10.1007/s12094-010-0492-x. [DOI] [PubMed] [Google Scholar]
- 16.Andolino DL, Johnson CS, Maluccio M, Kwo P, Tector AJ, Zook J, Johnstone PA, Cardenes HR. Stereotactic body radiotherapy for primary hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2011;81:e447–e453. doi: 10.1016/j.ijrobp.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Bujold A, Massey CA, Kim JJ, Brierley J, Cho C, Wong RK, Dinniwell RE, Kassam Z, Ringash J, Cummings B, et al. Sequential phase I and II trials of stereotactic body radiotherapy for locally advanced hepatocellular carcinoma. J Clin Oncol. 2013;31:1631–1639. doi: 10.1200/JCO.2012.44.1659. [DOI] [PubMed] [Google Scholar]
- 18.Kang JK, Kim MS, Cho CK, Yang KM, Yoo HJ, Kim JH, Bae SH, Jung da H, Kim KB, Lee DH, et al. Stereotactic body radiation therapy for inoperable hepatocellular carcinoma as a local salvage treatment after incomplete transarterial chemoembolization. Cancer. 2012;118:5424–5431. doi: 10.1002/cncr.27533. [DOI] [PubMed] [Google Scholar]
- 19.Choi BO, Jang HS, Kang KM, Lee SW, Kang YN, Chai GY, Choi IB. Fractionated stereotactic radiotherapy in patients with primary hepatocellular carcinoma. Jpn J Clin Oncol. 2006;36:154–158. doi: 10.1093/jjco/hyi236. [DOI] [PubMed] [Google Scholar]
- 20.Choi BO, Choi IB, Jang HS, Kang YN, Jang JS, Bae SH, Yoon SK, Chai GY, Kang KM. Stereotactic body radiation therapy with or without transarterial chemoembolization for patients with primary hepatocellular carcinoma: preliminary analysis. BMC Cancer. 2008;8:351. doi: 10.1186/1471-2407-8-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Louis C, Dewas S, Mirabel X, Lacornerie T, Adenis A, Bonodeau F, Lartigau E. Stereotactic radiotherapy of hepatocellular carcinoma: preliminary results. Technol Cancer Res Treat. 2010;9:479–487. doi: 10.1177/153303461000900506. [DOI] [PubMed] [Google Scholar]
- 22.Kwon JH, Bae SH, Kim JY, Choi BO, Jang HS, Jang JW, Choi JY, Yoon SK, Chung KW. Long-term effect of stereotactic body radiation therapy for primary hepatocellular carcinoma ineligible for local ablation therapy or surgical resection. Stereotactic radiotherapy for liver cancer. BMC Cancer. 2010;10:475. doi: 10.1186/1471-2407-10-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seo YS, Kim MS, Yoo SY, Cho CK, Choi CW, Kim JH, Han CJ, Park SC, Lee BH, Kim YH, et al. Preliminary result of stereotactic body radiotherapy as a local salvage treatment for inoperable hepatocellular carcinoma. J Surg Oncol. 2010;102:209–214. doi: 10.1002/jso.21593. [DOI] [PubMed] [Google Scholar]
- 24.Huang WY, Jen YM, Lee MS, Chang LP, Chen CM, Ko KH, Lin KT, Lin JC, Chao HL, Lin CS, et al. Stereotactic body radiation therapy in recurrent hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;84:355–361. doi: 10.1016/j.ijrobp.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 25.Honda Y, Kimura T, Aikata H, Kobayashi T, Fukuhara T, Masaki K, Nakahara T, Naeshiro N, Ono A, Miyaki D, et al. Stereotactic body radiation therapy combined with transcatheter arterial chemoembolization for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2013;28:530–536. doi: 10.1111/jgh.12087. [DOI] [PubMed] [Google Scholar]
- 26.Bae SH, Kim MS, Cho CK, Kim KB, Lee DH, Han CJ, Park SC, Kim YH. Feasibility and efficacy of stereotactic ablative radiotherapy for Barcelona Clinic Liver Cancer-C stage hepatocellular carcinoma. J Korean Med Sci. 2013;28:213–219. doi: 10.3346/jkms.2013.28.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xi M, Zhang L, Zhao L, Li QQ, Guo SP, Feng ZZ, Deng XW, Huang XY, Liu MZ. Effectiveness of stereotactic body radiotherapy for hepatocellular carcinoma with portal vein and/or inferior vena cava tumor thrombosis. PLoS One. 2013;8:e63864. doi: 10.1371/journal.pone.0063864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanuki N, Takeda A, Oku Y, Mizuno T, Aoki Y, Eriguchi T, Iwabuchi S, Kunieda E. Stereotactic body radiotherapy for small hepatocellular carcinoma: a retrospective outcome analysis in 185 patients. Acta Oncol. 2014;53:399–404. doi: 10.3109/0284186X.2013.820342. [DOI] [PubMed] [Google Scholar]
- 29.Blomgren H, Lax I, Näslund I, Svanström R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861–870. doi: 10.3109/02841869509127197. [DOI] [PubMed] [Google Scholar]
- 30.Tse RV, Hawkins M, Lockwood G, Kim JJ, Cummings B, Knox J, Sherman M, Dawson LA. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 31.Herfarth KK, Debus J, Lohr F, Bahner ML, Rhein B, Fritz P, Höss A, Schlegel W, Wannenmacher MF. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:164–170. doi: 10.1200/JCO.2001.19.1.164. [DOI] [PubMed] [Google Scholar]
- 32.Wulf J, Hädinger U, Oppitz U, Thiele W, Ness-Dourdoumas R, Flentje M. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol. 2001;177:645–655. doi: 10.1007/pl00002379. [DOI] [PubMed] [Google Scholar]
- 33.Méndez Romero A, Wunderink W, Hussain SM, De Pooter JA, Heijmen BJ, Nowak PC, Nuyttens JJ, Brandwijk RP, Verhoef C, Ijzermans JN, et al. Stereotactic body radiation therapy for primary and metastatic liver tumors: A single institution phase i-ii study. Acta Oncol. 2006;45:831–837. doi: 10.1080/02841860600897934. [DOI] [PubMed] [Google Scholar]
- 34.Iwata H, Shibamoto Y, Hashizume C, Mori Y, Kobayashi T, Hayashi N, Kosaki K, Ishikawa T, Kuzuya T, Utsunomiya S. Hypofractionated stereotactic body radiotherapy for primary and metastatic liver tumors using the novalis image-guided system: preliminary results regarding efficacy and toxicity. Technol Cancer Res Treat. 2010;9:619–627. doi: 10.1177/153303461000900610. [DOI] [PubMed] [Google Scholar]
- 35.Goodman KA, Wiegner EA, Maturen KE, Zhang Z, Mo Q, Yang G, Gibbs IC, Fisher GA, Koong AC. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys. 2010;78:486–493. doi: 10.1016/j.ijrobp.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Dewas S, Bibault JE, Mirabel X, Fumagalli I, Kramar A, Jarraya H, Lacornerie T, Dewas-Vautravers C, Lartigau E. Prognostic factors affecting local control of hepatic tumors treated by Stereotactic Body Radiation Therapy. Radiat Oncol. 2012;7:166. doi: 10.1186/1748-717X-7-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibarra RA, Rojas D, Snyder L, Yao M, Fabien J, Milano M, Katz A, Goodman K, Stephans K, El-Gazzaz G, et al. Multicenter results of stereotactic body radiotherapy (SBRT) for non-resectable primary liver tumors. Acta Oncol. 2012;51:575–583. doi: 10.3109/0284186X.2011.652736. [DOI] [PubMed] [Google Scholar]
- 38.Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18:215–222. doi: 10.1016/j.semradonc.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 40.Bibault JE, Dewas S, Vautravers-Dewas C, Hollebecque A, Jarraya H, Lacornerie T, Lartigau E, Mirabel X. Stereotactic body radiation therapy for hepatocellular carcinoma: prognostic factors of local control, overall survival, and toxicity. PLoS One. 2013;8:e77472. doi: 10.1371/journal.pone.0077472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Common NCI Terminology Criteria for Adverse Events (CTCAE) v.4 (n.d.) Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 42.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Kang J, Nie Q, DU R, Zhang L, Zhang J, Li Q, Li J, Qi W. Stereotactic body radiotherapy combined with transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis. Mol Clin Oncol. 2014;2:43–50. doi: 10.3892/mco.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radu P, Groza I, Iancu C, Al Hajjar N, Andreica V, Sparchez Z. Treatment of hepatocellular carcinoma in a tertiary Romanian center. Deviations from BCLC recommendations and influence on survival rate. J Gastrointestin Liver Dis. 2013;22:291–297. [PubMed] [Google Scholar]
- 45.Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, Zanus G, Neri D, Boccagni P, Srsen N, D’Amico F, et al. Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol. 2006;44:723–731. doi: 10.1016/j.jhep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Wörns MA, Koch S, Niederle IM, Marquardt JU, Nguyen-Tat M, Gamstätter T, Schuchmann M, Schulze-Bergkamen H, Galle PR, Weinmann A. The impact of patient and tumour baseline characteristics on the overall survival of patients with advanced hepatocellular carcinoma treated with sorafenib. Dig Liver Dis. 2013;45:408–413. doi: 10.1016/j.dld.2012.10.010. [DOI] [PubMed] [Google Scholar]


